Abstract
Recent studies have highlighted a potentially important role for Wnts as profibrotic mediators, and implicated increased Wnt activity in systemic sclerosis and other fibrotic diseases. Strikingly, new data indicates that Wnts have a central role in the profibrotic activity of TGF-β.
Systemic sclerosis (SSc) is a multiorgan, fibrotic autoimmune disease that is also known as scleroderma. The fibrotic features of SSc are currently untreatable, leading to considerable morbidity and mortality, making new therapeutic agents for this disease of particular importance. Wnts, known for their wide-ranging roles during development, have been increasingly investigated in disease processes. Now, their possible role in fibrosis has been highlighted by a new study by Akhmetshina et al.,1 the findings of which add to the growing literature describing Wnt activity in SSc and highlight Wnts as future therapeutic targets.
“…the profibrotic effect of TGF-β is mediated by endogenous Wnt activity…”
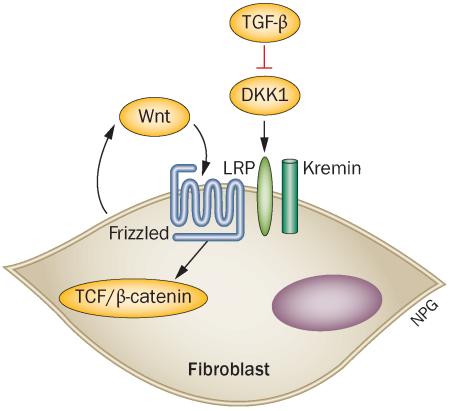
Using a transgenic mouse overexpressing Dickkopf-related protein 1 (DKK1), an endogenous Wnt inhibitor, Akhmetshina et al.1 showed that blocking Wnt proteins inhibits skin fibrosis in several mouse models of SSc; DKK1 blocked fibrosis in Tsk1 mice (characterized by progressive collagen and hypodermal thickening), bleomycin-induced skin fibrosis in mice and a novel murine model of skin fibrosis induced by subcutaneous injection of an adenoviruses expressing transforming growth factor (TGF)-β receptor 1. Furthermore, the researchers provide an intriguing link between Wnt and TGF-β, the profibrotic mediator most strongly implicated in SSc as well as other fibrotic diseases. They show that, in dermal fibroblasts, TGF-β inhibits DKK1 expression, whilst inducing intranuclear expression of β-catenin and axin-2, cellular events typically provoked by Wnt activation. These observations suggest that the profibrotic effect of TGF-β is mediated by endogenous Wnt activity, as revealed by downregulation of DKK1 in this setting. Completing this picture, recombinant DKK1 added to TGF-β-treated fibroblasts blocked the profibrotic effects of TGF-β.1 These data, together with the results of DKK1 in murine models, underline the well-established balance between Wnts and Wnt inhibitors in determining Wnt activity. This balance has been clearly shown in developmental settings; for example, in regulating hair follicle development and spacing. In addition, Wnt and TGF-β pathways have been shown to intersect both through coregulation of promoters and through binding to SMAD4 and LEF, indicating that these two molecules form a complex that affects downstream signalling, an interaction that signals formation of Spemann's organizer (a dorsal signalling centre).2
The notion that Wnts might be profibrotic is consistent with several other existing observations. Our group showed several years ago that Wnt3, another canonical Wnt, stimulates connective tissue growth factor (CTGF) and smooth muscle actin, a marker for myofibrobalsts.3 Moreover, Wei et al.4 have more recently shown that transgenic mice overexpressing Wntb10b develop skin fibrosis and lipoatrophy. These authors suggest that the effect of Wnts in skin, particularly Wnt10b, might be related to its developmental role in shifting fibroblast differentiation away from an adipogenic to profibrotic phenotype. Such a mechanism is reminiscent of early studies showing that Wnts control muscle stem cells in aging, pushing them toward a more fibrogenic phenotype.5 This finding is reinforced in a more recent report by the same group showing that Wnt3a stimulates a shift in preadipocytes from adipocyte to myofibroblast differentiation.6 The effect is mediated, at least in part, by TGF-β activation of SMAD2/3 phosphorylation and Wnt inhibition of peroxisome proliferator-activated receptor γ.
Wei and colleagues4 also showed that Wnt10b stimulates fibrosis in a mouse model of SSc independently of TGF-β. This finding is consistent with the new report by Akhmetshina and co-workers,1 which showed that TGF-β is effectively permissive to the effects of apparently endogenous Wnt(s) by downregulating DKK1. The source of this Wnt activity in fibroblast cultures in unclear, but might be produced by the fibroblasts themselves or be present in the serum used for fibroblast culture.
Interestingly, other cytokines have previously been implicated in mediating the profibrotic effects of TGF-β, namely CTGF and endothelin-1. Although it might seem implausible that all these mediators (Wnt, endothelin-1 and CTGF) are required for the profibrotic activity of TGF-β, perhaps regulation of fibrosis is so tightly controlled that multiple factors—that is a `per fect storm'—is required to permit progressive TGF-β-mediated fibrosis. Alternatively, Wnts could be part of a here-to-fore unrecognized cytokine cascade triggered by TGF-β.
Turning back to the effect of Wnt inhibition described by Akhmetshina et al.1 in murine models, the in vitro activity of DKK1 suggests that its overexpression in vivo is blocking a profibrotic Wnt signal, not necessarily requiring the involvement of TGF-β. The results are in agreement with another report showing that Wnt10b is upregulated in bleomycin-induced mouse models of skin fibrosis.4 The results reported here are also largely in agreement with our previous observations that fascial hypertrophy seen in Tsk1 mice, starting during late embryogenesis, is associated with striking upregulation of several Wnts including Wnt2 and Wnt11, though with only modestly increased Wnt10b expression.7 Thus, the specific Wnts involved in Tsk1 fascial hypertrophy are probably Wnt2 and Wnt11. Although Wnt11 was classically considered a noncanonical Wnt, on the basis of data indicating it did not activate the canonical β-catenin–TCF pathway, studies have suggested that this finding is an in vitro artefact and that Wnt11, and perhaps all of the 19 different Wnts, can activate canonical Wnt signalling though Frizzled receptors and LRP (low-density lipoprotein receptor-related protein) co-receptors.8
Applying these observations from studies in mice to the pathogenesis of human SSc is much more challenging. Akhmetshina et al.1 demonstrated increased expression of Wnt1 and Wnt10b, and decreased expression of DKK1, in skin from patients with SSc. However, the unknown status of the plethora of other Wnts and endogenous Wnt inhibitors complicates interpretation of these findings. Myself and others have previously reported in a comprehensive survey of Wnt mRNA levels in the skin of patients with SSc that only Wnt2 was statistically significantly upregulated (by a modest average increase of 2.2-fold compared with healthy controls).3 By contrast, the Wnt inhibitor, secreted frizzled-related protein 4 (SFRP4), was also strongly upregulated whereas DKK1 showed no change in expression and WIF1 (Wnt inhibitory factor 1, another Wnt inhibitor) was downregualted 7.9-fold.3 Thus, our data strongly implicate WIF1, rather than DKK1, in potentially permitting unrestrained Wnt activity in the skin of patients with SSc. On the other hand, consistent with the current report, Wei et al.4 have also shown the Wnt10b protein is upregulated in the skin of an SSc mouse model. Differences between mRNA and protein levels could explain the differences in these studies, but the ambiguities associated with immunohistochemical studies should provide some pause for thought regarding the specific Wnt(s) and Wnt inhibitors regulated in SSc skin.
Assessing cytokine activity in human pathology is difficult and continues to `plague' most human studies; it is particularly important for research into the effects of Wnts, owing to the large number of Wnts and Wnt antagonists. In the report by Akhmetshina et al.,1 increased levels of nuclear β-catenin staining supports upregulated Wnt activity in SSc skin, which has also been seen in lungs from patients with SSc.9 Cytokine-regulated genes provide another means to try and implicate a cytokine in pathogenesis and have been applied in SSc to infer a role for interferon and TGF-β activity in SSc.10 Increased AXIN2 expression in SSc skin supports Wnt activation occurring here, but provides a narrow `signature' for the effect of Wnt. My colleagues and I previously reported a modest, but statistically significant, increase in Wnt activity in sera from patients with SSc compared with healthy controls, further supporting the notion that increased Wnt activity could contribute to SSc fibrosis.3
An improved understanding of the role of Wnts in mediating the effect of TGF-β on fibrosis is crucial for considering the possibility of therapeutic intervention. If blocking Wnt activity is able to inhibit the profibrotic activity of TGF-β, then this approach becomes an attractive target for intervention in SSc. Global blockade of Wnt activity would clearly be harmful during development, but might be tolerated in adults. However, a more complete understanding of the specific defects involving Wnts and/or endogenous Wnt inhibitors in SSc would potentially permit more selective approaches to therapy targeting the Wnt pathway.
Footnotes
Competing interests The author declares no competing interests.
References
- 1.Akhmetshina A, et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishita M, et al. Interaction between Wnt and TGF-β signalling pathways during formation of Spemann's organizer. Nature. 2000;403:781–785. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- 3.Lemaire R, et al. Antagonistic effect of the matricellular signaling protein CCN3 on TGF-β-and Wnt-mediated fibrillinogenesis in systemic sclerosis and Marfan syndrome. J. Invest. Dermatol. 2010;130:1514–1523. doi: 10.1038/jid.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei J, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 2011;63:1707–1717. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 6.Wei J, et al. Wnt/β-catenin signaling is hyperactivated in systemic sclerois and induces smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. doi: 10.1002/art.34424. http://dx.doi.org/10.1002/art.34424. [DOI] [PMC free article] [PubMed]
- 7.Bayle J, et al. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J. Invest. Dermatol. 2008;128:871–881. doi: 10.1038/sj.jid.5701101. [DOI] [PubMed] [Google Scholar]
- 8.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 9.Lam AP, et al. Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am. J. Respir. Cell Mol. Biol. 2011;45:915–922. doi: 10.1165/rcmb.2010-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farina G, Lafyatis D, Lemaire R, Lafyatis R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2010;62:580–588. doi: 10.1002/art.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]