Abstract
Giardia lamblia is a protozoan parasite that is found worldwide and has both medical and veterinary importance. We applied the transcription start sequence (TSS-seq) and RNA sequence (RNA-seq) techniques to study the transcriptome of the assemblage A WB strain trophozoite. We identified 8000 transcription regions (TR) with significant transcription. Of these regions, 1881 TRs were more than 500 nucleotides upstream of an annotated ORF. Combining both techniques helped us to identify 24 ORFs that should be re-annotated and 60 new ORFs. From the 8000 TRs, we were able to identify an AT-rich consensus that includes the transcription initiation site. It is possible that transcription that was previously thought to be bidirectional is actually unidirectional.
Introduction
Giardia lamblia (also called Giardia intestinalis), a member of the family Hexamitidae, is a diplomonad parasitic protozoan that infects humans and that was discovered by Leeuwenhoek (1681). Giardia has a worldwide distribution and infects a wide range of animals in addition to humans. It is a common cause of diarrhea in both developed and developing countries. For example, Giardia is the most commonly detected human intestinal parasite in the United States [1]–[3].
The life cycle of Giardia is simple, containing only the trophozoite and cyst stages. The trophozoites are pear shaped, measuring 12–15 µm in length and 4–9 µm in width, with a ventral sucking disk, four pairs of flagella and two identical nuclei. The cysts are oval in shape, measuring 5×7–10 µm, and they contain four nuclei [1], [4]. The cysts are environmentally stable and can survive for weeks to months in water. The cyst is the naturally occurring infective stage [5]. Once reaching the duodenum, the cysts begin to excyst and give rise to trophozoites that attach themselves to the mucosa of the upper part of the small intestine. The symptoms vary widely from no symptoms to acute diarrhea. Although the exact stimulus for encystation remains unknown, trophozoites begin to encyst in the lower part of the small intestine, and cysts passed in stools are already mature and infective [6], [7].
Recurrent infection with Giardia occurs when the parasite escapes the immune response of the host due to the expression of variant surface proteins (VSP). Giardia has approximately 228 VSP genes, and all of them seem to be transcribed; however, only one is expressed at the cell membrane. The mechanism of selective expression seems to be related to RNA interference and post-transcription regulation. Switching between VSPs leads to changes in the cell wall antigens, allowing the parasite to escape the host’s immunity [8], [9].
There are seven different genetic assemblages known for Giardia [10]. Only assemblages A and B infect humans [1]. The genome of Giardia lamblia assemblage A was sequenced and published. The assemblage A genome is ∼12 Mb distributed over 5 chromosomes with nearly ∼ 77% representing open reading frames (ORF) with multiple repeats, short intergenic regions, few introns and short untranslated 5′ and 3′ regions (UTRs) [11]. The total count of genes is 9747, and 3766 of these are deprecated genes [12]. Giardia has the ability to translate mRNAs with very short 5′-UTRs, which may be as short as one nucleotide (nt) [13]. Most of the 5′-UTRs are less than 20 nt [14]. However, Knodler et al. [15] reported two long 5′-UTRs of 146 nt and 280 nt in an investigation of glucosamine-6-phosphate isomerase expression. The promoter system is thought to be a simple TATA box-like system. A study of a few selected genes suggested that several different sequences could be acting as promoters [14], [16]. Bidirectional transcription is believed to be an inherent feature of Giardia [17], leading to an abundance of sterile (non coding) antisense transcripts, which represent approximately 20% of the transcriptome [18]. New evidence suggests that Giardia might have some split genes [19], [20].
The availability of second-generation sequencers has made it easier and less expensive to study the gene expression profile of Giardia, improving our understanding of the unique features of these parasites and allowing us to identify expressed genes and verify annotated ones. Combining the oligo-capping method [21] and massive parallel sequencing technology, we established a method to collect genome-wide data for transcriptional start sites (TSS). This method is also effective to observe gene expression in a quantitative manner [22].
Another method is RNA sequencing (RNA-seq), whereby cDNA is directly sequenced, allowing for correct identification and quantitation of gene expression and detection of introns [23]. Because Giardia has a small genome and only two stages and it is an important human and animal pathogen with a widespread distribution of infection, it is a good target for such analysis. Determining which genes are transcribed and making these data available to the research community is important to understanding the encystation process and the mechanisms by which Giardia escapes immunity. Here, we applied both TSS-seq and RNA-seq methods to Giardia lamblia assemblage A trophozoites cultured in vitro. We aimed to study the relationship between transcription start sites and annotated genes, to confirm known data about Giardia, to identify possible new ORFs and to determine whether bidirectional transcription is due to conserved motifs.
Materials and Methods
Parasites and Culture
Giardia lamblia trophozoites (WB strain, ATCC 50803) of the same strain that was used to obtain the genome sequence were maintained in modified TYI-S-33 medium [24]. Mass culture was performed in 15 ml Falcon™ tubes, and the parasites were collected at a concentration of ∼1, 1×106/ml by centrifugation for at 1500 rpm for 4 minutes. Five volumes of Trizol (Invitrogen, CA, USA) were added to the parasite pellet, mixed by pipetting and then drop-frozen in liquid nitrogen. A pestle and a mortar were used to grind the Trizol pellets to ensure complete destruction of the cell walls and improve the RNA extraction yield. The RNA was then purified.
Oligo-capping and High Throughput Sequencing
A sample containing 200 µg of the obtained total RNA was subjected to oligo-capping by treatment of the RNA with bacterial alkaline phosphatase (TaKaRa, Japan) and tobacco acid pyrophosphatase (Ambion, USA), followed by ligation with RNA-oligo (5′-AAUGAUACGGCGACCACCGAGAUCUACACUCUUUCCCUACACGACGCUCUUCCGAUCUGG-3′) using RNA ligase (TaKaRa) [22]. The poly A-containing RNA was selected, and first strand cDNA was synthesised using random hexamer primers (5′-CAAGCAGAAGACGGCATACGANNNNNNC-3′) and SuperScript II (Invitrogen). Gene Amp PCR kits (PerkinElmer) were used with the PCR primers (5′-AATGATACGGCGACCACCGAG-3′) (5′-CAAGCAGAAGACGGCATACGA-3′) under the following reaction conditions: 15 cycles of 94°C for 1 min, 56°C for 1 min and 72°C for 2 min. The PCR fragments were size-fractionated and used for the sequencing reactions with the Illumina GA. In total, 36 cycles of the sequencing reactions were performed according to the manufacturer’s instructions.
Data Processing
The obtained sequences were mapped onto the Giardia lamblia ATCC50803 genomic sequencing (version 1.1 Giardiadb, http://Giardiadb.org/Giardiadb/) with the sequence alignment program Eland. Unmapped or redundantly mapped reads were removed from the data set. Reads with more than two mismatches were also removed. The reads were sorted into TSS sites, and the TSS sites were then clustered. Due to the compactness of the genome and the small intergenic distance, a size window of 10 nts was used to count the TSS sites. Overlapping transcription windows were merged and designated as transcription regions (TRs). TSS reads were considered to be in different TRs if they were separated by more than 10 bases without any TSS reads in between. The start position of a TR or the position of a TSS site with the highest sequence read number was used to evaluate the correlation between TRs and ORFs. For further details, see the supplementary material and methods, figure S1, figure S2, figure S3, and figureS4.
To calculate the background distribution, the Poisson distribution was used. The background distribution was estimated to be ≈1.3 reads for a 10-nt window. In this study, a TR was considered significant if it contained a TSS site with 5 or more reads.
RT-PCR
To verify the mapping results for the TSS reads, 20 TRs 100 nt downstream from the start site that were more than 500 nt away from any ORF and 10 TRs of 200 nt that were within 500 nt upstream an ORF were chosen to be amplified using RT-PCR. Primer-BLAST was used to design the specific primers [25]. Briefly, SuperScriptII™ (Invitrogen) was used to synthesise the cDNA, and then TaKaRa Ex Taq™ (TaKaRa) was used to amplify the targeted sequences using specific primers for 30 cycles. The primer list is provided in the table S1.
RNA-Seq
To verify the TSS reads and confirm their relationships to ORFs, high throughput RNA-seq was performed using TruSeq™ (Illumina) according to the manufacturer’s protocol. Briefly, 1 µg of total RNA was purified and fragmented and then used to synthesise the 1st strand cDNA, which was followed by synthesis of the 2nd strand. The ends were repaired, and the 3′ ends were adenylated. The fragments were ligated to adapters and amplified for 15 cycles.
The RNA-seq tags were mapped to the genome. Due to the presence of multiple repeats and the small genome size, Integrative Genomics Viewer (IGV) [26], [27] was used to visually evaluate the expression and verify the TSS reads. Any area of transcription that was not related to a gene was evaluated using NCBI BLAST® [28].
The metagenomic ORF finding tool Orphelia was used to read multiple ORFs in a 700 base pair model to identify ORFs when no ORFs were found within the 500-nt downstream TR [29], [30].
WebLogo, a web-based application that can be used to draw logos for conserved sequences in relation to their positions, was used to detect and draw a sequence consensus [31], [32].
Results and Discussion
For the first time, we have applied the TSS-seq technique to analyse the TSSs of trophozoites of the assemblage A strain of Giardia lamblia, WB clone, ATCC50803. A total of 6,343,253 34-base sequences was generated and mapped to the same strain as that used for genome version 1.1 (http://Giardiadb.org/common/downloads/release-1.1/Glamblia), allowing a 2-base mismatch. Reads were deposited at DDBJ Sequence Read Archive, accession number DRA001089.We obtained 2,600,245 uniquely mapped reads that were sorted into 404,331 TSS sites (the details of the mapping result and sorting are shown in table S2). These reads were clustered into 63,795 transcription regions (TRs) using a 10-base window, of which 8000 TRs were expressed at levels that were significantly higher than the background (see Materials and Methods).
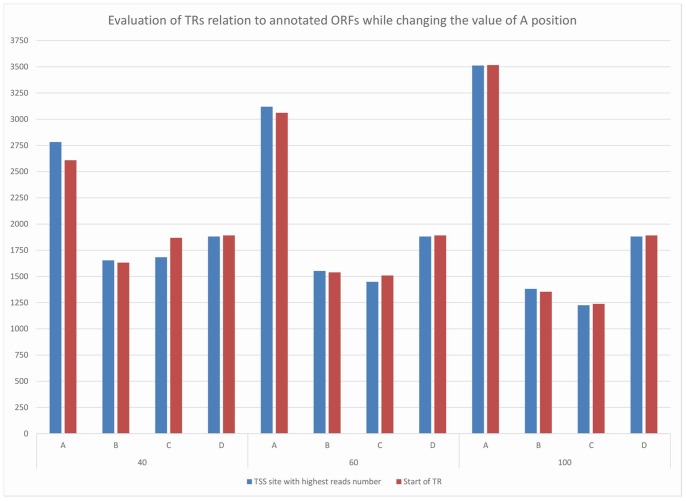
Next, we evaluated the correlation between the TRs and the ORFs, using the gene file from version 2.1 (http://Giardiadb.org/common/downloads/release-2.1/GintestinalisAssemblageA/), which contains 9747 ORFs. Of these, 3766 ORFs are deprecated. According to the distance from the first ATG, the TRs were categorised into four categories, A, B, C and D (figure 1). In agreement with the previous findings that the majority of the 5′-UTRs are short [14], we found that 2516 (31.5%) TRs out of 8000 are present within 40 nt of the start codons of 2448 of the ORFs. Increasing the distance from 40 nt to 60 nt and 100 nt lead to a gradual increase of 3–5.8% in the number of TRs counted at each step (the details are shown in table S3). We used the position of the TSS site with the highest read count as the reference position of the TRs for this analysis. Using the start position of a TR as a reference position, we obtained a slightly lower number of correlated TRs (2516 TRs to 2448 ORFs using the TSS site with the highest reads compared with 2336 TRs to 2285 ORFs) for TRs located 40 nt upstream. This difference disappeared at a 100-nt distance. Only 1918 (24%) of the TRs were located within ORFs. Of these, 536 were localised within the first 100 nt of the ORF. For the transcripts from these TRs, the translation start site was at the 2nd start codon or later.
Figure 1. Evaluation of the correlation between TRs and genes.
A: Perfectly positioned if the distance between the TR and the start codon is ±40, ±60 or ±100 nt. B: The TR is intragenic. C: If the TR was located between 500 nt up-stream of the first codon and distance A, it was considered as possibly related to the gene. D: If the TR was located more than 500 nt up-stream any annotated ORF.
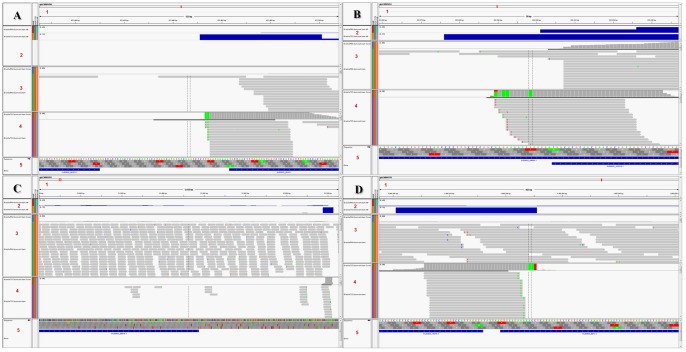
Interestingly, 3106 (38.8%) TRs were located more than 100 nt upstream of the 1st ATG, and 1881 (23.5%) of these were located more than 500 nt upstream (position D category). The transcripts from these further upstream TRs could correspond to transcripts with long 5′-UTRs or sterile polyadenylated transcripts. Elmendorf et al. [18], estimated that 20% of cDNA libraries are sterile polyadenylated transcripts. These transcripts could have a regulatory importance, either by directly interfering with the transcription of other genes or by initiating the RNAi pathways [8], [9]. Combining TSS analysis with RNA-seq (see below), we were able to identify 7 ORFs with long 5′-UTRs. One of them, GL50803_29595, had a 5′-UTR of 406 bases and was highly expressed in the RNA-seq (figure 2 B). The data are shown in table (1).
Figure 2. Combining TSS and RNA-seq with the use of IGV tool.
A: GL50803_23497 (deprecated gene) has a closely positioned TR and is highly expressed in RNA-seq. B: A long 5`-UTR is observed in GL50803_29595, which is highly expressed in RNA-seq. *Panel formation: 1- Scaffold browser scale. 2- Mapped RNA-seq and TSS-seq read counts in relation to the scaffold. 3- Mapped RNA-seq read distribution. 4- Mapped TSS-seq read distribution. 5- Annotated genes (including deprecated ones).
Table 1. List of genes with long 5′-UTR.
| Gene ID | 5′-UTR length | Location |
| GL50803_29096 | 226-nt long | CH991769∶343,205-343429 |
| GL50803_29595 | 406-nt long | CH991771∶260,060-260464 |
| GL50803_27713 | 216-nt long | CH991771∶260,060-260,751 |
| GL50803_28770 | 151-nt long | CH991768∶89,975-90,125 |
| GL50803_31608 | 212-nt long | CH991768∶676,797-677,008 |
| GL50803_15887 | 178-nt long | CH991782∶282,308-282,485 |
| GL50803_32766 | 178-nt long | CH991814∶234,320-234514 |
We used RT-PCR to verify some of the TSS data. Twenty targets were chosen from TRs located more than 500 nt upstream of annotated ORFs (Position D), and 10 targets were selected from the TRs that were within 500 nt of ORFs (Position A, B or C). In total, 17 out of the 20 position D category TRs and 5 out of 10 of the position A, B or C category TRs were amplified, as shown in figure 3. The finding that 17 targets among the position D category TRs were amplified could be an indicator that some ORFs are present downstream of TRs that are located more than 500 nt upstream of annotated ORFs. It was necessary to investigate whether these tags represent sterile antisense tags or whether they could represent ORFs that were missed during the annotation of genome.
Figure 3. Results of RT-PCR for 30 targets.
All samples were run in duplicate (template added and template free). For further details about the position and size of the target, see supplemental material and methods.
We used Orphelia [29], [30] to check the presence of the ORFs near such TRs. We took 3000 bases of the genome sequence downstream from the start of TRs and examined the presence of ORFs that are 300-nt or more in length. At the same time, we decided to conduct a full-scale RNA-seq to determine whether such ORFs can be expressed or not. To evaluate the RNA-seq data, we first looked at 30 TRs for which we performed RT-PCR. We were able to detect the presence of some transcription for 29 of these 30 TRs. Although we must use caution when interpreting RNA-seq data because they do not have strand specificity (TSS-seq data are strand-specific), RNA-seq can sometimes be more sensitive than RT-PCR. Of the 20 position D category TRs, 19 appeared to have new downstream ORFs that are 100 nt long or more (RNA-seq supports the presence of these transcripts). Two of the TRs had conserved domains and are described below (as re-annotated genes).
The Giardia genome contains 9747 ORFs, of which 3766 ORFs are deprecated. We found 4187 ORFs with TRs located from 500 nt upstream of the start codon to the end of ORF. Among them, 554 are deprecated ORFs. A total of 111 of the 575 deprecated ORFs had TRs within 100 nt upstream of the start codon. Thus, these 554 ORFs may be re-considered, or only the 369 ORFs that do not completely overlap with non-deprecated genes. From the RNA-seq data, we could evaluate the expression of 363 of the 575 deprecated ORFs. An example is the deprecated gene GL50803_23497 shown in figure 2 A.
We have identified a total of 84 ORFs that are either novel or that need to be re-annotated by combining TSS and RNA-seq data; omitting ORFs that had no or low similarity or no or low expression. We have examined all of these ORFs using both protein BLAST and nucleotide BLAST. A total of 24 ORFs should be re-annotated. These ORFs have TRs near the proposed start site, and they have RNA-seq tags covering the new annotated regions. Another 60 ORFs are new and were not detected during annotation of the genome. Among these, 13 ORFs belong to the variant surface protein (VSP) family; 5 are re-annotated ORFs and 8 are new ORFs that were expressed according to both TSS and RNA-seq. Another 6 ORFs with Ankyrin-like conserved domains were also found. Using BLAST®, we identified similar genes in either the same 50803 strain or in other Giardia strains that have been sequenced (Table 2).
Table 2. List of new genes and genes to be re-annotated.
| Position | Blast result | Conclusion |
| CH991763∶266007-267494_R | P23-like domain, similar to GL50581_3538 figGiardiaintestinalis ATCC 50581] | new gene, similar to other assemblage |
| CH991763∶264707-265966_R | IFT complex B, GL p15, re-annotate GL50803_40995 | gene to be re-annotated |
| CH991817∶18,017-18,463 | Hypothetical protein, re-annotate GL50803_25713 | gene to be re-annotated |
| CH991814∶252,697-256,896 | Hypothetical protein GL P15/kinase protein | new gene/conserved domain |
| CH991803∶2702-4543 | VSP, similar to GL50803_114065 | gene repeat/conserved domain, FU-like and VSP domains,re-annotate GL50803_102540 |
| CH991798∶30,210-30,809 | ribosomal protein S11 | re-annotate (GL50803_14827) 199AA instead of 154, similarto other assemblage |
| CH991782∶26,231-28,138_R | VSP, conserved domain | 635 instead of 195 AA, original gene GL50803-101380,gene to be re-annotated |
| CH991779∶250938-252914 -R | VSP, conserved domain | gene repeat |
| CH991779∶569,674-571,152 | VSP, conserved domain | new gene |
| CH991779∶1,223,042-1,223,698 | Hypothetical protein, GL P15 | new gene, similar to other assemblage |
| CH991779∶1,425,747-1,427,552 | VSP, conserved domain | gene to be re-annotated, re-annotate GL50803_40630 |
| CH991785∶11,532-11,867_R | SORL conserved domain, hypothetical protein GL P15 | new gene |
| CH991776∶59,721-59,930 | ribosomal S30 conserved domain | new gene |
| CH991771∶171,536-171,874_R(112AA) | similar to hypothetical protein GL50803_32738 | gene repeat |
| CH991769∶78,752-81,292 | hypothetical protein, two conserved domains | new gene, similar to other assemblage GL P15 |
| CH991769∶412,442-413,248 | similar to reverse transcriptase | gene repeat |
| CH991769∶624,472-624,627 | 50S ribosomal protein L39e | new gene |
| CH991769∶770,102-771,508 | hypothetical protein | similar gene/gene repeat, similar to other assemblageGL P15/hypothetical GL50803_17273 |
| CH991768∶744,494-745,936 | Hypothetical protein | new gene similar to similar to other assemblageGL P15 |
| CH991768∶1,281,692-1,282,111-R | GL P15, Ribosomal protein S19e domain conserved | new gene-reverse strand, CH991768∶1,281,692-1,282,111-R |
| CH991764∶147,549-148,025_R | partially similar to hypothetical protein GL50803_20672 | new gene |
| CH991764∶148,024-149,931 | VSP conserved domain, | new gene |
| ,CH991762∶115,657-116,463 | ANK conserved domain | new gene/gene repeat, similar to GL P15, Ser/Thrprotein kinase |
| CH991761∶101,085-102,872 | VSP conserved domain, similar GL50803_116477 | gene repeat, re-annotate GL50803_135831 |
| CH991761∶113,307-113,858 | VSP conserved domain | gene repeat |
| CH991761∶113,432-113,770_R | similar to hypothetical protein GL50803_105806 | gene repeat |
| CH991761∶295,809-301,103 | Hypothetical protein, GL P15, conserved domain WD40 | new gene, partially similar to Hypothetical protein(GL50803_113673) |
| CH991763∶4,689-7,532_R | conserved domain, Ankyrin-like and protein kinase | gene repeat, similar to GL50803_113094 |
| CH991763∶689121-689569 | partially similar to GL50803_101496 | partial gene repeat |
| CH991763∶688,749-688,942_R | partially similar to GL50803_137676, kinase | partial gene repeat |
| CH991767∶1667698-1667877 | conserved domain, Ferredoxin Fd1, Fd2 | partial gene repeat |
| CH991761∶301,967-303,142 | conserved domain, NEK, kinase-like | new gene/gene repeat |
| CH991761∶302,965-305,298 | ANK conserved domain, similar to kinase | new gene |
| CH991763∶1395469-1397541_R | VSP conserved domain, similar to High cysteine membraneprotein Group 1 (GL50803_91707) | new gene |
| CH991767∶885323-886138 | VSP domain, similar to P15 | partial gene repeat |
| CH991767∶1127974-1130037_R | VSP domain, similar to P15 | new gene |
| CH991767∶1130397-1135277 | hypothetical protein, similar to P15 | new gene |
| CH991767∶1135382-1140265 | conserved domains, chromosome segregation protein SMC, similar to Axoneme-associated protein GASP-180 | gene repeat |
| CH991767∶1140362-1145860 | re-annotate GL50803_32999 to be similar to P15 (GLP15_1881) | many conserved domains |
| CH991767∶1146036-1147784 | conserved ANK domain, Coiled-coil protein [Giardia intestinalis ATCC 50581], Hypothetical protein GL50803_41212 | new gene |
| CH991767∶1696020-1696778 | conserved ANK domain and zinc finger, similar to GL50803_113284 hypothetical protein and Protein 21.1 P15 | gene repeat |
| CH991793∶23497-23754 | ORF with low similarity | new gene, well expressed in RNA-seq |
| CH991763∶1,306,665-1,307,180 | ORF with low similarity | new gene, expressed in RNA-seq |
| CH991776∶157233-158693 | conserved ANK and kinase domains, partially similar to?NEK (GL50803_93221) | new gene/gene repeat |
| CH991762∶387,382-387,645 | partially similar to hypothetical protein GL50803_38965 | partial gene repeat |
| CH991763∶692,405-692,956 | partially similar to GL50803_31921, | new gene |
| CH991763∶692571-693002 | partially similar to hypothetical protein GL50803_5692 | new gene |
| CH991767∶340089-340346 | mostly retrotransposon | gene repeat/new gene |
| CH991767∶436,937-437,701_R | similar to VSP,(GL50803_111732) | gene repeat |
| CH991767∶435261-437231 | similar to high cysteine membrane protein EGF-like (GL50803_114626) | gene repeat |
| CH991782∶818,861-820,612 | similar to P15 and 50581 strains | re-annotate GL50803_40224 |
| CH991761∶20575-22203 | similar to P15 and 50581 strains | re-annotate GL50803_96616 |
| CH991767∶1,732,248-1,734,773 | similar to P15 and 50581 strains | re-annotate GL50803_39210 |
| CH991779∶262026-264188 | similar to P15 and 50581 strains | re-annotate GL50803_35276 |
| CH991776∶21991-23994 | similar to P15 and 50581 strains | re-annotate GL50803_34684 |
| CH991779∶1223042-1223698 | new hypothetical protein, conserved among 3 assemblages | new gene, expressed in in RNA-seq |
| CH991769∶937870-939393 | new hypothetical protein, conserved among 3 assemblages, conserved Ribophorin I domain | new gene, expressed in in RNA-seq |
| CH991814∶199061-199591 | similar to GL50803_114246, GTP-binding protein, putative | partial gene repeat |
| CH991779∶1,155,366-1,156,079_R | similar to Rossmann-fold protein [Giardia lamblia P15],conserved putative domain | new gene |
| CH991769∶2,224-3,885_R | similar to hypothetical protein GLP15_2551 | new gene |
| CH991769∶953,848-955,386_R | similar to P15 hypothetical protein | re-annotate GL50803_7035 |
| CH991763∶1385753-1387552_R | similar to hypothetical protein in P15 and 50581 strains | new gene |
| CH991779∶681,867-683,660_R | similar to P15 and 50581 strains | re-annotate GL50803_2822 |
| CH991776∶278,076-279,610_R | PTZ00382 conserved domain | re-annotate GL50803_97233, well expressed in RNA-seq |
| CH991769∶77,021-78,700 | new hypothetical protein, similar to P15 and 50581 | new gene |
| CH991769∶56,305-56,718_R | new hypothetical protein, similar to P15 and 50581 | new gene |
| CH991767∶707,305-707,724_R | new hypothetical protein, similar to hypothetical protein GLP15_3559 | new gene |
| CH991769∶334626-334943_R | Gene repeat, Giardia lamblia ATCC 50803 Pam18p (GL50803_300001) | gene repeat |
| CH991767∶707,305.707,724_R | similar to hypothetical protein GLP15_3559 | new gene |
| CH991776∶310,552-313,605_R | new hypothetical protein, similar to P15 and 50581 | new gene |
| CH991814∶296,825-303,154_R | new hypothetical protein, similar to P15 and 50581 | new gene |
| CH991769∶494114-494923 | new hypothetical protein, similar to P15 and 50581 | new gene |
| CH991814∶275,394-281,945 | similar to Kinase [Giardia lamblia P15], multiple conserved domain | new gene |
| CH991779∶986627-987556_R | similar to Kinase GL50803_101307 and GL50803_86934 | gene repeat |
| CH991793∶39014-40039_R | new hypothetical protein, similar to P15 and 50581 | new gene |
| CH991763∶195,962-199,271_R | similar to P15 and 50581 strains | re-annotate GL50803_32861 |
| CH991780∶48,596-52,006_R | similar to P15 and 50581 strains | re-annotate GL50803_41369 |
| CH991763∶487,379-487,747 | new hypothetical protein, similar to P15 and 50581 | new gene |
| CH991767∶1652396-1654378 | similar to P15 and 50581 strains | re-annotate GL50803_41311 |
| CH991771∶3728-4342 | similar to GLP15_4099 | re-annotate GL50803_40244 |
| CH991767∶473391-475715_R | similar toGLP15_5080 and GL50581_209 | re-annotate GL50803_36426 |
| CH991769∶546414-550226_R | similar to GLP15_5033 and GL50581_4447 | re-annotate GL50803_103205 |
| CH991776∶41095-43620_R | similar to GLP15_3901 | re-annotate GL50803_39904 |
| CH991768∶596,759-597,880_R | similar to P15 and 50581 strains | re-annotate GL50803_30448 |
Teodorovic et al. [17] estimated that 50% of transcription loci had bidirectional activity with no correlation between the sense and anti-sense copy numbers. The high frequency of the expected bidirectional transcription suggests that a certain definite bidirectional promoter element is present.
We evaluated our results to detect the bidirectional relationships of the 8000 significantly expressed TRs to the entire set of 63795 TRs. With up to 300 nt difference, we found total of 3686 bidirectional pairs of TRs. Of those pairs, 1175 pairs (2350 TRs, 29.4%) had significant expression in both directions, while 2521 pairs had significant expression in one direction and insignificant expression to the opposite direction. We attempted to determine whether there is any conserved sequence (consensus) for the highly expressed bidirectional TRs by examining the sequences 150 nt upstream and 150 nt downstream from the nucleotide positioned midway between the start sites of each pair. However, we were not able to find any conserved sequences for a promoter region.
As the 5`-UTR is very short, this result raised the possibility that there is no real bidirectional transcriptional consensus, but that unidirectional transcription events occur near each other. Thus, we decided to examined the presence of a consensus for the entire 8000 TRs. The use of motif search tools was unsuccessful as Giardia lack motifs patterns seen in other eukaryotes so we decide to use alignment tools to find any conserved sequences. Using the start position as a land mark, we failed to find any consensus within 100 nt upstream of the start position of the TR. We thought that the transcription start site with highest copy reads might be the most effective point of reference. We examined 50 nt up stream and 50 nt downstream from that position and identified an AT-rich consensus, which is shown in figure 4. This consensus occurs from −5 nt to +5 nt relative to the position with the highest TSS read number. An A in middle of the sequence should be the major transcription initiation site. This is the first such consensus for transcription initiation to be identified in the genome of Giardia. This finding demonstrates the importance of precise mapping of TSS.
Figure 4. Conserved consensus for the transcription initiation site in Giardia.

We created a weight matrix for this consensus and evaluated the TR regions again. We found that only 565 TRs lack this consensus. Table 3 shows the top 15 repeated variants of the consensus in Giardia. A variant of this consensus was predicted by Holberton and Marshall [33] while studying the promoters of cytoskeleton genes. They suggested that the transcription initiation site sequence is composed of nine bases (AATTAAAAA) and is associated with two other sequences of (CAATTT) and (CAAAAA,A/T,T/C,AGA,G/T,TC,C/T,GAA) that they detected using two algorithms and a weight matrix created for seven genes. Additionally, a variant of that consensus (ATTTTAAAAT) was among the sequences suggested by Yee et al., [34] who identified this sequence as the major transcription start site for the glutamate dehydrogenase gene. The authors demonstrated that altering the bases or the order of the bases will lead to severe down regulation of expression.
Table 3. Frequent transcription-initiation site consensus variants.
| ATTTTAAAATG | 21 |
| ATTTTAAAAAT | 17 |
| AATTTAAAATG | 16 |
| AAAATAAAAAT | 15 |
| AAAATAAAATG | 13 |
| AAATTAAAAAA | 13 |
| AAATTAAAAAT | 13 |
| AAATTAAAATG | 13 |
| AATTTAAAAAT | 13 |
| CTTTTAAAAAT | 13 |
| AAAATAAAAAG | 12 |
| TTTTTAAAATG | 12 |
| AATTCAAAAAA | 11 |
| ATTTTAAAAAA | 11 |
| AATTTAAAAAA | 10 |
| ATTTCAAAAAA | 10 |
| ATTTTAATTTT | 10 |
Although many researchers have predicted some sequences such as (CAAT) or (AG) as conserved motifs within 40-100 nt upstream of the transcription initiation site, we did not find any other conserved consensus within 150 nt upstream or downstream of the TSSs with highest reads. Furthermore, we examined up to 300 nt upstream of the TSSs with the highest reads for 565 TRs that lack the transcription initiation consensus. We did not detect any conserved consensus for these 565 TRs. We did find this consensus variant among the sequences reported by Teodorovic et al., [17] at the loci that have been suggested to be bidirectional. Those loci may have multiple transcription initiators rather than one bidirectional promoter.
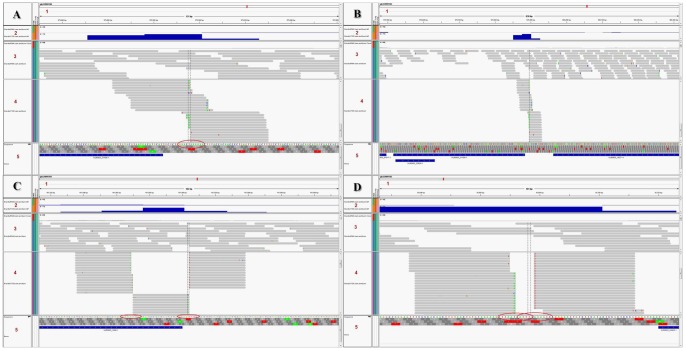
As the consensus is somewhat symmetrical, we investigated the possibility of true bidirectional transcription, allowing a ±5-nt difference. We found that 928 pairs of TRs (1856 TRs, 23.2%) were bi-directionally significantly expressed, while the antisense transcripts of 1195 TRs were insignificantly expressed. The occurrence of bidirectional transcription was not related to the symmetry of the consensus, as it is in some cases (figure 5 A); the symmetry of consensus was conserved with no bidirectional transcription. Other variants of the consensus were observed to have only unidirectional transcription (figure 5 B, C & D). In some cases, bidirectional transcription occurred at the same nucleotide within the symmetrical consensus between two adjacent genes (figure 6 A & B). In other cases, bidirectional transcription occurred due to the presence of two nearby transcription initiation sites (figure 6 C) or due to two overlapping transcription initiation sites (figure 6 D).
Figure 5. Transcription initiation site with only unidirectional transcription.
A: A nearly symmetrical consensus showing only unidirectional transcription. B, C & D: A variant of the consensus showing only unidirectional transcription with the presence of nearby genes. *Panel formation: 1- Scaffold browser scale. 2- Mapped RNA-seq and TSS-seq read counts in relation to the scaffold. 3- Mapped RNA-seq read distribution. 4- Mapped TSS-seq read distribution. 5- Annotated genes (including deprecated ones).
Figure 6. Transcription initiation site with bidirectional transcription.
A & B: Bidirectional transcription starting at the same nucleotide position at different distances from nearby genes. C: Bidirectional transcription occurring at the same nucleotide position as one starting at another close transcription initiation site. D: Bidirectional transcription occurring at two overlapping transcription initiation sites. *Red oval mark was used to mark the consensus. **Panel formation: 1- Scaffold browser scale. 2- Mapped RNA-seq and TSS-seq read counts in relation to the scaffold. 3- Mapped RNA-seq read distribution. 4- Mapped TSS-seq read distribution. 5- Annotated genes (including deprecated ones).
Combining the TSS-seq and RNA-seq techniques was a powerful approach for identifying new genes, confirming or re-annotating known genes and identifying unusually long 5`-UTRs. TSS-seq allowed us to identify the correct transcription sites, which helped us to find the transcription initiation consensus in Giardia. We failed to identify any other motifs in Giardia. This raises the question of how transcription starts in other places that lack the transcription initiation consensus. Further work is needed to address this question. The presence of the transcription initiation consensus for the majority of the genes shows how simple yet efficient the transcription mechanism of Giardia is.
Supporting Information
Mapping of TSS sequence copy and TR clustering.
(TIF)
How to measure Distance between Transcription regions (TR) and open reading frames (ORFs).
(TIF)
Relation between TR and ORFs if the TR overlap an ORF. *If ORF1 and ORF2 is in the same orientation: TR3 is Upstream-TR if ATG of ORF1 is nearer than ATG of ORF2. **If ORF1 and ORF2 is in the opposite orientation: TR4 is Always Upstream-TR irrespective nearness of ATG to ORF1 or ORF2
(TIF)
How to evaluate positions of TRs in relation to ORFs.
(TIF)
List of targets and primers used in RT-PCR.
(DOCX)
Statistics of TSS reads.
(DOCX)
Details of transcription regions (TRs) position in relation to annotated open reading frames(ORFs).
(DOCX)
Acknowledgments
We are grateful to Ms. Kazumi Abe for her expertise in TSS-seq and RNA-seq and Ms. Terumi Horiuchi for data analysis.
Funding Statement
Mohammed E. M. Tolba was supported by The Tokyo Biochemical Research Foundation (TBRF) for postdoctoral fellowship. This work was in part supported by KAKENHI (Grant-in-Aid for Scientific Research) in the Priority Area of “Genome Science” from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Adam R (2001) Biology of Giardia lamblia. Clinical microbiology reviews 14: 447–475. Available: http://cmr.asm.org/content/14/3/447.short. Accessed 11 July 2012. [DOI] [PMC free article] [PubMed]
- 2.Thompson RCA, Monis P (2012) Giardia–from genome to proteome. Advances in parasitology 78: 57–95. Available: http://www.ncbi.nlm.nih.gov/pubmed/22520441. Accessed 12 November 2012. [DOI] [PubMed]
- 3.Yoder JS, Gargano JW, Wallace RM, Beach MJ (2012) Giardiasis surveillance–United States, 2009–2010. Morbidity and mortality weekly report Surveillance summaries (Washington, DC: 2002) 61: 13–23. Available: http://www.ncbi.nlm.nih.gov/pubmed/22951494. Accessed 22 March 2013. [PubMed]
- 4.Erlandsen SL, Macechko PT, Van Keulen H, Jarroll EL (n.d.) Formation of the Giardia cyst wall: studies on extracellular assembly using immunogold labeling and high resolution field emission SEM. The Journal of eukaryotic microbiology 43: 416–429. Available: http://www.ncbi.nlm.nih.gov/pubmed/8822813. Accessed 2 March 2013. [DOI] [PubMed]
- 5.Plutzer J, Ongerth J, Karanis P (2010) Giardia taxonomy, phylogeny and epidemiology: Facts and open questions. International journal of hygiene and environmental health 213: 321–333. Available: http://www.ncbi.nlm.nih.gov/pubmed/20619729. Accessed 16 August 2011. [DOI] [PubMed]
- 6. Svärd SG, Hagblom P, Palm JED (2003) Giardia lamblia – a model organism for eukaryotic cell differentiation. FEMS microbiology letters 218: 3–7 Available: http://www.ncbi.nlm.nih.gov/pubmed/12583890. [DOI] [PubMed] [Google Scholar]
- 7.Ali SA, Hill DR (2003) Giardia intestinalis. Current opinion in infectious diseases 16: 453–460. Available: http://www.sciencedirect.com/science/article/pii/0020751989900064. Accessed 13 September 2011. [DOI] [PubMed]
- 8.Prucca CG, Slavin I, Quiroga R, Elías E V, Rivero FD, et al. (2008) Antigenic variation in Giardia lamblia is regulated by RNA interference. Nature 456: 750–754. Available: http://www.ncbi.nlm.nih.gov/pubmed/19079052. Accessed 18 July 2011. [DOI] [PubMed]
- 9.Rivero MR, Kulakova L, Touz MC (2010) Long double-stranded RNA produces specific gene downregulation in Giardia lamblia. The Journal of parasitology 96: 815–819. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2924914&tool=pmcentrez&rendertype=abstract. Accessed 22 November 2011. [DOI] [PMC free article] [PubMed]
- 10. Monis PT, Andrews RH, Mayrhofer G, Ey PL (1999) Molecular systematics of the parasitic protozoan Giardia intestinalis. Molecular biology and evolution 16: 1135–1144 Available: http://www.ncbi.nlm.nih.gov/pubmed/10486969. [DOI] [PubMed] [Google Scholar]
- 11.Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, et al. (2007) Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science (New York, NY) 317: 1921–1926. Available: http://www.ncbi.nlm.nih.gov/pubmed/17901334. Accessed 4 March 2013. [DOI] [PubMed]
- 12.Aurrecoechea C, Brestelli J, Brunk BP, Carlton JM, Dommer J, et al. (2009) GiardiaDB and TrichDB: integrated genomic resources for the eukaryotic protist pathogens Giardia lamblia and Trichomonas vaginalis. Nucleic acids research 37: D526–30. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2686445&tool=pmcentrez&rendertype=abstract. Accessed 2 March 2013. [DOI] [PMC free article] [PubMed]
- 13.Li L, Wang CC (2004) Capped mRNA with a single nucleotide leader is optimally translated in a primitive eukaryote, Giardia lamblia. The Journal of biological chemistry 279: 14656–14664. Available: http://www.ncbi.nlm.nih.gov/pubmed/14722094. Accessed 22 November 2011. [DOI] [PubMed]
- 14.Adam RD (2000) The Giardia lamblia genome. International Journal for Parasitology 30: 475–484. Available: 10.1016/S0020-7519(99)00191-5. Accessed 4 March 2013. [DOI] [PubMed]
- 15. Knodler L a, Svärd SG, Silberman JD, Davids BJ, Gillin FD (1999) Developmental gene regulation in Giardia lamblia: first evidence for an encystation-specific promoter and differential 5′ mRNA processing. Molecular microbiology 34: 327–340 Available: http://www.ncbi.nlm.nih.gov/pubmed/10564476. [DOI] [PubMed] [Google Scholar]
- 16.Yee J, Tang A, Lau W-L, Ritter H, Delport D, et al. (2007) Core histone genes of Giardia intestinalis: genomic organization, promoter structure, and expression. BMC molecular biology 8: 26. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1872034&tool=pmcentrez&rendertype=abstract. Accessed 11 August 2011. [DOI] [PMC free article] [PubMed]
- 17.Teodorovic S, Walls C, Elmendorf H (2007) Bidirectional transcription is an inherent feature of Giardia lamblia promoters and contributes to an abundance of sterile antisense transcripts throughout the genome. Nucleic acids research 35: 2544–2553. Available: http://nar.oxfordjournals.org/content/35/8/2544.short. Accessed 2 March 2013. [DOI] [PMC free article] [PubMed]
- 18.Elmendorf HG, Singer SM, Nash TE (2001) The abundance of sterile transcripts in Giardia lamblia. Nucleic acids research 29: 4674–4683. Available: http://nar.oxfordjournals.org/content/29/22/4674.short. Accessed 22 November 2011. [DOI] [PMC free article] [PubMed]
- 19.Kamikawa R, Inagaki Y, Tokoro M, Roger AJAJ, Hashimoto T (2011) Split introns in the genome of Giardia intestinalis are excised by spliceosome-mediated trans-splicing. Current biology: CB 21: 311–315. Available: http://www.sciencedirect.com/science/article/pii/S0960982211000480. Accessed 22 November 2011. [DOI] [PubMed]
- 20.Blumenthal T (2011) Split genes: another surprise from Giardia. Current biology: CB 21: R162–3. Available: http://www.ncbi.nlm.nih.gov/pubmed/21334298. Accessed 4 March 2013. [DOI] [PubMed]
- 21.Kazuo M, Sumio S (1994) Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides. Gene 138: 171–174. Available: http://www.sciencedirect.com/science/article/pii/0378111994908028. Accessed 2 April 2013. [DOI] [PubMed]
- 22.Tsuchihara K, Suzuki Y, Wakaguri H, Irie T, Tanimoto K, et al. (2009) Massive transcriptional start site analysis of human genes in hypoxia cells. Nucleic acids research 37: 2249–2263. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2673422&tool=pmcentrez&rendertype=abstract. Accessed 5 April 2012. [DOI] [PMC free article] [PubMed]
- 23.Nagalakshmi U, Waern K, Snyder M (2010) RNA-Seq: a method for comprehensive transcriptome analysis. Current protocols in molecular biology/edited by Frederick M Ausubel. [et al] Chapter 4: Unit 4.11.1–13. Available: http://www.ncbi.nlm.nih.gov/pubmed/20069539. Accessed 6 March 2012. [DOI] [PubMed]
- 24.Keister DI (1983) Axenic culture of Giardia lamblia in TYI-S-33 with bile medium supplemented. [DOI] [PubMed]
- 25.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, et al. (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC bioinformatics 13: 134. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3412702&tool=pmcentrez&rendertype=abstract. Accessed 3 Mar 2013. [DOI] [PMC free article] [PubMed]
- 26.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, et al. (2011) Integrative genomics viewer. Nature biotechnology 29: 24–26. Available: 10.1038/nbt.1754. Accessed 2 Mar 2013. [DOI] [PMC free article] [PubMed]
- 27.Thorvaldsdóttir H, Robinson JT, Mesirov JP (2012) Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Briefings in bioinformatics: bbs017–. Available: http://bib.oxfordjournals.org/content/early/2012/04/18/bib.bbs017.full?keytype=ref& ijkey = qTgjFwbRBAzRZWC. Accessed 28 Feb 2013. [DOI] [PMC free article] [PubMed]
- 28.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, et al. (2008) NCBI BLAST: a better web interface. Nucleic acids research 36: W5–9. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2447716&tool=pmcentrez&rendertype=abstract. Accessed 3 Mar 2013. [DOI] [PMC free article] [PubMed]
- 29.Hoff KJ, Tech M, Lingner T, Daniel R, Morgenstern B, et al. (2008) Gene prediction in metagenomic fragments: a large scale machine learning approach. BMC bioinformatics 9: 217. Available: http://www.biomedcentral.com/1471-2105/9/217. Accessed 10 Mar 2013. [DOI] [PMC free article] [PubMed]
- 30.Hoff KJ, Lingner T, Meinicke P, Tech M (2009) Orphelia: predicting genes in metagenomic sequencing reads. Nucleic acids research 37: W101–5. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2703946&tool=pmcentrez&rendertype=abstract. Accessed 10 Mar 2013. [DOI] [PMC free article] [PubMed]
- 31.Crooks GE, Hon G, Chandonia J, Brenner SE (2004) WebLogo: A Sequence Logo Generator: 1188–1190. 10.1101/gr.849004.1 [DOI] [PMC free article] [PubMed]
- 32.Schneider TD, Stephens RM (1990) Sequence logos: a new way to display consensus sequences. Nucleic acids research 18: 6097–6100. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=332411&tool=pmcentrez&rendertype=abstract. Accessed 4 Mar 2013. [DOI] [PMC free article] [PubMed]
- 33. Holberton D V, Marshall J (1995) Analysis of consensus sequence patterns in cytoskeleton gene promoters. Analysis 23: 2945–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yee J (2000) Transcriptional Analysis of the Glutamate Dehydrogenase Gene in the Primitive Eukaryote, Giardia lamblia. IDENTIFICATION OF A PRIMORDIAL GENE PROMOTER. Journal of Biological Chemistry 275: 11432–11439. Available: http://www.jbc.org/content/275/15/11432.full. Accessed 3 Mar 2013. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mapping of TSS sequence copy and TR clustering.
(TIF)
How to measure Distance between Transcription regions (TR) and open reading frames (ORFs).
(TIF)
Relation between TR and ORFs if the TR overlap an ORF. *If ORF1 and ORF2 is in the same orientation: TR3 is Upstream-TR if ATG of ORF1 is nearer than ATG of ORF2. **If ORF1 and ORF2 is in the opposite orientation: TR4 is Always Upstream-TR irrespective nearness of ATG to ORF1 or ORF2
(TIF)
How to evaluate positions of TRs in relation to ORFs.
(TIF)
List of targets and primers used in RT-PCR.
(DOCX)
Statistics of TSS reads.
(DOCX)
Details of transcription regions (TRs) position in relation to annotated open reading frames(ORFs).
(DOCX)