Abstract
TNF-related apoptosis-inducing ligand (TRAIL) is a promising agent for management of cancer because of its selective cytotoxicity to cancer cells. However, some cancer cells have resistance to TRAIL. Accordingly, novel treatment strategies are required to overcome TRAIL resistance. Here, we examined the synergistic apoptotic effect of apigenin in combination with TRAIL in Huh-7 cells. We found that combined treatment of TRAIL and apigenin markedly inhibited Huh-7 cell growth compared to either agent alone by inducing apoptosis. Combined treatment with apigenin and TRAIL induced chromatin condensation and the cleavage of poly (ADP-ribose) polymerase (PARP). In addition, enhanced apoptosis by TRAIL/apigenin combination was quantified by annexin V/PI flow cytometry analysis. Western blot analysis suggested that apigenin sensitizes cells to TRAIL-induced apoptosis by activating both intrinsic and extrinsic apoptotic pathway-related caspases. The augmented apoptotic effect by TRAIL/apigenin combination was accompanied by triggering mitochondria-dependent signaling pathway, as indicated by Bax/Bcl-2 ratio up-regulation. Our results demonstrate that combination of TRAIL and apigenin facilitates apoptosis in Huh-7 cells.
Keywords: TRAIL, Huh-7, Combination, Apoptosis, Caspase family, Bcl-2 family
INTRODUCTION
TNF-related apoptosis-inducing ligand (TRAIL), a member of the TNF-superfamily, is a promising agent since it selectively triggers tumor cell apoptosis but not in normal cells (Ash-kenazi et al., 1999; Walczak et al., 1999). However, resistance to TRAIL-induced apoptosis has been also reported in various cancer cells (Hinz et al., 2000; Armeanu et al., 2003; Zhang and Zhang, 2008). Therefore, effective strategies to overcome this resistance are required for therapeutic application of TRAIL. Resistance to TRAIL is associated to multiple factors in TRAIL-mediated apoptosis signaling pathway (Zhang and Fang, 2005). Low expression of pro-apoptotic molecules like death receptors, FADD, and caspase-8 could lead to TRAIL resistance. Conversely, high expression of anti-apoptotic proteins, such as decoy receptors, Bcl-2, Bcl-xl, FLIP, XIAP, and IAP could also be related to TRAIL resistance (Eggert et al., 2001; Van Geelen et al., 2004; Zhang and Fang, 2005). Thus, combination with agents that target these molecules could further enhance the TRAIL-induced apoptosis.
Previous studies showed that combination therapy with TRAIL and chemotherapeutic agents or radiation is effective to overcome resistance to TRAIL (Bhojani et al., 2003). Especially, it have been reported that various natural dietary products effectively enhances therapeutic efficacy of TRAIL by combination in TRAIL-resistant cancer cells. For example, curcumin potentiated TRAIL-induced apoptosis in vitro and in vivo through down regulation of p-Akt and NF-κB in prostate cancer (Deeb et al., 2004; Andrzejewski et al., 2008) or through ROS-mediated DR5 up-regulation (Jung et al., 2005). Resveratrol also has been diversely studied as an agent to overcome TRAIL-resistance in various cancer cells (Jacque-min et al., 2010). Keampferol is another natural compound that sensitizes resistant cancer cells to TRAIL-induced apoptosis. Co-treatment with kaempferol facilitated TRAIL-mediated apoptosis by DR4 and DR5 up-regulation in colon cancer cells (Yoshida et al., 2008) and through the suppression of survivin in human glioma cells (Siegelin et al., 2008).
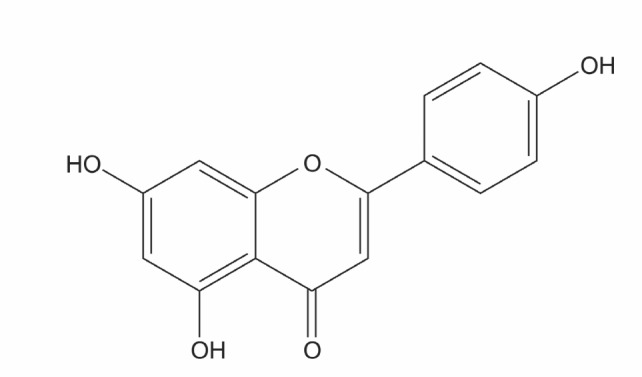
Apigenin, the naturally occurring dietary compound, is a kind of flavones as depicted in Fig. 1. It has been widely reported that apigenin possesses anti-tumor effect against various malignant cell lines. Chemical carcinogens or UV-induced mouse skin carcinogenesis was suppressed by apigenin application in the in vivo studies (Wei et al., 1990; Birt et al., 1997). Moreover, apigenin-induced cell growth inhibition, cell cycle arrest, and apoptosis have been estimated in prostate, breast, colon, leukemic, and liver cancer cell lines (Wang et al., 2000; Gupta et al., 2001; Way et al., 2004; Chiang et al.,2006)
Fig. 1. Chemical structure of apigenin.
Interestingly, apigenin selectively inhibited the cell growth in liver cancer cells at the concentrations that had no effect in normal murine liver cells (Chiang et al., 2006). Therefore, apigenin is considered as a promising anticancer agent.
In the present study, we examined the effects of apigenin in combination with TRAIL in Huh-7 human hepatocellular carcinoma (HCC) cells. We found that apigenin potentiates TRAIL-induced apoptosis through caspase family and Bcl-2 family regulation.
MATERIALS AND METHODS
Cell culture
Huh-7 human hepatocellular carcinoma cells were obtained from the Korean Cell Line Bank (Seoul, South Korea) and maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (Welgene, Deagu, South Korea) at 37℃ in a humidified condition of 5% CO2. The medium was supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin.
MTT assay
Cell viability was determined by 3-(4,5-Dimethylthiazoly-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Cells were seeded in 96-well plate and treated with indicated concentrations of apigenin and/or TRAIL for 24 h. After incubation with MTT solution for 4 h at 37℃, the formazan crystals were dissolved in dimethyl sulfoxide (DMSO). The absorbance at 546 nm and 650 nm were measured by a microplate reader (EL800, Bio-Tek Instrument Inc., Winooski, VT, USA).
DAPI staining
Apoptotic cell death was observed by DAPI (4,6-Diamidino-2-phenyl-indole) staining. Huh-7 cells were seeded in 6-well plate and treated with apigenin and/or TRAIL for 24 h. The cells were washed with cold PBS and fixed with methanol/DMSO (4:1) solution at 4℃. The cells were rehydrated with methanol and permeabilized with 0.2% Triton X-100 in PBS for 5 min. Then, cells were stained with 1 μg/ml DAPI solution for 30 min at room temperature and washed with PBS. Finally, the morphology of the cells’ nuclei was observed by fluorescence microscope (Olympus BH Series, Tokyo, Japan).
Flow cytometric analysis
Apoptosis was quantified by using annexin V/ propidium iodide (PI) detection kit (BD biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Cells were treated with apigenin and/or TRAIL for 24 h and harvested. The cells were resuspended in 1x binding buffer and stained
with annexin V and PI solution at room termperature. Samples were analyzed by BD FACSCanto II Flow Cytometer (BD Biosciences, San Jose, CA, USA).
Western blotting
Cells were incubated with apigenin and/or TRAIL for 24 h and lysed with RIPA buffer (50 mM Tris-HCL, pH 8.0, with 150 mM NaCl, 0.1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing protease inhibitor (Roche). Lysates were centrifuged at 17,000 rpm for 20 min. Protein contents was measured with Bio-Rad protein assay kit. The equal amount of protein was loaded on 10% SDS-PAGE gels and transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking with 5% nonfat dry milk in TBS-T (25 mM Tris, 137 mM NaCl and 0.1% Tween 20) for 1 h at room temperature, the blots were incubated overnight at 4℃ with primary antibodies. After washing with TBS-T, the membrane was incubated with secondary horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies. Finally, the protein signals were visualized by enhanced chemiluminescence (ECL) advance detection kit. Multi Gauge software (Fuji Photo Film, Tokyo, Japan) was used to quantify the protein signals.
Statistical analysis
Values are expressed as the mean ± SD. Statistical significance was evaluated by using one-way analysis of variance (ANOVA) followed by Turkey’s test. p value of less than 0.05 was considered statistically significant.
RESULTS
Apigenin potentiates TRAIL-induced cell growth inhibition in Huh-7 cells
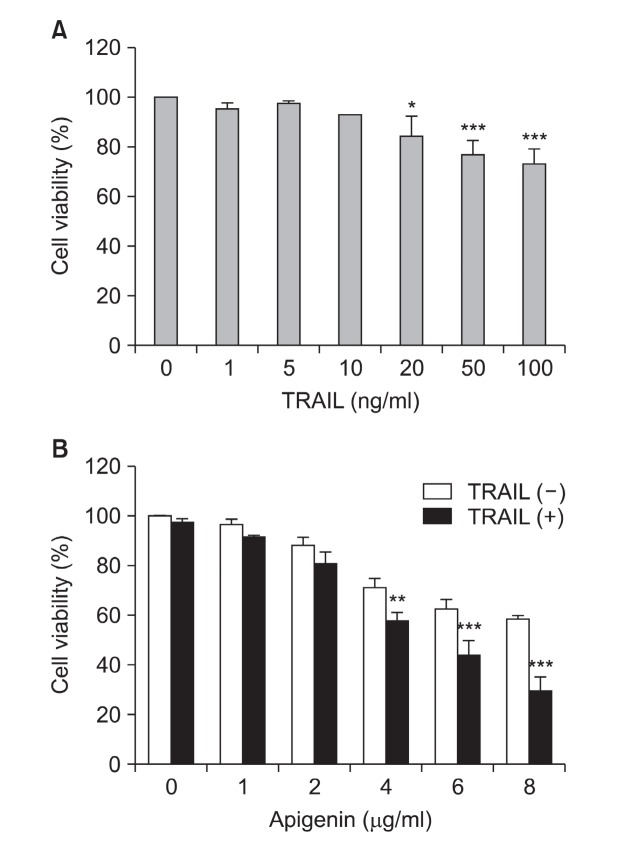
To investigate the effect of TRAIL alone, Huh-7 cells were treated with increasing concentrations of TRAIL (0-100 ng/ml) for 24 h and cell viability was determined by MTT assay. As indicated in Fig. 2A, there was no significant change of cell viability up to 10 ng/ml TRAIL. Therefore, a concentration of 5 ng/ml TRAIL that has no effect on Huh-7 cell viability, was chosen for the subsequent experiments.
Fig. 2. The effects of TRAIL and apigenin on Huh-7 cell viability. (A) Cells were incubated with various concentrations of TRAIL (0-100 ng/ml) for 24 h. (B) Cells were treated with various concentrations of apigenin (0-8 μg/ml) with or without TRAIL (5 ng/ml) for 24 h. Cell viability was measured by MTT assay. Data represent mean ± SD of three independent experiments. Significant difference: *p<0.05, **p<0.01, ***p<0.001 versus control untreated cells.
Next, to determine the dose capable of potentiate the effect of TRAIL, the cells were treated with the indicated concentrations of apigenin with or without TRAIL (Fig. 2B). As a result, cell proliferation was reduced significantly by TRAIL/apigenin combined treatment when compared with control or single treated group.
Apigenin sensitizes Huh-7 cells to TRAIL-induced apoptosis
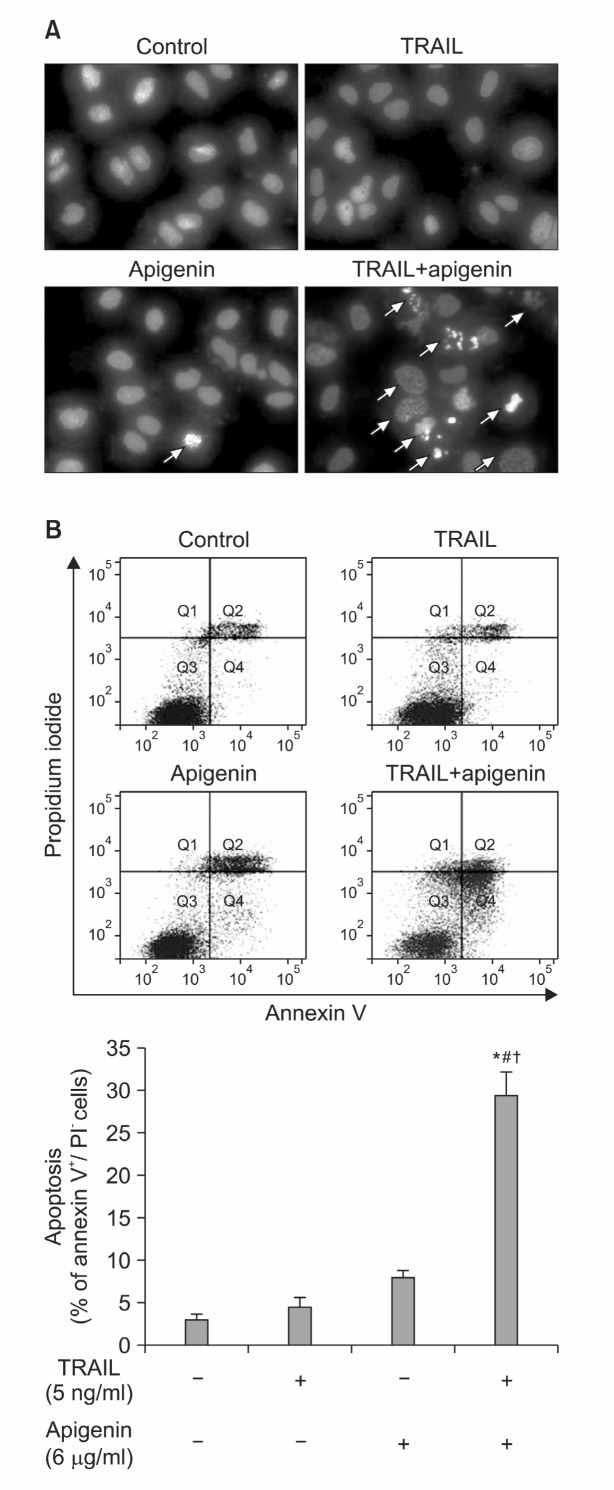
During apoptosis, cells display typical morphological changes. To determine whether TRAIL/apigenin-induced cell death occur through apoptosis, apoptotic morphological changes such as fragmented nuclei and chromatin condensation were observed by DAPI staining. As shown in Fig. 3A, nuclear fragmentation was markedly increased in TRAIL/apigenin combination treated group, whereas treatment with apigenin or TRAIL alone did not.
Fig. 3. Effects of TRAIL and apigenin co-treatment on Huh-7 cell apoptosis. (A) Cells were treated with TRAIL (5 ng/ml) and api-genin (6 μg/ml) for 24 h and apoptotic cell death was evaluated by fluorescence microscopy after DAPI staining. Arrows indicate the apoptotic cells with fragmented nuclei. (B) Cells were incubated with TRAIL (5 ng/ml) and apigenin (6 μg/ml) for 24 h. Flow cyto-metric analysis was used to quantify the early apoptotic cell death. Data from three separate experiments (± SD) was also shown as a bar graph. *p<0.001 compared with control group; #p<0.001 compared with 5 ng/ml TRAIL; †p<0.001 compared with 6 μg/ml apigenin.
Then, to evaluate the quantitative induction of apoptosis, we measured the annexin V-stained cells using flow cytometric analysis. Consistent with the results showed above, the combined treatment resulted in distinct increase of apoptosis (Fig. 3B). These results indicate that apigenin sensitizes Huh-7 cells to TRAIL-mediated apoptosis.
Augmented apoptosis by TRAIL and apigenin combination is induced via caspase activation
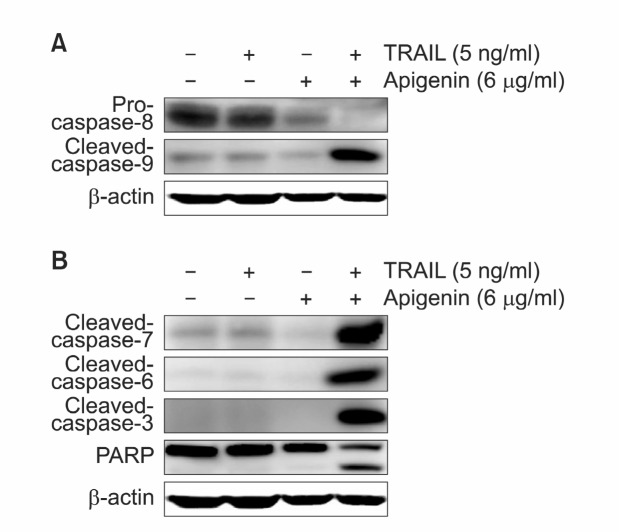
Caspase family members are well known proteases that play central role in mammalian apoptosis. During apoptosis, activated caspases induce diverse changes in cells including cleavage of cytoskeletal proteins, a decrease in DNA damage repair capacity and down regulation of proteins related to cell survival (Cohen, 1997). In general, apoptotic caspases are divided into two groups, the initiator caspases (caspase-2, -8, -9, and -10) and effector caspases (caspase-3, -6, and -7) (Wolf and Green, 1999). To elucidate the signaling pathway associated with synergy effect of TRAIL and apigenin, we examined the involvement of caspase proteases by western blot analysis. As indicated in Fig. 4A, TRAIL/apigenin combined treatment led to a significant increase of both extrinsic (caspase-8) and intrinsic (caspase-9) initiator caspase activation compared to TRAIL or apigenin alone treated group. We next examined the activation of effector caspases such as caspase-3, -6, and -7. Although TRAIL or apigenin alone did not cause any effect on effector caspases, TRAIL/apigenin combination
Fig. 4. TRAIL/apigenin combined treatment induces activation of caspase family members and PARP cleavage. Cells were incubated for 24 h with 6 μg/ml apigenin with or without 5 ng/ml TRAIL and cell lysates were prepared for western blotting. (A) Western blot analysis was performed with initiator caspase antibodies. (B) Western blot analysis of effector caspases and PARP. β-actin was used as loading control.
induced obvious increase of active caspases. Furthermore, PARP, one of the substrate for caspase-3, -6, and -7, was also activated by exposure to TRAIL/apigenin combined treatment (Fig. 4B). Taken together, our results indicate that the synergy effect of co-treatment of TRAIL and apigenin is associated with caspase family activation.
Combined treatment of apigenin with TRAIL regulates the Bcl-2 family proteins
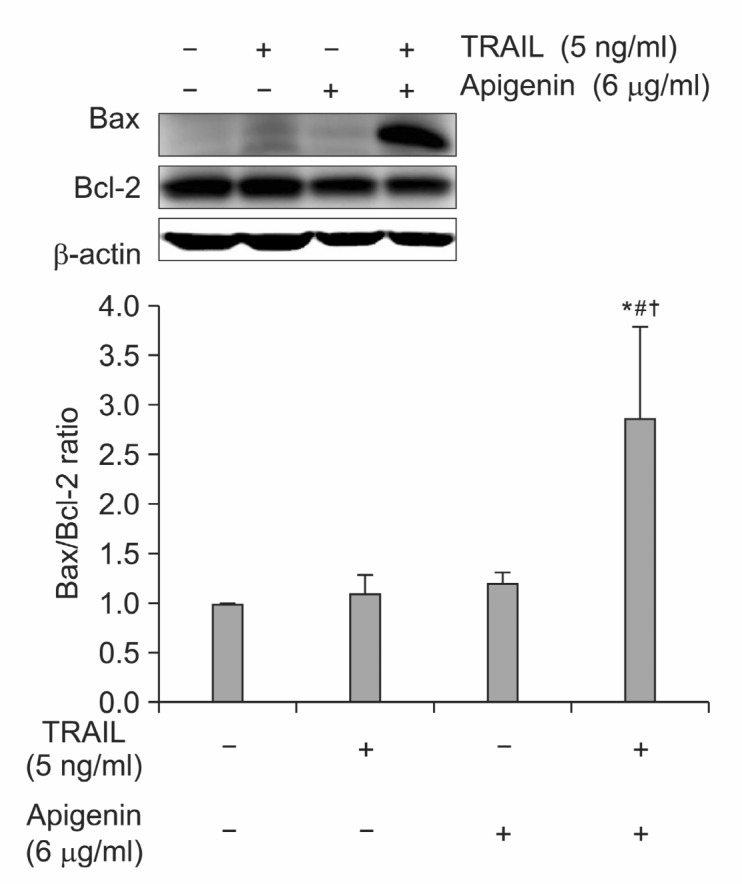
It has been reported that Bcl-2 family is another important regulator of inducing apoptosis. Particularly, the balance between pro-apoptotic Bax and anti-apoptotic Bcl-2 protein is the key determinant of cell’s fate for undergoing apoptosis (Gross et al., 1999). According to our result, the ratio of Bax/Bcl-2 was markedly enhanced by co-treatment of TRAIL and apigenin, indicating that the synergy effect of TRAIL/apigenin combination is related to Bcl-2 family modulation (Fig. 5).
Fig. 5. Effects of TRAIL/apigenin combination on Bcl-2 family regulation. (A) Cells were treated with 6 μg/ml apigenin with or without 5 ng/ml TRAIL for 24 h and whole cell extracts were prepared. Western blot analysis was carried out using anti-Bax and anti-Bcl-2 antibody. β-actin was used as loading control. (B) Bax/Bcl-2 ratio from three independent experiments (± SD) was also shown as a bar graph. *p<0.05 compared with control group; #p<0.05 compared with 5 ng/ml TRAIL; †p<0.05 compared with 6 μg/ml apigenin.
DISCUSSION
Failing in apoptosis is the major obstacle in clinical use of anticancer agents because a lot of chemotherapeutic treatments mediate apoptosis (Johnstone et al., 2002). Thus, new therapeutic strategies to overcome resistance are needed for effective cancer management. As an agent for cancer therapy, cytokine TRAIL is considered to be a promising strategy since its ability to inducing apoptotic cell death in cancer cells without normal cell death (Ashkenazi et al., 1999; Roth et al., 1999; Walczak et al., 1999). Although the early studies showed that tagged forms of recombinant human TRAIL (rhTRAIL) were toxic to several normal human cells (Walczak et al., 1999; Nitsch et al., 2000), further in vivo and in vitro studies have revealed that non-tagged rhTRAIL (Apo2L/TRAIL) is nontoxic to normal human cells (Lawrence et al., 2001; Hao et al., 2004)
However, it is not enough to use TRAIL as a single agent because some cancer cells are resistant against TRAIL. Hence, current studies are in progress to sensitize the TRAIL-resistant cancer cells by combined regimens (Koschny et al., 2007; Kruyt, 2008). In the present study, we investigated whether the resistant Huh-7 cells could be sensitized by combination of TRAIL and apigenin and examined the underlying mechanisms. In our results, staining cells with DAPI showed the obviously increased cells with fragmented and condensed nuclei in TRAIL and apigenin combination treated group. Also, the markedly increased percentage of the annexin V-stained cells in combined treatment indicated that TRAIL/apigenin combination causes increased apoptosis significantly at the concentration that either agent alone did not.
There are at least two ways for inducing apoptosis that one is initiated from the death receptors and the other is triggered by cellular stress. Both pathways eventually reach to the activation of caspases, the aspartate-specific cysteine proteases (Sun et al., 1999). Among the diverse possible mechanisms in TRAIL-resistance, down-regulation or loss of caspase activity could be a potential factor of resistance to TRAIL because TRAIL mediates apoptosis in cancer cells through caspase-dependent manner (Yano et al., 2003; Zhang and Fang, 2005). In this study, we found that TRAIL/apigenin co-treatment potentiates both intrinsic (caspase-9) and extrinsic (caspase-8) initiator caspase activity in Huh-7 cells. Increased activation of effector caspases (caspase-3, -6, -7) and their substrate PARP was also observed in TRAIL/apigenin co-treated group, suggesting that the combined effect of TRAIL plus apigenin is the result of the caspase cascade pathway induction.
It has been proposed that the balance between Bax/Bcl-2 also could be an important factor to determine susceptibility of cancer cells to undergo TRAIL-induced apoptosis (Kim et al., 2006). Bax, the pro-apoptotic protein, translocates to mitochondria in response to cytotoxic signals and acts through permeabilizing the mitochondrial outer membrane. Whereas, the pro-survival Bcl-2 protein protects cells from various cytotoxic stimuli through inhibiting Bax activation (Adams and Cory, 2007). In our study, we hypothesized that Bcl-2 family regulation might be associated with the enhanced apoptotic death in TRAIL/apigenin treated cells because intrinsic apoptosis pathway is mainly involved in Bcl-2 family alterations. According to our results, Bax/Bcl-2 ratio was noticeably increased by combined treatment of TRAIL and apigenin at the concentration that either agent alone did not induce the marked change of Bax/Bcl-2 level.
The p53 protein, a well-known tumor suppressor protein, is also related to promote apoptosis. As an important regulator of apoptosis, p53 works in many steps in apoptotic signaling pathways (Moll and Zaika, 2001). Therefore, p53 could be an effective target for cancer therapy. However, p53 mutation is frequently found in approximately 50% of human cancers (Higashitsuji et al., 2007). Accordingly, searching agents with ability to inducing apoptosis in p53-independent manner could be an effective strategy to kill the p53 gene-mutated cancer cells. Here, we showed that combination of TRAIL with apigenin induced marked increase of apoptosis in Huh-7 cells, in which the status of p53 is mutant type. Our results suggest that enhanced apoptosis by TRAIL/apigenin combination is mediated by p53-independent pathway. Therefore, apigenin could be a challenging agent to enhance TRAIL-induced apoptosis even in p53-mutated hepatocarcinoma.
In conclusion, we demonstrated that apigenin potentiates TRAIL-induced inhibition of Huh-7 cell proliferation. The augmented inhibition was mediated through inducing apoptosis by regulating initiator/effector caspases (caspase-8, -9, -3, -6, -7) and cleavage of PARP. The effect of TRAIL/apigenin combination was also involved in up-regulation of Bax/Bcl-2 ratio by p53-independent manner. Overall, our results provide a possibility of TRAIL application on liver cancer therapy by combination with apigenin.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0010538).
References
- 1.Adams J. M. Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. (2007);26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrzejewski T. Deeb D. Gao X. Danyluk A. Arbab A. S. Dulchavsky S. A. Gautam S. C. Therapeutic efficacy of curcumin/TRAIL combination regimen for hormone-refractory prostate cancer. Oncol. Res. (2008);17:257–267. doi: 10.3727/096504008786991611. [DOI] [PubMed] [Google Scholar]
- 3.Armeanu S. Lauer U. M. Smirnow I. Schenk M. Weiss T. S. Gregor M. Bitzer M. Adenoviral gene transfer of tumor necrosis factor-related apoptosis-inducing ligand overcomes an impaired response of hepatoma cells but causes severe apoptosis in primary human hepatocytes. Cancer Res. (2003);63:2369–2372. [PubMed] [Google Scholar]
- 4.Ashkenazi A. Pai RC. Fong S. Leung S. Lawrence DA. Marsters SA. Blackie C. Chang L. McMurtrey A. E. Hebert A. DeForge L. Koumenis I. L. Lewis D. Harris L. Bussiere J. Koeppen H. Shahrokh Z. Schwall R. H. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. (1999);104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhojani M. S. Rossú B. D. Rehemtulla A. TRAIL and anti-tumor responses. Cancer Biol. Ther. (2003);2(4 Suppl 1):S71–S78. [PubMed] [Google Scholar]
- 6.Birt D. F. Mitchell D. Gold B. Pour P. Pinch H. C. Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin a plant flavonoid. Anticancer. Res. (1997);17:85–91. [PubMed] [Google Scholar]
- 7.Chiang L. C. Ng L. T. Lin I. C. Kuo P. L. Lin C. C. Anti-proliferative effect of apigenin and its apoptotic induction in human Hep G2 cells. Cancer Lett. (2006);237:207–214. doi: 10.1016/j.canlet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Cohen G. M. Caspases: the executioners of apoptosis. Biochem. J. (1997);326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeb D. Jiang H. Gao X. Hafner M. S. Wong H. Divine G. Chapman R. A. Dulchavsky S. A. Gautam S. C. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-κB through suppression of IκBα phosphorylation. Mol. Cancer Ther. (2004);3:803–812. [PubMed] [Google Scholar]
- 10.Eggert A. Grotzer M. A. Zuzak T. J. Wiewrodt B. R. Ho R. Ikegaki N. Brodeur G. M. Resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Cancer Res. (2001);61:1314–1319. [PubMed] [Google Scholar]
- 11.Gross A. McDonnell J. M. Korsmeyer S. J. BCL-2 family members and the mitochondria in apoptosis. Genes. Dev. (1999);13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S. Afaq F. Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem. Biophys. Res. Commun. (2001);287:914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 13.Hao C. Song J. H. Hsi B. Lewis J. Song D. K. Petruk K. C. Tyrrell D. L. Kneteman N. M. TRAIL inhibits tumor growth but is nontoxic to human hepatocytes in chimeric mice. Cancer Res. (2004);64:8502–8506. doi: 10.1158/0008-5472.CAN-04-2599. [DOI] [PubMed] [Google Scholar]
- 14.Higashitsuji H. Higashitsuji H. Masuda T. Liu Y. Itoh K. Fujita J. Enhanced deacetylation of p53 by the anti-apoptotic protein HSCO in association with histone deacetylase 1. J. Biol. Chem. (2007);282:13716–13725. doi: 10.1074/jbc.M609751200. [DOI] [PubMed] [Google Scholar]
- 15.Hinz S. Trauzold A. Boenicke L. Sandberg C. Beckmann S. Bayer E. Walczak H. Kalthoff H. Ungefroren H. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene. (2000);19:5477–5486. doi: 10.1038/sj.onc.1203936. [DOI] [PubMed] [Google Scholar]
- 16.Jacquemin G. Shirley S. Micheau O. Combining naturally occurring polyphenols with TNF-related apoptosis-inducing ligand: a promising approach to kill resistant cancer cells? Cell Mol. Life Sci. (2010);67:3115–3130. doi: 10.1007/s00018-010-0407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnstone R. W. Ruefli A. A. Lowe S. W. Apoptosis: a link between cancer genetics and chemotherapy. Cell. (2002);108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 18.Jung E. M. Lim J. H. Lee T. J. Park J. W. Choi K. S. Kwon T. K. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5). Carcinogenesis. (2005);26:1905–1913. doi: 10.1093/carcin/bgi167. [DOI] [PubMed] [Google Scholar]
- 19.Kim D. S. Jeong Y. M. Moon S. I. Kim S. Y. Kwon S. B. Park E. S. Youn S. W. Park K. C. Indole-3-carbinol enhances ultraviolet B-induced apoptosis by sensitizing human melanoma cells. Cell Mol. Life Sci. (2006);63:2661–2668. doi: 10.1007/s00018-006-6306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koschny R. Ganten T. M. Sykora J. Haas T. L. Sprick M. R. Kolb A. Stremmel W. Walczak H. TRAIL/bortezomib cotreatment is potentially hepatotoxic but induces cancer-specific apoptosis within a therapeutic window. Hepatology. (2007);45:649–658. doi: 10.1002/hep.21555. [DOI] [PubMed] [Google Scholar]
- 21.Kruyt F. A. TRAIL and cancer therapy. Cancer Lett. (2008);263:14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence D. Shahrokh Z. Marsters S. Achille K. Shih D. Mounho B. Hillan K. Totpal K. DeForge L. Schow P. Hooley J. Sherwood S. Pai R. Leung S. Khan L. Gliniak B. Bussiere J. Smith C. A. Strom S. S. Kelley S. Fox J. A. Thomas D. Ashkenazi A. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat. Med. (2001);7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 23.Moll U. M. Zaika A. Nuclear and mitochondrial apoptotic pathways of p53. FEBS. Lett. (2001);493:65–69. doi: 10.1016/s0014-5793(01)02284-0. [DOI] [PubMed] [Google Scholar]
- 24.Nitsch R. Bechmann I. Deisz R. A. Haas D. Lehmann T. N. Wendling U. Zipp F. Human brain-cell death induced by tumour-necrosis-factor-related apoptosis-inducing ligand (TRAIL). Lancet. (2000);356:827–828. doi: 10.1016/S0140-6736(00)02659-3. [DOI] [PubMed] [Google Scholar]
- 25.Roth W. Isenmann S. Naumann U. Kügler S. Bähr M. Dichgans J. Ashkenazi A. Weller M. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem. Biophys. Res. Commun. (1999);265:479–483. doi: 10.1006/bbrc.1999.1693. [DOI] [PubMed] [Google Scholar]
- 26.Siegelin M. D. Reuss D. E. Habel A. Herold-Mende C. vonDeimling A. The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Mol. Cancer Ther. (2008);7:3566–3574. doi: 10.1158/1535-7163.MCT-08-0236. [DOI] [PubMed] [Google Scholar]
- 27.Sun X. M. MacFarlane M. Zhuang J. Wolf B. B. Green D. R. Cohen G. M. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J. Biol. Chem. (1999);274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- 28.Van Geelen C. M. de Vries E. G. de Jong S. Lessons from TRAIL-resistance mechanisms in colorectal cancer cells: paving the road to patient-tailored therapy. Drug Resist. Updat. (2004);7:345–358. doi: 10.1016/j.drup.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Walczak M. Miller R. E. Ariail K. Gliniak B. Griffith T.S. Kubin M. Chin W. Jones J. Woodward A. Le T. Smith C. Smolak P. Goodwin R. G. Rauch C. T. Schuh J C. Lynch D. H. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. (1999);5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 30.Wang W. Heideman L. Chung C. S. Pelling J. C. Koehler K. J. Birt D. F. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol. Car-cinog. (2000);28:102–110. [PubMed] [Google Scholar]
- 31.Way T. D. Kao M. C. Lin J. K. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. (2004);279:4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 32.Wei H. Tye L. Bresnick E. Birt D. F. Inhibitory effect of apigenin a plant flavonoid on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. (1990);50:499–502. [PubMed] [Google Scholar]
- 33.Wolf B. B. Green D. R. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J. Biol. Chem. (1999);274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 34.Yano Y. Hayashi Y. Nakaji M. Nagano H. Seo Y. Ninomiya T. Yoon S. Wada A. Hirai M. Kim S. R. Yokozaki H. Kasuga M. Different apoptotic regulation of TRAIL-caspase pathway in HBV- and HCV-related hepatocellular carcinoma. Int. J. Mol. Med. (2003);11:499–504. [PubMed] [Google Scholar]
- 35.Yoshida T. Konishi M. Horinaka M. Yasuda T. Goda A. E. Taniguchi H. Yano K. Wakada M. Sakai T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun. (2008);375:129–133. doi: 10.1016/j.bbrc.2008.07.131. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L. Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. (2005);12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y. Zhang B. TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol. Cancer Res. (2008);6:1861–1871. doi: 10.1158/1541-7786.MCR-08-0313. [DOI] [PubMed] [Google Scholar]