Abstract
This study was conducted to evaluate the effect of Grateloupia elliptica, a seaweed native to Jeju Island, Korea, on the prevention of hair loss. When immortalized rat vibrissa dermal papilla cells were treated with extract of G. elliptica, the proliferation of dermal papilla cells significantly increased. In addition, the G. elliptica extract significantly inhibited the activity of 5α-reductase, which converts testosterone to dihydrotestosterone (DHT), a main cause of androgenetic alopecia. On the other hand, the G. elliptica extract promoted PGE2 production in HaCaT cells in a dose-dependent manner. The G. elliptica extract exhibited particularly high inhibitory effect on LPS-stimulated IL-12, IL-6, and TNF-α production in lipopolysaccharide (LPS)-stimulated bone marrow-derived dendritic cells. The G. elliptica extract also showed inhibitory activity against Pityrosporum ovale, a main cause of dandruff. These results suggest that G. elliptica extract has the potential to treat alopecia via the proliferation of dermal papilla, 5α-reductase inhibition, increase of PGE2 production, decrease of LPS-stimulated pro-inflammatory cytokines and inhibitory activity against Pityrosporum ovale.
Keywords: Prevention of hair loss, Grateloupia elliptica, Dermal papilla cell, 5α-reductase, PGE2, LPS-stimulated pro-inflammatory cytokine, Pityrosporum ovale
INTRODUCTION
Alopecia is a distressing condition for an increasing number of men and women and includes androgrenetic alopecia (AGA), alopecia areata (AA), telogen effluvium and so on (Kaufman et al., 1998). Hair loss is emerged from psychological and physical stress, and dandruff. However, the underlying causes of baldness are poorly understood and only two FDA-approved drugs (finasteride and minoxidil) have been available for nearly 50 years (Burton and Marshall, 1979; Kaufman et al., 1998). Finasteride, a type II 5α-reductase inhibitor, was initially used for curing prostatic hypertrophy (Gormley, 1995), but later found to stimulate hair growth in men with AGA, which is the most common type of alopecia (Van Neste et al., 2000; Kaufman et al., 2008a; Kaufmanx et al., 2008b). Nevertheless, its use is limited because of potential side effects, especially in women (Whiting et al., 1999). Minoxidil, an anti-hypertensive, has been reported to stimulate hair growth by the opening of ATP-sensitive K+-channel (Hamaoka et al., 1997; Shorter et al., 2008), the up-regulation of vascular endothelial growth factor (VEGF) (Lachgar et al., 1998) and the activation of β-catenin pathway (Kwack et al., 2011) in dermal papilla cells (DPCs). Han et al. reported that minoxidil has proliferative and anti-apoptotic effects on DPCs (Han et al., 2004). The DPCs consists of a cluster of specialized fibroblasts that play important roles in the regulation of the hair cycle through the secretion of diffusible proteins such as insulin-like growth factor-1 (IGF-1) (Itami et al., 1995), hepatocyte growth factor (HGF) (Shimaoka et al., 1994), VEGF (Lachgar et al., 1996) and transforming growth factor-β (TGF-β) (Soma et al., 2002; Soma et al., 2003). Several reports have also described implication of PG pathway in hair growth and PGE2 is also described as a possible modulator of hair growth (Coleman et al., 1994b; Colombe et al., 2007; Colombe et al., 2008).
On the other hand, AA is one of the most common autoimmune diseases which is characterized by autoimmune assault on the hair follicle resulting in hair loss (Alkhalifah et al., 2010). Many reports suggest that AA is mediated by T-lymphocytes with type-1 helper T-cell (Th1) cytokine profile. TNF-α, IL-6 and IL-12, pro-inflammatory cytokines, are involved in Th1-mediated inflammation as part of the normal immune response, as well as infl ammatory diseases, including AA (Gately et al.,1998; Gilhar and Kalish, 2006).
The association between hair loss and dandruff has been reported (Nematian et al., 2006; Pierard-Franchimont et al., 2006). Hair shedding increases with cutaneous infection of Pityrosporum ovale (Nematian et al., 2006).
To develop new therapies to enhance hair growth, we screened the extracts of Jeju seaweeds and discovered that Grateloupia elliptica (Grateloupiaceae) has the potential to promote hair growth. G. elliptica, a red seaweed, was reported to have anti-inflammatory effects by the decrease of production of pro-inflammatory mediators (Yang et al., 2010). Bromo-phenols of G. elliptica showed high α-glucosidase activity and seem to have potential to prevent diabetes mellitus (Kim et al., 2008). However, the effect of G. elliptica on the prevention of hair loss has not yet been reported. Therefore, the present study was carried out to investigate the preventing effect of G.elliptica extact on the hair loss.
MATERIALS AND METHODS
Extract and HPLC analysis
G. elliptica were collected along the coast of Sungsanpo in Jeju Island, Korea, between March and June 2009. The seaweed was washed three times with tap water to remove the salt, epiphytes, and sand attached to the surface, then carefully rinsed with fresh water and maintained in a medical refrigerator at −20℃. Thereafter, the frozen whole body was lyophilized and homogenized with a grinder prior to extraction. The seaweed sample was pulverized into powder using a grinder. The powder (1 g) was extracted with 70% aqueous ethanol (100 ml) at room temperature for 24 h and filtrated. After filtration, the ethanol extract was evaporated to dryness under vacuum.
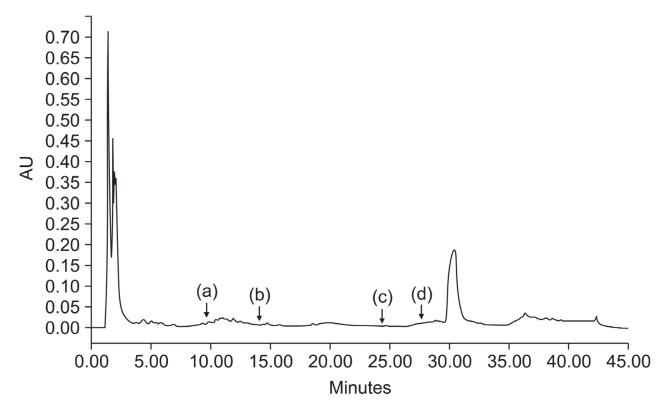
The amount of polyphenols in the ethanol extract of G. elliptica measured by the Folin-Ciocalteu colorimetric method was 13.5%. HPLC analysis was performed on the HPLC (Alliance 2695 system with PDA, Waters Co., Milford, MA, USA);Used column was XTerra® C18 (100×4.6 mm, i.d. 3.5 um; Waters Co., Milford, MA, Ltd. USA), mobile phase, linear-gradient mixture of A (aqueous 0.5 % acetic acid) and B (0.5% acetic acid in acetonitrile) for 0-45 min; injection volume 10 ul; flow rate, 1 ml/min; and detection, UV at 254 nm (Fig. 1). The G. elliptica extract used in the study was not identified to have antioxidant compounds such as catechin, rutin, quercetin and flavone (Fig. 1).
Fig. 1. HPLC profile of G. elliptica extract. HPLC analysis was performed on the HPLC (Alliance 2695 system with PDA Waters Co., Milford, MA, USA); Used column was XTerra® C18 (100× 4.6 mm, i.d. 3.5 um; Waters Co. Ltd. USA), mobile phase, linear-gradient mixture of A (aqueous 0.5% acetic acid) and B (0.5% acetic acid in acetonitrile) for 0-45 min; injection volume 10 ul; flow rate, 1 ml/min; and detection, UV at 254 nm. Each arrow indicates catechin (a), rutin (b), quercetin (c) and flavone (d).
This extract was dissolved in dimethyl sulfoxide (DMSO)(Sigma, St. Louis, MO, USA) for subsequent treatment; the final concentration of DMSO was adjusted to 0.2% (v/v) in the following experiments.
Assay for the proliferation of dermal papilla cells
Rat vibrissa immortalized dermal papilla cell line (Filsell et al., 1994) was donated by the Skin Research Institute, Amore Pacific Corporation R&D Center, Korea. The dermal papilla cells were cultured in DMEM (Hyclone Inc, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL, Rockville, NY, USA) and penicillin/streptomycin (100 unit/ml and 100 μg/ml, respectively) at 37℃ in a humidified atmosphere under 5% CO2.
The proliferation of dermal papilla cells was evaluated by measuring the metabolic activity using a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (Carmichael et al., 1987). Briefly, dermal papilla at 1.0×104 cells/ml were seeded into 96-well plate, cultured 24h in serum-free DMEM, and then treated with vehicle (DMSO diluted 1:500 in serum-free DMEM) as a control and G. elliptica extract of 0.1, 1, 10 and 100 μg/ml for 4 days. After incubation, 0.1 mg (50μl of a 2 mg/ml solution) of MTT (Sigma, St. Louis, MO, USA) was added to each well, and the cells were then incubated at 37℃ for 4 h. Next, the plates were centrifuged at 1,000 rpm for 5 min at room temperature and the media was then carefully aspirated. DMSO 200 μl was then added to each well to dissolve the formazan crystals and the absorbance of the plate at 540 nm was then read immediately on a microplate reader (BioTek Instrument, Inc., Winooski, VT, USA). All experiments were performed three times and the mean absorbance values were calculated. The results are expressed as the percentage in the absorbance caused by treatment with G. elliptica extract compared to that of the untreated controls. Minoxidil (Sigma, St. Louise, CA, USA) was used as a positive control.
Assay for prostatic 5α-reductase activity
Male Spargue-Dawley (SD) rats (8 wk old) were purchased from Dae-Han Biolink (Eumseong, South Korea), and given a standard laboratory diet with water ad libitum. All animals were cared for by using protocols (20100031) approved by the IACUC of Jeju National University. Male SD rats (8 wk old) were sacrificed with CO2. The rat prostates were removed from their capsules, washed with saline, and stored at −80°C. Frozen tissues were thawed on ice and procedures were carried out at 4℃. The tissues were homogenized with a Polytron homogenizer (Brinkman Instruments, Westbury, NY, USA) in 5-6 tissue volumes of medium A (0.32 M sucrose, 1 mM dithiothreitol (DTT), 0.2 mM phenylmethylsulfonylfluoride (PMSF); and 20 mM potassium phosphate buffer, pH 6.6). The homogenates were centrifuged at 1,500 g for 20 min. The pellets were recovered, washed with three tissue volumes of medium A, and centrifuged two additional times at 400 g for 10 min.The washed pellets were suspended in medium A and stored at −80℃ until use. The suspension (2.5 mg protein/ml as determined by the Bradford assay using Bio-Rad reagents) was used as source of 5α-reductase. 5α-reductase activities were analyzed as previously described (Hirosumi, et al. 1995). The reaction mixture had a final volume of 500 μl and contained 1 mM DTT, 40 mM potassium phosphate buffer, pH 6.6, 2 mM NADPH, and 120 nCi [1,2,6,7-3H] testosterone. Triplicate re-actions were initiated when the reaction mixture was added to the rat prostatic enzyme fraction (250 μg of protein) containing 0.2% DMSO (as a control), or G. elliptica extract (0.1, 1 and 10 μg/ml). Finasteride 2 nM (Merck-Sharpe-Dohme, White-house Station, NJ, USA) was used as a positive control. The mixture was incubated at 37℃ for 60 min, and then stopped by adding 1 ml of ethyl acetate and mixing for 1 min. After centrifugation at 1,000 g for 5 mim, the organic phase was removed, dried under a heating plate, dissolved in 50 μl of ethyl acetate containing 500 μg/ml of testosterone and 500 μg/ml dihydrotestosterone (DHT), and applied to a silica gel 60 F254 TLC plate (Merck). The plate was developed in a solvent system consisting of an ethyl acetate: cyclohexane (1:1) solution, and the plate was air dried. Testosterone was visualized under UV light (254 nm) and DHT was detected using a 10% H2SO4 solution and posteriorly heating the plate. Under these conditions, DHT develops a classical dark yellow color. Areas containing androgen were removed and the strips were soaked in 5 ml of ULTIMA GOLDTM Cocktail (PerkinElmer, Waltham, MA, USA) and radioactivity was measured by a liquid scintillation counter (Packard Bioscience, Meriden, CT, USA). The activity of 5α-reductase was expressed as a ratio calculated by the equation [DHT/(T+DHT)]×100.
Assay for PGE2 production in HaCaT keratinocyte cells
The immortalized human keratinocyte cell line, HaCaT, were cultured in RPMI-1640 (GIBCO, Grand Island, NY, USA) supplemented with 10% FBS (GIBCO, Grand Island, NY, USA) and penicillin/streptomycin (100 unit/ml and 100 μg/ml, respectively) at 37℃ in a humidified CO2 incubator.
HaCaT cells (2.0×105 cells/ml) were pre-incubated for 18 h and treated with various concentrations of G. elliptica extract (50, 25 and 12.5 μg/ml) for 24 h. PGE2 amount in the culture supernatant was measured with human PGE2 enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Briefly, PGE2 antibodies and cell culture supernatants were added to each well and incubated for 2 h at room temperature on an orbital shaker. After washing, para-nitrophenylphosphate (pNPP) solution was added to each well. The plates were incubated for 1 h at room temperature on an orbital shaker. The optical density of each well was measured at 450 nm using an ELISA reader.
Measurement of cytokine production
Bone marrow-derived dendritic cells (BMDCs) were grown from wild-type C57BL/6 mice (Taconic Farm, Germantown, NY, USA) as described previously (Koh et al., 2010). Briefly, the mouse tibia and femur was obtained by flushing with DMEM to yield bone marrow cells. The cells were cultured in RPMI 1640 medium containing 10% FBS (Gibco, Rockville, NY, USA), 50 μM β-mercaptoethanol, 2 mM glutamine supplemented with 3% J558L hybridoma cell culture supernatant containing granulocyte-macrophage colony-stimulating factor. The culture medium was replaced with fresh medium every second day. At day 6 of culture, non-adherent cells and loosely adherent DC aggregates were harvested, washed, and resuspended in RPMI 1640 supplemented with 5% FBS.
The BMDCs were incubated in 48-well plates in 0.5 ml containing 1×105 cells per well treated with G. elliptica extract of 0 to 50 μg/ml for 1 h before stimulation with 10 ng/ml LPS from Salmonella minnesota (Alexis, NY, USA). Supernatants were harvested 16 h after stimulation. Concentrations of murine TNF-α, IL-6 and IL-12 p40 in the culture supernatant were determined by ELISA (Pharmingen, San Diego, CA, USA) according to the manufacture’s instructions. The inhibitory activity was expressed as the inhibition rate (%), which was calculated from the following formula: Inhibitory activity (%)=[(cytokine production in DMSO-treated DC–cytokine production in compound-treated DC)/ cytokine production in DMSO-treated DC] ×100
Culture of Pityrosporum ovale and determination of the antifungal activity
P. ovale was purchased from the Korean Culture Center of Microorganisms (KCCM, Seoul, Korea). The strain was cultured in modified Dixon’s broth (1.5% Malt extract, 2% Ox-bile, 1% Tween 40, 0.25% Glycerol) at 37℃. To assess the antifungal activity of extracts, the agar-well diffusion method was used (Anesini and Perez, 1993). The antifungal activity was evaluated by measuring the inhibition-zone diameter observed after 48 h of incubation. Inoculums suspensions were adjusted by spectrophotometer (SpectraMAX 190, Molecular devices, Sunnyvale, CA, USA) to an absorbance 0.6 of at 550 nm. Two-layer plates were prepared with 20 ml of lower agar followed by, 10 ml of upper agar inoculated with 200 μl of P.ovale (5×105 CFU/ml) in Petri dishes. Forty five microliter aliquots of 40 mg/ml were individually dispensed onto 6-mm-diameter sterile filter paper discs (Adventec, Tokyo, Japan). Each disc was placed on nutrient agar that had been previously seeded with the target P. ovale and incubated for 2 days at 37℃. Inhibition zones (including the disc diameter) were measured using a ruler. The values were the average (mm) of 3 measurements per disk, taken at 3 different directions. Zinc pyrithione was used as a positive control, and DMSO was used as negative control. The experiment was performed in triplicate.
Statistical analyses
Student’s t-test was used to determine the statistical significance of differences between values for the experimental and control groups. The data were presented as means ± standard deviation (SD) of at least three independent experiments performed in triplicate.
RESULTS
Effect of G. elliptica extract on the proliferation of dermal papilla cells
To evaluate the effect of G. elliptica extract on cell proliferation of hair follicles, immortalized rat vibrissa dermal papilla cells were treated with various concentrations of G. elliptica extract, and proliferation of dermal papilla cells was examined.
G. elliptica extract promoted the proliferation of dermal papilla cells by 102.1, 104.7, 114.4 and 169.5% at the concentration of 0.1, 1, 10 and 100 μg/ml compared with the vehicle-treated control, respectively (Fig. 2). Specifically, 100 μg/ml of G. elliptica extract was found to induce a greater increase in
Fig. 2. Proliferation effect of G. elliptica extract on cultured dermalpapilla cells. Immortalized dermal papilla cells (DPCs) from rat vibrissa follicles (1.0×104 cells/ml) were plated in 96 well plates. DPCs were treated with various concentration of G. elliptica extract extract or minoxidil (MXD), as indicated. Cell proliferation was measured using a MTT assay for 4 days. All experiments were performed in triplicate. Data are presented as the mean ± the S.D. * p < 0.05 vs. control.
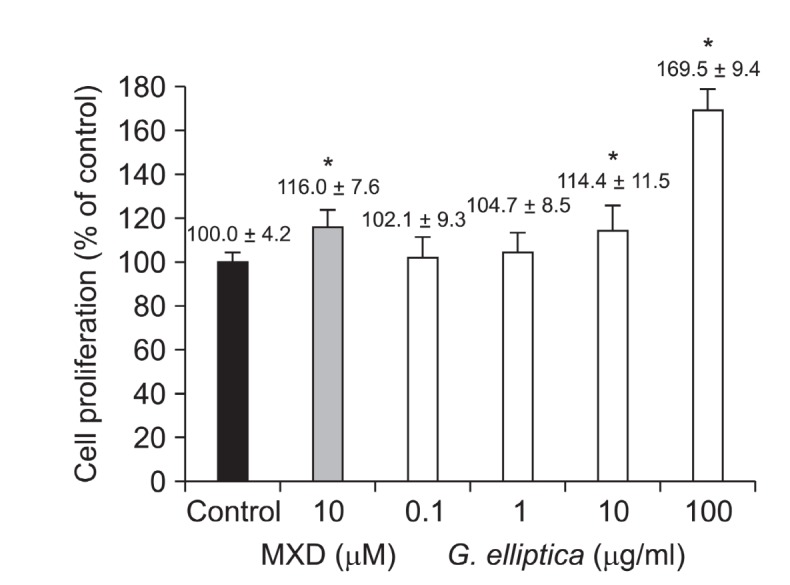
proliferation of dermal papilla cells than 10 μM of minoxidil, a positive control. The results suggest that G. elliptica extract might have hair-growth promoting effect via the proliferation of dermal papilla cells.
Effect of G. elliptica extract on 5α-reductase activity
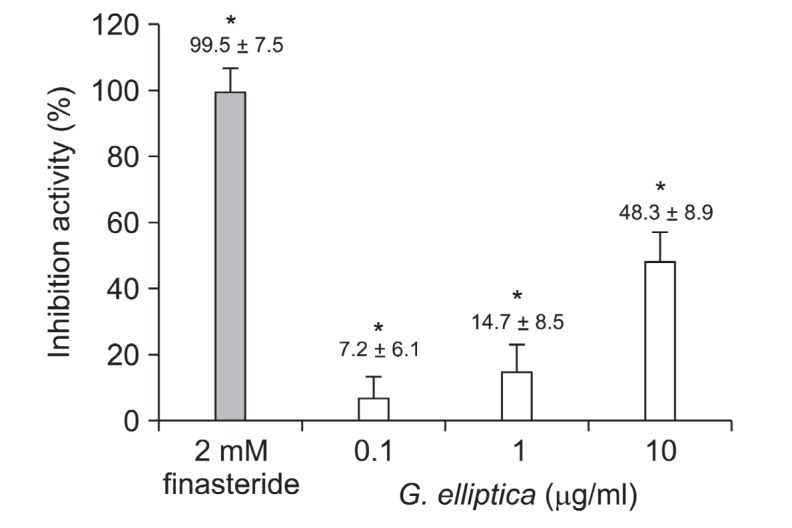
Conversion of testosterone to DHT is important in hair loss and, whether G. elliptica extract could inhibit 5α-reductase activity, we examined the 5α-reductase activity with crude enzyme from rat prostate. As shown in Fig. 3, G. elliptica extract inhibited 5α-reductase activities by 7.2%, 14.7% and 48.3% at the concentration of 0.1, 1 and 10 μg/ml in dose-dependent manner. Finasteride, a positive control, inhibited 5α-reductase activities by 98% at 2 nM concentration. The result suggests that G. elliptica extract could have the potential for the treatment of AGA via the 5α-reductase inhibition.
Fig. 3. The effect of G. elliptica extract on the inhibition of 5α-reductase. Assay of 5α-reductase inhibition was performed using crude extract of rat prostate as described in “Materials and Methods”. The conversion rate of testosterone (T) to dihydrotestosterone (DHT) was calculated by the equation [DHT/(T+DHT)]×100. Inhibition activity (%) was expressed as a percentage of reduced conversion rate compared to the control. The inhibition activity of control group was regarded as 0% (not shown). Finasteride was used as a positive control. Data are presented as the mean ± the S.D of three independent experiments. * p < 0.05 vs. control.
Effect of G. elliptica extract on the PGE2 production in HaCaT cells
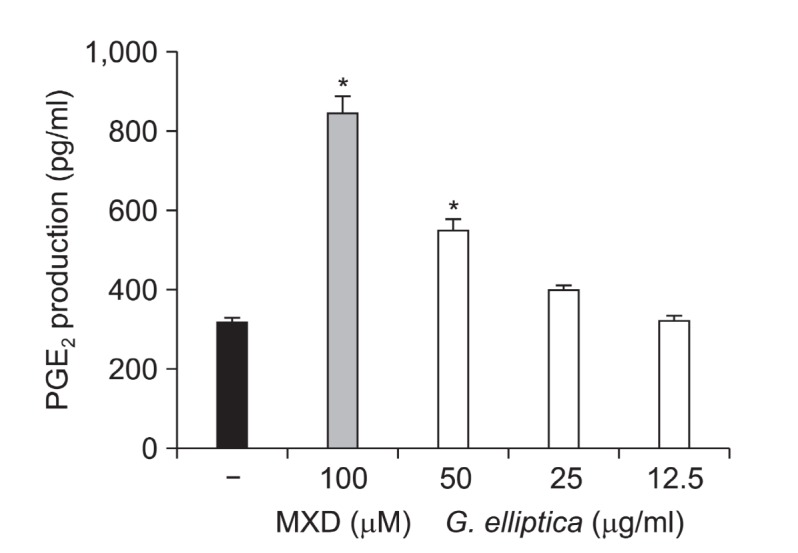
The PGE2 production effect of G. elliptica extract was measured in HaCaT human keratinocytes. G. elliptica extract at 12.5, 25, and 50 μg/ml increased PGE2 production in a dose dependent manner (Fig. 4). Minoxidil, a positive control, significantly increased PGE2 production at 100 μM. This result indicates that G. elliptica extract might have hair-growth promoting effect via the increase of PGE2 production.
Fig. 4. Effect of G. elliptica extract on PGE2 production in HaCaT human keratinocytes. HaCaT cells (2.0×105 cells/ml) were pre-incubated for 18 h, and cells were treated with G. elliptica extract for 24 h. PGE2 amount was determined from the culture supernatant by ELISA method. Data are presented as the mean ± the S.D of three independent experiments. * p < 0.05 vs. control.
Inhibitory effect of G. elliptica extract on the production of pro-inflammatory cytokines in LPS-stimulated bone marrow-derived dendritic cells
To evaluate the G. elliptica extract for anti-inflammatory activity, G. elliptica extract was tested for the inhibitory effect on LPS-stimulated IL-12 p40 production in BMDCs (Fig. 5). G. elliptica extract inhibited IL-12 p40 production by LPS-stimulated BMDCs with the inhibition values of 47.8% at the concentration of 50 μg/ml. In addition, G. elliptica extract exhibited significantly inhibitory effect on IL-6 production in LPS- stimulated DC with the inhibition value of 31.4%. In order to confirm the anti-inflammatory activity of G. elliptica extract, cell viability was simultaneously determined by using the colorimetric
Fig. 5. Inhibitory activity of G. elliptica extract on IL-12 p40 (A), IL-6 (B), and TNF-α (C) production by LPS-stimulated BMDCs. DCs were treated with the indicated concentration of the extract for 1 h before stimulation with LPS (10 ng/ml). Supernatants were harvested 16 h after stimulation. Concentrations of murine IL-12 p40, IL-6, and TNF-α in the culture supernatants were determined by ELISA. The data were presented as inhibition activity (%) compared to the value of vehicle-treated BMDCs.
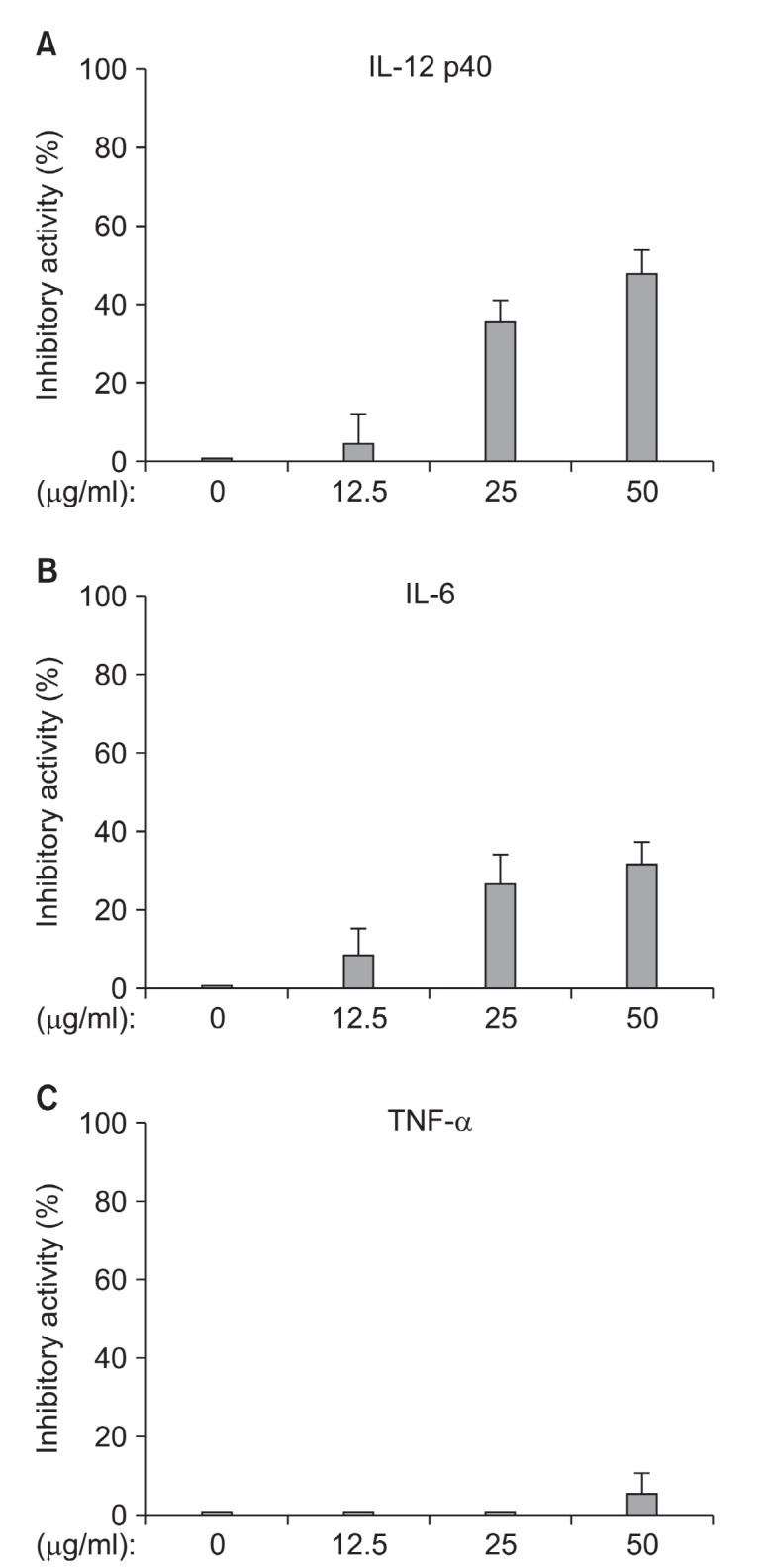
MTT assay and as a result, the extract had little or no effect at the concentration tested (data not shown). These results suggest that G. elliptica extract could have the potential for the treatment of AA via the decrease of pro-inflammatory cytokine production.
Antifungal activity of G. elliptica extract
Hair shedding has been reported to increase with cutaneous infection of P. ovale (Nematian et al., 2006). We examined whether G. elliptica extract could inhibit the growth of P.
ovale, normal flora yeast in dandruff. G. elliptica extract and zinc pyrithione, a positive control, exhibited 10 mm and 35 mm inhibition-zone diameter, whereas inhibitory-zone was 0 mm in the negative control (Table 1). The result shows that G. elliptica extract may prevent hair loss via inhibitory activity against P. ovale.
Table 1.
Inhibitory activity of G. elliptica extract against P. ovale
| Sample | Diameter (mm) |
|---|---|
|
| |
| G. elliptica extract | 10 |
| DMSO | - |
| Zinc pyrithione | 35 |
DMSO: Dimethyl sulfoxide.
DISCUSSION
In this study, the hair-loss preventing effects of G. elliptica extract were investigated. To the best of our knowledge, this study is the first to demonstrate that G. elliptica extract has the potential to treat alopecia via the proliferation of dermal papilla, 5α-reductase inhibition, increase of PGE2 production, decrease of LPS-stimulated pro-inflammatory cytokines and inhibitory activity against P. ovale.
The mesenchyme-derived dermal papilla cells play a pivotal role in hair growth regulation. The morphology of dermal papilla cells can be altered through the hair growth cycle, being maximal in volume in the growing phase (anagen) and least in the resting phase (telogen). Evidence has shown that the size of dermal papilla cells is well correlated with hair growth,and the cell number of dermal papilla cells is increased in the growing phase of hair cycle (Jahoda et al., 1984; Elliott et al.,1999).
In the continuing search for new treatment of alopecia from natural sources, we examined several seaweed extracts on the proliferation of dermal papilla cells. We found that the G. elliptica extract significantly increased the proliferation of dermal papilla cells by 169.5% at 100 μg/ml concentration compared with the control group, whereas extracts of Sargassum coreanum, Halymeni adilatata and Laurencia pinnata did not show proliferation activity of dermal papilla cells (data not shown). Specifically, 100 μg/ml of G. elliptica extract was found to induce a greater increase in proliferation of dermal papilla cells than 10 μM of minoxidil, a positive control.
AGA, the most common type of alopecia, may be modulated by the inhibition of 5α-reductase, which converts testosterone to DHT (Kaufman, 1996). Finasteride is known to repress the progression of AGA through inhibition of 5α-reductase (Kaufman, 1996). We found that G. elliptica extract could inhibit the activity of 5α-reductase by 48% at 10 μg/ml concentration. Previous studies suggest that AGA may be caused by DHT in different ways: The miniaturization of dermal papilla and hair follicles is induced by DHT, which leads to transition from anagen to catagen (Sinclair, 1998). DHT increases the levels of transforming growth factor-β1 (TGF-β1) and TGF-β2 in dermal papilla cells, which leads to decreased proliferation of epithelial cells (Inui et al., 2002; Hibino and Nishiyama, 2004). Up-regulation of dickkopf related protein-1 (DKK-1) by DHT can cause repression of the growth of epithelial cells in hair follicles (Kwack et al., 2008). In further study, we need to examine whether G. elliptica extract can regulate the levels of TGF-β1/β2 and DKK-1 in dermal papilla cells.
Hair follicle growth and cycle could be controlled by prostaglandins, under autocrine and paracrine loops. Minoxidil increases the activity of purified COX-1, suggesting a positive role of PG in hair growth onset (Coleman et al., 1994a; Johnstone and Albert, 2002; Colombe et al., 2007; Colombe et al., 2008). Lantanoprost, a PGF2α analogue, is widely used in the treatment of glaucoma and has a hypertrichotic side effect such as the increased number, length, thickness, and darkening of eyelashes hair (Johnstone and Albert, 2002; Uno et al., 2002; Messenger and Rundegren, 2004). PGE2 is described as a possible factor of hair growth (Colombe et al.,2007). Indeed, viprostol, a PGE2 analog, is an effective anti-hypertensive agent and was reported to increase human hair growth (Johnstone and Albert, 2002; Colombe et al., 2007). G. elliptica extract was found to increase PGE2 production in a dose dependent manner. By the way, there is a report that PGE2 was found in various species of seaweed Gracilaria. asiatica, G. lichenoides and G. rhodocaudata (Bernard, 2008). We thus examined the PGE2 amount in G. elliptica through the processing in a condition without HaCaT cells. PGE2 in G.elliptica couldn’t be detected at 50 μg/ml concentration (data not shown).
Cyclosporin A (CsA), a T cell-specific immunosuppressant, has the hair growth stimulating effect, which is observed not only in normal but also in patients with alopecia areata (Yamamoto and Kato, 1994) . Recent studies suggest that CsA may induce hair growth by inhibiting calcineurin which is needed for nuclear localization of NFATc1 in the bulge region. NFATc1 is activated by bone morphogenic protein (BMP) signaling, which is required for the maintenance of stem cell quiescence (Horsley et al., 2008). Therefore, when calcineurin/NFATc1 signaling is suppressed, stem cells are activated prematurely, resulting in hair follicular growth. In addition, TNF-α is required for a timely anagen-catagen transition in mouse pelage follicles (Tong and Coulombe, 2006). In the other hand, because IL-12 is a key cytokine in Th1-mediated autoimmune responses, down-regulation of IL-12 production may ameliorate the autoimmune diseases such as alopecia areata (Taki et al., 1997). Fig. 5 shows that G. elliptica extract may have potent anti-inflammatory action and can be useful for amelioration of the autoimmune diseases such as alopecia areata.
The non-pathogenic yeast P. ovale can undergo transition to a pathogenic form under favorable conditions. At high concentrations, this opportunistic organism diminishes the normal protective barrier of skin and affects the body’s ability to control inflammation. P. ovale-related diseases such as Seborrheic dermatitis, psoriasis are often difficult to treat. In particular, hair shedding is known to increase with dandruff by infection of P. ovale (Nematian et al., 2006). G. elliptica extract may have a potential as a treatment for P. ovale-associated hair diseases including hair loss.
Overall, the results of this study demonstrated that G. elliptica extract is capable of preventing hair loss via the proliferation of dermal papilla, 5α-reductase inhibition, increase of PGE2 production, decrease of pro-inflammatory cytokines and inhibitory activity against P. ovale. In further studies, active compounds from G. elliptica extract should be elucidated.
Acknowledgments
This research was a part of the project titled "Development of product and material promoting hair-growth from Jeju marine algae" funded by the Ministry of Land, Transport and Mari-time Affairs, Korea.
References
- 1.Alkhalifah A. Alsantali A. Wang E. McElwee K. J. Shapiro J. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J. Am. Acad. Dermatol. 2010;62:177–188. doi: 10.1016/j.jaad.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Anesini C. Perez C. Screening of plants used in Argentine folk medicine for antimicrobial activity. J. Ethnopharmacol. 1993;39:119–128. doi: 10.1016/0378-8741(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 3.Bernard B. A. Factors affecting PGE2 production in seaweed Gracilaria tenuistipitata. J. Food Drug Anal. 2008;59:59–65. [Google Scholar]
- 4.Burton J. L. Marshall A. Hypertrichosis due to minoxidil. Br. J. Dermatol. 1979;101:593–595. doi: 10.1111/j.1365-2133.1979.tb11892.x. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael J. DeGraff W. G. Gazdar A. F. Minna J. D. Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 6.Coleman R. A. Grix S. P. Head S. A. Louttit J. B. Mallett A. Sheldrick R. L. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994a;47:151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 7.Coleman R. A. Smith W. L. Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994b;46:205–229. [PubMed] [Google Scholar]
- 8.Colombe L. Michelet J. F. Bernard B. A. Prostanoid receptors in anagen human hair follicles. Exp. Dermatol. 2008;17:63–72. doi: 10.1111/j.1600-0625.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- 9.Colombe L. Vindrios A. Michelet J. F. Bernard B. A. Prostaglandin metabolism in human hair follicle. Exp. Dermatol. 2007;16:762–769. doi: 10.1111/j.1600-0625.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- 10.Elliott K. Stephenson T. J. Messenger A. G. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J. Invest. Dermatol. 1999;113:873–877. doi: 10.1046/j.1523-1747.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 11.Filsell W. Little J. C. Stones A. J. Granger S. P. Bayley S. A. Transfection of rat dermal papilla cells with a gene encoding a temperature-sensitive polyomavirus large T antigen generates cell lines retaining a differentiated phenotype. J. Cell. Sci. 1994;107(Pt7):1761–1772. doi: 10.1242/jcs.107.7.1761. [DOI] [PubMed] [Google Scholar]
- 12.Gately M. K. Renzetti L. M. Magram J. Stern A. S. Adorini L. Gubler U. Presky D. H. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 13.Gilhar A. Kalish R. S. Alopecia areata: a tissue specific autoimmune disease of the hair follicle. Autoimmun. Rev. 2006;5:64–69. doi: 10.1016/j.autrev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Gormley G. J. Finasteride: a clinical review. Biomed. Pharmacother. 1995;49:319–324. doi: 10.1016/0753-3322(96)82658-8. [DOI] [PubMed] [Google Scholar]
- 15.Hamaoka H. Minakuchi K. Miyoshi H. Arase S. Chen C. H. Nakaya Y. Effect of K+ channel openers on K+ channel in cultured human dermal papilla cells. J. Med. Invest. 1997;44:73–77. [PubMed] [Google Scholar]
- 16.Han J. H. Kwon O. S. Chung J. H. Cho K. H. Eun H. C. Kim K. H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004;34:91–98. doi: 10.1016/j.jdermsci.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Hibino T. Nishiyama T. Role of TGF-beta2 in the human hair cycle. J. Dermatol. Sci. 2004;35:9–18. doi: 10.1016/j.jdermsci.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Hirosumi J. Nakayama O. Fagan T. Sawada K. Chida N. Inami M. Takahashi S. Kojo H. Notsu Y. Okuhara M. FK143 a novel nonsteroidal inhibitor of steroid 5 alpha-reductase:(1) In vitro effects on human and animal prostatic enzymes. J. Steroid Biochem. Mol. Biol. 1995;52:357–363. doi: 10.1016/0960-0760(94)00187-Q. [DOI] [PubMed] [Google Scholar]
- 19.Horsley V. Aliprantis A. O. Polak L. Glimcher L. H. Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inui S. Fukuzato Y. Nakajima T. Yohikawa K. Itami S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. J. Investig. Dermatol. Symp. Proc. 2002;16:1967–1969. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- 21.Itami S. Kurata S. Takayasu S. Androgen induction of follicular epithelial cell growth is mediated via insulin-like growth factor-I from dermal papilla cells. Biochem. Biophys. Res. Commun. 1995;212:988–994. doi: 10.1006/bbrc.1995.2067. [DOI] [PubMed] [Google Scholar]
- 22.Jahoda C. A. Horne K. A. Oliver R. F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- 23.Johnstone M. A. Albert D. M. Prostaglandin-induced hair growth. Surv. Ophthalmol. 2002;47 Suppl 1:S185–202. doi: 10.1016/S0039-6257(02)00307-7. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman K. D. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol. Clin. 1996;14:697–711. doi: 10.1016/S0733-8635(05)70396-X. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman K. D. Girman C. J. Round E. M. Johnson-Levonas A.O. Shah A. K. Rotonda J. Progression of hair loss in men with androgenetic alopecia (male pattern hair loss): long-term (5-year) controlled observational data in placebo-treated patients. Eur. J. Dermatol. 2008a;18:407–411. doi: 10.1684/ejd.2008.0435. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman K. D. Olsen E. A. Whiting D. Savin R. DeVillez R. Bergfeld W. Price V. H. Van Neste D. Roberts J. L. Hordinsky M. Shapiro J. Binkowitz B. Gormley G. J. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J. Am. Acad. Dermatol. 1998;39:578–589. doi: 10.1016/S0190-9622(98)70007-6. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman K. D. Rotonda J. Shah A. K. Meehan A. G. Long-term treatment with finasteride 1 mg decreases the likelihood of developing further visible hair loss in men with androgenetic alopecia (male pattern hair loss). Eur. J. Dermatol. 2008b;18:400–406. doi: 10.1684/ejd.2008.0436. [DOI] [PubMed] [Google Scholar]
- 28.Kim K. Y. Nam K. A. Kurihara H. Kim S. M. Potent alpha-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry. 2008;69:2820–2825. doi: 10.1016/j.phytochem.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Koh Y. S. Koo J. E. Biswas A. Kobayashi K. S. MyD88-dependent signaling contributes to host defense against ehrlichial infection. PLoS One. 2010;5:e11758. doi: 10.1371/journal.pone.0011758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwack M. H. Sung Y. K. Chung E. J. Im S. U. Ahn J. S. Kim M.K. Kim J. C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Invest. Dermatol. 2008;128:262–269. doi: 10.1038/sj.jid.5700999. [DOI] [PubMed] [Google Scholar]
- 31.Kwack M. H. Kang B. M. Kim M. K. Kim J. C. Sung Y. K. Minoxidil activates beta-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011;62:154–159. doi: 10.1016/j.jdermsci.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Lachgar S. Charveron M. Gall Y. Bonafe J. L. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1998;138:407–411. doi: 10.1046/j.1365-2133.1998.02115.x. [DOI] [PubMed] [Google Scholar]
- 33.Lachgar S. Moukadiri H. Jonca F. Charveron M. Bouhaddioui N. Gall Y. Bonafe J. L. Plouet J. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J. Invest. Dermatol. 1996;106:17–23. doi: 10.1111/1523-1747.ep12326964. [DOI] [PubMed] [Google Scholar]
- 34.Messenger A. G. Rundegren J. Minoxidil: mechanisms of action on hair growth. Br. J. Dermatol. 2004;150:186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 35.Nematian J. Ravaghi M. Gholamrezanezhad A. Nematian E. Increased hair shedding may be associated with the presence of Pityrosporum ovale. Am. J. Clin. Dermatol. 2006;7:263–266. doi: 10.2165/00128071-200607040-00008. [DOI] [PubMed] [Google Scholar]
- 36.Pierard-Franchimont C. Xhauflaire-Uhoda E. Loussouarn G. SaintLeger D. Pierard G. E. Dandruff-associated smouldering alopecia: a chronobiological assessment over 5 years. Clin. Exp. Dermatol. 2006;31:23–26. doi: 10.1111/j.1365-2230.2005.02026.x. [DOI] [PubMed] [Google Scholar]
- 37.Shimaoka S. Imai R. Ogawa H. Dermal papilla cells express hepatocyte growth factor. J. Dermatol. Sci. 1994;7 Suppl:S79–83. doi: 10.1016/0923-1811(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 38.Shorter K. Farjo N. P. Picksley S. M. Randall V. A. Human hair follicles contain two forms of ATP-sensitive potassium channels only one of which is sensitive to minoxidil. FASEB. J. 2008;22:1725–1736. doi: 10.1096/fj.07-099424. [DOI] [PubMed] [Google Scholar]
- 39.Sinclair R. Male pattern androgenetic alopecia. BMJ. 1998;317:865–869. doi: 10.1136/bmj.317.7162.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soma T. Dohrmann C. E. Hibino T. Raftery L. A. Profile of transforming growth factor-beta responses during the murine hair cycle. J. Invest. Dermatol. 2003;121:969–975. doi: 10.1046/j.1523-1747.2003.12516.x. [DOI] [PubMed] [Google Scholar]
- 41.Soma T. Tsuji Y. Hibino T. Involvement of transforming growth factor-beta2 in catagen induction during the human hair cycle. J. Invest. Dermatol. 2002;118:993–997. doi: 10.1046/j.1523-1747.2002.01746.x. [DOI] [PubMed] [Google Scholar]
- 42.Taki S. Sato T. Ogasawara K. Fukuda T. Sato M. Hida S. Suzuki G. Mitsuyama M. Shin E. H. Kojima S. Taniguchi T. Asano Y. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity. 1997;6:673–679. doi: 10.1016/S1074-7613(00)80443-4. [DOI] [PubMed] [Google Scholar]
- 43.Tong X. Coulombe P. A. Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion. Genes Dev. 2006;20:1353–1364. doi: 10.1101/gad.1387406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uno H. Zimbric M. L. Albert D. M. Stjernschantz J. Effect of latanoprost on hair growth in the bald scalp of the stump-tailed macacque: a pilot study. Acta Derm. Venereol. 2002;82:7–12. doi: 10.1080/000155502753600803. [DOI] [PubMed] [Google Scholar]
- 45.Van Neste D. Fuh V. Sanchez-Pedreno P. Lopez-Bran E. Wolff H. Whiting D. Roberts J. Kopera D. Stene J. J. Calvieri S. Tosti A. Prens E. Guarrera M. Kanojia P. He W. Kaufman K. D. Finasteride increases anagen hair in men with androgenetic alopecia. Br. J. Dermatol. 2000;143:804–810. doi: 10.1046/j.1365-2133.2000.03780.x. [DOI] [PubMed] [Google Scholar]
- 46.Whiting D. A. Waldstreicher J. Sanchez M. Kaufman K. D. Measuring reversal of hair miniaturization in androgenetic alopecia by follicular counts in horizontal sections of serial scalp biopsies: results of fi nasteride 1 mg treatment of men and postmenopausal women. J. Investig. Dermatol. Symp. Proc. 1999;4:282–284. doi: 10.1038/sj.jidsp.5640230. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto S. Kato R. Hair growth-stimulating effects of cyclosporin A and FK506 potent immunosuppressants. J. Dermatol. Sci. 1994;7 Suppl:S47–54. doi: 10.1016/0923-1811(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 48.Yang E. J. Moon J. Y. Kim M. J. Kim D. S. Kim C. S. Lee W.J. Lee N. H. Hyun C. G. Inhibitory effect of Jeju endemic seaweeds on the production of pro-infl ammatory mediators in mouse macrophage cell line RAW 264.7. J. Zhejiang Univ. Sci.B. 2010;11:315–322. doi: 10.1631/jzus.B0900364. [DOI] [PMC free article] [PubMed] [Google Scholar]