Albert de la Chapelle
Victories
More than 1,000 disease genes have been cloned and their mutations characterized, including those that account for the most common Mendelian disorders. While this represents one glorious consequence of the global genome initiatives, it also is a mere beginning.
Challenges
Clinical benefit from gene discoveries has so far been limited. With the first great gene discoveries, such as the cloning of the hemophilia A gene and the dystrophin gene, large-scale germline molecular diagnostics was born, and benefits to affected families were immediate and substantial. However, did we imagine that in the year 2002 there would still be no specific, gene-related therapy, let alone cure? I did not.
Disappointments
The a priori expectation was that once the gene defect was pinpointed, a drug or strategy would be designed that overcomes the defect. Let us face the fact that this has not yet occurred (with the notable exception of the recent Gleevec story).
In the absence of breakthroughs that are directly therapeutic, positive development has occurred as a result of improved diagnostics. For instance, early and precise germline mutation detection in multiple endocrine neoplasia saves lives by way of preventative surgery. However, these are relatively rare or very rare disorders. The dream of larger scale prevention of some adult-onset Mendelian diseases may soon become possible. In his presidential address to this Society in 1998, A. Beaudet outlined in detail how such might be the case with hemochromatosis (Beaudet 1998). Dr. Beaudet also mentioned hereditary nonpolyposis colorectal cancer (HNPCC) as a possible candidate for population-based diagnosis and prevention. Below, I shall outline my view of this possibility.
HNPCC—A Multiorgan Cancer Syndrome
Much of our present knowledge about HNPCC comes from Dr. Henry Lynch, who pioneered the clinical study of inherited forms of the common cancers. Characteristics of HNPCC include dominant Mendelian inheritance of predisposition, early-onset (usually believed to have a mean age of ∼44 years), synchronous tumors (more than one primary tumor simultaneously), metachronous tumors (more that one cancer during lifetime), cancer in various organs (colorectum, endometrium, stomach, ovary, uroepithelium, brain, biliary tract, lymphoma), and relatively good prognosis (Lynch and de la Chapelle 1999).
Predisposition to HNPCC is caused by mutations in one of several DNA mismatch-repair (MMR) genes. The majority of clinically typical HNPCC is caused by mutations in either MLH1 or MSH2, while mutations in MSH6 and PMS2 are believed to be rare and give rise to clinical features that are less typical (ICG-HNPCC Web site). The MMR gene mutations obey the Knudson principle, in that heterozygous carriers of a germline mutation do not have disease. Somatic inactivation of the wild-type allele (e.g., by deletion, mutation, or promoter methylation) leads to MMR deficiency, which triggers malignant transformation, presumably by allowing spontaneously arising mutations in other genes to accumulate. Why MMR deficiency–associated tumors occur in some organs (e.g., colon, endometrium) and not in others (e.g., lung, prostate) is debated. Likewise, there is no definitive answer to the question why the clinical outcome of colorectal cancer is more favorable in HNPCC than in sporadic cases (Lynch and de la Chapelle 1999).
Clinical surveillance of HNPCC mutation carriers is beneficial. A 15-year study initiated in 1982 suggests that surveillance by colonoscopy every 3 years significantly reduces cancer morbidity (Järvinen et al. 2000). The death rate was reduced by 66%. In the study of a total of 252 individuals, there were, for instance, nine deaths from colorectal cancer among those not surveilled (n=119) and no deaths from colorectal cancer in those surveilled (n=133). Thus, it follows logically that more lives can be saved the more carriers of HNPCC can be diagnosed and clinically surveilled.
Detection of HNPCC
Previous diagnostic criteria were based on a combination of family aggregation and early onset of colorectal cancer (Vasen et al. 1991). In well-developed health care systems, individuals fulfilling these criteria will be noted and offered genetic testing. When a proband with a mutation is identified, at-risk family members will be offered genetic testing, and further carriers (affected or healthy) will be diagnosed.
The above scenario might be seen as traditional or passive. It relies on a well-functioning, sophisticated health care system and high awareness of genetics in cancer. Even under ideal circumstances, it is capable of detecting just a fraction of all HNPCC. The sensitivity may be on the order of 30%.
Here, an untraditional or active approach to HNPCC detection is proposed instead. It relies on microsatellite instability (MSI) of the tumor as a molecular marker. MSI arises as a result of MMR deficiency. MSI is found in 12%–18% of all colorectal cancers and is readily detectable by a simple test (Boland et al. 1998; de la Chapelle 1999). MSI is highly sensitive for HNPCC (80%–95%) (Aaltonen et al. 1994), but as many sporadic tumors also show MSI, the specificity is only ∼20%.
The proposed method is to test all colorectal cancers for MSI and all individuals with an MSI-positive tumor for MMR gene mutations. This scenario has been implemented in Finland, where MSI was determined in the tumors of 1,044 unselected consecutive colorectal cancer patients. The 129 MSI-positive cases (12%) were studied for germline mutations in MLH1 and MSH2. There were 28 mutation-positive individuals (i.e., new cases of HNPCC) (Aaltonen et al. 1998; Salovaara et al. 2000). Those 28 patients represented 22% of the 129 MSI-positive cases and 2.7% of all 1,044 patients screened. Interestingly, only 9/28 (32%) patients had a family history that fulfilled the traditional diagnostic criteria for HNPCC (Vasen et al. 1991), confirming that the sensitivity of these criteria to detect HNPCC is low. The study revealed that at least 2.7% of all colorectal cancer patients had HNPCC. Although this figure is an underestimate for multiple reasons, it provides a first estimate of the population frequency of MMR mutations, 1:740. Finally, the study showed that the proposed active approach was feasible.
Molecular Screening of All Colorectal Cancer Patients as a Means of Diagnosing HNPCC
The above findings were obtained in Finland, a small country with a relatively centralized health care system, obligatory medical insurance, high general-education level, and positive attitudes toward modern, including genetic, medicine. It remains to be determined whether a similar program can be implemented in circumstances that are less favorable. In particular, does the American health care system allow such studies? Are attitudes among the population and health care professionals conducive to similar programs? Finally, even if such studies could be carried out, are they deemed as desirable as the surveillance figures from Finland suggest?
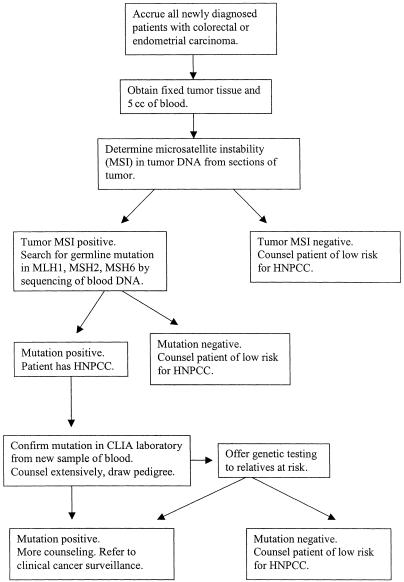
There is probably no other way of answering this question than by performing pilot studies in the United States. One such study is under way in the region of metropolitan Columbus, Ohio. Its ∼1.5 million population is reasonably representative of the U.S. population ethnically and socioeconomically. Most colorectal cancer care is provided by six major hospitals, all located in Columbus. The project is designed to accrue as many newly diagnosed colorectal or endometrial carcinoma patients as possible in the six hospitals. The design of the study is shown in figure 1. A total of 1,500 colorectal cancer patients and 450 endometrial cancer patients will be entered into the study. Presently, 75% of the accrual has occurred. Preliminary observations can be reported as follows.
Figure 1.
Molecular screening for HNPCC in the Columbus, Ohio, metropolitan area.
Accrual is ∼65% of all eligible patients. Differences in accrual between the hospitals are large and decidedly due to differences in the degree of dedication to the study by the health professionals. The main reason for noninclusion is not patient refusal but unintentional noninclusion by personnel. Refusal to consent is most often associated with old to very old age. The laboratory procedures are highly standardized and the results readily interpretable, with the exceptions discussed below. Preliminary results, after near-complete analysis of ∼30% of the cases, suggest that the proportion of patients with germline MMR mutations will be equal to or higher than in Finland (>2.7%). This appears to be the case both in colorectal and endometrial cancer.
Particular emphasis is given to the educational, counseling, and family aspects of the study. Participating professionals are regularly informed about the study. Patients whose tumors are MSI negative, and patients whose tumors are MSI positive but whose germline is negative for MMR mutations, receive detailed written feedback, including recommendations about clinical surveillance.
Patients with confirmed or suspected disease-causing mutations are seen by a genetic counselor and counseled. The mutation is confirmed in a CLIA laboratory from a new blood sample. A pedigree is drawn; family members at 25% or higher a priori risk are offered testing for the mutation found in the probands. So far, some 100 relatives of 12 probands have been tested; ∼40% are mutation positive. Mutation-positive and mutation-negative individuals are given recommendations regarding clinical surveillance and referred back to their original physician.
Obstacles
Compliance, Feasibility, and Ethical Considerations
Subjective experience, so far, from the Columbus study clearly suggests that patient attitudes are highly positive. Fear of legal, professional, and insurance discrimination is rare. A major factor determining inclusion in the study is the degree of organizational involvement and motivation of the hospital administrators, physicians, and nurses. Another major factor is how the study is presented to the patient; in other words, the professional skill and personality of the person dealing with the patient during the consent procedure.
Accuracy and Interpretation of Mutation Detection
Mutations in MLH1, MSH2 and MSH6 are searched for by genomic exon-by-exon sequencing. While this does represent the present gold standard, notably, several types of mutations are missed, including large deletions, other structural abnormalities, and many changes causing splicing errors. Mutations missed in this way may constitute as many as 20% of all disease-causing mutations. Another major problem is the interpretation of missense mutations, which account for some 30% of all mutations in MLH1 and MSH6. Unfortunately, there are no easy ways of dealing with the interpretation of missense changes. One recent major technical advance is the use of allele separation, also known as “conversion to haploidy” (Yan et al. 2000). This often allows deletions and splice-site mutations to be identified and interpreted (Nakagawa et al. 2002). In some instances, allele separation helps interpret missense mutations as well.
Ease and Accuracy of Clinical Surveillance
Experience from previous and ongoing studies suggests that the sensitivity of colonoscopy to detect cancer is high; however, the desirable interval between colonoscopies in mutation-positive individuals may be just one year. Clearly, this creates a major logistic, economic, and emotional burden. One alternative approach is prophylactic subtotal colectomy, a relatively drastic procedure. A further method is the fecal occult blood test, which is marred by low sensitivity and specificity.
Better methods must be sought. For instance, virtual colonoscopy, if improved, could offer an alternative, perhaps in conjunction with other tests, such as the determination of gene mutations in DNA extracted from the stools. HNPCC mutation carriers may be particularly important targets of such tests because MSI is detectable, with high sensitivity and specificity (Traverso et al. 2002), and tumors are predominantly right sided.
Clinical surveillance of HNPCC gene carriers is challenging also because of the high risk of cancer in organs other than the colorectum, in particular, the endometrium, stomach, and ovaries.
Nationwide Screening for HNPCC
Applying the above-referenced data from Finland to the U.S. population, a nationwide screening program of all newly diagnosed colorectal cancer patients could lead to the detection of 21,880 new carriers of HNPCC mutations each year (table 1). Is this realistic? Experience gathered in the ongoing Columbus study suggests that the scenario is conceptually, logistically, and clinically possible to carry out. This does not mean that all cases can be accrued. Economic issues are difficult to solve, no matter how favorable the figures might look in a cost-efficiency analysis. Finally, the health care system would need to be reformed in order to accommodate and improve the massive surveillance effort that would be required.
Table 1.
Theoretical Outline of Molecular Screening for HNPCC in the United States:Annual Outcome
| Population | |
| No. of patients newly diagnosed with colorectal cancer | 140,000 |
| No. of tumors tested for MSI | 140,000 |
| No. of patients with MSI of tumor (12%) | 16,800 |
| Search for mutations in MLH1, MSH2, MSH6 | 16,800 |
| No. of patients with germline mutation (3%)= new HNPCC probands | 4,200 |
| No. of relatives at risk tested (10 per proband; test for proband’s mutation only) | 42,000 |
| No. of relatives with germline mutation (40%) | 16,800 |
| Total no. of new cases of HNPCC (4,200 + 16,800) | 21,000 |
Conclusion
Dealing with HNPCC is a real challenge to our society. If the only choice in treatment and prevention were a drug or vaccine that restores deficient mismatch repair or gene therapy that “cures” the germline mutations, hopes of success would be slim. The second-line approach described here, including improved diagnosis, population-based molecular screening, and clinical surveillance targeted at the highest risk groups, is realistic and provides hope.
Acknowledgments
I thank Heather Hampel, CGC, for critical reading of the manuscript. The author's research is supported by grants CA67941 and CA16058 from the National Cancer Institute.
Footnotes
Previously presented at the annual meeting of The American Society of Human Genetics, in Baltimore, on October 18, 2002.
Electronic-Database Information
Accession number and URL for data presented herein are as follows:
- http://www.nfdht.nl/ (for International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer Web site) [Google Scholar]
References
- Aaltonen LA, Salovaara R, Kristo P, Canzian F, Hemminki A, Peltomäki P, Chadwick RB, Kääriänen H, Percesepe A, Ahtola H, Härkönen N, Julkunen R, Kangas E, Ojala S, Tulikoura J, Valkamo E, Eskelinen M, Järvinen H, Mecklin JP, de la Chapelle A (1998) Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. New Engl J Med 338:1481–1487 [DOI] [PubMed] [Google Scholar]
- Beaudet AL (1999) 1998 ASHG presidential address: making genomic medicine a reality. Am J Hum Genet 64:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S (1998) A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248–5257 [PubMed] [Google Scholar]
- de la Chapelle A (1999) Testing tumors for microsatellite instability. Eur J Hum Genet 7:407–408 [DOI] [PubMed] [Google Scholar]
- Järvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomäki P, de la Chapelle A, Mecklin J-P (2000) Controlled 15-year trial on screening for colorectal cancer in hereditary nonpolyposis colorectal cancer families. Gastroenterology 118:829–834 [DOI] [PubMed] [Google Scholar]
- Lynch HT, de la Chapelle A (1999) Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet 36:801–818 [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Yan H, Lockman J, Hampel H, Kinzler KW, Vogelstein B, de la Chapelle A (2002) Allele separation facilitates interpretation of potential splicing alterations and genomic rearrangements. Cancer Res 62:4579–4582 [PubMed] [Google Scholar]
- Salovaara R, Loukola A, Kristo P, Kääriäinen H, Ahtola H, Eskelinen M, Härkönen N, Julkunen R, Kangas E, Ojala S, Tulikoura J, Valkamo E, Järvinen H, Mecklin J-P, Aaltonen LA, de la Chapelle A (2000) Population-based molecular detection of hereditary nonpolyposis colorectal cancer. J Clin Oncol 18:2193–2200 [DOI] [PubMed] [Google Scholar]
- Traverso G, Shuber A, Olsson L, Levin B, Johnson C, Hamilton SR, Boynton K, Kinzler KW, Vogelstein B (2002) Detection of proximal colorectal cancers through analysis of faecal DNA. Lancet 359:403–404 [DOI] [PubMed] [Google Scholar]
- Vasen HF, Mecklin JP, Khan PM, Lynch HT (1991) The international collaborative group on hereditary non-polyposis colorectal cancer. Dis Colon Rectum 34:424–425 [DOI] [PubMed] [Google Scholar]
- Yan H, Papadopoulos N, Marra G, Perrera C, Jiricny J, Boland CR, Lynch HT, Chadwick RB, de la Chapelle A, Berg K, Eshleman JR, Yuan W, Markowitz S, Laken SJ, Lengauer C, Kinzler KW, Vogelstein B (2000) Conversion of diploidy to haploidy. Nature 403:723–724 [DOI] [PubMed] [Google Scholar]