Abstract
Oroxylin A is a flavone isolated from a medicinal herb reported to be effective in reducing the inflammatory and oxidative stresses. It also modulates the production of brain derived neurotrophic factor (BDNF) in cortical neurons by the transactivation of cAMP response element-binding protein (CREB). As a neurotrophin, BDNF plays roles in neuronal development, differentiation, synaptogenesis, and neural protection from the harmful stimuli. Adenosine A2A receptor colocalized with BDNF in brain and the functional interaction between A2A receptor stimulation and BDNF action has been suggested. In this study, we investigated the possibility that oroxylin A modulates BDNF production in cortical neuron through the regulation of A2A receptor system. As ex-pected, CGS21680 (A2A receptor agonist) induced BDNF expression and release, however, an antagonist, ZM241385, prevented oroxylin A-induced increase in BDNF production. Oroxylin A activated the PI3K-Akt-GSK-3β signaling pathway, which is inhibited by ZM241385 and the blockade of the signaling pathway abolished the increase in BDNF production. The physiological roles of oroxylin A-induced BDNF production were demonstrated by the increased neurite extension as well as synapse formation from neurons. Overall, oroxylin A might regulate BDNF production in cortical neuron through A2A receptor stimulation, which promotes cellular survival, synapse formation and neurite extension.
Keywords: Oroxylin A, BDNF, CREB, Adenosine A2A receptor, CGS21680, ZM241385
INTRODUCTION
Oroxylin A (5,7-dihidrixy-6-methoxyflavone) is a flavonoid originated from the root of Scutellaria baicalensis Georgi, which acts as a γ-aminobutyric acid (GABAA) receptor antagonist (Huen et al., 2003). Oroxylin A ameliorated memory dysfunction induced by scopolamine (Kim et al., 2007), and Aβ (25-35) (Kim et al., 2008) and also increased the number of phosphorylated cAMP response element-binding protein (CREB) and brain derived neurotrophic factor (BDNF) positive cells (Kim et al., 2006), which might be associated with the neuroprotective effects of oroxylin A in cultured rat primary neuron (Jeon et al., 2011).
BDNF is a member of the neurotrophin family (Lewin, 1996) which plays important roles in central nervous system (CNS) such as protection of neuronal degeneration (Lindholm et al., 1993), differentiation of hippocampal and cortical neurons (Ip et al., 1993; Croll et al., 1994; Nawa et al., 1994; Marty et al., 1996) and synaptogenesis (Shen et al., 2006). It is also well known that BDNF is involved in the regulation of neurite out-growth as well as directional movement via a variety of different mechanisms (Bartrup et al., 1997; Winckler, 2007; Sasaki et al., 2010).
Adenosine is a purine nucleoside, which transmits its physiological signal through adenosine receptors. Four subtypes of adenosine receptors have been described to date, A1, A2A, A2B, and A3 subtypes (Tucker and Linden, 1993), which are classified as 2 categories by functions; A1 and A3 which are negatively coupled to adenylate cyclase via G proteins and A2A and A2B which are positively coupled to the same effectors (Proll et al., 1986; Moser et al., 1991). Among them, A2A receptor modulates tonic expression of BDNF as well as synaptic actions of BDNF on hippocampal neurons (Diógenes et al., 2004; Tebano et al., 2008). A2A receptor also activates one of BDNF receptors, tropomyosin-related kinase B (TrkB), and Akt signaling molecule, which promotes motor neuron survival (Wiese et al., 2007) and modulates neurite outgrowth in several different cell types (Cheng et al., 2002; Canals et al., 2005; O'Driscoll and Gorman, 2005). Greengard group showed that A2A receptor-mediated modulation of PC12 cell differentiation and neurite extension in collaboration with FGF receptors (Flajolet et al., 2008). These strong trophic actions of A2A receptor activation make it one of the promising targets for several psychiatric and neurodegerative diseases (Cunha et al., 2008).
The positive relationship of A2A receptor activation and in-creased BDNF action as well as our preliminary results suggesting that oroxylin A may bind to A2A receptor (our unpublished results) prompted us to investigate whether oroxylin A might regulate BDNF production via modulating A2A receptor on cultured rat primary neurons using a pharmacological agonist and antagonist. We also investigated the possible intracellular signaling pathway mediating the increased BDNF production by oroxylin A as well as the role of oroxylin A on neurite extension.
MATERIALS AND METHODS
Materials
Neurobasal medium was purchased from GIBCO BRL (NY, USA) and B-27 supplements was obtained from Invitrogen (CA, USA). The BDNF ELISA kit was from Promega (Madison, WI). Specific primary antibody against BDNF was purchased from Santa Cruz Biotechnology Inc. (sc-546, Santa Cruz, CA), and Tuj-1 was from Covance (Richmond, CA). Other phospho-and total- form of antibodies (CREB, ERK, Akt, and GSK-3β) were obtained from Cell Signaling Technology (Beverly, MA, USA). Oroxylin A was obtained from Korea food & drug administration. U0126 and wortmannin were obtained from Calbiochem (San Diego, CA). CGS21680 and ZM241385 were purchased from Tocris Bioscience (Bristol, UK), and all other chemical reagents were purchased from Sigma (St.Louis, MO, USA).
Methods
Cell culture: Primary cortical neurons were isolated from embryonic cerebral cortex of Sprague-Dawley rat (SD rat) as previously described (Jeon et al., 2011). Cerebral cortex obtained from E16 pups was digested and re-suspended in NBM containing B-27 and then cells were placed on poly-D-lysine (PDL) pre-coated plates. The cultures were kept in a humidified 10% CO2 atmosphere at 37℃ for 10 days and media were half-replaced with fresh media every 3 days.
Drug treatment: Inhibitors were pre-treated 1 hr before the oroxylin A treatment. As an ERK1/2 phosphorylation inhibitor, U0126 was used at 10 μM and wortmannin was used as an Akt inhibitor at 100 nM. To modulate the activity of adenosine A2A receptor, an antagonist (50 nM of ZM241385) and an agonist (20 nM of CGS21680) was used.
Western blot:Treated cells were lysed with 2× sample buf-fer (4% w/v SDS, 20% glycerol, 200 mM DTT, 0.1 M Tris-HCl, pH 6.8, and 0.02% bromophenol blue) and the samples were fractionated by 8-12% SDS-PAGE and electrotransferred to nitrocellulose (NC) membrane. The NC membrane was blocked with 1 μg/ml polyvinyl alcohol (PVA) for 0.5 hr at room temperature (RT) and incubated overnight at 4℃ with the appropriate primary antibodies which were diluted at 1:5000 in 5% skim milk (Roth, Germany). After washing with Tris-buffered saline containing 0.1% Tween20 (TBS-T), NC membranes were incubated with peroxidase conjugated secondary antibody for 2 hr at RT. Following three times of washings with TBS-T, blots were detected by enhanced chemiluminescence (Amersham, Buckinghampshire, UK).
Reverse transcription polymerase chain reaction (RT-PCR): Cellular total RNA was extracted from primary neurons using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and 2 μg of them was converted to cDNA (Maxime RT PreMix Kit, iNtRON Biotechnology, Seoul) according to the manufacturer’s protocol. The PCR amplification was performed using Maxime PCR premix Kit (iNtRON Biotechnology, Seoul) and was consisted of 26 cycles with the oligonucleotide primers for BDNF (ac-cession number EF125679.1, Tm=60℃, (Kobayashi et al., 2008)), GABAAR2 (accession number NM_001135779.1, Tm=60℃), GABAAR5 (accession number NM_017295.1, Tm=55℃), A2AR (accession number L08102, Tm=55℃), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, accession number M17701, Tm=60℃). The following primers were used for amplification reactions:
for BDNF,
forward primer : 5’-ATA GGA GAC CCT CCG CAA CT-3’
reverse primer : 5’-CTG CCA TGC ATG AAA CAC TT-3’
for GAPDH,
forward primer : 5’-TCC CTC AAG ATT GTC AGC AA-3’
reverse primer : 5’-AGA TCC ACA ACG GAT ACA TT-3’
for GABAA R2,
forward primer : 5’- CGG TGC CAG CGA GAA CTG TGT-3’
reverse primer : 5’- GGG CGT AGT TGG CAA CGG CT-3’
for GABAA R5,
forward primer : 5’-GCC CGG AAT TCG CTG CCC AA-3’
reverse primer : 5’- GTC CCG CCT GGA AGC TGC TC -3’
for A2A R,
forward primer : 5’-CCA TGC TGG GCT GGA ACA-3’
reverse primer : 5’-GAA GGG GCA GTA ACA CGA -3’
The amplified products were analyzed on 1% agarose gel and stained with EtBr. The expected size of the amplified DNA fragments was 280 base pairs for BDNF, 297 base pairs for GABAA R alpha subunit 2, 275 base pairs for GABAA R alpha subunit 5, and 308 base pairs for GAPDH.
ELISA assay: The amount of released BDNF was quantified from medium of treated neuron using the Emax ImmunoAssay system (Promega, Madison, WI). BDNF enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer's manual. Briefly, 96 well plates were pre-coated with anti-BDNF mAb diluted with carbonate coating buffer at 4℃ for 24 hr. After 1 hr blockade with supplied Blocking buffer,
the plates were incubated with standard and culture medium sample for 2 hr at RT followed by incubation with anti-human BDNF pAb. After 1 hr incubation of Anti-IgY HRP conjugate, the reaction was developed with tetramethylbenzidine (TMB One Solution) and the absorbance was read at 450 nm with a microplate reader (Tecan Trading AG, Switzerland) after stopping the reaction with 1N HCl.
Immunocytochemistry: DIV 2 primary cortical neurons plated on the PDL pre-coated cover glasses (Fisher Scientific, PA) were treated with 20 μM of oroxylin A for 24 hr. Glasses were washed twice with PBS and fixed with 4% paraformaldehyde (PFA) for 0.5 hr. After three times of washing, samples were
permeabilized using 0.3% Triton X-100 solution for 15 min at RT and blocked by blocking buffer (1% BSA, 5% FBS in PBS) for 30 min at RT. Samples were incubated overnight at 4℃ with the primary antibody against neuron (Tuj-1, 1:500 in blocking buffer). Next day, after washing, samples were incubated for 2 hr with secondary antibodies conjugated with TMRE (diluted at 1:500 in blocking buffer). Then samples were washed and mounted using Vectashield (Vector laboratories, Burlingame, CA, USA). Cellular images were observed by fluorescence microscope (motorized research microscope bx61, Olympus, Japan) and analyzed by Image J software (NIH, USA).
Statistics
Data are expressed as the mean ± standard error of mean (S.E.M.) and analyzed for statistical significance by using one way analysis of variance (ANOVA) followed by Newman-Keuls test as a post hoc test and a p value<0.05 was considered significant.
RESULTS
Oroxylin A stimulated BDNF expression and release in rat primary neurons through Adenosine A2A receptor activation
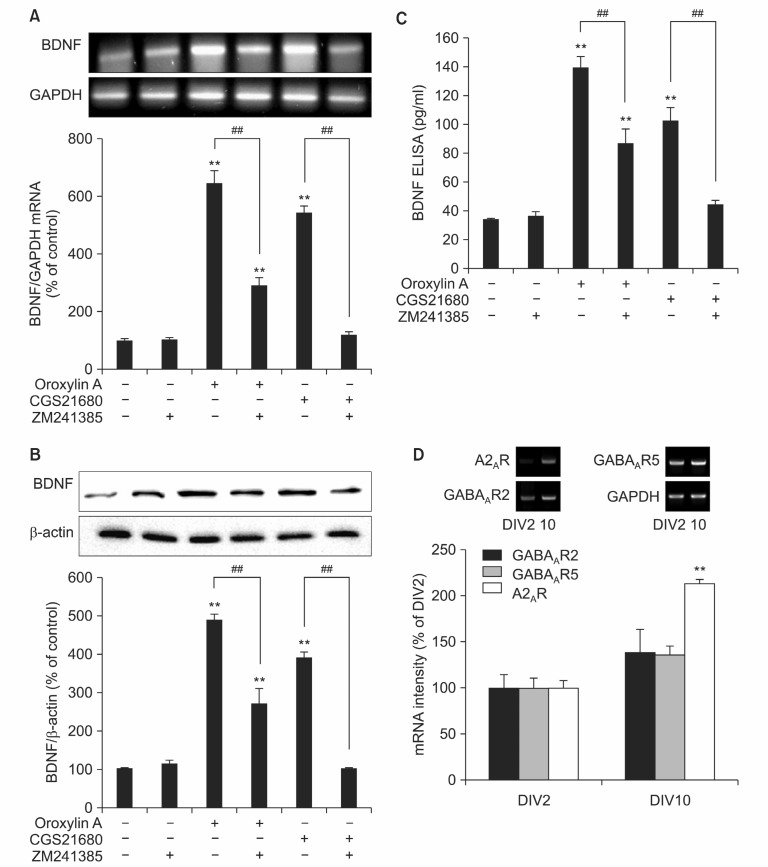
Treatment of oroxylin A or an A2A receptor agonist, CGS21680 increased BDNF mRNA level 6.24 ± 0.44 and 4.65 ± 0.26 folds compared with control, respectively (Fig. 1A). Co-treatment with an A2A antagonist ZM241385 inhibited the increased BDNF mRNA expression induced by oroxylin A or CGS21680. Similar pattern of changes was also observed for BDNF protein level (Fig. 1B) as well as BDNF release (Fig. 1C), which was determined by Western blot and ELISA, respectively. nterestingly, while A2A receptor antagonist ZM241385 completely prevented the increased expression of BDNF induced by A2A receptor agonist CGS21680, ZM241385 only partially inhibited oroxylin A-mediated increase in BDNF expression. To investigate the expression of A2A receptor in rat primary neuron, we performed RT-PCR analysis. The expression of A2A receptor was confirmed ar DIV 2 neuron, which was in-creased in DIV 10. Similar pattern was also observed with GABAA receptor (Fig. 1D). These results suggest that oroxylin A may induce BDNF expression by A2A receptor activation as well as other intracellular signaling pathways.
Fig. 1. Oroxylin A induced BDNF production in cortical neurons through adenosine A2A receptor. (A) Oroxylin A (20 μM), CGS21680 (20 nM), and ZM241385 (50 nM) was treated to primary cortical neurons for 24 hr and cells were lysed and analyzed by RT-PCR. (B) In case of BDNF protein level samples were analyzed by Western blot. (C) Released BDNF was quantified by ELISA assay. (D) Cortical neurons were collected at DIV2, and DIV10 to confirm the expression of A2AR and GABAAR respectively. Each graph represents quantification of RT-PCR and Western blot band intensity respectively. Data represent Mean ± S.E.M. **Significantly different as compared with control and ##significantly different as compared with oroxylin A or CGS21680 stimulation (p≺0.01, n=4).
Oroxylin A-induced BDNF production was mediated by Akt and GSK-3β phosphorylation in rat primary neurons
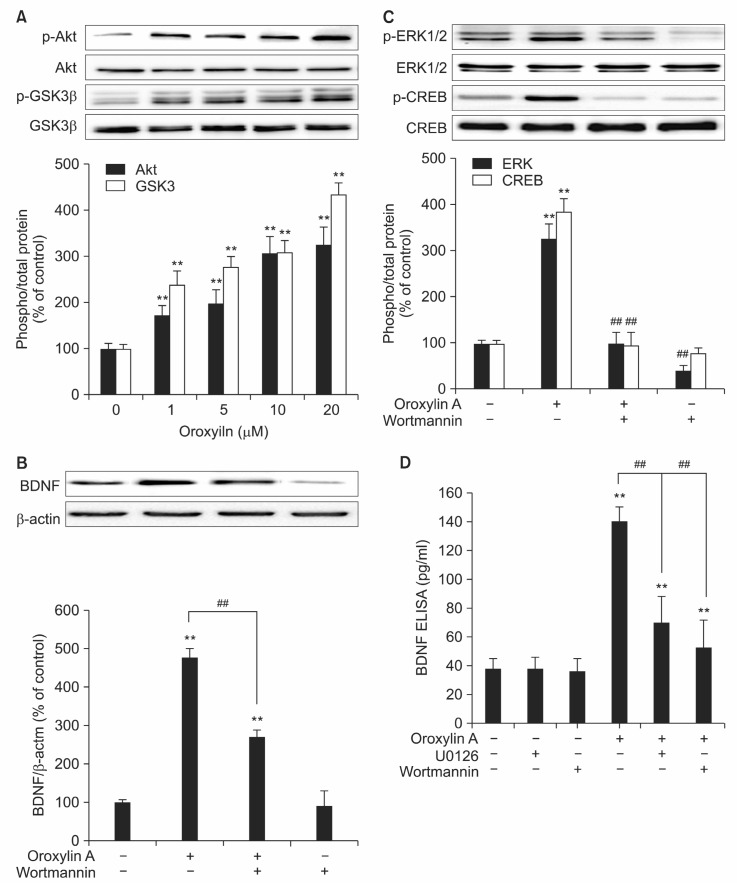
We previously reported that oroxylin A-induced BDNF production was mediated by CREB and ERK1/2 phosphorylation (Kim et al., 2008; Jeon et al., 2011). Because Akt-GSK3β pathway is a downstream pathway of A2A receptor activation and is also involved in the modulation of BDNF production (Mai et al., 2002), we next investigated the activation of Akt-GSK3β pathway by oroxylin A (Fig. 2A). Oroxylin A induced phosphorylation of Akt and its downstream target GSK-3β in a concentration dependent manner (Fig. 2A). At the highest concentration of oroxylin A (20 μM), phosphorylation of Akt and GSK-3β reached 319.07 ± 36% and 431.20 ± 28.31% of control level, respectively (Fig. 2A). Oroxylin A-induced BDNF protein expression determined by Western blot was inhibited by pretreatment of an Akt inhibitor, wortmannin (Fig. 2B) and in this condition, ERK1/2 and CREB phosphorylation, a whole mark of transcriptional activation of oroxylin A-induced BDNF expression, was also inhibited as reported previously (Fig. 2C) (Jeon et al., 2011). We next measured the release of BDNF using ELISA assay and either Akt inhibitor wortmannin or
Fig. 2. Oroxylin A induced the activation of Akt which was necessary for the phosphorylation of ERK1/2-CREB. (A) Rat primary cortical neurons were treated with oroxylin A (1, 5, 10, 20 μM) for 3 hr and analyzed by Western blot. Phospho/total- Akt and GSK-3β ratio was determinedby densitometric quantification of Western blot. (B) A PI3K activation inhibitor wortmannin was used to investigate the role of Akt and GSK-3β signaling on BDNF production. After 1 hr treatment of wortmannin (100 nM), cells were treated with oroxylin A for 24 hr and the level of BDNF protein expression was measured by Western blot. (C) ERK1/2 and CREB phosphorylation was determined after wortmannin and oroxylin A treatment. (D) The level of BDNF release was measured using ELISA assay from U0126-, wortmannin-, or oroxylin A-treated cell culture supernatants. Detailed protocol was described in Materials and Methods. Each graph represents quantification of Western blot band intensity. Data represent Mean ± S.E.M. **Significantly different as compared with control and ##significantly different as compared withoroxylin A or CGS21680 stimulation (p≺0.01, n=4).
ERK1/2 inhibitor, U0126 treatment (Fig. 2D) inhibited oroxylin-induced BDNF release. These results suggest that oroxylin A induces phosphorylation of Akt-GSK3β pathway which may modulate, at least in part, the phosphorylation of ERK1/2 and CREB pathways.
Oroxylin A activates Akt-GSK-3β pathways through adenosine A2A receptor
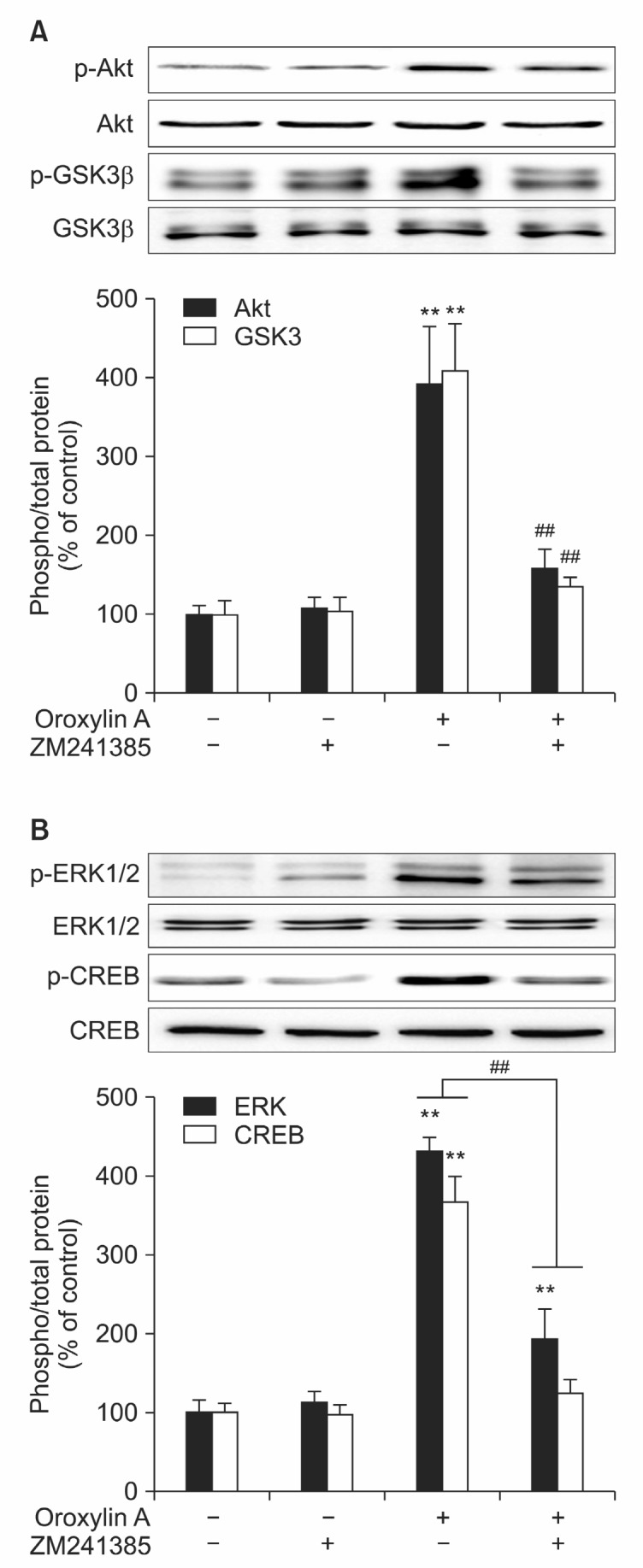
Next, we investigated whether adenosine A2A receptor is involved in the activation of Akt pathway induced by oroxylin A (Fig. 3). Pretreatment of adenosine A2A receptor antagonist, ZM241385, inhibited oroxylin A-induced phosphorylation of Akt and GSK-3β (Fig. 3A) and in this condition, ERK1/2-CREB phosphorylation was also significantly inhibited (Fig. 3B). Taken together with the decreased oroxylin-induced BDNF production by ZM241385 (Fig. 1B), these results suggest that oroxylin A induces phosphorylation of Akt-GSK3β and down-stream pathways leading to the BDNF production by modulating the activation of A2A receptor.
Fig. 3. Adenosine A2A receptor mediates oroxylin A-induced Akt and ERK1/2 phosphorylation and BDNF production. To identify the signaling pathway involved in the Adenosine A2A receptor-mediated stimulation of BDNF production, phosphorylation level of Aktand ERK1/2 were analyzed using Western blot. (A, B) Cells were treated with ZM241385 (50 nM) for 1 hr before the treatment oforoxylin A (20 μM). After 1 hr, cells were harvested to analyze the level of phospho/total Akt-GSK-3β (A) and ERK1/2- CREB (B) by Western blot. Each graph represents quantification of Western blot band intensity. Data represent the mean ± S.E.M. **Significantly different as compared with control and ##significantly different as compared with oroxylin A stimulation (p≺0.01, n=4).
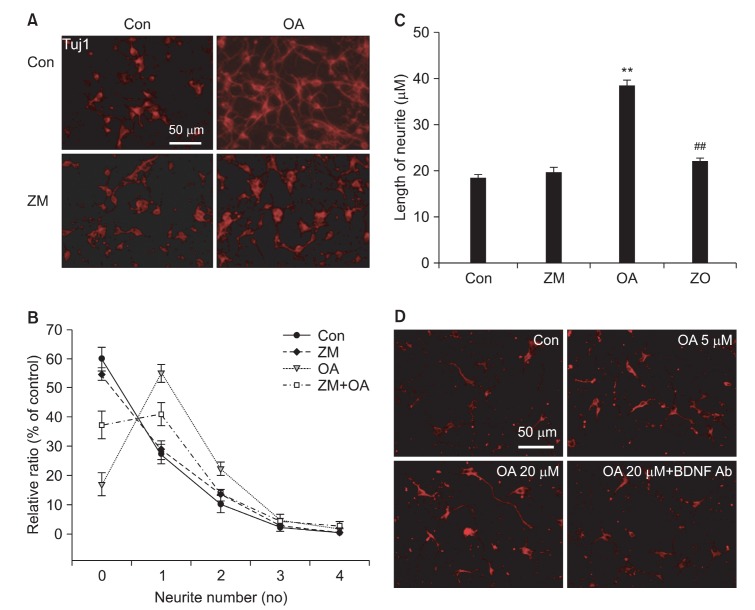
Oroxylin A induces neurite outgrowth in cultured rat primary neuron
Considering the roles of BDNF in neurons, we finally investigated the role of oroxylin A on the neurite outgrowth in immature rat primary cortical neuron. We previously reported the neuroprotective effect of oroxylin A against glutamate, pre-sumably via the increased production of BDNF, in rat primary cortical neurons (Jeon et al., 2011). DIV 2 rat primary cortical neurons were treated with oroxylin A and visualized by a neuronal marker, Tuj-1 immunostaining (Fig. 4). Oroxylin A increased the average number of neurite branches extending from a single neuron as well as the length of individual neurite. Interestingly, pre-incubation of ZM241385 prevented oroxylin-induced neurite outgrowth, again suggesting the essential role of A2A receptor activation in this process (Fig. 4). The increased neurite extension by oroxylin A was also inhibited by the addition of anti-BDNF antibody (Fig. 4D). Overall, these results suggest that oroxylin A enhances neurite out-growth by regulating BDNF expression and release, at least in part, through A2A receptor.
Fig. 4. Oroxylin A-induced up-regulation of BDNF facilitated differentiation of rat primary cortical neuron. Neurons were treated with oroxylin A and were immunostained against neuronal marker Tuj-1 (A). The number of neurite extending from each neuron was analyzed by Image J (B) as described in materials and methods. The length of neurite (C) was also analyzed by Image J analysis of immunostaining data. (D) At DIV2, cortical neurons were treated with oroxylin A (5, 20 μM) and immunostained with Tuj-1 antibody to visualize the neurite extension. Anti-BDNF antibody (2 μg/ml) was used to block BDNF action from oroxylin A treated neurons. Data represent the mean ± S.E.M. Scale bar represents 50 μm. **Significantly different as compared with control and ##significantly different as compared with oroxylin A treated group (p≺0.01, n=4).
DISCUSSION
In this study, we provided evidences that oroxylin A increased BDNF production and neurite outgrowth at least in part by A2A receptor stimulation followed by activation of Akt-GSK3β pathway. In our previous reports, oroxylin A improved working memory in scopolamine treated animals (Kim et al., 2007) as well as in transiently bilateral common carotid artery occluded animals (Kim et al., 2006), which may also be related to the increased BDNF production and neurogenesis in brain. In addition, we observed increased synaptogenesis by oroxylin A as evidenced by increased expression of synaptic marker proteins in immature rat primary cortical neuron (Jeon et al., 2011). Gasiorowski et al. recently showed oroxylin A dramatically increased cognition and memory from aged animals, which was consistent with our data (Gasiorowski et al., 2011). However they did not elucidate the exact mechanism of neuroprotective effects in their report. Nevertheless, these results suggest that using the flavonoids like oroxylin A, as a potential neuroprotective agent may be a potential neuroprotective booster (food additive, drug) target. Our data suggesting the possible role of oroxylin A in neuroprotection, neurogenesis and neural differentiation via modulation of BDNF expression by mechanism involving regulation of A2A activation may support and strengthen this view.
Previously, oroxylin A is reported to suppress nitric oxide generation (Jiwajinda et al., 2002) and inhibit LPS induced iNOS and COX-2 expression by modulating NF-κB activation (Chen et al., 2000; Chen et al., 2001). The effect of oroxylin A on the prevention of uterine contraction was also suggested. Oroxylin A may inhibit uterine contractions by opening calcium dependent potassium channels or adenosine triphosphate dependent potassium channel (Shih et al., 2009), which suggest that oroxylin A may modulate cellular membrane channels or receptors, in our cases, A2A adenosine receptor.
Adenosine receptors control essential brain functions like synaptic plasticity, neurotransmitter transport, and astrogliosis (Sebastião and Ribeiro, 2009) by receptor dimerization. Especially, A2A receptor-dopamine D2 receptor heterodimers may exist in the striatal GABA pathways, where activation of A2A receptors inhibits D2 receptor action. As a result of the A2A receptor-induced reduction of D2 receptor signaling, the activity of GABA neurotransmission is increased, which may provide novel tools to treat Parkinson’s disease, schizophrenia, and addiction (Francesco et al., 2008). However, it will be needed further investigations to define whether oroxylin A will be able to modulate the pathophysiology of these disorders.
In addition to the regulation of BDNF expression, the activation of A2A receptor increases calcium dependent protein secretion by modulating cAMP-PKA, ERK1/2 and PKC pathways in PC12 cells (Huang et al., 2001; Cheng et al., 2002; Flajolet et al., 2008), which is consistent with the increase in BDNF release by oroxylin A as observed in this study. Pousinha et al. also showed that increased BDNF affected synaptic transmission from CGS21680 administered Wistar rats (Pousinha et al., 2006), and this A2A receptor-mediated BDNF action was coupled to phospholipase C-γ (PLC-γ), which was regulated by A2A-activated PKA pathway to act on neuromuscular transmission (Pousinha et al., 2006). It has been also reported that A2A receptor is coupled to metabotropic glutamate receptor 5 (mGluR5), which facilitates the activation of N-methyl-D-aspartate (NMDA) receptor in hippocampus (Tebano et al., 2005; Rebola et al., 2008). In this regard, we showed NMDA receptor antagonist (MK801) treatment significantly reduced the oroxylin A induced BDNF production as well as MAPK phosphorylation (Jeon et al., 2011). Overall, these suggest that activation of A2A receptor by oroxylin A may be positively coupled to NMDA receptor activation, which plays a role in the up-regulation of BDNF expression.
A2A receptor stimulation inhibits primary neurosphere formation and the proliferation of human and rodent neural precursor cells (Scemes et al., 2003; Stafford et al., 2007) suggesting that it may play a regulatory role in neuronal differentiation, which is one of the actions of BDNF during developmental period and is well presented in the increase of neurogenesis by oroxylin A (Terashima et al., 2010; Jeon et al., 2011; Noble et al., 2011).
Interestingly, it has been suggested that removal of endogenous adenosine inhibited the excitatory stimulation of BDNF activation, and on the contrary, administration of CGS21680 potentiated the BDNF actions on LTP suggesting that proper level of endogenous adenosine should be maintained to trigger BDNF action (Fontinha et al., 2008). These results suggest that facilitatory actions of BDNF are essentially dependent on adenosine and adenosine A2A receptor activation, which underscores the importance of A2A receptor on BDNF actions.
BDNF protects cortical and hippocampal neurons from the injury (Cheng et al., 1997; Han and Holtzman, 2000) and induces growth and differentiation of new neurons and synapses (Choi et al., 2009; Lee and Son, 2009). In clinical aspects, BDNF has been implicated in the regulation of learning and memory (Lynch et al., 2008; Cowansage et al., 2010), and reported in the therapeutic effects in psychiatric disorders such as depression (Hashimoto et al., 2004; Schmidt and Duman, 2007) and schizophrenia (Angelucci et al., 2005) as well as neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis (Pezet and Malcangio, 2004; Pardon, 2010). Considering inefficient transport of BDNF through BBB, one possible approach to take advantage of BDNF as a therapeutic target is to use small molecules to boost endogenous level of BDNF. Interestingly, daily administration of A2A agonist CGS21680 ameliorates the symptoms of Huntington’s disease animal models (Chou et al., 2005). Whether oroxylin A may provide clinical efficacy against neurological diseases such as mood disorder and neurodegenerative diseases by inducing BDNF expression and release through its action on A2A receptor should be further investigated in future studies.
Acknowledgments
This work was supported by Konkuk University in 2008 (Seung Hwa Park) and in part by the National Research Foun-dation of Korea (NRF) grant (2010-0023394) from the Korea government.
Contributor Information
Seung Hwa Park, Phone: +82-2-2049-6322, FAX: +82-2-2030-7899.
Chan Young Shin, Phone: +82-2-2030-7834, FAX: +82-2-2030-7899.
References
- 1.Angelucci F. Brenè S. Mathé A. A. BDNF in schizophrenia, depression, and corresponding animal models. Mol. Psychiatry. (2005);10:345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- 2.Bartrup J. T. Moorman J. M. Newberry N. R. BDNF enhances neuronal growth and synaptic activity in hippocampal cellcultures. Neuroreport. (1997);8:3791–3794. doi: 10.1097/00001756-199712010-00027. [DOI] [PubMed] [Google Scholar]
- 3.Canals M. Angulo E. Casadó V. Canela E. I. Mallol J. Viñals F. Staines W. Tinner B. Hillion J. Agnati L. Fuxe K. Ferré S. Lluis C. Franco R. Molecular mechanisms involved in the adenosine A and A receptor-induced neuronal differentiation in neuroblastoma cells and striatal primary cultures. J. Neurochem. (2005);92:337–348. doi: 10.1111/j.1471-4159.2004.02856.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y. Yang L. Lee T. J. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-kappaB activation. Biochem. Pharmacol. (2000);59:1445–1457. doi: 10.1016/S0006-2952(00)00255-0. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y. C. Shen S. C. Chen L. G. Lee T. J. Yang L. L. Wogonin, baicalin, and baicalein inhibition of inducible nitric oxide synthase and cyclooxygenase-2 gene expressions induced by nitric oxide synthase inhibitors and lipopolysaccharide. Biochem. Pharmacol. (2001);61:1417–1427. doi: 10.1016/S0006-2952(01)00594-9. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H. C. Shih H. M. Chern Y. Essential role of cAMP-response element-binding protein activation by A2A adenosine receptors in rescuing the nerve growth factor-induced neurite outgrowth impaired by blockage of the MAPK cascade. J. Biol. Chem. (2002);277:33930–33942. doi: 10.1074/jbc.M201206200. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y. Gidday J. M. Yan Q. Shah A. R. Holtzman D. M. Marked age-dependent neuroprotection by brain-derived neurotrophic factor against neonatal hypoxic-ischemic brain injury. Ann. Neurol. (1997);41:521–529. doi: 10.1002/ana.410410416. [DOI] [PubMed] [Google Scholar]
- 8.Choi S. H. Li Y. Parada L. F. Sisodia S. S. Regulation of hippocampal progenitor cell survival, proliferation and dendritic development by BDNF. Mol. Neurodegener. (2009);4:52. doi: 10.1186/1750-1326-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou S. Y. Lee Y. C. Chen H. M. Chiang M. C. Lai H. L. Chang H. H. Wu Y. C. Sun C. N. Chien C. L. Lin Y. S. Wang S. C. Tung Y. Y. Chang C. Chern Y. CGS21680 attenuates symptoms of Huntington's disease in a transgenic mouse model. J. Neurochem. (2005);93:310–320. doi: 10.1111/j.1471-4159.2005.03029.x. [DOI] [PubMed] [Google Scholar]
- 10.Cowansage K. K. LeDoux J. E. Monfils M. H. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr. Mol. Pharmacol. (2010);3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- 11.Croll S. D. Wiegand S. J. Anderson K. D. Lindsay R. M. Nawa H. Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur. J. Neurosci. (1994);6:1343–1353. doi: 10.1111/j.1460-9568.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 12.Cunha R. A. Ferré S. Vaugeois J. M. Chen J. F. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr. Pharm. Des. (2008);14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diógenes M. J. Fernandes C. C. Sebastião A. M. Ribeiro J. A. Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J. Neurosci. (2004);24:2905–2913. doi: 10.1523/JNEUROSCI.4454-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flajolet M. Wang Z. Futter M. Shen W. Nuangchamnong N. Ben-dor J. Wallach I. Nairn A. C. Surmeier D. J. Greengard P. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat. Neurosci. (2008);11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontinha B. M. Diógenes M. J. Ribeiro J. A. Sebastião A. M. Enhancement of long-term potentiation by brain-derived neurotrophic factor requires adenosine A2A receptor activation by endogenous adenosine. Neuropharmacology. (2008);54:924–933. doi: 10.1016/j.neuropharm.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Francesco A. L. Diego G. Susanna G. Giuseppina L. Amina W. S. Sergi F. Rafael F. Kjell F. Integrative action of receptor mosaics: relevance of receptor topology and allosteric modulators. J. Recept. Signal. Transduct. Res. (2008);28:543–565. doi: 10.1080/10799890802590420. [DOI] [PubMed] [Google Scholar]
- 17.Gasiorowski K. Lamer-Zarawska E. Leszek J. Parvathaneni K. Yendluri B. B. Błach-Olszewska Z. Aliev G. Flavones from root of Scutellaria baicalensis Georgi: drugs of the future in neurodegeneration? CNS. Neurol. Disord. Drug. Targets. (2011);10:184–191. doi: 10.2174/187152711794480384. [DOI] [PubMed] [Google Scholar]
- 18.Han B. H. Holtzman D. M. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J. Neurosci. (2000);20:5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto K. Shimizu E. Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain. Res. Brain. Res. Rev. (2004);45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Huang N. K. Lin Y. W. Huang C. L. Messing R. O. Chern Y. Activation of protein kinase A and atypical protein kinase C by A(2A) adenosine receptors antagonizes apoptosis due to serum deprivation in PC12 cells. J. Biol. Chem. (2001);276:13838–13846. doi: 10.1074/jbc.M008589200. [DOI] [PubMed] [Google Scholar]
- 21.Huen M. S. Leung J. W. Ng W. Lui W. S. Chan M. N. Wong J. T. Xue H. 5,7-Dihydroxy-6-methoxyflavone, a benzodiazepine site ligand isolated from Scutellaria baicalensis Georgi, with selective antagonistic properties. Biochem. Pharmacol. (2003);66:125–132. doi: 10.1016/S0006-2952(03)00233-8. [DOI] [PubMed] [Google Scholar]
- 22.Ip N.Y. Li Y. Yancopoulos G.D. Lindsay R.M. Cultured hippocampal neurons show responses to BDNF, NT-3 and NT-4, but not NGF. J. Neurosci. (1993);13:3394–3405. doi: 10.1523/JNEUROSCI.13-08-03394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon S. J. Rhee S. Y. Seo J. E. Bak H. R. Lee S. H. Ryu J. H. Cheong J. H. Shin C. Y. Kim G. H. Lee Y. S. Ko K. H. Oroxylin A increases BDNF production by activation of MAPK-CREB pathway in rat primary cortical neuronal culture. Neurosci. Res. (2011);69:214–222. doi: 10.1016/j.neures.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Jiwajinda S. Santisopasri V. Murakami A. Kim O. K. Kim H. W. Ohigashi H. Suppressive effects of edible thai plants on superoxide and nitric oxide generation. Asian. Pac. J. Cancer. Prev. (2002);3:215–223. [PubMed] [Google Scholar]
- 25.Kim D. H. Jeon S. J. Son K. H. Jung J. W. Lee S. Yoon B. H. Choi J. W. Cheong J. H. Ko K. H. Ryu J. H. Effect of the flavonoid, oroxylin A, on transient cerebral hypoperfusion-induced memory impairment in mice. Pharmacol. Biochem. Behav. (2006);85:658–668. doi: 10.1016/j.pbb.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Kim D. H. Jeon S. J. Son K. H. Jung J. W. Lee S. Yoon B. H. Lee J. J. Cho Y. W. Cheong J. H. Ko K. H. Ryu J. H. The ameliorating effect of oroxylin A on scopolamine-induced memory impairment in mice. Neurobiol. Learn. Mem. (2007);87:536–546. doi: 10.1016/j.nlm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Kim D. H. Kim S. Jeon S. J. Son K. H. Lee S. Yoon B. H. Cheong J. H. Ko K. H. Ryu J. H. The effects of acute and repeated oroxylin A treatments on Abeta(25-35)-induced memory impairment in mice. Neuropharmacology. (2008);55:639–647. doi: 10.1016/j.neuropharm.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi H. Yokoyama M. Matsuoka Y. Omori M. Itano Y. Kaku R. Morita K. Ichikawa H. Expression changes of multiple brain-derived neurotrophic factor transcripts in selective spinal nerve ligation model and complete Freund's adjuvant model. Brain. Res. (2008);1206:13–19. doi: 10.1016/j.brainres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Lee E. Son H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB. Rep. (2009);42:239–244. doi: 10.5483/BMBRep.2009.42.5.239. [DOI] [PubMed] [Google Scholar]
- 30.Lewin G. R. Neurotrophins and the specification of neuronal phenotype. Philos. Trans. R. Soc. Lond. B. Biol. Sci. (1996);351:405–411. doi: 10.1098/rstb.1996.0035. [DOI] [PubMed] [Google Scholar]
- 31.Lindholm D. Dechant G. Heisenberg C. P. Thoenen H. Brain-derived neurotrophic factor is a survival factor for cultured rat cerebellar granule neurons and protects them against glutamate-induced neurotoxicity. Eur. J. Neurosci. (1993);5:1455–1464. doi: 10.1111/j.1460-9568.1993.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 32.Lynch G. Rex C. S. Chen L. Y. Gall C. M. The substrates of memory: defects, treatments, and enhancement. Eur. J. Pharmacol. (2008);585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mai L. Jope R. S. Li X. BDNF-mediated signal transduction is modulated by GSK3beta and mood stabilizing agents. J. Neurochem. (2002);82:75–83. doi: 10.1046/j.1471-4159.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- 34.Marty S. Carroll P. Cellerino A. Castrén E. Staiger V. Thoenen H. Lindholm D. Brain-derived neurotrophic factor promotes the differentiation of various hippocampal nonpyramidal neurons including Cajal-Retzius cells in organotypic slice cultures. J. Neurosci. (1996);16:675–687. doi: 10.1523/JNEUROSCI.16-02-00675.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moser A. Liebetrau A. Cramer H. Adenosine receptor-coupled adenylate cyclase in the caudate nucleus of the rat brain. Neuropharmacology. (1991);30:769–773. doi: 10.1016/0028-3908(91)90185-E. [DOI] [PubMed] [Google Scholar]
- 36.Nawa H. Pelleymounter M. A. Carnahan J. Intraventricular administration of BDNF increases neuropeptide expression in newborn rat brain. J. Neurosci. (1994);14:3751–3765. doi: 10.1523/JNEUROSCI.14-06-03751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble E. E. Billington C. J. Kotz C.M. Wang C. The lighter side of BDNF. Am. J. Physiol. Regul. Integr. Comp. Physiol. (2011);300:R1053–1069. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Driscoll C. M. Gorman A. M. Hypoxia induces neurite outgrowth in PC12 cells that is mediated through adenosine A2A receptors. Neuroscience. (2005);131:321–329. doi: 10.1016/j.neuroscience.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Pardon M. C. Role of neurotrophic factors in behavioral processes:implications for the treatment of psychiatric and neurodegenerative disorders. Vitam. Horm. (2010);82:185–200. doi: 10.1016/S0083-6729(10)82010-2. [DOI] [PubMed] [Google Scholar]
- 40.Pezet S. Malcangio M. Brain-derived neurotrophic factor as a drug target for CNS disorders. Expert. Opin. Ther. Targets. (2004);8:391–399. doi: 10.1517/14728222.8.5.391. [DOI] [PubMed] [Google Scholar]
- 41.Pousinha P. A. Diogenes M. J. Ribeiro J. A. Sebastião A. M. Triggering of BDNF facilitatory action on neuromuscular transmission by adenosine A2A receptors. Neurosci. Lett. (2006);404:143–147. doi: 10.1016/j.neulet.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 42.Proll M. A. Clark R. B. Butcher R. W. A1 and A2 adenosine receptors regulate adenylate cyclase in cultured human lung fibroblasts. Mol. Cell. Endocrinol. (1986);44:211–217. doi: 10.1016/0303-7207(86)90126-7. [DOI] [PubMed] [Google Scholar]
- 43.Rebola N. Lujan R. Cunha R.A. Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. (2008);57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki Y. Welshhans K. Wen Z. Yao J. Xu M. Goshima Y. Zheng J. Q. Bassell G. J. Phosphorylation of zipcode binding protein 1 is required for brain-derived neurotrophic factor signaling of local beta-actin synthesis and growth cone turning. J. Neurosci. (2010);30:9349–9358. doi: 10.1523/JNEUROSCI.0499-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scemes E. Duval N. Meda P. Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J. Neurosci. (2003);23:11444–11452. doi: 10.1523/JNEUROSCI.23-36-11444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt H. D. Duman R. S. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav. Pharmacol. (2007);18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 47.Sebastião A. M. Ribeiro J. A. Triggering neurotrophic factor actions through adenosine A2A receptor activation: implications for neuroprotection. Br. J. Pharmacol. (2009);158:15–22. doi: 10.1111/j.1476-5381.2009.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen W. Wu B. Zhang Z. Dou Y. Rao Z. R. Chen Y. R. Duan S. Activity-induced rapid synaptic maturation mediated by presynaptic cdc42 signaling. Neuron. (2006);50:401–414. doi: 10.1016/j.neuron.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Shih H. C. Hsu C. S. Yang L. L. In vitro study of the tocolytic effect of oroxylin A from Scutellaria baicalensis root. J. Biomed. Sci. (2009);16:27. doi: 10.1186/1423-0127-16-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stafford M. R. Bartlett P. F. Adams D. J. Purinergic receptor activation inhibits mitogen-stimulated proliferation in primary neurospheres from the adult mouse subventricular zone. Mol. Cell. Neurosci. (2007);35:535–548. doi: 10.1016/j.mcn.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Tebano M. T. Martire A. Potenza R. L. Grò C. Pepponi R. Armida M. Domenici M. R. Schwarzschild M. A. Chen J. F. Popoli P. Adenosine A(2A) receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. J. Neurochem. (2008);104:279–286. doi: 10.1111/j.1471-4159.2007.05046.x. [DOI] [PubMed] [Google Scholar]
- 52.Tebano M. T. Martire A. Rebola N. Pepponi R. Domenici M. R. Grò M. C. Schwarzschild M. A. Chen J. F. Cunha R. A. Popoli P. Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-D-aspartate effects. J. Neurochem. (2005);95:1188–1200. doi: 10.1111/j.1471-4159.2005.03455.x. [DOI] [PubMed] [Google Scholar]
- 53.Terashima M. Kobayashi M. Motomiya M. Inoue N. Yoshida T. Okano H. Iwasaki N. Minami A. Matsuoka I. Analysis of the expression and function of BRINP family genes during neuronal differentiation in mouse embryonic stem cell-derived neural stem cells. J. Neurosci. Res. (2010);88:1387–1393. doi: 10.1002/jnr.22315. [DOI] [PubMed] [Google Scholar]
- 54.Tucker A. L. Linden J. Cloned receptors and cardiovascular responses to adenosine. Cardiovasc. Res. (1993);27:62–67. doi: 10.1093/cvr/27.1.62. [DOI] [PubMed] [Google Scholar]
- 55.Wiese S. Jablonka S. Holtmann B. Orel N. Rajagopal R. Chao M. V. Sendtner M. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc. Natl. Acad. Sci. USA. (2007);104:17210–17215. doi: 10.1073/pnas.0705267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winckler B. BDNF instructs the kinase LKB1 to grow an axon. Cell. (2007);129:459–460. doi: 10.1016/j.cell.2007.04.021. [DOI] [PubMed] [Google Scholar]