Abstract
Schizophrenia is a devastating psychiatric illness that afflicts 1% of the population worldwide, resulting in substantial impact to patients, their families, and health care delivery systems. For many years, schizophrenia has been felt to be associated with dysregulated dopaminergic neurotransmission as a key feature of the pathophysiology of the illness. Although numerous studies point to dopaminergic abnormalities in schizophrenia, dopamine dysfunction cannot completely account for all of the symptoms seen in schizophrenia, and dopamine-based treatments are often inadequate and can be associated with serious side effects. More recently, converging lines of evidence have suggested that there are abnormalities of glutamate transmission in schizophrenia. Glutamatergic neurotransmission involves numerous molecules that facilitate glutamate release, receptor activation, glutamate reuptake, and other synaptic activities. Evidence for glutamatergic abnormalities in schizophrenia primarily has implicated the NMDA and AMPA subtypes of the glutamate receptor. The expression of these receptors and other molecules associated with glutamate neurotransmission has been systematically studied in the brain in schizophrenia. These studies have generally revealed region- and molecule-specific changes in glutamate receptor transcript and protein expression in this illness. Given that glutamatergic neurotransmission has been implicated in the pathophysiology of schizophrenia, recent drug development efforts have targeted the glutamate system. Much effort to date has focused on modulation of the NMDA receptor, although more recently other glutamate receptors and transporters have been the targets of drug development. These efforts have been promising thus far, and ongoing efforts to develop additional drugs that modulate glutamatergic neurotransmission are underway that may hold the potential for novel classes of more effective treatments for this serious psychiatric illness.
Keywords: Ionotropic, Metabotropic, Antipsychotics, Modulators, Accessory proteins
Schizophrenia is a devastating illness that afflicts 1% of the population, or more than 60 million people worldwide. This is one of the most serious of all psychiatric illnesses: more hospital beds (psychiatric and medical combined) are filled by persons with schizophrenia than due to any other medical condition (Buchanan and Carpenter, 2000). This disorder is characterized by a constellation of clinical findings, often divided into positive and negative symptoms. Positive symp-toms include dramatic hallucinations, which are often auditory. Patients report that they hear voices that are clearly located outside of their heads, most often engaged in a running commentary on their thoughts and behaviors. Other common positive symptoms include paranoid delusions and disorders of thought processes. Much more insidious and debilitating are the negative symptoms, which are associated with the diminution of normal social behaviors, and include withdrawal, decreased spontaneous communication, decreased eye contact, decreased or muted facial expression and vocal inflection, and diminished spontaneous movement (Buchanan and Carpenter, 2000).
For decades, schizophrenia research focused on the dopamine hypothesis of schizophrenia, which postulates that dysregulated dopaminergic neurotransmission is a key feature of the pathophysiology of the illness. The dopamine hypothesis is based on the observation that antipsychotics block D2 receptors, and their affinity for these receptors highly correlates with their ability to ameliorate some psychotic symptoms. Second, psychostimulants that enhance dopaminergic activity can elicit positive symptoms of schizophrenia, including delusions, hallucinations, and thought disorder. Although numerous studies point to dopaminergic abnormalities in schizophrenia (Joyce and Meador-Woodruff, 1997; Laruelle et al., 1999), dopamine dysfunction cannot completely account for all of the symptoms seen in schizophrenia, since neuroleptics typically are effective only for the positive symptoms of the illness, while negative symptoms and cognitive deficits are relatively refractory to treatment with antipsychotics. Consequently, alternative neurotransmitter systems that may be involved in the pathophysiology of schizophrenia have been sought.
A growing body of evidence now implicates glutamatergic dysfunction in schizophrenia. The most widely held hypothesis propose that schizophrenia is associated with decreased glutamate activity in limbic brain structures, likely involving the postsynaptic NMDA and/or AMPA subtypes of glutamate receptors (Itil et al., 1967; Aanonsen and Wilcox, 1986; Javitt and Zukin, 1991; Krystal et al., 1994; Coyle, 1996; Goff and Wine, 1997; Tamminga, 1999). Some of the most compelling evidence implicating glutamate dysfunction in schizophrenia is the fact that phencyclidine (PCP) and similar compounds, which are uncompetitive antagonists of the NMDA receptor, can induce both the positive and negative symptoms of schizophrenia, including cognitive deficits (Javitt and Zukin,1991; Tamminga, 1999). Moreover, these compounds can exacerbate both positive and negative symptoms in schizophrenia (Lahti et al., 1995). Chronic administration of PCP-like compounds may provide a more valid model of schizophrenia than acute treatment, since it induces a persistent psychotic symptomatology (Javitt and Zukin, 1991), and reduces frontal lobe blood flow and glucose utilization, which is remarkably similar to the “hypofrontality” described in schizophrenia (Hertzmann et al., 1990). Acute PCP administration leads to increased glutamate neurotransmission in the prefrontal cortex of rodents (Moghaddam et al., 1997; Moghaddam and Ad-ams, 1998); however, relatively little is known of the impact of chronic PCP exposure on the glutamate system. Electro-physiological data show heightened depolarization of pre-frontal cortical pyramidal neurons of rats chronically treated with PCP following local application of NMDA (Arvanov and Wang, 1999; Yu et al., 2002). Chronic PCP treatment alters expression of NMDA receptor subunits, as well as the subunit stoichiometry of the NMDA receptor (Yu et al., 2002). A possible interpretation of these data is that chronic PCP treatment leads to a prolonged reduction in glutamate transmission, re-sulting in increased NMDA receptor expression and an amplified response to exogenous NMDA application (Jentsch and Roth, 1999). These observations, taken together, suggest that glutamatergic abnormalities in schizophrenia likely involve the NMDA receptor or its downstream signaling pathways. Given that NMDA receptor firing requires partial predepolarization of the postsynaptic membrane by activation of AMPA receptors, abnormal AMPA receptor expression or function may be manifested as an NMDA receptor abnormality. Accordingly, the AMPA receptor has also been implicated in the pathophysiology of this illness.
As candidate genes associated with neurotransmission have been sought as possible substrates for aspects of the pathophysiology of schizophrenia, a number of proteins and pathways have been identified. A high level of complexity of the cell biology of neurotransmission is recognized, resulting in numerous candidate genes for involvement in schizophrenia. Neurotransmission involves myriad molecules, including pre-and post- synaptic receptors; intracellular receptor-interacting proteins that link receptors to signal transduction pathways, cytoskeletal elements, and other receptors; signal transduction cascades; membrane- and vesicle-bound transporters; synthetic and catabolic enzymes; and machinery that regulates expression of all of these molecules at both transcriptional and translational levels. To further appreciate the role that glutamate may have in the pathophysiology of schizophrenia, and the potential molecular targets for new drug discovery, we briefly review the physiology of glutamatergic transmission.
GLUTAMATERGIC NEUROTRANSMISSION
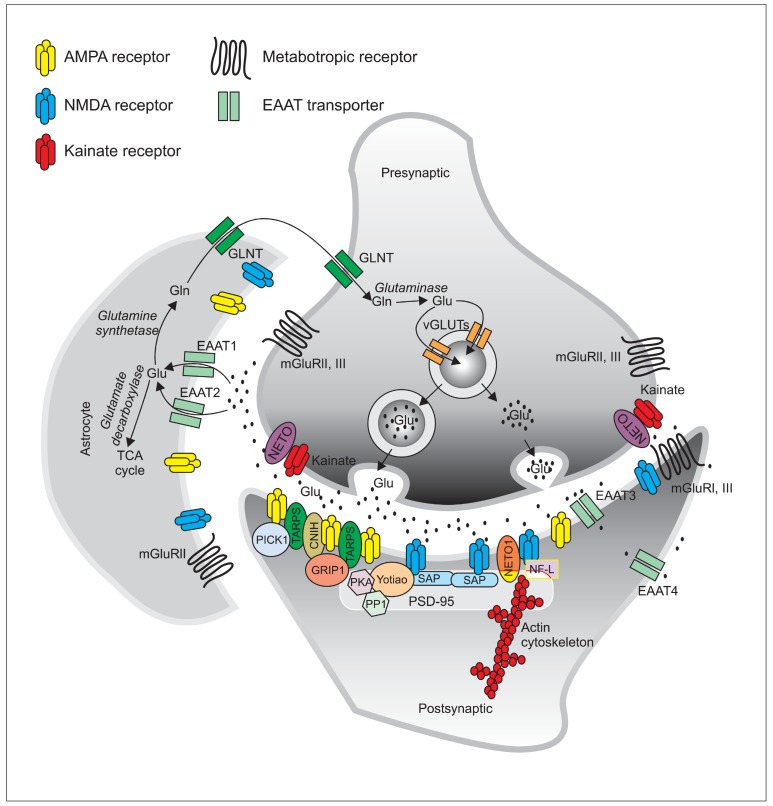
The tripartite glutamatergic synapse is characterized by bidirectional communications between the presynaptic neuron, the post-synaptic neuron and the surrounding astrocytes (Araque et al., 1999; Ni et al., 2007). Initially, glutamate is synthesized from glutamine in the presynaptic neuron, where it is packaged into secretory vesicles by one of at least three vesicular glutamate transporters (VGLUT 1-3) (Aihara et al., 2000; Bellocchio et al., 2000; Takamori et al., 2000; Fremeau et al., 2002). Upon excitation of the pre-synaptic neuron, the glutamatergic vesicles fuse with the pre-synaptic membrane and release their contents into the synaptic cleft. Synaptic glutamate then acts upon different glutamatergic receptors in the pre- and post- synaptic membranes and on astrocytes (Kanai et al., 1993; Masson et al., 1999; Danbolt, 2001). The rapid clearance of extracellular glutamate is mediated by high affinity membrane excitatory amino acid transporters (EAAT1-EAAT5), located on both neurons and astrocytes (Kanai et al.,1993; Rothstein et al., 1994; Lehre et al., 1995; Bar-Peled et al., 1997; Milton et al., 1997; Nagao et al., 1997; Gesemann et al., 2010; Neuhauss et al., 2010; Rico et al., 2010). Recovered glutamate in astrocytes either enters the TCA cycle as α-ketoglutarate, or is converted into glutamine by glutamine synthethase. Glutamine is then released from astrocytes for uptake into the pre-synaptic neuron (Danbolt, 2001). In this neuron, the enzyme glutaminase can oxidize glutamine into glutamate, which is then repackaged into vesicles for its subsequent release (Danbolt, 2001) (Fig. 1).
Fig. 1. The glutamatergic synapse. Glutamate is packaged into presynaptic vesicles and released to the synaptic cleft where it acts upon ionotropic and metabotropic receptors and is rapidly cleared by EAATs. In the astrocyte, glutamate either enters the TCA cycle or is converted to glutamine by the enzyme glutamine synthetase. Glutamine is then released to the presynaptic neuron where it is converted to glutamate and packaged into vesicles for further release.
Once released into the synaptic cleft, glutamate acts upon glutamate receptors. These include ionotropic glutamate receptors, which are ligand-gated ion channels (NMDA, AMPA and kainate subtypes, Table 1) that mediate fast excitatory transmission, and G-protein coupled metabotropic receptors (mGluR1-8, Table 2) responsible for modulating and fine tuning the synapse (Hollmann and Heinemann, 1994; Bleakman and Lodge, 1998).
Table 1.
Classification and features of ionotropic glutamate receptors
| Family | Subunit* | Characteristics | Alternative nomenclature* |
|---|---|---|---|
|
| |||
| NMDA | GluN1 | - Glycine binding | GLUN1, NMDA-R1, NR1, GluRζ1 |
| - Obligatory subunit | |||
| GluN2A | - Primarily synaptic | GLUN2A, NMDA-R2A, NR2A, GluRε1 | |
| - Ubiquitous expression in adult brain | |||
| - High channel conductance | |||
| - High sensitivity to Mg2+ | |||
| GluN2B | - Glutamate and polyamine binding | GLUN2B, NMDA-R2B, NR2B, hNR3, GluRε2 | |
| - Primarily extrasynaptic | |||
| - Highly expressed in early development | |||
| - Primarily expressed in adult forebrain | |||
| - High channel conductance | |||
| - High sensitivity to Mg2+ | |||
| GluN2C | - Glutamate binding | GLUN2C, NMDA-R2C, NR2C, GluRε3 | |
| - Primarily expressed in adult cerebellum | |||
| - Low channel conductance | |||
| - Low sensitivity to Mg2+ | |||
| GluN2D | - Glutamate binding | GLUN2D, NMDA-R2D, NR2D, GluRε4 | |
| - Primarily extrasynaptic | |||
| - Highly expressed in early development | |||
| Low channel conductance | |||
| - Low sensitivity to Mg2+ | |||
| GluN3A | - Primarily extrasynaptic | GLUN3A, NMDA-R3A, NMDAR-L, chi-1 | |
| - Glycine binding | |||
| - Highly expressed in early development | |||
| - Low channel conductance | |||
| - Low sensitivity to Mg2+ | |||
| GluN3B | - Glycine binding | GLUN3B, NMDA-R3B | |
| - Primarily extrasynaptic | |||
| - Highly expressed in the spinal cord, pons, midbrain and medulla. | |||
| - Low channel conductance | |||
| - Low sensitivity to Mg2+ | |||
| AMPA | GluA1 | - Ca2+ permeable | GluA1,GluR1, GluRA, GluR-A, GluR-K1, HBGR1 |
| - Impermeable when coupled to edited GluA2 | |||
| - Higher conductance | |||
| GluA2 | - Q/R edited: Linear current-voltage relationship Impermeable to Ca2+ Low single-channel conductance | GluA2,GluR2, GluRB, GluR-B, GluR-K2, HBGR2 | |
| - Q/R unedited: Inwardly rectifying when blocked by endogenous intracellular polyamines Ca2+ permeable Higher conductance | |||
| GluA3 | - Ca2+ permeable | GLUA3, GluR3, GluRC, GluR-C, GluR-K3 | |
| - Impermeable when coupled to edited GluA2 | |||
| - Higher conductance | |||
| GluA4 | - Ca2+ permeable | GLUA4, GluR4, GluRD, GluR-D | |
| - Impermeable when coupled to edited GluA2 | |||
| - Higher conductance | |||
| Kainate | GluK1 | - Can form homomeric channels | GLUK5, GluR5, GluR-5, EAA3 |
| - Required for functional heteromeric receptors | |||
| - Found in cortex, striatum, hippocampus, dorsal root ganglia and cerebellum. | |||
| - Q/R edited: Linear current-voltage relationship Impermeable to Ca2+ Low single-channel conductance | |||
| - Q/R unedited: Inwardly rectifying when blocked by endogenous intracellular polyamines Ca2+ permeable Higher conductance | |||
| GluK2 | - Can form homomeric channels | GLUK6, GluR6, GluR-6, EAA4 | |
| - Required for functional heteromeric receptors | |||
| - Found in cortex, striatum, hippocampus, dorsal root ganglia, retina and cerebellum | |||
| - Q/R edited: Linear current-voltage relationship Impermeable to Ca2+ Low single-channel conductance | |||
| - Q/R unedited: Inwardly rectifying when blocked by endogenous intracellular polyamines Ca2+ permeable Higher conductance | |||
| GluK3 | - Can form homomeric channels | GLUK7, GluR7, GluR-7, EAA5 | |
| - Required for functional heteromeric receptors | |||
| - Found in cortex, hippocampus, retina and cerebellum. | |||
| GluK4 | - Only forms heteromeric receptors | GLUK1, KA1, KA-1, EAA1 | |
| - Primarily located at hippocampal CA3 and dentate granule neurons and purkinje cells in cerebellum | |||
| GluK5 | - Only forms heteromeric receptors | GLUK2, KA2, KA-2, EAA2 | |
| - Found in cortex, hippocampus, retina and cerebellum. | |||
*Collingridge et al., 2009.
Table 2.
Classification and features of metabotropic glutamate receptors
| Family and Receptors | Coupling | Characteristics |
|---|---|---|
|
| ||
| Group I | Excitatory | |
| mGluR1 | Gq coupled | - Primarily postsynaptic |
| - Expressed in neurons | ||
| mGluR5 | Gq coupled | - Primarily postsynaptic |
| - Expressed in neurons and astrocytes | ||
| Group II | Inhibitory | |
| mGluR2 | Gi coupled | - Primarily presynaptic |
| - Expressed in neurons and astrocytes | ||
| mGluR3 | Gi coupled | - Pre- and postsynaptic |
| - Expressed in neurons and astrocytes | ||
| Group III | Inhibitory | |
| mGluR4 | Gi coupled | - Pre- and postsynaptic |
| - Expressed in neurons and reactive astrocytes | ||
| mGluR6 | Gi coupled | - Exclusively in postsynapses of retinal bipolar metabotropic (ON-center) cells |
| mGluR7 | Gi coupled | - Pre- and postsynapses |
| - Expressed in neurons | ||
| mGluR8 | Gi coupled | - Primarily presynaptic |
| - Expressed in neurons and reactive astrocytes | ||
NMDA receptors
NMDA receptors (Table 1) are heterotetramers composed of two obligatory GluN1 subunit and two regulatory GluN2 or GluN3 subunits (Collingridge et al., 2009). GluN1 is alternatively spliced into eight forms while four different genes encode for GluN2 (GluN2A-D) and two genes encode for GluN3 (Glu-N3A-B). The properties and localization of the NMDA receptor vary according to the subunit composition of the channel. The typical NMDA receptor is composed of a dimer of GluN1 and a dimer of GluN2 subunits that have glycine and glutamate binding properties, respectively. Receptors containing two GluN1 and two GluN3 subunits, on the other hand, only bind glycine and their activity is independent from glutamate. The subunit composition of NMDA receptors not only differs within areas of the human brain and in different stages of development, but also within synaptic regions (Rao and Craig, 1997; Thomas et al., 2006). GluN1/2A, for example, predominates in adult synaptic sites while GluN1/2B is more abundant in extrasynaptic sites during development (Cull-Candy et al., 2001).
NMDA receptor subunits are synthesized in the endoplasmic reticulum (ER), where they assemble into heterotetramers. GluN1/2 and 1/3 complexes exit the ER, are modified in the Golgi apparatus and sorted in the trans-Golgi network (TGN). The mature complexes leave the TGN packaged into vesicles that are either delivered to the cell surface or to endosomes. NMDA receptor vesicles are initially trafficked on microtubules by KIF17 (protein kinesin family member 17) and delivered to the dendritic spine surface membrane in a later stage by myosin cargo proteins sliding on actin filaments (Lau and Zukin, 2007; Stephenson et al., 2008). At the synaptic site, there is subunit specific recycling which also contributes to the pool of available receptors (Lavezzari et al., 2004).
NMDA receptors form large complexes with intracellular proteins that regulate their trafficking, delivery and anchoring
to the synapse (Sans et al., 2003; Prybylowski et al., 2005; Sans et al., 2005). These proteins link the receptors to the underlying cytoskeletal machinery and allow the activation of downstream molecular pathways. Intracellular proteins bind to the C-terminus of specific subunits. GluN1, for example, is phosphorylated on its C-terminus by PKA and PKC and this modulation promotes NMDA receptor trafficking to the membrane (Tingley et al., 1993; Tingley et al., 1997; Chung et al., 2004). GluN2, on the other hand, is phosphorylated by Fyn, an event that prevents the receptor’s endocytosis from the synapse (Sala and Sheng, 1999). The membrane associated guanylate kinase (MAGUK) family of scaffolding proteins is involved in the trafficking and clustering of GluN2 subunits. This family includes chapsyn-110, postsynaptic density (PSD) -95, PSD-93, synapse associated protein (SAP) 97 and SAP102.
Each of these proteins differentially interact with and modulate specific GluN2 isoforms (Cousins et al., 2008). Another novel auxiliary protein, neuropilin tolloid-like 1 (NETO1), is thought to be involved in maintenance and/or insertion of GluN2A NMDA receptors at the synapse of the CA1 region of the hippocampus (Ng et al., 2009). Two intracellular proteins that interact with the GluN1 subunit have been described to date: the neuronal intermediate filament protein, neurofilament-light (NF-L) that prevents ubiquitination and subsequent degradation of GluN1 (Ehlers et al., 1998; Ratnam and Teichberg, 2005), and Yotiao (Lin et al., 1998) that regulates channel activity by link-ing NMDA receptors to PKA and PP1 (Westphal et al., 1999).
Because of the well-established role of NMDA receptors in neurotoxicity and synaptic plasticity, novel drugs primar-ily target NMDA receptor modulation through interactions at the glycine binding co-agonist site of this receptor. However, NMDA receptor synthesis, trafficking delivery and anchoring to the synaptic site involves a large number of molecules that have recently been identified and characterized. All of these molecules are potential sites for drug development to treat the symptoms of schizophrenia.
AMPA receptors
Similar to NMDA receptors, AMPA-type glutamate receptors (Table 1) are involved in fast glutamate transmission, neuronal circuit remodeling and higher order cognitive functions such as learning and memory (Malinow and Malenka, 2002; Kessels and Malinow, 2009; Keifer and Zheng, 2010). AMPA receptors are homotetramers assembled as dimers of GluA subunits dimers (Collingridge et al., 2009; Sobolevsky et al., 2009). GluA subunits 1-4 are encoded by independent genes (Boulter et al., 1990; Keinanen et al., 1990) and assemble into homodimers in the ER, where they undergo conformational changes necessary for the proper function of the receptors
and quality control of folding and dimerization (Mah et al., 2005). Further post translational modifications of AMPA recep-tor subunits occur in the Golgi apparatus, involving phosphorylation and glycosylation (Greger and Esteban, 2007). The cellular and subcellular localization and biophysical properties of the different AMPA receptors vary according to their subunit composition (Hayashi et al., 2000; Shi et al., 2001). The GluA1 subunit, for example, is required for trafficking of receptors in response to activity and participates in basal neurotransmission, while the GluA2 subunit participates in basal conditions regulating Ca2+ permeability, homeostasis, and trafficking to the synapse (Granger et al., 2011).
AMPA receptor subunits contain specific PDZ-binding domains on their cytoplasmic carboxy-termini (C-termini) consisting of approximately 90 amino acids. These domains enable their interactions with other PDZ-containing intracel-lular proteins within the postsynaptic density (PSD), which are important for AMPA receptor expression, function and localization within the cell (Malinow and Malenka, 2002). PDZ interactions occurring between the C-termini of AMPA receptor subunits and accessory PSD proteins such as PICK1, involved in GluA2 sorting and recycling (Steiner et al., 2005), and GRIP1, which regulates trafficking, anchoring and endocytosis of AMPA receptors (Citri et al., 2010; Clem et al., 2010; Anggono et al., 2011), can facilitate specific patterns of AMPA receptor trafficking to the PSD (Malinow and Malenka, 2002). Similarly, these domains enable AMPA receptors to colocalize with NMDA receptors at the synaptic membrane via anchoring proteins such as the SAP family proteins, or have their recycling pool regulated by another accessory protein, NSF (Song et al., 1998; Chen et al., 2000).
Another AMPA receptor modulatory protein, transmembrane AMPA regulatory protein 2 (TARP2, or stargazin), was the first transmembrane protein found to interact with AMPA receptors (Chen et al., 2000; Diaz, 2010). TARP2 was first identified in the naturally occurring mutant stargazer mouse that lacks functional AMPA receptors at cerebellar granule cell synapses (Noebels et al., 1990; Letts et al., 1998; Hashimoto et al., 1999; Chen et al., 2000). TARP2 is a member of a family of eight closely related AMPA receptor auxiliary proteins that are widely expressed in the brain and share homology with the γ-1 accessory subunit of the voltage dependent calcium channels found in skeletal muscle. TARPs have biophysical effects on AMPA receptors, such as controlling channel gating, kinetics, glutamate binding affinity, activation and desensitization rates, and receptor stability (Tomita, 2010). Class I TARPS, (TARP2, 3, 4 and 8) and Class II TARPs (TARP5 and 7) have distinct properties. The members of Class I have slow deactivation and desensitization rates, increase glutamate and kainate efficacy, and increase steady-state currents and receptor trafficking (Kato et al., 2010). TARP7 also decreases deactivation kinetics and increases kainate efficacy and steady-state currents, but has been shown to have less effect on glutamate affinity or receptor trafficking (Kato et al., 2010). TARP5 shows even more differences, and has been reported to increase de-activation, decrease glutamate affinity and steady-state current, and have little effect on kainate efficacy and receptor trafficking (Kato et al., 2010).
AMPA receptors and TARPs are coassembled in the ER, and it is here that they form their first direct interaction (Coombs and Cull-Candy, 2009; Tomita, 2010). After assembly, the AMPA receptor/TARP complex is trafficked through the Golgi apparatus and to the synaptic membrane before being laterally translocated to the PSD (Bredt and Nicoll, 2003; Beneyto and Meador-Woodruff, 2006; Coombs and Cull-Candy, 2009). At the PSD, the PDZ domain of the TARP protein binds to the PDZ domain of a PSD scaffolding protein, PSD-95, anchoring the complex to the membrane (Chen et al., 2000; Chen et al., 2003; Bats et al., 2007). AMPA receptors cannot bind directly to PSD-95, and rely on TARP proteins to provide this important structural role for AMPA receptor stability (Tomita, 2010).
The cornichon protein family has also been recently identified as auxiliary proteins of AMPA receptors that have similar functions to TARPs. CNIH2 and CNIH3 are coassembled with an estimated 70% of native AMPA receptors in the rat brain, and have been shown to enhance surface expression and gating of AMPA receptors (Schwenk et al., 2009). As with the TARPs, cornichons have also been reported to slow deactivation and desensitization of AMPA receptors (Schwenk et al., 2009)
The cornichon protein family has also been recently identified as auxiliary proteins of AMPA receptors that have similar functions to TARPs. CNIH2 and CNIH3 are coassembled with an estimated 70% of native AMPA receptors in the rat brain, and have been shown to enhance surface expression and gating of AMPA receptors (Schwenk et al., 2009). As with the TARPs, cornichons have also been reported to slow deactivation and desensitization of AMPA receptors (Schwenk et al., 2009).
AMPA receptors may be coassembled with both TARPs and cornichons. Additionally, there are other AMPA receptors accessory molecules that bind to AMPA receptors or that complex with TARPs and cornichons to affect the trafficking and kinetics of these receptors. The cystine-knot AMPA receptor modulating protein (CKAMP44) associates with TARP-associated AMPA receptors in synaptic spines and increases their deactivation and desensitization (von Engelhardt et al., 2010). SynDIG1, or synapse differentiation induced gene 1, has recently been shown to regulate the development of excitatory synapses and is colocalized with AMPA receptors in heterologous cells (Kalashnikova et al., 2010). Similarly, NARP, EphB2 and SALM2 are other molecules known to be involved with
targeting AMPA receptors to the developing excitatory synapse, and nPIST, a Golgi protein, promotes AMPA receptor clustering and may be important for AMPA receptor trafficking to the plasma membrane (Cuadra et al., 2004; Kalashnikova et al., 2010).
Kainate receptors
Different from other ionotropic receptors, kainate receptors (Table 1) show strong desensitization kinetics and slow response recovery (Lerma, 2003), serve primarily a modulatory function at the synapse, and are predominantly located pre-synaptically throughout the brain. Kainate receptors subunits 1-5 (GluK1-5) are encoded by different genes and undergo alternative splicing. GluK1-3 can form functional homomeric ion channels and are required for functional heteromeric receptors. In contrast, GluK4 and 5 are not able to form homomeric channels but do assemble with GluK1-3 into functional heteromeric receptor complexes (Bettler et al., 1990; Egeb-jerg et al., 1991; Schiffer et al., 1997). The kinetics properties of kainate receptors vary according to their subunit composition, localization and developmental stage. Several accessory subunits have been recently identified for kainate receptors. Among them, NETO1 has been characterized as an auxiliary protein participating in both kainate and NMDA receptor regulation (Ng et al., 2009; Copits et al., 2011; Straub et al., 2011; Tang et al., 2011). While NETO1 controls kainate receptor current kinetics, NETO2 drives receptor insertion into the plasma membrane, targets GluK1 receptors to the synapse, and significantly reduces receptor desensitization to glutamate (Zhang et al., 2009; Copits et al., 2011; Straub et al., 2011). Because NETO proteins regulate kainate receptor activity and localization, they are potential targets for drug discovery.
Metabotropic glutamate receptors
The eight metabotropic glutamate receptors (mGluRs) members are divided into three groups (group I [mGluR1 and 5], group II [mGluR2 and 3] and group III [mGluR4, 6, 7, 8]), based on their pharmacological properties and the nature of their downstream signaling pathways (Table 2). Group I mGluRs are primarily localized on postsynaptic membranes, often close to NMDA receptors and modulating their activity. They activate Gαq proteins, resulting in increased intracellular calcium levels. Presynaptic group II and III receptors activate Gαi, decreasing cAMP levels and modulating presynaptic glutamate release (Schoepp, 2001). Different mGluRs have unique regional and subcellular localization, as well as specific positive and negative modulators (Olive, 2009) (Table 2). Recently, drugs targeted towards group II mGluRs have proven successful in early stage clinical trials in schizophrenia as discussed below.
RECEPTOR ABNORMALITIES IN SCHIZOPHRENIA
Recent postmortem brain studies have found alterations of ionotropic glutamate receptors (NMDA, AMPA, and kainate, Table 3) and metabotropic glutamate receptors in schizophrenia. Accordingly, these pharmacologically distinct receptors have become targets for new drug discovery. Changes in subunit expression and binding sites in schizophrenia appear to be specific to limbic, cortical and hippocampal brain regions and have been interpreted to suggest abnormal subunit stoichiometry of glutamate receptors. Examination of glutamate receptor accessory molecules has revealed robust changes in their expression levels in schizophrenia as well, suggesting that regulation of glutamatergic neurotransmission and intracellular signaling is compromised in this illness.
Table 3.
Glutamate receptor abnormalities in schizophrenia versus comparison subjects
| Family | Subunit | Cortex | Hippocampus | Thalamus | B. G. | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| T | P | T | P | T | P | T | P | ||
|
| |||||||||
| NMDA | GluN1 | ↓1-3/↑4,5/↔6 | ↑/7↔8 | ↓9/↔6 | ↓10 | ↓11,12/↔13,14 | ↔15 | ↔16/↑17 | |
| GluN2A | ↑4/↓3/↔6 | ↔7 | ↔6,9 | ↔11,13,14 | ↔15 | ↔16/↑17 | |||
| GluN2B | ↔3,6,18 | ↔7/ ↓8 | ↑9/↔6 | ↑14/↓11/↔13 | ↑15 | ↔16/↑17 | |||
| GluN2C | ↓3/↔6,1 | ↔7 | ↔6 | ↓11/↔13,14 | ↔16/↑17 | ||||
| GluN2D | ↑18/↔3,6 | ↔7 | ↔6 | ↔11,13,14/↓19 | ↔16/↑17 | ||||
| GluN3A | ↑20 | ||||||||
| AMPA | GluA1 | ↓1/↑21/↔3,6 | ↑22/↓23 | ↓24,25/↔6 | ↓26/↔27 | ↓11/↔13 | ↔16/↑17 | ||
| GluA2 | ↓28/↑18/↔6,21 | ↓23/↔27 | ↓25,29/↔6 | ↓25,26/↔27 | ↔11,13 | ↔16/↑17 | |||
| GluA3 | ↔6,21,28 | ↔27 | ↔6 | ↓26/↔27 | ↓11,19/↔13 | ↔16/↑17 | |||
| GluA4 | ↓28/↑21/↔6 | ↔6 | ↔11 | ↔16/↑17 | |||||
| Kainate | GluK1 | ↓6,30/↔31 | ↔6 | ↓32/↔37 | ↔11,13 | ↔16,31/↑17 | |||
| GluK2 | ↔6,31 | ↓33/↔6 | ↓32 | ↓19/↔13 | ↔16,17,31 | ||||
| GluK3 | ↑31/↓1/↔6 | ↔6 | ↓32 | ↓19/↔13 | ↔16,17,31 | ||||
| GluK4 | ↓1/↔6,31 | ↔6 | ↔11 | ↔16,17,31 | |||||
| GluK5 | ↓31/↔6 | ↓33/↔6 | ↓11/↔13 | ↔16,17,31 | |||||
| mGluR | mGluR1 | ↑34 | ↑35 | ↔11,36 | ↔35 | ||||
| mGluR2 | ↑37/↓38 | ↑35/↔39,40 | ↔11,36 | ↔35 | |||||
| mGluR3 | ↔41 | ↑35/↓39 | ↔11,36 | ↔35 | |||||
| mGluR4 | ↔35 | ↔11,36 | ↔35 | ||||||
| mGluR5 | ↑41/↔34 | ↔35 | ↔11,36 | ↔35 | |||||
| mGluR6 | ↔11 | ||||||||
| mGluR7 | ↔11,36 | ||||||||
| mGluR8 | ↔11,36 | ||||||||
T: Transcript, P: Protein, B. G.: Basal ganglia, ↑: increased, ↓: decreased, ↔: unchanged. 1Sokolov, 1998; 2Humphries et al., 1996; 3Beneyto and Meador-Woodruff, 2008; 4Dracheva et al., 2001; 5Le Corre et al., 2000; 6Beneyto et al., 2007; 7Kristiansen et al., 2006; 8Kristiansen et al., 2010a; 9Gao et al., 2000; 10Vrajova et al., 2010; 11Ibrahim et al., 2000; 12Clinton et al., 2003; 13Dracheva et al., 2008; 14Clinton and Meador-Woodruff, 2004; 15Clinton et al., 2006; 16Meador-Woodruff et al., 2001a; 17Mueller et al., 2004; 18Akbarian et al., 1996; 19Sodhi et al., 2011; 20Mueller and Meador-Woodruff, 2004; 21Dracheva et al., 2005; 22Hammond et al., 2010; 23Corti et al., 2011; 24Harrison et al., 1991; 25East-wood et al., 1995; 26Eastwood et al., 1997a; 27Breese et al., 1995; 28Beneyto and Meador-Woodruff, 2006; 29Eastwood et al., 1997a; 30Scarr et al., 2005; 31Meador-Woodruff et al., 2001a; 32Benes et al., 2001; 33Porter et al., 1997; 34Volk et al., 2010; 35Gupta, et al., 2005; 36Richardson-Burns et al., 2000; 37Ghose et al., 2009; 38Gonzalez-Maeso et al., 2008; 39Ghose et al., 2008; 40Crook et al., 2002; 41Ohnuma et al., 1998.
NMDA receptors
Converging evidence suggests decreased function of NMDA receptors in schizophrenia, which has resulted in numerous studies examining NMDA receptor expression in this illness (Table 3). Conflicting findings have been reported in different cortical regions. In the prefrontal cortex, transcript studies found increased GluN2D and decreased GluN2C subunit expression (Akbarian et al., 1996). In the dorsolateral prefrontal cortex (DLPFC), studies have reported both increased GluN1 and GluN3A transcript expression (Mueller and Meador-Woodruff, 2004; Dracheva et al., 2008) and decreased GluN1, GluN2A and GluN2C expression (Beneyto and Meador-Woodruff, 2008). Relatively fewer changes have been noted when proteins rather than transcripts have been studied in this area. Total cell homogenates and autoradiography studies found no differences in NMDA receptor subunit expression in the DLPFC, but when ER subfractions were studied, a selective decrease of GluN2B expression was found (Beneyto and Meador-Woodruff, 2008; Kristiansen et al., 2010a). In the anterior cingulated cortex (ACC), increased NMDA binding and increased protein expression of the GluN1-C2′ GluN1 isoform was found (Zavitsanou et al., 2002; Kristiansen et al., 2006). A selective increase of NMDA receptor binding was found in the posterior cingulate cortex (Newell et al., 2005). In the occipital cortex, increased GluN1 and GluN2A subunit transcripts have been reported (Humphries et al., 1996; Soko-lov, 1998; Le Corre et al., 2000; Dracheva et al., 2001). In the cerebellum, findings vary with polymorphisms of the neuregulin (NRG) gene. Gene expression of the GluN2D subunit was increased in the cerebellum of homozygous patients, while GluN2C subunit expression was decreased in patients with a NRG polymorphism (Schmitt et al., 2010).
In the hippocampal region, reduced MK801 binding suggested NMDA receptor abnormalities in schizophrenia (Beney-to et al., 2007). Supporting this, a study on pan and isoform specific GluN1 proteins reported decreased in total GluN1 and GluN1-4b isoform expression in the left hippocampus, and of the GluN1-2b isoform in the right hippocampus (Vrajova et al., 2010). Another study, focused on the identification of receptor transcript abnormalities in hippocampal subregions, found a selective decrease in GluN1 transcripts in the dentate gyrus in schizophrenia, and increased GluN2B transcripts in the CA2 area (Gao et al., 2000). These transcript data, however, conflict with other studies that show no change in GluN1 and GluN2 isoforms in hippocampal subregions in this illness (Mc-Cullumsmith et al., 2007). These data suggest the possibility of region and isoform specific GluN abnormalities in the hippocampus in schizophrenia.
Contradictory findings have been reported in the thalamus. GluN1 transcript has been reported to be decreased or un-changed, while GluN1 protein expression was found unchanged (Ibrahim et al., 2000; Clinton and Meador-Woodruff, 2004; Clinton et al., 2006). Similarly, GluN2B transcripts were reported to be decreased or increased in schizophrenia (Ibra-him et al., 2000; Clinton and Meador-Woodruff, 2004), while protein expression studies in this area have found increased GluN2B (Clinton et al., 2006). More recently, a comprehensive study of ionotropic glutamate receptor transcript expression in thalamic nuclei found no differences in GluN1 or GluN2 isoforms in schizophrenia (Dracheva et al., 2008). In the striatum, no abnormal ionotropic glutamate receptor transcript expression has been found (Meador-Woodruff et al., 2001b) but in the substantia nigra pars compacta, GluN1 was reported to be increased in schizophrenia (Mueller et al., 2004).
AMPA receptors
Examination of the expression of AMPA receptors in post-mortem brain in schizophrenia has yielded subunit-specific findings that also appear to be region specific (Table 3). As with NMDA receptors, variable and often contradictory data have been reported. In the prefrontal cortex of schizophrenia patients, a recent study found decreased GluA1 and GluA2 protein expression (Corti et al., 2011), while binding studies did not find changes in AMPA receptor expression in this area (Healy et al., 1998). At the transcript level, no changes in AMPA subunits were found (Healy et al., 1998). A recent report focusing on AMPA receptor trafficking in the DLPFC found an increase in GluA1 subunit protein expression in an early endosome compartment, suggesting trafficking abnormalities in this area (Hammond et al., 2010). Discrepancies have been found in mRNA studies in DLPFC in schizophrenia. While some studies report decreased GluA2 and GluA4 transcripts (Beneyto and Meador-Woodruff, 2006), others found an increase in GluA2 and GluA4 in this same area (Dracheva et al., 2005). In the occipital cortex, one study found increased GluA4 transcript levels (Dracheva et al., 2005).
In the hippocampus, decreased AMPA receptor binding in CA4/CA3 and CA2 regions have been reported in schizophrenia (Kerwin et al., 1990; Eastwood et al., 1997b; Gao et al., 2000), together with decreased expression of GluA1 and GluA2 AMPA receptor transcripts (Harrison et al., 1991; East-wood et al., 1995; Eastwood et al., 1997a). Another study, however, failed to show AMPA receptor binding changes in this area (Beneyto et al., 2007).
While some studies found no change in mRNA expression in the thalamus in schizophrenia (Dracheva et al., 2008), others report abnormal GluA1 and GluA3 subunit transcript ex-pression (Ibrahim et al., 2000). In the striatum, no changes in AMPA receptor have been reported in this illness (Meador-Woodruff et al., 2001b; Noga and Wang, 2002). In the caudate, however, two binding studies found opposite outcomes, where one found an increase in binding (Noga et al., 1997), and another found a decrease in binding in this area and in the nucleus accumbens (Noga and Wang, 2002).
Kainate receptors
Kainate receptor expression changes in schizophrenia are relatively less consistent than those seen for NMDA receptors (Table 3). In the prefrontal cortex, binding studies have found an increase, decrease or no change in kainate receptors (Nishikawa et al., 1983; Meador-Woodruff et al., 2001a; Zavitsanou et al., 2002; Scarr et al., 2005), while transcript studies report decreased GluK2 and GluK3 mRNA in this area (Soko-lov, 1998). In the DLPFC, conflicting transcript data has been reported, finding decreased or no change in GluK1 and decreased GluK5 levels (Meador-Woodruff et al., 2001a; Scarr et al., 2005). Transcript analysis also suggested altered GluK1 expression in the perirhinal cortex in schizophrenia (Beneyto et al., 2007; Woo et al., 2007), but several studies have failed to find any changes in protein expression of kainate receptor subunits in this area or in the ACC (Breese et al., 1995; Zavitsanou et al., 2002). A study measuring co-expression of GluK1 and GAD67 transcripts in the ACC in schizophrenia reported a decrease in GluK1 in GAD67 containing neurons (Beneyto et al., 2007). In the orbitofrontal cortex, a decrease in the number of cells expressing kainate receptors has been reported in this illness (Garey et al., 2006).
In the hippocampus, conflicting data have been reported. Binding studies report decreased or no change in kainate receptors (Kerwin et al., 1990; Gao et al., 2000); while decreased transcript expression of GluK2 and GluK5 subunits was found (Porter et al., 1997). At the protein level, a study in apical dendrites reported decreased dendritic expression of GluK1, K2 and K3 subunits in the CA1, CA2 and CA3 areas of the hippocampus (Benes et al., 2001), but another study in crude hippocampal extracts found no changes in the same kainate subunits in this area (Breese et al., 1995).
In the thalamus, a decrease in neuronal GluK2 and GluK3 and a decrease in GluK5 transcript have been reported (Ibra-him et al., 2000; Sodhi et al., 2011). No changes in kainate receptors were found in the striatum (Meador-Woodruff et al., 2001b).
Metabotropic glutamate receptors
There has been recent interest in the role of the metabotropic glutamate receptors in schizophrenia, especially focusing in the group II family (Table 3). Because of the lack of specific antibodies against mGluR2 and mGluR3, there have been conflicting reports on the expression of these receptors. Most recently, using specific antibodies targeted to mGluR3, the monomeric form of this receptor was shown to be decreased in prefrontal cortex in schizophrenia (Ghose et al., 2009). Another study with specific antibodies found no change of mGluR3 monomers in prefrontal cortex but a decrease in mGluR3 dimers (Corti et al., 2007). Most studies done at the transcript level have found no change in mGluR3 mRNA (Ohnuma et al., 1998; Richardson-Burns et al., 2000; Gupta et al., 2005; Ghose et al., 2008). Unlike mGluR3, mGluR2 protein expression appears to remain unchanged in the prefrontal cortex in schizophrenia (Crook et al., 2002; Ghose et al., 2008), while transcript level studies have produced conflicting results, showing an increase in the white matter of the prefrontal cortex or a decrease in mGluR2 expression in this same area (Ghose et al., 2008; Gonzalez-Maeso et al., 2008).
Group I metabotropic receptors modulate NMDA receptors and have been studied as possible candidates disrupted in schizophrenia. Genetic linkage studies suggest that mGluR5 is involved in schizophrenia, and increased mGluR5 and mGluR1 transcript, and mGluR1 protein expression have been found in prefrontal cortex in this illness (Ohnuma et al., 1998; Devon et al., 2001; Gupta et al., 2005; Volk et al., 2010), further supporting a possible role of group I metabotropic receptors in the pathophysiology of schizophrenia.
Group III metabotropic receptors have been less studied. Human polymorphism studies failed to find an association between mGluR4 and schizophrenia (Ohtsuki et al., 2001; Shibata et al., 2009), while more promising results were found for mGluR8 and mGluR7 in single nucleotide polymorphism screenings, where at least one susceptibility locus was described in each gene (Takaki et al., 2004; Shibata et al., 2009).
ASSOCIATED INTRACELLULAR PROTEINS ABNORMALITIES IN SCHIZOPHRENIA
NMDA associated proteins
Proteins that associate specifically with NMDA receptors, such as PSD95, PSD93, SAP102, and NF-L modulate NMDA receptor function by promoting clustering and anchoring at the synaptic membrane and regulating intracellular signaling (Sheng and Pak, 2000). These proteins have been found to be abnormal in multiple brain regions in schizophrenia (Table 4). In the thalamus, the transcript levels of SynGAP, an NMDA receptor interacting protein underlying signaling and trafficking of these receptors, were reported decreased in this area (Sodhi et al., 2011), but PSD95, SAP102, and NF-L transcripts were found to be either elevated or decreased (Clinton et al., 2003; Clinton and Meador-Woodruff, 2004; Clinton et al.,2006). PSD95 protein expression was increased in the thalamus, while in the prefrontal cortex no changes were seen in SAP102 protein expression (Toyooka et al., 2002; Clinton et al., 2003)
Table 4.
Glutamate receptor accessory protein abnormalities in schizophrenia versus comparison subjects
| Family | Subunit | Cortex | Hippocampus | Thalamus | B. G. | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| T | P | T | P | T | P | T | P | ||
|
| |||||||||
| NMDA | PSD-93 | ↑1 | ↓1 | ↔2 | ↔3,4 | ||||
| accessory | PSD-95 | ↑1,5/↓6/↔7 | ↓1,8,9 | ↔6 | ↑2/↓10 | ↑11 | ↔3,4 | ||
| proteins | SAP102 | ↔7 | ↓12 | ↑2/↓10 | ↔11 | ↓3,4 | |||
| NF-L | ↓7/↑1 | ↓1 | ↑2/↓10 | ↔11 | ↔3,4 | ||||
| SynGAP | ↓8 | ↓13 | |||||||
| APBA1 | ↑14 | ↔14 | |||||||
| mLin2/CASK | ↑14 | ↓14 | |||||||
| mLin7A/Veli-1 | ↑14 | ↔14 | |||||||
| mLin7C/Veli-3 | ↑14 | ↓14 | |||||||
| Kif17 | ↔14 | ↔14 | |||||||
| Yotiao | ↔4 | ||||||||
| AMPA | GRIP1 | ↑15 | ↑16/↔12 | ↓13 | |||||
| accessory | TARP2 | ↑17 | |||||||
| proteins | PICK1 | ↓17 | |||||||
| SAP97 | ↓12 | ↓12/↑16 | |||||||
| NSF | ↓18,19/↔17 | ↔17 | |||||||
| Syntenin | ↔17 | ||||||||
T: Transcript, P: Protein, B. G.: Basal ganglia, ↑: increased, ↓: decreased, ↔: unchanged. 1Kristiansen et al., 2006; 2Clinton et al., 2003; 3Kristiansen et al., 2010a; 4Mueller et al., 2004; 5Dracheva et al., 2001; 6Ohnuma et al., 2000; 7Beneyto and Meador-Wood-ruff, 2008; 8Funk et al., 2009; 9Kristiansen et al., 2010b; 10Clinton and Meador-Woodruff, 2004; 11Clinton et al., 2006; 12Toyooka et al., 2002; 13Sodhi et al., 2011; 14Kristiansen et al., 2010a; 15Dracheva et al., 2005; 16Hammond et al., 2010; 17Beneyto and Meador-Woodruff, 2006; 18Mirnics et al., 2000; 19Whiteheart and Matveeva, 2004.
At the transcript level, PSD95 was found decreased, increased or exhibiting no change in the cortex, while the pro-tein level was decreased (Ohnuma et al., 1998; Kristiansen et al., 2006; Beneyto and Meador-Woodruff, 2008; Funk et al., 2009; Kristiansen et al., 2010b). NF-L transcript expression was shown either decreased or increased in DLPFC (Beneyto and Meador-Woodruff, 2008), while it’s protein expression was reported to be decreased in this area (Kristiansen et al., 2006). SAP97, which is associated with AMPA GluA1 subunits but also targets NMDA and kainate receptors to dendritic spines, was found to be decreased in the prefrontal cortex in schizophrenia (Toyooka et al., 2002; Li et al., 2011). In the ACC, the protein expression of SynGAP was found decreased (Funk et al., 2009). Several microtubule-associated trafficking complex proteins, including KIF17, APBA1, CASK, and mLin7 were also found to be abnormally expressed at the transcript and protein level in the cortex in schizophrenia, suggesting abnormal NMDA receptor trafficking in this illness (Kristiansen et al., 2010a). In the hippocampus, only SAP102 protein has been found to be decreased (Toyooka et al., 2002).
AMPA associated proteins
Recent investigations have moved beyond studies of glutamate receptors to include direct measurement of glutamate receptor auxiliary molecules that regulate receptor function and localization (Table 4). Significant and region-specific changes in these molecules have been reported in schizophrenia. In the DLPFC, decreased transcript expression of PICK1, a protein that interacts with GluA2-4 containing AMPA receptors, was found (Dev et al., 1999; Lu and Ziff, 2005; Beneyto and Meador-Woodruff, 2006). Decreased transcript expression of SAP97, a GluA1-interacting protein, was reported in the DLPFC, while protein studies of this molecule in this area yielded conflicting results, being increased in one study, but decreased in another (Toyooka et al., 2002; Ham-mond et al., 2010). TARP2 transcript was also reported to be increased in the DLPFC in schizophrenia, suggesting abnormal intracellular receptor localization in this illness (Beneyto, 2006). The transcript expression of NSF, a GluA2 interacting protein, and SAP97, a GluA1 associated protein, were found to be decreased in the prefrontal cortex (Mirnics et al., 2000; Toyooka et al., 2002; Whiteheart and Matveeva, 2004), while SAP97 protein expression was found to be either increased or decreased in this area (Hammond et al., 2010). GRIP, which interacts with the GluA2 subunit, is increased at both the transcript and protein level in the cortex (Dracheva et al., 2005; Hammond et al., 2010). mRNA levels of GRIP and ABP, another receptor interacting protein, were also increased in the occipital cortex (Dracheva et al., 2005). In the thalamus, a decrease in transcript expression of GRIP1 has been reported (Sodhi et al., 2011).
ANTIPSYCHOTIC DRUGS TARGETING GLUTAMATERGIC RECEPTORS
Given that multiple studies in the brain in schizophrenia have revealed abnormalities in the molecules associated with glutamate neurotransmission in this illness, new drug development targeting the glutamate synapse has become an area of great interest. Glutamatergic neurotransmission involves many molecules, including pre- and post-synaptic receptors, intracellular receptor-interacting proteins that link glutamate receptors to signal transduction pathways, and membrane-and vesicle-bound glutamate transporters. All of these molecules are potential targets for drug development. Recent efforts have focused on modulation of the glutamate receptors, especially the NMDA subtype, as reviewed below. Given the complexity of the glutamate synapse, however, there is great potential for further new drug development that could profitably target other molecules associated with glutamate neuro-transmission for use in treating this psychiatric illness.
NMDA receptor co-agonist site modulators
Because NMDA receptor hypofunction has been implicated in schizophrenia, treatment of this illness by modulating this system by the administration of glutamate or a related agonist would superficially seem to be an obvious starting point. However, excessive activation of glutamate receptors can lead to neurotoxicity. Toxicity and pharmacodynamic/kinetic issues have led to the development of alternative pharmacological approaches to modulate NMDA and other glutamate receptors.
D-Serine is a selective, full agonist at the glycine co-agonist site on NMDA receptors, and as such it increases the activation of the receptor in the presence of endogenous glutamate. Patients with schizophrenia showed an improvement in positive, negative and cognitive symptoms when 30 mg/kg/day of D-serine was added as an adjuvant to non-clozapine antipsychotic regimes (Tsai et al., 1998; Heresco-Levy et al., 2005). In contrast, another trial showed no differences between placebo and D-serine adjuvant therapy (Lane et al., 2010). A recent meta-analysis taking into consideration the number of trials and sample size in subgroup analyses, concluded than when added to non-clozapine antipsychotic treatment, D-Serine is therapeutically beneficial, resulting in improvement of negative and total schizophrenia symptoms (Singh and Singh, 2011).
Glycine, another full agonist of the NMDA co-agonist site, has been tested by itself and as also an adjuvant for treatment of schizophrenia. The most compelling evidence of the benefits of glycine adjuvant treatment in schizophrenia comes from a series of glycine augmentation studies done by Javitt et al. (Javitt et al., 1994; Javitt, 1996; Javitt et al., 2001; Javitt, 2002). Increasing doses of glycine from 30 g/day to 60 g/day have found improvement of both negative and cognitive symptoms (Javitt et al., 1994; Javitt, 1996; Javitt et al., 2001; Javitt, 2002). Similarly, a meta-analysis showed that glycine improved positive and total symptom scores when used as an adjuvant to non-clozapine antipsychotics, but worsened these symptoms when added to clozapine (Singh and Singh, 2011). Overall, glycine appears to be effective in the treatment of schizophrenia when given as an adjuvant to non-clozapine antipsychotic treatment.
D-cycloserine is an NMDA glycine binding site partial agonist that when added to non-clozapine antipsychotic drugs can be effective for treating the negative symptoms of schizophrenia but within a narrow therapeutic window (Goff et al., 1999a; Evins et al., 2002; Heresco-Levy and Javitt, 2004; Goff et al., 2005). Optimal doses of D-cycloserine were established at 50 mg/kg, with larger doses of 250 mg/kg resulting in worsening of positive symptoms (Buchanan et al., 2007). Interestingly, worsening of negative symptoms was seen when D-cycloserine was added to atypical antipsychotics such as clozapine (Goff et al., 1996; Goff et al., 1999b). More recently, a trial comparing chronic administration of D-cycloserine in addition to conventional antipsychotics found no improvement (Goff et al., 2005). Because of the conflicting outcomes of these studies, the efficacy of D-cycloserine in the treatment of schizophrenia remains controversial.
One study has investigated the effect of D-alanine, an endogenous full agonist of the co-agonist site of the NMDA receptor, in schizophrenia (Tsai et al., 2006). This study was a double blind placebo-controlled trial of 100 mg/kg/day of D-alanine added to antipsychotic treatment. This resulted in significant improvement in positive, negative and cognitive symptoms, making D-alanine another promising adjuvant for schizophrenia treatment.
Sarcosine, a glycine transporter-1 inhibitor, activates the NMDA receptor co-agonist site by increasing the concentration of glycine in the synaptic cleft (Bergeron et al., 1998; Javitt, 2006). Similar to other glycine modulators, sarcosine had no effect when added to clozapine (Lane et al., 2006). However, when 2 g/day were added to conventional antipsychotic drugs or risperidone, there was a significant improvement of positive, negative, and depressive symptoms and general psychopathology scores in schizophrenia (Tsai et al., 2004). A recent meta-analysis has shown that sarcosine improves negative and total symptom scores in schizophrenia (Singh and Singh, 2011). Sarcosine was also beneficial for acute exacerbation of schizophrenia symptoms, improving the response to standard medication (Lane et al., 2008) and possibly being more beneficial than D-serine adjuvant therapy in this phase of the illness (Lane et al., 2005).
Milacemide is a prodrug of glycine which readily crosses the blood brain barrier and was studied as a possible NMDA receptor agonist. Studies in schizophrenia have not yielded promising results, since low doses of this compound have no effect in schizophrenia symptoms while high doses exacerbate negative symptoms (Rosse et al., 1990; Rosse et al., 1991).
In summary, NMDA receptor glycine co-agonist site modulators are promising targets for adjuvants of an ongoing conventional antipsychotic drug treatment. Their use with atypical antipsychotics, however, may result in worsening of symptoms in schizophrenia, which may be due to unique effects of atypical antipsychotics on NMDA receptors.
AMPA receptor potentiators
Given the importance of AMPA receptors for fast excitatory neurotransmission and alterations of AMPA receptor subunits and associated proteins in schizophrenia, targeting these receptors may have potential therapeutic roles in the treatment of this illness. Ampakines are a novel class of compounds that function by decreasing desensitization and deactivation rates of the AMPA channel, effectively increasing the opening time of the channel and the receptor response to glutamate (Arai et al., 1996). There are several families of AMPA receptor potentiators: pyrrolidinones, which include piracetam and aniracetam; the ampakines 1-BCP, Org 26576, Org 24448, CX516 and CX546; benzothiadiazides, such as cyclothiazide; and the biarylpropylsulfonamide, LY404187 (Jordan et al., 2005). Preclinical studies have shown these drugs can facilitate LTP, learning and memory, hippocampal neurogenesis and neurotrophic factor expression. They also act in an antidepressant-like manner and affect serotonin neurotransmission (Jordan et al., 2005; Goff et al., 2008).
Piracetam was the first ampakine to be studied in schizophrenia, and was found to improve positive symptoms when combined with haloperidol during an 8-week trial (Noorbala et al., 1999). A preliminary trial performed by Goff et al. (Goff et al., 2001) evaluated the effect of CX516 on 19 schizophrenia patients being simultaneously treated with clozapine, and found improvements in executive brain functions such as memory and attention. However, a follow-up study by the same group found no change in cognition or other schizophrenia symptoms when patients were treated with CX516 and either clozapine, olanzapine, or risperidone for 4 weeks (Goff et al., 2008). Although ampakines may theoretically be a promising therapeutic approach, there is currently no clear clinical evidence of their effectiveness in treating the symptoms of schizophrenia.
mGluR modulators
Given the modulatory action of mGluRs on NMDA receptors, a number of positive allosteric modulators targeted to type II mGluRs are currently in development (Imre, 2007). Because the binding sites for ligands across members of the mGluR subtypes are often highly conserved, it is difficult to achieve subtype specific selectivity. A glutamate analog that selectively binds to mGluR2/3, LY-404039, has displayed promising anti-psychotic effects improving positive and negative symptoms in schizophrenia (Chaki, 2010; Chaki and Hikichi, 2011). Studies in a rodent model have shown that LY-404039 acts through the activation of mGluR2 rather than mGluR3, suggesting an association of mGluR2 with antipsychotic-like properties. This, however, has not yet been evaluated clinically (Fell et al., 2008). Further suggesting a promising role for mGluR2 modulators in the treatment of psychotic symptoms, a selective potentiator of mGluR2, LY-487379, has shown promising results in rodents models (Galici et al., 2005). More recently, several highly selective allosteric modulators of mGluR5 have been developed (Kanuma et al., 2010), and together with other mGluR activators are targets of studies for the treatment of psychiatric and neurological disorders.
Kainate receptor antagonists
While NMDA and AMPA receptors have been the targets for most drug discovery around glutamate receptors, relatively little has been done to target the kainate receptor. The AMPA/kainate receptor antagonist LY293558 has been shown to ameliorate psychotic-like symptoms induced by ketamine (Moghaddam et al., 1997), but no clinical trials have been done with this compound or any other selective kainate receptor antagonist. Topiramate, a drug with partial activity, is a kai-nate receptor antagonist, reduces mood symptoms but does not have additional effects on positive or negative symptoms in schizophrenia when administered with other antipsychotics (Tiihonen et al., 2005).
Other glutamatergic drugs
Memantine is an NMDA receptor antagonist, used for the treatment of Alzheimer’s disease. It acts as a non-competitive, low-affinity, voltage-dependent NMDA receptor antagonist that binds to the magnesium blocking site with higher affinity than magnesium, thus inhibiting the influx of calcium ions (Kornhu-ber et al., 1989). Several studies have found that memantine given together with clozapine improves positive and negative symptoms in refractory schizophrenia patients (Gama et al., 2005; Krivoy et al., 2008; de Lucena et al., 2009). Another large study, however, did not find an improvement in schizophrenia symptoms with adjuvant memantine treatment but rather found an increase in adverse effects (Lieberman et al., 2009). Although the effects of memantine on positive and negative symptoms of schizophrenia have conflicting results, memantine has proven useful for the treatment of catatonia (Thomas et al., 2005; Carpenter et al., 2006; Carroll et al.,2006).
First used to treat alcoholic patients in Europe, acamprosate is currently being considered for the treatment of schizophrenia due to its modulatory action on synaptic glutamate release and NMDA receptor function (Naassila et al., 1998; De Witte et al., 2005; Paz et al., 2008). Interestingly, acamprosate may act through mGluR5 without abolishing normal synaptic transmission. Acamprosate can also bind to the poly-amine modulator site of the NMDA receptor and may be able to act as either an agonist or antagonist at the NMDA receptor (Paz et al., 2008). Studies have yet to be reported examining the effectiveness of this drug on treating schizophrenia-like symptoms; however, in patients with schizophrenia and alcohol dependence, Ralevski et al. found that treatment with acamprosate did not negatively affect cognitive outcome or levels of alcohol consumption in these patients (Tek et al., 2008; Ralevski et al., 2011).
SUMMARY
Abnormalities of dopaminergic neurotransmission have long been held to be critical to understanding the pathophysiology of schizophrenia, and most treatments have targeted dopamine receptors. Recent advances have pointed to abnormalities of other neurotransmitters being involved in schizophrenia, especially dysregulation of glutamate transmission in this illness. Numerous studies have now been reported that have measured molecules associated with glutamate neurotransmission in the brain in schizophrenia, and abnormalities of multiple receptors and receptor-associated proteins have now been identified. Glutamatergic neurotransmission involves myriad molecules, including pre- and post-synaptic receptors, intracellular receptor-interacting proteins that link glutamate receptors to signal transduction pathways, and membrane- and vesicle-bound glutamate transporters which remove glutamate from the synaptic cleft and then package it into vesicles for release. Of the large number of molecules associated with glutamatergic neurotransmission, the strongest evidence for abnormalities of this transmitter system in schizophrenia involves disturbances of the glutamate receptors, specifically the NMDA and AMPA subtypes. Recent efforts at new treatment development have focused on modulation of the NMDA and AMPA receptors, and more recently the metabotropic glutamate receptors have been targeted as well. Early results, especially strategies that target the glycine-binding co-agonist site of the NMDA receptor, have been promising thus far. Given the complexity of the glutamate synapse, many other molecules are potential targets for new drug discovery in attempts to develop more effective treatments for this illness.
References
- 1.Aanonsen L. M. Wilcox G. L. Phencyclidine selectively blocks a spinal action of N-methyl-D-aspartate in mice. Neurosci. Lett. (1986);67:191–197. doi: 10.1016/0304-3940(86)90396-4. [DOI] [PubMed] [Google Scholar]
- 2.Aihara Y. Mashima H. Onda H. Hisano S. Kasuya H. Hori T. Yamada S. Tomura H. Yamada Y. Inoue I. Kojima I. Takeda J. Molecular cloning of a novel brain-type Na(+)-dependent inorganic phosphate cotransporter. J. Neurochem. (2000);74:2622–2625. doi: 10.1046/j.1471-4159.2000.0742622.x. [DOI] [PubMed] [Google Scholar]
- 3.Akbarian S. Sucher N. J. Bradley D. Tafazzoli A. Trinh D. Het-rick W. P. Potkin S. G. Sandman C. A. Bunney W. E. Jr. Jones E. G. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J. Neurosci. (1996);16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anggono V. Clem R. L. Huganir R. L. PICK1 loss of function occludes homeostatic synaptic scaling. J. Neurosci. (2011);31:2188–2196. doi: 10.1523/JNEUROSCI.5633-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai A. Kessler M. Rogers G. Lynch G. Effects of a memory-enhancing drug on DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and synaptic transmission in hippocampus. J. Pharmacol. Exp. Ther. (1996);278:627–638. [PubMed] [Google Scholar]
- 6.Araque A. Parpura V. Sanzgiri R. P. Haydon P. G. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. (1999);22:208–215. doi: 10.1016/S0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 7.Arvanov V. L. Wang R. Y. Clozapine but not haloperidol prevents the functional hyperactivity of N-methyl-D-aspartate receptors in rat cortical neurons induced by subchronic administration of phencyclidine. J. Pharmacol. Exp. Ther. (1999);289:1000–1006. [PubMed] [Google Scholar]
- 8.Bar-Peled O. Ben-Hur H. Biegon A. Groner Y. Dewhurst S. Furuta A. Rothstein J. D. Distribution of glutamate transporter subtypes during human brain development. J. Neurochem. (1997);69:2571–2580. doi: 10.1046/j.1471-4159.1997.69062571.x. [DOI] [PubMed] [Google Scholar]
- 9.Bats C. Groc L. Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. (2007);53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Bellocchio E. E. Reimer R. J. Fremeau R. T. Jr. Edwards R. H. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. (2000);289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- 11.Benes F. M. Todtenkopf M. S. Kostoulakos P. GluR5,6,7 subunit immunoreactivity on apical pyramidal cell dendrites in hippocampus of schizophrenics and manic depressives. Hippocampus. (2001);11:482–491. doi: 10.1002/hipo.1065. [DOI] [PubMed] [Google Scholar]
- 12.Beneyto M. Kristiansen L. V. Oni-Orisan A. McCullumsmith R. E. Meador-Woodruff J. H. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. (2007);32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 13.Beneyto M. Meador-Woodruff J. H. Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse. (2006);60:585–598. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- 14.Beneyto M. Meador-Woodruff J. H. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. (2008);33:2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- 15.Bergeron R. Meyer T. M. Coyle J. T. Greene R.W. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc. Natl. Acad. Sci. USA. (1998);95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettler B. Boulter J. Hermans-Borgmeyer I. O'Shea-Greenfield A. Deneris E. S. Moll C. Borgmeyer U. Hollmann M. Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. (1990);5:583–595. doi: 10.1016/0896-6273(90)90213-Y. [DOI] [PubMed] [Google Scholar]
- 17.Bleakman D. Lodge D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology. (1998);37:1187–1204. doi: 10.1016/S0028-3908(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 18.Boulter J. Hollmann M. O'Shea-Greenfield A. Hartley M. Deneris E. Maron C. Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. (1990);249:1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- 19.Bredt D. S. Nicoll R. A. AMPA receptor trafficking at excitatory synapses. Neuron. (2003);40:361–379. doi: 10.1016/S0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 20.Breese C. R. Freedman R. Leonard S. S. Glutamate receptor subtype expression in human postmortem brain tissue from schizophrenics and alcohol abusers. Brain Res. (1995);674:82–90. doi: 10.1016/0006-8993(94)01384-T. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan R. W. Carpenter W. T. Schizophrenia: Introduction and Overview. In Comprehensive Textbook of Psychiatry, 7th Edition (B. J. Sadock, V. A. Sadock, Eds) Lippin-cott, Williams, and Wilkins; Philadelphia.: (2000). pp. 1096–1110. [Google Scholar]
- 22.Buchanan R. W. Javitt D. C. Marder S. R. Schooler N. R. Gold J. M. McMahon R. P. Heresco-Levy U. Carpenter W. T. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am. J. Psychiatry. (2007);164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter S. S. Hatchett A. D. Fuller M. A. Catatonic schizophrenia and the use of memantine. Ann. Pharmacother. (2006);40:344–346. doi: 10.1345/aph.1G297. [DOI] [PubMed] [Google Scholar]
- 24.Carroll B. T. Thomas C. Jayanti K. Amantadine and memantine in catatonic schizophrenia. Ann. Clin. Psychiatry. (2006);18:133–134. doi: 10.1080/10401230600614710. [DOI] [PubMed] [Google Scholar]
- 25.Chaki S. Group II metabotropic glutamate receptor agonists as a potential drug for schizophrenia. Eur. J. Pharmacol. (2010);639:59–66. doi: 10.1016/j.ejphar.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 26.Chaki S. Hikichi H. Targeting of metabotropic glutamate receptors for the treatment of schizophrenia. Curr. Pharm. Des. (2011);17:94–102. doi: 10.2174/138161211795049570. [DOI] [PubMed] [Google Scholar]
- 27.Chen L. El-Husseini A. Tomita S. Bredt D. S. Nicoll R. A. Stargazin differentially controls the trafficking of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate and kainate receptors. Mol. Pharmacol. (2003);64:703–706. doi: 10.1124/mol.64.3.703. [DOI] [PubMed] [Google Scholar]
- 28.Chen L. Chetkovich D. M. Petralia R. S. Sweeney N. T. Kawasaki Y. Wenthold R. J. Bredt D. S. Nicoll R. A. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. (2000);408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 29.Chung H. J. Huang Y. H. Lau L. F. Huganir R. L. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J. Neurosci. (2004);24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Citri A. Bhattacharyya S. Ma C. Morishita W. Fang S. Rizo J. Malenka R. C. Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. J. Neurosci. (2010);30:16437–16452. doi: 10.1523/JNEUROSCI.4478-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clem R. L. Anggono V. Huganir R. L. PICK1 regulates incorporation of calcium-permeable AMPA receptors during cortical synaptic strengthening. J. Neurosci. (2010);30:6360–6366. doi: 10.1523/JNEUROSCI.6276-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinton S. M. Haroutunian V. Davis K. L. Meador-Woodruff J. H. Altered transcript expression of NMDA receptor-associated postsynaptic proteins in the thalamus of subjects with schizophrenia. Am. J. Psychiatry. (2003);160:1100–1109. doi: 10.1176/appi.ajp.160.6.1100. [DOI] [PubMed] [Google Scholar]
- 33.Clinton SM. Haroutunian V. Meador-Woodruff JH. Up-regulation of NMDA receptor subunit and post-synaptic density protein expression in the thalamus of elderly patients with schizophrenia. J. Neurochem. (2006);98:1114–1125. doi: 10.1111/j.1471-4159.2006.03954.x. [DOI] [PubMed] [Google Scholar]
- 34.Clinton S. M. Meador-Woodruff J. H. Abnormalities of the NMDA Receptor and Associated Intracellular Molecules in the Thalamus in Schizophrenia and Bipolar Disorder. Neuropsychopharmacology. (2004);29:1353–1362. doi: 10.1038/sj.npp.1300451. [DOI] [PubMed] [Google Scholar]
- 35.Collingridge G. L. Olsen R. W. Peters J. Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. (2009);56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coombs I. D. Cull-Candy S. G. Transmembrane AMPA receptor regulatory proteins and AMPA receptor function in the cerebellum. Neuroscience. (2009);162:656–665. doi: 10.1016/j.neuroscience.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Copits B. A. Robbins J. S. Frausto S. Swanson G. T. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J. Neurosci. (2011);31:7334–7340. doi: 10.1523/JNEUROSCI.0100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corti C. Crepaldi L. Mion S. Roth A. L. Xuereb J. H. Ferraguti F. Altered dimerization of metabotropic glutamate receptor 3 in schizophrenia. Biol. Psychiatry. (2007);62:747–755. doi: 10.1016/j.biopsych.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Corti C. Xuereb J. H. Crepaldi L. Corsi M. Michielin F. Ferraguti F. Altered levels of glutamatergic receptors and Na+/K+ ATPase-alpha1 in the prefrontal cortex of subjects with schizophrenia. Schizophr. Res. (2011);128:7–14. doi: 10.1016/j.schres.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Cousins S. L. Papadakis M. Rutter A. R. Stephenson F. A. Differential interaction of NMDA receptor subtypes with the post-synaptic density-95 family of membrane associated guanylate kinase proteins. J. Neurochem. (2008);104:903–913. doi: 10.1111/j.1471-4159.2007.05067.x. [DOI] [PubMed] [Google Scholar]
- 41.Coyle J. T. The glutamatergic dysfunction hypothesis for schizophrenia. Harv. Rev. Psychiatry. (1996);3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 42.Crook J. M. Akil M. Law B. C. Hyde T. M. Kleinman J. E. Comparative analysis of group II metabotropic glutamate receptor immunoreactivity in Brodmann's area 46 of the dorsolat-eral prefrontal cortex from patients with schizophrenia and normal subjects. Mol. Psychiatry. (2002);7:157–164. doi: 10.1038/sj.mp.4000966. [DOI] [PubMed] [Google Scholar]
- 43.Cuadra A. E. Kuo S. H. Kawasaki Y. Bredt D. S. Chetkovich D. M. AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST. J. Neurosci. (2004);24:7491–7502. doi: 10.1523/JNEUROSCI.1255-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cull-Candy S. Brickley S. Farrant M. NMDA receptor subunits: diversity development and disease. Curr. Opin. Neurobiol. (2001);11:327–335. doi: 10.1016/S0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 45.Danbolt N. C. Glutamate uptake. Prog. Neurobiol. (2001);65:1–105. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 46.de Lucena D. Fernandes B. S. Berk M. Dodd S. Medeiros D.W. Pedrini M. Kunz M. Gomes F. A. Giglio L. F. Lobato M. I. Belmonte-de-Abreu P. S. Gama C. S. Improvement of negative and positive symptoms in treatment-refractory schizo-phrenia:a double-blind, randomized, placebo-controlled trial with memantine as add-on therapy to clozapine. J. Clin. Psychiatry. (2009);70:1416–1423. doi: 10.4088/JCP.08m04935gry. [DOI] [PubMed] [Google Scholar]
- 47.De Witte P. Littleton J. Parot P. Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs. (2005);19:517–537. doi: 10.2165/00023210-200519060-00004. [DOI] [PubMed] [Google Scholar]
- 48.Dev K. K. Nishimune A. Henley J. M. Nakanishi S. The protein kinase C alpha binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. (1999);38:635–644. doi: 10.1016/S0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 49.Devon R. S. Anderson S. Teague P. W. Muir W. J. Murray V. Pe-losi A. J. Blackwood D. H. Porteous D. J. The genomic organisation of the metabotropic glutamate receptor subtype 5 gene and its association with schizophrenia. Mol. Psychiatry. (2001);6:311–314. doi: 10.1038/sj.mp.4000848. [DOI] [PubMed] [Google Scholar]
- 50.Diaz E. Regulation of AMPA receptors by transmembrane accessory proteins. Eur. J. Neurosci. (2010);32:261–268. doi: 10.1111/j.1460-9568.2010.07357.x. [DOI] [PubMed] [Google Scholar]
- 51.Dracheva S. McGurk S. R. Haroutunian V. mRNA expression of AMPA receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J. Neurosci. Res. (2005);79:868–878. doi: 10.1002/jnr.20423. [DOI] [PubMed] [Google Scholar]
- 52.Dracheva S. Byne W. Chin B. Haroutunian V. Ionotropic glutamate receptor mRNA expression in the human thalamus: absence of change in schizophrenia. Brain Res. (2008);1214:23–34. doi: 10.1016/j.brainres.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dracheva S. Marras S. A. Elhakem S. L. Kramer F. R. Davis K. L. Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am. J. Psychiatry. (2001);158:1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- 54.Eastwood S. L. Burnet P. W. Harrison P. J. GluR2 glutamate receptor subunit flip and flop isoforms are decreased in the hippocampal formation in schizophrenia: a reverse transcriptase-polymerase chain reaction (RT-PCR) study. Brain Res. Mol. Brain Res. (1997a);44:92–98. doi: 10.1016/S0169-328X(96)00195-7. [DOI] [PubMed] [Google Scholar]
- 55.Eastwood S. L. Kerwin R. W. Harrison P. J. Immuno-autoradiographic evidence for a loss of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate-preferring non-N-methyl-D-aspartate glutamate receptors within the medial temporal lobe in schizophrenia. Biol. Psychiatry. (1997b);41:636–643. doi: 10.1016/S0006-3223(96)00220-X. [DOI] [PubMed] [Google Scholar]
- 56.Eastwood S. L. McDonald B. Burnet P. W. Beckwith J. P. Kerwin R. W. Harrison P. J. Decreased expression of mRNAs encoding non-NMDA glutamate receptors GluR1 and GluR2 in medial temporal lobe neurons in schizophrenia. Brain Res. Mol. Brain Res. (1995);29:211–223. doi: 10.1016/0169-328X(94)00247-C. [DOI] [PubMed] [Google Scholar]
- 57.Egebjerg J. Bettler B. Hermans-Borgmeyer I. Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. (1991);351:745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- 58.Ehlers M. D. Fung E. T. O'Brien R. J. Huganir R. L. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J. Neurosci. (1998);18:720–730. doi: 10.1523/JNEUROSCI.18-02-00720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evins A. E. Amico E. Posever T. A. Toker R. Goff D. C. D-Cycloserine added to risperidone in patients with primary negative symptoms of schizophrenia. Schizophr. Res. (2002);56:19–23. doi: 10.1016/S0920-9964(01)00220-1. [DOI] [PubMed] [Google Scholar]
- 60.Fell M. J. Svensson K. A. Johnson B. G. Schoepp D. D. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039). J.Pharmacol. Exp. Ther. (2008);326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- 61.Fremeau R. T. Jr. Burman J. Qureshi T. Tran C. H. Proctor J. Johnson J. Zhang H. Sulzer D. Copenhagen D. R. Storm-Mathisen J. Reimer R. J. Chaudhry F. A. Edwards R. H. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc. Natl. Acad. Sci. USA. (2002);99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Funk A. J. Rumbaugh G. Harotunian V. McCullumsmith R. E. Meador-Woodruff J. H. Decreased expression of NMDA receptor-associated proteins in frontal cortex of elderly patients with schizophrenia. Neuroreport. (2009);20:1019–1022. doi: 10.1097/WNR.0b013e32832d30d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galici R. Echemendia N. G. Rodriguez A. L. Conn P. J. A selective allosteric potentiator of metabotropic glutamate (mGlu)2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J. Pharmacol. Exp. Ther. (2005);315:1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- 64.Gama C. S. Antunes P. Moser C. Belmonte-de-Abreu P. S. [Memantine as an adjunctive therapy for schizophrenia negative symptoms]. Rev. Bras. Psiquiatr. (2005);27:257–258. doi: 10.1590/s1516-44462005000300023. [DOI] [PubMed] [Google Scholar]
- 65.Gao X. M. Sakai K. Roberts R. C. Conley R. R. Dean B. Tamminga C. A. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am. J. Psychiatry. (2000);157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 66.Garey L. J. Von Bussmann K. A. Hirsch S. R. Decreased numerical density of kainate receptor-positive neurons in the orbitofrontal cortex of chronic schizophrenics. Exp. Brain Res. (2006);173:234–242. doi: 10.1007/s00221-006-0396-8. [DOI] [PubMed] [Google Scholar]
- 67.Gesemann M. Lesslauer A. Maurer C. M. Schonthaler H. B. Neuhauss S. C. Phylogenetic analysis of the vertebrate excitatory/neutral amino acid transporter (SLC1/EAAT) family reveals lineage specifi c subfamilies. BMC Evol. Biol. (2010);10:117. doi: 10.1186/1471-2148-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghose S. Crook J. M. Bartus C. L. Sherman T. G. Herman M. M. Hyde T. M. Kleinman J. E. Akil M. Metabotropic glutamate receptor 2 and 3 gene expression in the human prefrontal cortex and mesencephalon in schizophrenia. Int. J. Neurosci. (2008);118:1609–1627. doi: 10.1080/00207450802330702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghose S. Gleason K. A. Potts B. W. Lewis-Amezcua K. Tamminga C. A. Differential expression of metabotropic glutamate receptors 2 and 3 in schizophrenia: a mechanism for antipsychotic drug action? Am. J. Psychiatry. (2009);166:812–820. doi: 10.1176/appi.ajp.2009.08091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goff D. C. Henderson D. C. Evins A. E. Amico E. A placebo-controlled crossover trial of D-cycloserine added to clozapine in patients with schizophrenia. Biol. Psychiatry. (1999a);45:512–514. doi: 10.1016/S0006-3223(98)00367-9. [DOI] [PubMed] [Google Scholar]
- 71.Goff D. C. Herz L. Posever T. Shih V. Tsai G. Henderson D. C. Freudenreich O. Evins A. E. Yovel I. Zhang H. Schoenfeld D. A six-month, placebo-controlled trial of D-cycloserine co-administered with conventional antipsychotics in schizophrenia patients. Psychopharmacology (Berl) (2005);179:144–150. doi: 10.1007/s00213-004-2032-2. [DOI] [PubMed] [Google Scholar]
- 72.Goff D. C. Lamberti J. S. Leon A. C. Green M. F. Miller A. L. Patel J. Manschreck T. Freudenreich O. Johnson S. A. A placebo-controlled add-on trial of the Ampakine, CX516, for cognitive deficits in schizophrenia. Neuropsychopharmacology. (2008);33:465–472. doi: 10.1038/sj.npp.1301444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goff D. C. Leahy L. Berman I. Posever T. Herz L. Leon A. C. Johnson S. A. Lynch G. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J. Clin. Psychopharmacol. (2001);21:484–487. doi: 10.1097/00004714-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Goff D. C. Tsai G. Levitt J. Amico E. Manoach D. Schoenfeld D. A. Hayden D. L. McCarley R. Coyle J. T. A placebocontrolled trial of D-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch. Gen. Psychiatry. (1999b);56:21–27. doi: 10.1001/archpsyc.56.1.21. [DOI] [PubMed] [Google Scholar]
- 75.Goff D. C. Tsai G. Manoach D. S. Flood J. Darby D. G. Coyle J. T. D-cycloserine added to clozapine for patients with schizophrenia. Am. J. Psychiatry. (1996);153:1628–1630. doi: 10.1176/ajp.153.12.1628. [DOI] [PubMed] [Google Scholar]
- 76.Goff D. C. Wine L. Glutamate in schizophrenia: clinical and research implications. Schizophr. Res. (1997);27:157–168. doi: 10.1016/S0920-9964(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Maeso J. Ang R. L. Yuen T. Chan P. Weisstaub N. V. Lopez-Gimenez J. F. Zhou M. Okawa Y. Callado L. F. Milli-gan G. Gingrich J. A. Filizola M. Meana J. J. Sealfon S.C. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. (2008);452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Granger A. J. Gray J. A. Lu W. Nicoll R. A. Genetic analysis of neuronal ionotropic glutamate receptor subunits. J. Physiol. (2011);589:4095–4101. doi: 10.1113/jphysiol.2011.213033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greger I. H. Esteban J. A. AMPA receptor biogenesis and trafficking. Curr. Opin. Neurobiol. (2007);17:289–297. doi: 10.1016/j.conb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Gupta D. S. McCullumsmith R. E. Beneyto M. Haroutunian V. Davis K. L. Meador-Woodruff J. H. Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse. (2005);57:123–131. doi: 10.1002/syn.20164. [DOI] [PubMed] [Google Scholar]
- 81.Hammond J. C. McCullumsmith R. E. Funk A. J. Haroutunian V. Meador-Woodruff J. H. Evidence for abnormal forward trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology. (2010);35:2110–2119. doi: 10.1038/npp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harrison P. J. McLaughlin D. Kerwin R. W. Decreased hippocampal expression of a glutamate receptor gene in schizophrenia. Lancet. (1991);337:450–452. doi: 10.1016/0140-6736(91)93392-M. [DOI] [PubMed] [Google Scholar]
- 83.Hashimoto K. Fukaya M. Qiao X. Sakimura K. Watanabe M. Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J. Neurosci. (1999);19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hayashi Y. Shi S. H. Esteban J. A. Piccini A. Poncer J. C. Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. (2000);287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 85.Healy D. J. Haroutunian V. Powchik P. Davidson M. Davis K. L. Watson S. J. Meador-Woodruff J. H. AMPA receptor binding and subunit mRNA expression in prefrontal cortex and striatum of elderly schizophrenics. Neuropsychopharmacology. (1998);19:278–286. doi: 10.1016/S0893-133X(98)00014-1. [DOI] [PubMed] [Google Scholar]
- 86.Heresco-Levy U. Javitt D. C. Comparative effects of glycine and D-cycloserine on persistent negative symptoms in schizophrenia: a retrospective analysis. Schizophr. Res. (2004);66:89–96. doi: 10.1016/S0920-9964(03)00129-4. [DOI] [PubMed] [Google Scholar]
- 87.Heresco-Levy U. Javitt D. C. Ebstein R. Vass A. Lichtenberg P. Bar G. Catinari S. Ermilov M. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol. Psychiatry. (2005);57:577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 88.Hertzmann M. Reba R. C. Kotlyarov E. V. Single photon emission computed tomography in phencyclidine and related drug abuse. Am. J. Psychiatry. (1990);147:255–256. doi: 10.1176/ajp.147.2.255b. [DOI] [PubMed] [Google Scholar]
- 89.Hollmann M. Heinemann S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 90.Humphries C. Mortimer A. Hirsch S. de Belleroche J. NMDA receptor mRNA correlation with antemortem cognitive impairment in schizophrenia. Neuroreport. (1996);7:2051–2055. doi: 10.1097/00001756-199608120-00040. [DOI] [PubMed] [Google Scholar]
- 91.Ibrahim H. M. Hogg A. J. Jr. Healy D. J. Haroutunian V. Davis K. L. Meador-Woodruff J. H. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am. J. Psychiatry. (2000);157:1811–1823. doi: 10.1176/appi.ajp.157.11.1811. [DOI] [PubMed] [Google Scholar]
- 92.Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. (2007);13:444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Itil T. Keskiner A. Kiremitci N. Holden J. M. Effect of phencyclidine in chronic schizophrenics. Can Psychiatr. Assoc. J. (1967);12:209–212. doi: 10.1177/070674376701200217. [DOI] [PubMed] [Google Scholar]
- 94.Javitt D. C. Glycine modulators in schizophrenia. Curr. Opin. Investig. Drugs. (2002);3:1067–1072. [PubMed] [Google Scholar]
- 95.Javitt D. C. Glycine therapy of schizophrenia. Biol. Psychiatry. (1996);40:684–686. doi: 10.1016/0006-3223(96)00269-7. [DOI] [PubMed] [Google Scholar]
- 96.Javitt D. C. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr. Opin. Psychiatry. (2006);19:151–157. doi: 10.1097/01.yco.0000214340.14131.bd. [DOI] [PubMed] [Google Scholar]
- 97.Javitt D. C. Silipo G. Cienfuegos A. Shelley A. M. Bark N. Park M. Lindenmayer J. P. Suckow R. Zukin S. R. Adjunctive high-dose glycine in the treatment of schizophrenia. Int. J. Neuropsychopharmacol. (2001);4:385–391. doi: 10.1017/S1461145701002590. [DOI] [PubMed] [Google Scholar]
- 98.Javitt D. C. Zukin S. R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. (1991);148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 99.Javitt D. C. Zylberman I. Zukin S. R. Heresco-Levy U. Lindenmayer J. P. Amelioration of negative symptoms in schizophrenia by glycine. Am. J. Psychiatry. (1994);151:1234–1236. doi: 10.1176/ajp.151.8.1234. [DOI] [PubMed] [Google Scholar]
- 100.Jentsch J. D. Roth R. H. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. (1999);20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 101.Jordan G. R. McCulloch J. Shahid M. Hill D. R. Henry B. Horsburgh K. Regionally selective and dose-dependent effects of the ampakines Org 26576 and Org 24448 on local cerebral glucose utilisation in the mouse as assessed by 14C-2-deoxyglucose autoradiography. Neuropharmacology. (2005);49:254–264. doi: 10.1016/j.neuropharm.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 102.Joyce J. N. Meador-Woodruff J. H. Linking the family of D2 receptors to neuronal circuits in human brain: insights into schizophrenia. Neuropsychopharmacology. (1997);16:375–384. doi: 10.1016/S0893-133X(96)00276-X. [DOI] [PubMed] [Google Scholar]
- 103.Kalashnikova E. Lorca R. A. Kaur I. Barisone G. A. Li B. Ishimaru T. Trimmer J. S. Mohapatra D. P. Diaz E. SynDIG1: an activity-regulated, AMPA- receptor-interacting transmembrane protein that regulates excitatory synapse development. Neuron. (2010);65:80–93. doi: 10.1016/j.neuron.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kanai Y. Smith C. P. Hediger M. A. A new family of neurotransmitter transporters: the high-affinity glutamate transporters. FASEB J. (1993);7:1450–1459. doi: 10.1096/fasebj.7.15.7903261. [DOI] [PubMed] [Google Scholar]
- 105.Kanuma K. Aoki T. Shimazaki Y. Recent patents on positive allosteric modulators of the metabotropic glutamate 5 receptor as a potential treatment for schizophrenia. Recent Pat. CNS Drug Discov. (2010);5:23–34. doi: 10.2174/157488910789753512. [DOI] [PubMed] [Google Scholar]
- 106.Kato A. S. Gill M. B. Yu H. Nisenbaum E. S. Bredt D. S. TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci. (2010);33:241–248. doi: 10.1016/j.tins.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 107.Keifer J. Zheng Z. AMPA receptor trafficking and learning. Eur. J. Neurosci. (2010);32:269–277. doi: 10.1111/j.1460-9568.2010.07339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Keinanen K. Wisden W. Sommer B. Werner P. Herb A. Verdoorn T. A. Sakmann B. Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. (1990);249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- 109.Kerwin R. Patel S. Meldrum B. Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience. (1990);39:25–32. doi: 10.1016/0306-4522(90)90219-t. [DOI] [PubMed] [Google Scholar]
- 110.Kessels H. W. Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. (2009);61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kornhuber J. Bormann J. Retz W. Hubers M. Riederer P. Memantine displaces [3H]MK-801 at therapeutic concentrations in postmortem human frontal cortex. Eur. J. Pharmacol. (1989);166:589–590. doi: 10.1016/0014-2999(89)90384-1. [DOI] [PubMed] [Google Scholar]
- 112.Kristiansen L. V. Beneyto M. Haroutunian V. Meador-Woodruff J. H. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol. Psychiatry. (2006);11:737–747, 705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- 113.Kristiansen L. V. Bakir B. Haroutunian V. Meador-Woodruff J. H. Expression of the NR2B-NMDA receptor trafficking complex in prefrontal cortex from a group of elderly patients with schizophrenia. Schizophr. Res. (2010a);119:198–209. doi: 10.1016/j.schres.2010.02.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kristiansen L. V. Patel S. A. Haroutunian V. Meador-Woodruff J. H. Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. (2010b);64:495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- 115.Krivoy A. Weizman A. Laor L. Hellinger N. Zemishlany Z. Fischel T. Addition of memantine to antipsychotic treatment in schizophrenia inpatients with residual symptoms: A preliminary study. Eur. Neuropsychopharmacol. (2008);18:117–121. doi: 10.1016/j.euroneuro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 116.Krystal J. H. Karper L. P. Seibyl J. P. Freeman G. K. Delaney R. Bremner J. D. Heninger G. R. Bowers M. B. Jr. Charney D. S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. (1994);51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 117.Lahti A. C. Holcomb H. H. Medoff D. R. Tamminga C. A. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. (1995);6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- 118.Lane H. Y. Chang Y. C. Liu Y. C. Chiu C. C. Tsai G. E. Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study. Arch. Gen. Psychiatry. (2005);62:1196–1204. doi: 10.1001/archpsyc.62.11.1196. [DOI] [PubMed] [Google Scholar]
- 119.Lane H. Y. Lin C. H. Huang Y. J. Liao C. H. Chang Y. C. Tsai G. E. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and D-serine add-on treatment for schizophrenia. Int. J. Neuropsychopharmacol. (2010);13:451–460. doi: 10.1017/S1461145709990939. [DOI] [PubMed] [Google Scholar]
- 120.Lane H. Y. Liu Y. C. Huang C. L. Chang Y. C. Liau C. H. Perng C. H. Tsai G. E. Sarcosine (N-methylglycine) treatment for acute schizophrenia: a randomized, double-blind study. Biol. Psychiatry. (2008);63:9–12. doi: 10.1016/j.biopsych.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 121.Lane H. Y. Huang C. L. Wu P. L. Liu Y. C. Chang Y. C. Lin P.Y. Chen P. W. Tsai G. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia. Biol. Psychiatry. (2006);60:645–649. doi: 10.1016/j.biopsych.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 122.Laruelle M. Abi-Dargham A. Gil R. Kegeles L. Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol. Psychiatry. (1999);46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 123.Lau C. G. Zukin R. S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. (2007);8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 124.Lavezzari G. McCallum J. Dewey C. M. Roche K. W. Subunit-specific regulation of NMDA receptor endocytosis. J. Neurosci. (2004);24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Le Corre S. Harper C. G. Lopez P. Ward P. Catts S. Increased levels of expression of an NMDARI splice variant in the superior temporal gyrus in schizophrenia. Neuroreport. (2000);11:983–986. doi: 10.1097/00001756-200004070-00017. [DOI] [PubMed] [Google Scholar]
- 126.Lehre K. P. Levy L. M. Ottersen O. P. Storm-Mathisen J. Danbolt N. C. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J. Neurosci. (1995);15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat. Rev. Neurosci. (2003);4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- 128.Letts V. A. Felix R. Biddlecome G. H. Arikkath J. Mahaffey C. L. Valenzuela A. Bartlett F. S., 2nd. Mori Y. Campbell K. P. Frankel W. N. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat. Genet. (1998);19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 129.Li D. Specht C. G. Waites C. L. Butler-Munro C. Leal-Ortiz S. Foote J. W. Genoux D. Garner C. C. Montgomery J. M. SAP97 directs NMDA receptor spine targeting and synaptic plasticity. J. Physiol. (2011);589:4491–4510. doi: 10.1113/jphysiol.2011.215566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lieberman J. A. Papadakis K. Csernansky J. Litman R. Volavka J. Jia X. D. Gage A. A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacology. (2009);34:1322–1329. doi: 10.1038/npp.2008.200. [DOI] [PubMed] [Google Scholar]
- 131.Lin J. W. Wyszynski M. Madhavan R. Sealock R. Kim J. U. Sheng M. Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. J. Neurosci. (1998);18:2017–2027. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lu W. Ziff E. B. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. (2005);47:407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 133.Mah S. J. Cornell E. Mitchell N. A. Fleck M. W. Glutamate receptor trafficking: endoplasmic reticulum quality control involves ligand binding and receptor function. J. Neurosci. (2005);25:2215–2225. doi: 10.1523/JNEUROSCI.4573-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Malinow R. Malenka R. C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. (2002);25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 135.Masson J. Sagne C. Hamon M. El Mestikawy S. Neurotransmitter transporters in the central nervous system. Pharmacol. Rev. (1999);51:439–464. [PubMed] [Google Scholar]
- 136.McCullumsmith R. E. Kristiansen L. V. Beneyto M. Scarr E. Dean B. Meador-Woodruff J. H. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res. (2007);1127:108–118. doi: 10.1016/j.brainres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meador-Woodruff J. H. Davis K. L. Haroutunian V. Abnormal kainate receptor expression in prefrontal cortex in schizophrenia. Neuropsychopharmacology. (2001a);24:545–552. doi: 10.1016/S0893-133X(00)00189-5. [DOI] [PubMed] [Google Scholar]
- 138.Meador-Woodruff J. H. Hogg A. J. Jr. Smith R. E. Striatal ionotropic glutamate receptor expression in schizophrenia bipolar disorder and major depressive disorder. Brain Res. Bull. (2001b);55:631–640. doi: 10.1016/s0361-9230(01)00523-8. [DOI] [PubMed] [Google Scholar]
- 139.Milton I. D. Banner S. J. Ince P. G. Piggott N. H. Fray A. E. Thatcher N. Horne C. H. Shaw P. J. Expression of the glial glutamate transporter EAAT2 in the human CNS: an immunohistochemical study. Brain Res. Mol. Brain Res. (1997);52:17–31. doi: 10.1016/s0169-328x(97)00233-7. [DOI] [PubMed] [Google Scholar]
- 140.Mirnics K. Middleton F. A. Marquez A. Lewis D. A. Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. (2000);28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 141.Moghaddam B. Adams B. W. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. (1998);281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 142.Moghaddam B. Adams B. Verma A. Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. (1997);17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mueller H. T. Haroutunian V. Davis K. L. Meador-Woodruff J. H. Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res. Mol. Brain Res. (2004);121:60–69. doi: 10.1016/j.molbrainres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 144.Mueller H. T. Meador-Woodruff J. H. NR3A NMDA receptor subunit mRNA expression in schizophrenia depression and bipolar disorder. Schizophr. Res. (2004);71:361–370. doi: 10.1016/j.schres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 145.Naassila M. Hammoumi S. Legrand E. Durbin P. Daoust M. Mechanism of action of acamprosate. Part I. Characterization of spermidine-sensitive acamprosate binding site in rat brain. Alcohol. Clin. Exp. Res. (1998);22:802–809. [PubMed] [Google Scholar]
- 146.Nagao S. Kwak S. Kanazawa I. EAAT4, a glutamate transporter with properties of a chloride channel, is predominantly localized in Purkinje cell dendrites, and forms parasagittal compartments in rat cerebellum. Neuroscience. (1997);78:929–933. doi: 10.1016/s0306-4522(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 147.Neuhauss S. C. Rico E. P. Gesemann M. Nomenclature of glutamate transporters in zebrafish and other vertebrates. Brain Res. Bull. (2010);83:297. doi: 10.1016/j.brainresbull.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 148.Newell K. A. Zavitsanou K. Huang X. F. Ionotropic glutamate receptor binding in the posterior cingulate cortex in schizophrenia patients. Neuroreport. (2005);16:1363–1367. doi: 10.1097/01.wnr.0000174056.11403.71. [DOI] [PubMed] [Google Scholar]
- 149.Ng D. Pitcher G. M. Szilard R. K. Sertie A. Kanisek M. Clapcote S. J. Lipina T. Kalia L. V. Joo D. McKerlie C. Cortez M. Roder J. C. Salter M. W. McInnes R. R. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. (2009);7:e41. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ni Y. Malarkey E. B. Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J. Neurochem. (2007);103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- 151.Nishikawa T. Takashima M. Toru M. Increased [3H]kainic acid binding in the prefrontal cortex in schizophrenia. Neurosci. Lett. (1983);40:245–250. doi: 10.1016/0304-3940(83)90046-0. [DOI] [PubMed] [Google Scholar]
- 152.Noebels J. L. Qiao X. Bronson R. T. Spencer C. Davisson M.T. Stargazer: a new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy. Res. (1990);7:129–135. doi: 10.1016/0920-1211(90)90098-g. [DOI] [PubMed] [Google Scholar]
- 153.Noga J. T. Hyde T. M. Herman M. M. Spurney C. F. Bigelow L. B. Weinberger D. R. Kleinman J. E. Glutamate receptors in the postmortem striatum of schizophrenic, suicide, and control brains. Synapse. (1997);27:168–176. doi: 10.1002/(SICI)1098-2396(199711)27:3<168::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 154.Noga J. T. Wang H. Further postmortem autoradiographic studies of AMPA receptor binding in schizophrenia. Synapse. (2002);45:250–258. doi: 10.1002/syn.10106. [DOI] [PubMed] [Google Scholar]
- 155.Noorbala A. A. Akhondzadeh S. Davari-Ashtiani R. Amini-Nooshabadi H. Piracetam in the treatment of schizophrenia: implications for the glutamate hypothesis of schizophrenia. J.Clin. Pharm. Ther. (1999);24:369–374. doi: 10.1046/j.1365-2710.1999.00238.x. [DOI] [PubMed] [Google Scholar]
- 156.Ohnuma T. Augood S. J. Arai H. McKenna P. J. Emson P. C. Expression of the human excitatory amino acid transporter 2 and metabotropic glutamate receptors 3 and 5 in the prefrontal cortex from normal individuals and patients with schizophrenia. Brain Res. Mol. Brain Res. (1998);56:207–217. doi: 10.1016/s0169-328x(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 157.Ohtsuki T. Toru M. Arinami T. Mutation screening of the metabotropic glutamate receptor mGluR4 (GRM4) gene in patients with schizophrenia. Psychiatr. Genet. (2001);11:79–83. doi: 10.1097/00041444-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 158.Olive M. F. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr. Drug Abuse. Rev. (2009);2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Paz R. D. Tardito S. Atzori M. Tseng K. Y. Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology. Eur. Neuropsychopharmacol. (2008);18:773–786. doi: 10.1016/j.euroneuro.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Porter R. H. Eastwood S. L. Harrison P. J. Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res. (1997);751:217–231. doi: 10.1016/s0006-8993(96)01404-7. [DOI] [PubMed] [Google Scholar]
- 161.Prybylowski K. Chang K. Sans N. Kan L. Vicini S. Wenthold R. J. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. (2005);47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ralevski E. O'Brien E. Jane J. S. Dean E. Dwan R. Petrakis I. Effects of acamprosate on cognition in a treatment study of patients with schizophrenia spectrum disorders and comorbid alcohol dependence. J. Nerv. Ment. Dis. (2011);199:499–505. doi: 10.1097/NMD.0b013e3182214297. [DOI] [PubMed] [Google Scholar]
- 163.Rao A. Craig A. M. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. (1997);19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- 164.Ratnam J. Teichberg V. I. Neurofilament-light increases the cell surface expression of the N-methyl-D-aspartate receptor and prevents its ubiquitination. J. Neurochem. (2005);92:878–885. doi: 10.1111/j.1471-4159.2004.02936.x. [DOI] [PubMed] [Google Scholar]
- 165.Richardson-Burns S. M. Haroutunian V. Davis K. L. Watson S. J. Meador-Woodruff J. H. Metabotropic glutamate receptor mRNA expression in the schizophrenic thalamus. Biol. Psychiatry. (2000);47:22–28. doi: 10.1016/s0006-3223(99)00207-3. [DOI] [PubMed] [Google Scholar]
- 166.Rico E. P. de Oliveira D. L. Rosemberg D. B. Mussulini B. H. Bonan C. D. Dias R. D. Wofchuk S. Souza D. O. Bogo M.R. Expression and functional analysis of Na(+)-dependent glutamate transporters from zebrafish brain. Brain Res. Bull. (2010);81:517–523. doi: 10.1016/j.brainresbull.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 167.Rosse R. B. Schwartz B. L. Davis R. E. Deutsch S. I. An NMDA intervention strategy in schizophrenia with "low-dose" milacemide. Clin. Neuropharmacol. (1991);14:268–272. doi: 10.1097/00002826-199106000-00012. [DOI] [PubMed] [Google Scholar]
- 168.Rosse R. B. Schwartz B. L. Leighton M. P. Davis R. E. Deutsch S. I. An open-label trial of milacemide in schizophrenia:an NMDA intervention strategy. Clin. Neuropharmacol. (1990);13:348–354. doi: 10.1097/00002826-199008000-00010. [DOI] [PubMed] [Google Scholar]
- 169.Rothstein J. D. Martin L. Levey A. I. Dykes-Hoberg M. Jin L. Wu D. Nash N. Kuncl R. W. Localization of neuronal and glial glutamate transporters. Neuron. (1994);13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 170.Sala C. Sheng M. The fyn art of N-methyl-D-aspartate receptor phosphorylation. Proc. Natl. Acad. Sci. USA. (1999);96:335–337. doi: 10.1073/pnas.96.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sans N. Prybylowski K. Petralia R. S. Chang K. Wang Y. X. Racca C. Vicini S. Wenthold R. J. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat. Cell Biol. (2003);5:520–530. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- 172.Sans N. Wang P. Y. Du Q. Petralia R. S. Wang Y. X. Nakka S. Blumer J. B. Macara I. G. Wenthold R. J. mPins modulates PSD-95 and SAP102 trafficking and influences NMDA receptor surface expression. Nat. Cell Biol. (2005);7:1179–1190. doi: 10.1038/ncb1325. [DOI] [PubMed] [Google Scholar]
- 173.Scarr E. Beneyto M. Meador-Woodruff J. H. Dean B. Cortical glutamatergic markers in schizophrenia. Neuropsychopharmacology. (2005);30:1521–1531. doi: 10.1038/sj.npp.1300758. [DOI] [PubMed] [Google Scholar]
- 174.Schiffer H. H. Swanson G. T. Heinemann S. F. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. (1997);19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 175.Schmitt A. Koschel J. Zink M. Bauer M. Sommer C. Frank J. Treutlein J. Schulze T. Schneider-Axmann T. Parlapani E. Rietschel M. Falkai P. Henn F. A. Gene expression of NMDA receptor subunits in the cerebellum of elderly patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. (2010);260:101–111. doi: 10.1007/s00406-009-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schoepp D. D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. (2001);299:12–20. [PubMed] [Google Scholar]
- 177.Schwenk J. Harmel N. Zolles G. Bildl W. Kulik A. Heimrich B. Chisaka O. Jonas P. Schulte U. Fakler B. Klocker N. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. (2009);323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
- 178.Sheng M. Pak D. T. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu. Rev. Physiol. (2000);62:755–778. doi: 10.1146/annurev.physiol.62.1.755. [DOI] [PubMed] [Google Scholar]
- 179.Shi S. Hayashi Y. Esteban J. A. Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. (2001);105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 180.Shibata H. Tani A. Chikuhara T. Kikuta R. Sakai M. Ninomiya H. Tashiro N. Iwata N. Ozaki N. Fukumaki Y. Association study of polymorphisms in the group III metabotropic glutamate receptor genes, GRM4 and GRM7, with schizophrenia. Psychiatry Res. (2009);167:88–96. doi: 10.1016/j.psychres.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 181.Singh S. P. Singh V. Meta-Analysis of the Efficacy of Adjunctive NMDA Receptor Modulators in Chronic Schizophrenia. CNS Drugs. (2011);25:859–885. doi: 10.2165/11586650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 182.Sobolevsky A. I. Rosconi M. P. Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. (2009);462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sodhi M. S. Simmons M. McCullumsmith R. Haroutunian V. Meador-Woodruff J. H. Glutamatergic gene expression is specifically reduced in thalamocortical projecting relay neurons in schizophrenia. Biol. Psychiatry. (2011);70:646–654. doi: 10.1016/j.biopsych.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sokolov B. P. Expression of NMDAR1 GluR1 GluR7 and KA1 glutamate receptor mRNAs is decreased in frontal cortex of "neuroleptic-free" schizophrenics: evidence on reversible up-regulation by typical neuroleptics. J. Neurochem. (1998);71:2454–2464. doi: 10.1046/j.1471-4159.1998.71062454.x. [DOI] [PubMed] [Google Scholar]
- 185.Song I. Kamboj S. Xia J. Dong H. Liao D. Huganir R. L. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. (1998);21:393–400. doi: 10.1016/S0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 186.Steiner P. Alberi S. Kulangara K. Yersin A. Sarria J. C. Regulier E. Kasas S. Dietler G. Muller D. Catsicas S. Hirling H. Interactions between NEEP21, GRIP1 and GluR2 regulate sorting and recycling of the glutamate receptor subunit GluR2. EMBO J. (2005);24:2873–2884. doi: 10.1038/sj.emboj.7600755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stephenson F. A. Cousins S. L. Kenny A. V. Assembly and forward trafficking of NMDA receptors (Review). Mol. Membr. Biol. (2008);25:311–320. doi: 10.1080/09687680801971367. [DOI] [PubMed] [Google Scholar]
- 188.Straub C. Hunt D. L. Yamasaki M. Kim K. S. Watanabe M. Castillo P. E. Tomita S. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nat. Neurosci. (2011);14:866–873. doi: 10.1038/nn.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Takaki H. Kikuta R. Shibata H. Ninomiya H. Tashiro N. Fukumaki Y. Positive associations of polymorphisms in the metabotropic glutamate receptor type 8 gene (GRM8) with schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. (2004);128B:6–14. doi: 10.1002/ajmg.b.20108. [DOI] [PubMed] [Google Scholar]
- 190.Takamori S. Rhee J. S. Rosenmund C. Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. (2000);407:189–194. doi: 10.1016/j.psychres.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 191.Tamminga C. Glutamatergic aspects of schizophrenia. Br. J. Psychiatry Suppl. (1999);37:12–15. doi: 10.1002/ajmg.b.20108. [DOI] [PubMed] [Google Scholar]
- 192.Tang M. Pelkey K. A. Ng D. Ivakine E. McBain C. J. Salter M. W. McInnes R. R. Neto1 is an auxiliary subunit of native synaptic kainate receptors. J. Neurosci. (2011);31:10009–10018. doi: 10.1038/35025070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tek C. Srihari V. Tek E. Successful acamprosate treatment of alcohol dependence in schizophrenia. Schizophr. Res. (2008);106:373. doi: 10.1016/j.schres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 194.Thomas C. Carroll B. T. Maley R. T. Jayanti K. Koduri A. Memantine and catatonic schizophrenia. Am. J. Psychiatry. (2005);162:626. doi: 10.1523/JNEUROSCI.6617-10.2011. [DOI] [PubMed] [Google Scholar]
- 195.Thomas C. G. Miller A. J. Westbrook G. L. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J. Neurophysiol. (2006);95:1727–1734. doi: 10.1016/j.schres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 196.Tiihonen J. Halonen P. Wahlbeck K. Repo-Tiihonen E. Hyvarinen S. Eronen M. Putkonen H. Takala P. Mehtonen O. P. Puck M. Oksanen J. Koskelainen P. Joffe G. Aer J. Hallikainen T. Ryynanen O. P. Tupala E. Topiramate add-on in treatment-resistant schizophrenia: a randomized, double-blind, placebo-controlled, crossover trial. J. Clin. Psychiatry. (2005);66:1012–1015. doi: 10.4088/jcp.v66n0808. [DOI] [PubMed] [Google Scholar]
- 197.Tingley W. G. Roche K. W. Thompson A. K. Huganir R. L. Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature. (1993);364:70–73. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- 198.Tingley W. G. Ehlers M. D. Kameyama K. Doherty C. Ptak J. B. Riley C. T. Huganir R. L. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-Daspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J. Biol. Chem. (1997);272:5157–5166. doi: 10.4088/JCP.v66n0808. [DOI] [PubMed] [Google Scholar]
- 199.Tomita S. Regulation of ionotropic glutamate receptors by their auxiliary subunits. Physiology (Bethesda) (2010);25:41–49. doi: 10.1038/364070a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Toyooka K. Iritani S. Makifuchi T. Shirakawa O. Kitamura N. Maeda K. Nakamura R. Niizato K. Watanabe M. Kakita A. Takahashi H. Someya T. Nawa H. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J. Neurochem. (2002);83:797–806. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 201.Tsai G. Lane H. Y. Yang P. Chong M. Y. Lange N. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry. (2004);55:452–456. doi: 10.1152/physiol.00033.2009. [DOI] [PubMed] [Google Scholar]
- 202.Tsai G. E. Yang P. Chang Y. C. Chong M. Y. D-alanine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry. (2006);59:230–234. doi: 10.1046/j.1471-4159.2002.01181.x. [DOI] [PubMed] [Google Scholar]
- 203.Tsai G. Yang P. Chung L. C. Lange N. Coyle J. T. D-serine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry. (1998);44:1081–1089. doi: 10.1016/j.biopsych.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 204.Volk D. W. Eggan S. M. Lewis D. A. Alterations in metabotropic glutamate receptor 1alpha and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am. J. Psychiatry. (2010);167:1489–1498. doi: 10.1016/j.biopsych.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.von Engelhardt J. Mack V. Sprengel R. Kavenstock N. Li K. W. Stern-Bach Y. Smit A. B. Seeburg P. H. Monyer H. CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science. (2010);327:1518–1522. doi: 10.1016/S0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- 206.Vrajova M. Stastny F. Horacek J. Lochman J. Sery O. Pekova S. Klaschka J. Hoschl C. Expression of the hippocampal NMDA receptor GluN1 subunit and its splicing isoforms in schizophrenia: postmortem study. Neurochem. Res. (2010);35:994–1002. doi: 10.1176/appi.ajp.2010.10030318. [DOI] [PubMed] [Google Scholar]
- 207.Westphal R. S. Tavalin S. J. Lin J. W. Alto N. M. Fraser I. D. Langeberg L. K. Sheng M. Scott J. D. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. (1999);285:93–96. doi: 10.1126/science.1184178. [DOI] [PubMed] [Google Scholar]
- 208.Whiteheart S. W. Matveeva E. A. Multiple binding proteins suggest diverse functions for the N-ethylmaleimide sensitive factor. J. Struct. Biol. (2004);146:32–43. doi: 10.1007/s11064-010-0145-z. [DOI] [PubMed] [Google Scholar]
- 209.Woo T. U. Shrestha K. Amstrong C. Minns M. M. Walsh J. P. Benes F. M. Differential alterations of kainate receptor subunits in inhibitory interneurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Schizophr. Res. (2007);96:46–61. doi: 10.1126/science.285.5424.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yu B. Wang C. Liu J. Johnson K. M. Gallagher J. P. Adaptation to chronic PCP results in hyperfunctional NMDA and hypofunctional GABA(A) synaptic receptors. Neuroscience. (2002);113:1–10. doi: 10.1016/j.jsb.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 211.Zavitsanou K. Ward P. B. Huang X. F. Selective alterations in ionotropic glutamate receptors in the anterior cingulate cortex in schizophrenia. Neuropsychopharmacology. (2002);27:826–833. doi: 10.1016/j.schres.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 212.Zhang W. St-Gelais F. Grabner C. P. Trinidad J. C. Sumioka A. Morimoto-Tomita M. Kim K. S. Straub C. Burlingame A. L. Howe J. R. Tomita S. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. (2009);61:385–396. doi: 10.1016/S0306-4522(02)00163-X. [DOI] [PMC free article] [PubMed] [Google Scholar]