Abstract
Matrix metalloproteinases (MMPs) are a subfamily of zinc-dependent proteases that are responsible for degradation and remodeling of extracellular matrix proteins. The activity of MMPs is tightly regulated at several levels including cleavage of prodomain, allosteric activation, compartmentalization and complex formation with tissue inhibitor of metalloproteinases (TIMPs). In the central nervous system (CNS), MMPs play a wide variety of roles ranging from brain development, synaptic plasticity and repair after injury to the pathogenesis of various brain disorders. Following general discussion on the domain structure and the regulation of activity of MMPs, we emphasize their implication in various brain disorder conditions such as Alzheimer’s disease, multiple sclerosis, ischemia/reperfusion and Parkinson’s disease. We further highlight accumulating evidence that MMPs might be the culprit in Parkinson’s disease (PD). Among them, MMP-3 appears to be involved in a range of pathogenesis processes in PD including neuroinflammation, apoptosis and degradation of α-synuclein and DJ-1. MMP inhibitors could represent potential novel therapeutic strategies for treatments of neurodegenerative diseases.
Keywords: Matrix metalloproteinases, MMP-3, Parkinson’s disease, Microglia, Neurodegenerative disorders
The matrix metalloproteinases (MMPs) are zinc and calcium-dependent endopeptidases which belong to the metzincin superfamily like the astacins, serralysins, reprolysins, and adamalysins or disintegrin metalloproteinases (ADAMs). Since its first discovery by Jerome Gross and Charles Lapiere in 1962 (Gross and Lapiere, 1962), MMPs have constituted a large family of proteases. Currently, 24 MMP genes and 23 MMP proteins have been reported because two identical genes in chromosome 1 encode MMP-23. They are a group of proteolytic enzymes that are involved in degradation and remodeling of extracellular matrix (ECM) and basement membrane proteins. Accumulating evidence, however, suggests that MMPs are also participating in a range of physiological processes such as inflammation, immunity, neurite growth and bone remodeling through processing bioactive molecules including cell surface receptors, apoptotic ligands, pro-neurotrophic factors and chemokines/cytokines (Yamamoto et al., 1999; Lee et al., 2001; Van Lint and Libert, 2007).
In the central nervous system (CNS), MMPs play a fundamental role in CNS development including neurogenesis, myelogenesis, and axonal guidance as well as maintaining normal brain functions such as synaptic plasticity, learning and memory. The basic biology and roles of MMPs in the CNS have been extensively discussed by recent reviews (Yong, 2005; Agrawal et al., 2008). In this review, we provide an overview of the basic biochemical characteristics, regulation of activation and biological functions of MMPs, and then discuss their role in the CNS mainly focusing on brain pathologic conditions including neurodegeneration, neuroinflammation and ischemia.
MODULAR DOMAIN STRUCTURE AND SUBFAMILIES
The MMP members share structural homologies including common N-terminal propeptide and catalytic domains. In addition, subfamilies are categorized by other domains such as fibronectin-like repeats, C-terminal hemopexin-like domains, Ig-like domain and transmembrane domains (Fig.1 ). All MMPs have an N-terminal signal peptide directing them to the secretory pathway. Except membrane-type MMPs (MT-MMPs), all MMPs are destined to be released into the extracellular space as inactive pro-enzyme forms called zymogens. The propeptide domain, consisting of about 80 amino acids, has a conserved PRCG (V/N)PD amino acid sequence. The cysteine contained within this sequence interacts with zinc
Fig. 1. Domain structure of matrix metalloproteinases family. All MMPs consist of a N-terminal signal peptide and a propeptide domain followed by a C-terminal catalytic domain. A propeptide domain contains a cysteine switch which forms complex with catalytic zinc in a catalytic domain inhibiting their enzymatic activity. MMP-7 and -26 have only the minimal domain. Most of MMPs have a linker (hinge-region) and hemopexin like domain at the C-terminal to a catalytic domain. MMP-11, -21 and -28 have a furin-activating motif, RX[K/R]R, at the C-terminal end of their propeptide domains. Two gelatinases, MMP-2 and -9, contain three fibronectin II like repeats in the catalytic domains. MMP-9 is the only MMP which has a heavily O-glycosylated hinge region. All membrane-anchored MMPs contain a furin-activating motif. MT4, -6-MMPs are anchored to the plasma membrane through GPI-anchor. MT1, -2, -3 and-5-MMPs are bound to the cell membrane through type I transmembrane domain while MMP-23 is through type II transmembrane domain. The C-terminal of MMP-23 contains cysteine array (Ca) and immunoglobulin (Ig)-like domain replacing hemopexin domain.
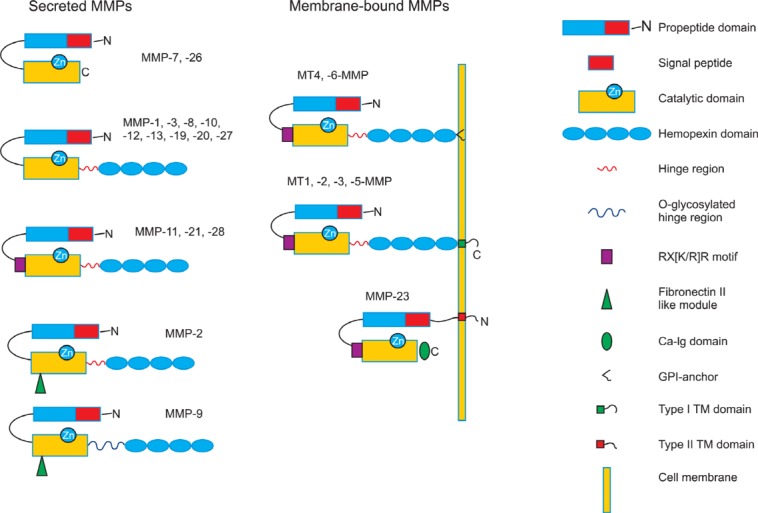
in the catalytic domain to suppress its own proteolytic activity (Cysteine-switch) (Van Wart and Birkedal-Hansen, 1990; Becker et al., 1995). The catalytic domain consisting of about 160-170 amino acids has a HEXXHXXGXXH, catalytic zinc-binding motif and a conserved methionine, which form a unique “Met-turn” structure (Bode et al., 1993). The catalytic domain also requires an additional structural zinc and 2 to 3 calcium ions for the stability and the enzymatic activity. MMP-7 and -26 consist of this minimal domain, propeptide and catalytic domains. C-terminal structures after catalytic domains are much more variable conferring MMPs substrate specificity and the interface for interaction with TIMPs (tissue inhibitor of metalloproteinases). Most MMPs have a linker (hinge region)and a hemopexin-like domain in addition to a minimal domain.The hinge region of most MMPs consists of 10-30 amino acids that connects a catalytic domain and a hemopexin-like domain.C-terminus hemopexin domain in collagenase (MMP-1, interstitial collagenase) is crucial for cleavage of triple helical interstitial collagen (Bode, 1995). The two gelatinases (MMP-2 and MMP-9) have additional three fibronectin-like repeats in their catalytic domain that interact with collagens and gelatins, respectively (Allan et al., 1995; Steffensen et al., 1995). Additionally, MMP-9 has a heavily O-glycosylated hinge region that is responsible for fine tuning its bioavailability (Van den Steen et al., 2006). The membrane-type MMP subfamily has a trans-membrane domain that anchors MMPs to the cell surface. MT1 through 6-MMPs and MMP23 belong to this subfamily. Three secreted MMPs (MMP-11, -21, -28) and all MT-MMPs have a basic RX[K/R]R motif at the C-terminal end of propeptide domain which can be cleaved by intracellular furin.
Regulation of MMPs
Since MMPs are able to degrade all the protein constituents in the extracellular matrix, their proteolytic activity has to be tightly controlled under normal conditions to prevent tissue destruction (Yong et al., 1998). There are the following ways to regulate the activity of MMPs: 1) gene transcriptional regulation; 2) activation of proenzyme by removing the propeptide domain; 3) the interaction with tissue inhibitor of metallopro-teinases (TIMPs); 4) pericellular or intracellular compartmentalization; 5) allosteric activation; 6) oxidative modification. Many MMP genes are inducible by a wide variety of effectors including growth factors, cytokines such as TNF-α and IL-1β, chemical agents, physical stress, oncogen products and interestingly, cell-cell or cell-ECM interaction (Ries and Petrides, 1995; Vincenti, 2001; Vincenti and Brinckerhoff, 2007). Their gene expression also can be suppressed by other factors such as TGF-β, retinoic acids and glucocorticoids (Osteen et al., 1996; Li et al., 2011; Ye et al., 2011). These external effectors trigger the various intracellular signal transduction pathways including MAPKs, JAK-STAT or NF-κB pathways leading to the induction of MMP genes (Korzus et al., 1997; Kheradmand et al., 1998; Reunanen et al., 1998). Although the activator protein-1 (AP-1) binding site in the MMP promoters has been considered as a main transcriptional regulator in most MMPs (Auble and Brinckerhoff, 1991; Kim et al., 2008; Liu et al., 2010; Singh et al., 2010), MMP-8, -11 and -21 are lack of AP-1 cis-element in their promoter. Another group of promoters of MMP-2, -14, and -28 does not have TATA box and are mainly regulated by ubiquitous Sp-1 family of transcriptional factor (Yan and Boyd, 2007).
The MMPs are initially expressed as inactive zymogens in which a zinc atom in the catalytic domain interacts with a cysteine residue (Cysteine switch) (Van Wart and Birkedal-Hansen, 1990). Activating factors cause the disruption of the Cys-Zn2+ interaction, which renders pro-MMPs partially active. Then, the partially active enzyme auto-catalyzes the propeptide region and make the enzyme fully active (Van Wart and Birkedal-Hansen, 1990). Activation of all MT-MMPs and furin-activated MMPs is well characterized intracellular event which leads to immediate catalytic activation of MMPs upon appearing on the cell surface or secretion. This process is achieved by serine proteinase, furin that is localized in the trans-Golgi network. Remaining MMPs are secreted as inactive zymogens and activated by serine proteinases like plasmin or other MMPs (MMP-3 and -14). Plasmin that is activated from plasminogen by the action of tissue- or urokinaseplasminogen activator is an important physiologic activator of pro-MMPs. Activation of proMMP could be achieved without involving proteolytic cleavage of prodomain by the mechanism called allosteric activation. Both proMMP-9 and -2 could be activated upon binding to their substrates, gelatin or collagen IV and α2 chain of collagen VI, respectively (Bannikov et al., 2002; Freise et al., 2009). Reactive oxygen species (ROS), peroxynitrite, glutathione could activate MMPs without removal of prodomains through modification of thiol in their catalytic core which lead to disruption of thiol-zinc interaction (Okamoto et al., 2001; Gu et al., 2002; McCarthy et al., 2008).
Tissue inhibitors of metalloproteinases (TIMPs) are the endogenous regulators of MMP activities in the tissue (Vincenti, 2001). Following activation, the activities of MMPs are regulated by the formation of non-covalent complexes with TIMPs. Four homologous TIMPs have been identified to date (Yong et al., 1998). They have about 190 amino acids with longer N-terminal and shorter C-terminal domain. N-terminal domain itself is fully functional in terms of inhibition of MMPs by chelating their catalytic zinc atom. The expression of TIMPs is also regulated at the transcriptional level. For example, TIMP-1 contains AP-1 site in its promoter (Ulisse et al., 1994). This implies that the expression of MMPs and TIMPs can be regulated coordinately by the same signal. However, the MMPs and TIMPs can also be regulated in opposite patterns. It has been shown that TGF-β induces the increased expression of TIMP-1 and suppresses collagenase and stromelysin (MMP-3) expression in fibroblasts and endothelial cells (Edwards et al., 1987). Since a number of studies have demonstrated that excessive production of MMPs are involved in the pathology of many inflammatory and malignant diseases, the balance between the MMPs and their inhibitors is thought to be important in the maintenance of normal physiologic conditions.
Subcellular localization and regulation
Accumulating evidence suggests that they are localized to various intracellular sites including nucleus, cytoplasm and mitochondria. Recent studies have identified diverse intracellular substrates for MMPs and its novel biological roles. Although the molecular mechanisms are poorly understood, nuclear localization of MMPs has been widely observed in various types of cells including cardiac myocytes, fibroblasts, neuronal cells, pulmonary artery endothelial cells and hepatocytes. Currently nuclear localization of MMP-2, -3, -9,-13 and MT1-MMP has been reported. MMP-2 and -3 have nuclear localization sequence (NLS) in the C-terminus and catalytic domain, respectively (Kwan et al., 2004; Si-Tayeb et al., 2006). Nuclear localization of MMP-2 in cardiac myocytes has been observed, highlighting its role in degradation of Poly (ADP-ribose) polymerase (PARP) (Kwan et al., 2004). More recently, it has been reported that cigarette-smoke induced MMP-2 expression in the nucleus of pulmonary artery endothelial cells, causing apoptosis (Aldonyte et al., 2009). In the ischemic neurons, increased proteolytic activity of MMP-2 and-9 in the ischemic neuronal nuclei at the early phase is responsible for DNA fragmentation after reperfusion. This is caused by MMP-mediated degradation of PARP-1 and X-ray cross-complementary factor 1 (XRCC1) (Yang et al., 2010). Exclusive expression of cleaved active MMP-3 in the nucleus was demonstrated, suggesting removal of prodomain is crucial for nuclear translocation of MMP-3 (Si-Tayeb et al., 2006). Interestingly, transcription factor-like function of MMP-3 was shown in chondrocytes. Binding of nuclear MMP-3 to a transcription enhance sequence in the connective tissue growth factor (CCN2/CTGF) enhances transcriptional activity CCN2/CTGF (Eguchi et al., 2008). Extensive analysis of nuclear MMP-3 associated proteins (NuMAPs) identified several candidates such as HP1γ and NCoR1 whose function on transcriptional regulation of CCN2/CTGF could be modulated by MMP-3.Nuclear localization of MMP-13 was also reported in oxygen and glucose deprived neuronal cells and after cerebral ischemia of rats and humans (Cuadrado et al., 2009). Both MMP-2 and MT1-MMP was observed in the nucleus of hepatocellular carcinoma (HCC) and aggressiveness of HCC including poor prognosis and large tumor expands was associated with nuclear localization of MT1-MMP (Ip et al., 2007).
Increasing number of studies indicate that various MMPs have been also found in the cytosol. We have recently demonstrated that active MMP-3 is expressed in the cytosol of dopaminergic cells and plays role in apoptosis and is involved in cleavage of α-synuclein and DJ-1, modulating their functions (Choi et al., 2008; Choi et al., 2011a; Choi et al., 2011b). This will be discussed more in detail later in this review. Mitochondrial localization and perinuclear accumulation of MMP-1 was shown in epithelial cells conferring resistance to apoptosis (Limb et al., 2005). Intracellular role of MMP-2 has been highlighted in acute myocardial ischemia and reperfusion model demonstrating that MMP-2 is responsible for cleavage of troponin I (TnI), the contractile protein regulatory element, and the cytoskeletal protein α-actinin. Peroxynitrite is a key mediator for the activation of MMP-2 in this model (Wang et al., 2002a; Wang et al., 2002b; Sung et al., 2007). Another example is MMP-26 which is mainly retained inside cells despite of its N-terminal signal peptide. Prodomain of MMP-26 contains a unique motif, PHCGVPD, that is assumed to increase autocatalytic activity leading intracellular activation (Marchenko et al., 2004).
MMPs in the Central Nervous System (CNS)
A range of MMPs play multiple roles in the development of the CNS, maintaining normal physiological functions, recovery after injury and the pathogenesis of brain diseases. During development of the CNS, various MMPs and TIMPs are expressed in various types of cells including neurons, astrocytes, oligodendrocytes and microglia, being involved in neurogenesis, axonal guidance, angiogenesis and myelinogenesis (Cañete Soler et al., 1995; Vaillant et al., 2003; Larsen et al., 2006). In the adult brain, MMPs are likely involved in a range of pivotal processes like migration of neurons and glia, synaptic plasticity, learning and memory, myelin turnover and angiogenesis through extracellular matrix (ECM) remodeling (Szklarczyk et al., 2002; Meighan et al., 2006; Ogier et al., 2006; Bozdagi et al., 2007). They are also important in the repair of adult brain after injuries such as spinal cord injury and stroke (Larsen et al., 2003; Lee et al., 2006). The normal functions of MMPs in the CNS were extensively discussed in the recent review by Agrawal et al. (2008).
MMPs in Neurodegenerative diseases
Recently, an increasing amount of evidence suggests that MMPs may play an crucial role in the pathogenesis of several neurodegenerative disorders including multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, malignant glioma, neuroinflammation and ischemia (Forsyth et al., 1999; Lorenzl et al., 2002; Lorenzl et al., 2003; Yong et al., 2007; Candelario-Jalil et al., 2009; Choi et al., 2011b; Shin et al., 2012).
The roles for MMPs in the pathogenesis of multiple sclerosis (MS) have been widely studied. MS is a brain inflammatory disorder demonstrating destruction of myelin sheath in the brain and spinal cord. Infiltration of various types of peripheral immune cells such as T cell, dendritic cells and monocyte/macrophages into the brain parenchyma accompanied with breakdown of blood-brain barrier (BBB) are major pathologic characteristics of MS. Upon entering in the CNS, they result in severe destruction of myelin and axon in cooperation with parenchymal resident cells including astrocytes and microglia. Alterations of various MMPs like MMP-1, -2, -3, -7, -9, -12, -13, -14 and -19 have been reported in MS. The MMPs were detected in the cerebrospinal fluid (CSF) of patients with MS or its animal model, experimental allergic encephalomyelitis (EAE) (Yushchenko et al., 2000; Fainardi et al., 2009). MMP-9 has been most extensively studied among them. Enhanced expression of both MMP-7 and -9 in parenchymal macrophages and small blood vessels were demonstrated in postmortem human MS brain (Cossins et al., 1997). The ratio of MMP-9/TIMP-1 in serum was elevated in relapsing-remitting MS patients and is correlated with increase in positive MRI lesions (Waubant et al., 1999). In EAE model, it has been shown that MMP-9 plays a key role in BBB disruption and trafficking of leukocytes into the brain parenchyma (Agrawal et al., 2006). Higher level of MMP-2 in serum and CSF of MS patients was also reported (Avolio et al., 2003; Benesová et al., 2009). The increased expressions of MMPs were shown in microglia and astrocytes in the brain lesions of MS patients (Cuzner et al.,1996; Maeda and Sobel, 1996). In animal studies, the application of several MMP inhibitors elicit reduced symptoms and severity of EAE (Hewson et al., 1995). The molecular mechanisms of MMPs in the pathogenesis of MS include the direct destruction of myelin protein, BBB disruption and chemokine/cytokine activation. Myelin protein could be a direct proteolytic substrate for MMPs. Activated leukocytes from peripheral blood or CNS resident cells could release MMPs which target myelin protein resulting in fragments. Fragmented myelin protein further activate neighboring immune cells releasing MMPs. This forms a vicious loop for activation/destruction leading to demyelinated axons (Starckx et al., 2003; Opde-nakker et al., 2006; Candelario-Jalil et al., 2009). MMP-mediated cleavage of cytokines/chemokines and its role in immune modulation have been investigated in MS patients and EAE model (Sellebjerg and Sørensen, 2003). MMPs are also involved in the conversion of pro-TNF-α to mature secreted protein and blood-brain barrier (BBB) disruption (Gearing et al., 1994; Gasche et al., 2001).
The role of MMPs in cerebral ischemia and stroke was also investigated both in animal models and human stroke. Dual roles of MMPs have been observed after brain ischemia: propagating neuronal death and apoptosis at the early phase of injury through disruption of ECM and opening the BBB; late-phase repairing by promoting angiogenesis and neurogenesis. At the early stage after stroke, transient activity of MMP-2 which is responsible for reversible opening of the BBB has been reported in rodent and non-human primates (Chang et al., 2003; Yang et al., 2007). Tight junction protein, claudin-5 is degraded by MMP-2. MMP-9 is activated and implicated in more extensive cerebral vascular damage at the later phase. Thus, early intervention within 3 h post reperfusion with MMP inhibitors showed the prevention of BBB disruption (Yang et al., 2007). In addition, cerebral infarct size was signifi cantly reduced by treatment with MMP inhibitors or in MMP-9 knockout but not in MMP-2 knockout mice (Asahi et al., 2000; Asahi et al., 2001). Interestingly, NO produced in cerebral ischemia and reperfusion can activate pro-MMP-9 by S-nitrosylation which in turn, causes direct neuronal apoptosis (Gu et al., 2002). In reperfusion injury following ischemia in rat brain, the expression of MMP-3 is induced in both microglia and apoptotic neurons and it is suggested that MMP-3 may play an important role in disrupting the BBB together with other MMPs such as MMP-2 and -9 (Rosenberg et al., 2001).
Malignant gliomas are the most common malignant brain tumors that are extremely invasive. The strong correlation between the invasiveness of glioma cells and MMPs such as MMP-2, MMP-9 and MT-MMPs has been shown both in vitro and in vivo (Rao et al., 1996; Uhm et al., 1996; Yamamoto et al., 1996). The expression of MMPs are up-regulated in many gliomas (Forsyth et al., 1999). In contrast to MMPs, the expression of TIMP-1 and -2 is decreased, suggesting disruption of the balance between MMPs and their inhibitors may contribute to pathology of malignant gliomas (Mohanam et al., 1995). It was also shown that MMPs inhibitor induces apoptosis of malignant gliomas (Yoshida et al., 2003). MMP-9 plays a key role in regulating invasiveness of malignant glioma cells and invasiveness is largely attributed to the poor prognosis. Recent study indentifi ed miRNAs, miR-491-5p, as a direct regulator of MMP-9 expression in U251 and U87 glioma cells, demonstrating that miR-491-5p reduces MMP-9 expression and inhibits cellular invasion (Yan et al., 2011). Recently, other MMPs like MMP-1, -11 and -19 were appeared to be of importance for the development of high-grade astrocytic tumor and may be promising targets for therapy (Stojic et al., 2008).
Alzheimer’s disease (AD) is the most common neurodegenerative disease that accounts for 50-80% of dementia. Pathologically, AD is characterized by gross atrophy of affected cerebral cortex resulted from neuronal loss and synaptic degeneration. The temporal, parietal lobe and parts of the frontal cortex and cingulate gyrus are most widely affected (Wenk, 2003). The presence of extracellular amyloid plaques and intracellular neurofibrillary tangles is the most characteristic pathologic feature of AD (Tiraboschi et al., 2004). Extracellular plaques consist of about 40 amino-acid long small peptides called beta-amyloid (Aβ). Aβ is generated by enzymatic cleavage of amyloid precursor protein (APP), a transmembrane protein. The process involved in proteolytic cleavage of APP is still waiting for elucidation. One of these fragments, Aβ1-42, gives rise to fibrils of Aβ, which further aggregates outside neurons in dense masses of protein known as senile plaques (Ohnishi and Takano, 2004; Tiraboschi et al., 2004). It has been shown that MMPs play a dual role in the pathogenesis of AD. MMPs may directly degrade Aβ resulting in reduction in Aβ deposit (Yan et al., 2006; Miners et al., 2008). On the other hand, MMPs such as MMP-2, -3 and -9 could be induced by Aβ in microglia, astrocytes or vascular smooth muscle cells contributing to brain parenchymal destruction. MMP-9 is overexpressed in AD brain tissue compared to controls and it can degrade synthetic 40-residue long Aβ protein in vitro (Backstrom et al., 1992; Roher et al., 1994). MMP-2 has also been shown to degrade 40- to 42-residue long Aβ purifi ed from AD brain tissue (Roher et al., 1994). In AD brains, MMP-3 is expressed predominantly in brain white matter. Double immunostaining of MMP-3 and GFAP in the white matter suggests that astrocytes may be a major source of MMP-3 in AD brain (Yoshiyama et al., 2000). MMP-3 immunoreactivity was also detected in the interstitium between myelinated axons and senile plaques of patients with AD (Yoshiyama et al., 2000). It has been also demonstrated that Aβ1-42 induces MMP-3, -12 and -13 expressions in microglia in PI3K-dependent manner (Ito et al., 2007). Recent analysis of CSF from AD patients indicated that MMP-3 is significantly elevated while MMP-2 is decreased (Horstmann et al., 2010). These data suggest that MMPs may be involved in the processing of APP and the pathogenesis of AD.
Parkinson’s disease (PD) is the second most common neurodegenerative disorders characterized by motor symptoms including resting tremor, rigidity, bradykinesia and postural instability resulting from selective degeneration of dopaminergic neurons in the substantia nigra pars compacta. Accumulating evidence suggests MMPs as a major culprit in the pathogenesis of PD. Expression of MMPs such as MMP-1, -2 and -9 as well as TIMP-1 and -2 in the substantia nigra (SN) of postmortem PD brain tissue was first reported by Lorenzl et al. showing alterations in MMP-2 and TIMP-1 in the SN of PD patients (Lorenzl et al., 2002). Since then, a number of studies including our own have demonstrated that MMPs are implicated in a range of pathophysiological processes of PD such as microglial activation, inflammation, direct dopaminergic apoptosis, disruption of the BBB and modulation of α-synuclein pathology by cleavage (Kim et al., 2005; Choi et al., 2008; Joo et al., 2010; Kim and Hwang, 2011).
MMP-3 AND PARKINSON’S DISEASE
MMP-3 was first noted as a neutral proteinase in human cartilage in 1974 (Sapolsky et al., 1974) and in rabbit bone fibroblast culture (Werb and Reynolds, 1974). The name‘stromelysin’ was introduced by Chin et al. (1985), but it has also been named ‘transin’ and ‘collagenase activating protein’(Chin et al., 1985; Matrisian et al., 1985; Treadwell et al., 1986). MMP-3 has a broad spectrum of substrates such as collagens, gelatin, elastin, laminin, casein, fibronectin, α1-AT, MBP and TNF-α precursor (Chandler et al., 1997). In addition, MMP-3 also can play a central role in the cleavage of other pro-form of MMPs including MMP-1, -2, -7, -8, -9, -13 leading to active forms (Chandler et al., 1997). MMP-3 has been implicated in inflammatory disorders including rheumatoid arthritis (RA), multiple sclerosis and AD (Zucker et al., 1994; Maeda and Sobel, 1996; Yoshiyama et al., 2000). MMP-3 levels are significantly increased both in serum and synovial fluid in RA patients (Zucker et al., 1994; Ishiguro et al., 1996). Serum MMP-3 levels are decreased by anti-TNF-α therapy in RA, suggesting that TNF-α may induce MMP-3 expression (Ca-trina et al., 2002). It has also been shown that MMP-3 levels in synovial fluid (SF) are significantly related with the concentration of soluble FasL (sFasL) in SF of patients with RA, which indicates that MMP-3 may regulate the shedding of FasL (Matsuno et al., 2001). The role of MMP-3 as a mediator of neuroinflammation responsible for dopaminergic neuronal degeneration was demonstrated. Active MMP-3 is released from neurons undergoing apoptosis after stress. Then it activates microglia to secrete pro-inflammatory cytokines such as IL-1β, TNF-α and IL-6 as well as reactive oxygen species (ROS) that subsequently cause neighboring neuronal death. NNGH, a specific inhibitor of MMP-3, largely attenuates microglial activation and neuronal death (Kim et al., 2005). MMP-3 is also involved in LPS-mediated microglial activation. MMP-3 and -9 are responsible for mitogen-activated protein kinases (MAPKs)- and NF-κB-mediated proinflammatory cytokines release from LPS-stimulated microglia (Woo et al., 2008).
Expression and activity of MMP-3 have been shown in a range of rodent PD models and postmortem PD brain. Increase in MMP-3 expression in the SN was observed in rodent model of PD, rats injected with 6-hydroxydopamine (6-OHDA) (Sung et al., 2005). Recent study showed that MMP-3 is expressed in Lewy bodies (LBs), a pathologic hallmark of PD (Choi et al., 2011b). Increased immunoreactivity of MMP-3 was also observed in tyrosine hydroxylase (TH)-positive dopaminergic neurons in the SN of mice administered with MPTP(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), a selective dopaminergic neurotoxin. In this study, significant reductions in MPTP-mediated dopaminergic neuronal degeneration as well as microglial activation were observed in MMP-3 knockout mice suggesting that MMP-3 is a key player in dopaminergic neuronal degeneration (Kim et al., 2007). Rat injected with LPS in the SN shows microglial acti-vation and dopaminergic neuronal degeneration. In this model, MMP-3 expression in the SN was significantly increased 24 h and 48 h after LPS injection (McClain et al., 2009). In contrary to the general belief that the inactive proform of MMP3 is activated in extracellular space, emerging evidence implies the existence of a mechanism of intracellular activation of MMP-3. Under apoptotic stress, MMP-3 is induced and generates the proform which is subsequently cleaved to the catalytically active MMP-3 by a serine protease other than furin. Intracellular enzymatic activity of MMP-3 is directly responsible for apoptosis of dopaminergic cells (Choi et al., 2008). Recently, intracellular targets for MMP-3 that are clearly linked to the pathogenesis of PD have been investigated (Sung et al., 2005; Choi et al., 2011a; Choi et al., 2011b). Mutations in SNCA that encodes α-synuclein were the first reported genetic cause in familiar forms of PD (Polymeropoulos et al., 1997). Later, α-synuclein aggregates were identified as the major component in Lewy bodies (LB), intracellular protein inclusions, a pathologic hallmark of PD (Spillantini et al., 1997). α-synuclein is cleaved by MMP-3 generating fragmented peptides that forms more toxic aggregates than the intact α-synuclein. MMP3-cleaved species (N-terminal) in extra-cellular space were cytotoxic when they were added to the cell culture media (Sung et al., 2005). The presence of C-terminally truncated α-synuclein has been reported in Lewy bodies of sporadic PD and Lewy body dementia (Baba et al., 1998; Liu et al., 2005). C-terminal truncation is also observed preferentially in A53T transgenic mice showing motor symptoms (Lee et al., 2002). Various lengths of the truncated forms of α-synuclein were reported (Li et al., 2005; Liu et al., 2005). About 15% of α-synuclein in Lewy bodies is truncated forms and incomplete degradation produced highly
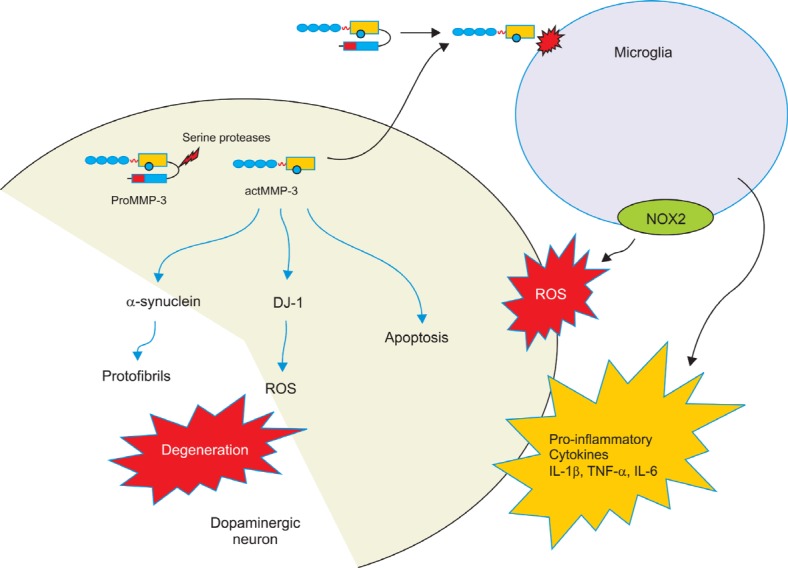
amyloidogenic fragments (Baba et al., 1998; Campbell et al., 2001). More recently, it has been shown that MMP-3 cleaves WT and mutant α-synuclein generating slightly different fragmented peptide profiles. MMP-3 gives rise to C-terminally truncated peptides of amino acids 1-78, 1-91, and 1-93 and that A53T mutant α-synuclein generates significant increase of these peptides. Both in vivo and in vitro experiments shows that these peptides cause stronger dopaminergic neuronal death compared to the intact α-synuclein despite less aggregation formation (Choi et al., 2011b). The results suggest that MMP-3 could modulate the aggregation property of α-synuclein contributing to the pathogenesis of PD. In addition, DJ-1 is also fragmented by MMP-3. DJ-1 is a protein belonging to ThiJ/PfpI/DJ-1 superfamily. Two mutations were identified within the DJ-1 gene in two families linked to the recessive PD PARK7 locus (Bonifati et al., 2003). One is a chromosomal deletion of 4 kb leading to the absence of DJ-1 expression, while the other is a L166P point mutation in which a highly conserved leucine was substituted for a proline. The latter destabilizes DJ-1 protein and promotes its degradation through the ubiquitin-proteasomal system (Miller et al., 2003). Active MMP-3 cleaves DJ-1 causing impairment of its antioxidant function. While MPTP administration significantly diminished DJ-1 expression in the SN of mice, its degradation was largely attenuated in MMP-3 knockout mice. This study suggests that cleavage of DJ-1 by the intracellular MMP-3 in response to cell stress impairs the protective role of DJ-1 against oxidative damage (Choi et al., 2011a). The proposed roles of MMP-3 in the pathogenesis of PD is illustrated in Fig. 2.
Fig. 2. The role of MMP-3 in the pathogenesis of Parkinson’s disease. Emerging evidence suggests that MMP-3 plays a key role in dopaminergic neuronal degeneration. Under stress conditions, MMP-3 is induced in dopaminergic neurons generating proMMP-3. Activation of MMP-3 might be achieved in cytoplasm as well as extracellular space. Catalytically active MMP-3 (actMMP-3) triggers microglial activation resulting in release of proinflammatory cytokine such as IL-1β, TNF-α and IL-6. Microglial substrates for act MMP-3 and signaling event leading to microglial activation are yet to be elucidated. NADPH oxidase (NOX2)-mediated ROS generation was also observed in microglia treated with actMMP-3. In addition, MMP-3 could be also activated intracellularly by unknown serine proteases upon stress such as 6-OHDA or MPP+, a selective dopaminergic toxin. Activated MMP-3 could cleave α-synuclein into several fragments by C-terminal truncation. These fragmented peptides are prone to aggregate and result in increased cytotoxicity. MMP-3 could also degrade DJ-1 and impair its antioxidant function resulting in increased oxidative stress. Intracellular actMMP-3 is directly linked apoptotic pathway in dopaminergic cells as well.
MMP inhibitors and Neuronal disorders
As emerging evidence indicates MMPs as a major culprit for a number of disease conditions including cancers, inflammation and neurodegenerative disorders, the importance of MMPs as a therapeutic target has been highlighted. MMP inhibitors could be categorized into two groups, macromolecular inhibitors such as TIMPs and monoclonal antibodies and small molecules including natural and synthetic inhibitors (Sang et al., 2006; Hu et al., 2007). TIMPs are the most thoroughly studied natural MMP inhibitors. Physiological balance between MMPs and TIMPs are considered important to prevent multiple disease conditions. Long-chain fatty acids, epigallocatechin gallate (EGCG) extracted from green tea and flavonoids also belong to natural MMP inhibitors. Since the first endeavor to develop smallmolecule MMP inhibitors for the treatment of arthritis, a number of synthetic MMP inhibitors have been tested for various diseases over the past three decades. The first generation of synthetic MMP inhibitors was designed based on mimicking natural peptide substrates, thus so called peptidomimetic MMP inhibitors. Later on, structure-based MMP inhibitors with a zinc-binding group (ZBG), a backbone that chelates zinc ion in the catalytic core of MMPs, have been extensively developed and tested. Four major ZBGs have been exploited for the development of MMP inhibitors: carboxylates, thiolates, phosphinyls and hydroxamates. Of these, hydroxamates-based MMP inhibitors have been studied most widely. A ZBG of hyroxamate acts as a bidentate ligand with the catalytic zinc ion and it also forms hydrogen bonds with enzyme backbones, resulting in potent inhibitory effect. Batimastat is one of the first generation of broad-spectrum hydroxamates inhibitors. Because of the similarity of catalytic core structure between MMPs, it has been challenging to develop highlyselective MMP inhibitors. As crystallographic structures of more MMPs were revealed, the nextgeneration MMP inhibitors with greater target selectivity were designed. Prinomastat is a second-generation hydroxamates MMP inhibitor that has much higher IC50 value against MMP-1 and -7 (Sang et al., 2006). Due to their numerous normal physiological functions including tissue remodeling, repression of tumor angiogenesis and inactivation of chemokines, therapeutic inhibitions of MMPs have potential to accompany side effects. Musculoskeletal syndrome (MSS) is the most common side effect caused by many MMP inhibitors, which is characterized by joint pain, stiffness and tendinitis (Cho et al., 2006). Despite the effort toward developing MMP inhibitors with high selectivity and therapeutic efficacy, tetracycline derivative, doxycycline, remains the only FDA approved MMP inhibitor (Hu et al., 2007). In spite of their low potency, non-hydroxamates MMP inhibitors containing other ZBGs such as carboxylates with greater target specifi city have been developed (Walker and Rosenberg, 2010).
It has been reported that MMP inhibitors bring about beneficial effects in animal studies of multiple sclerosis, vascular dementia, meningitis, Guillain-Barre syndrome and stroke. Of these, MMP inhibitors have been most intensively tested in acute cerebral ischemia. Studies on monoclonal antibodies against MMPs and broad spectrum MMP inhibitors such as GM-6001, BB-94 and BB-1101 demonstrate that BBB damage, infarct volume and neuronal death are significantly reduced by MMP inhibitions (Romanic et al., 1998; Gu et al., 2005). As discussed, MMPs have a dual role after stroke, aggravating neuronal damage at the early phase and tissue repair at the later stage, suggesting that short-term administration during the early stage would be effective. Patients with MS treated with minocycline, tetracycline derivative, demonstrate reduced number of gadolinium-enhancing lesions on MRI (Metz et al., 2004). In EAE, MMP inhibitors reduce damage to the BBB and low-dose tetracycline administration with interferon-beta effectively reduces inflammation (Giuliani et al., 2005). In vascular cognitive impairment which is characterized by progressive white matter damage cause by ischemic injury or hypoxic hypoperfusion, MMP inhibitors reduce white matter damage (Cho et al., 2006; Walker and Rosenberg, 2010). Use of MMP inhibitors in the treatment of AD is controversial since they are involved in the generation of Aβ from APP as well as clearance of Aβ As discussed earlier, a couple of MMPs, especially MMP-3, play crucial roles in the pathogenesis of PD, suggesting that MMP inhibitors might ameliorate dopaminergic neuronal degeneration.
CONCLUSION
In this review, we discuss general structure, activation, regulation and functions of MMPs and subtilize their roles in the CNS. Finally, we emphasize on the implication of MMPs in a range of neurodegenerative conditions. Based on an increasing number of studies, we further discuss the link between MMP-3 and the pathogenesis of PD. Lastly, current status on development of MMP inhibitors for treatment of neurodegenerative diseases is discussed. We are anticipating much more exciting works that potentially lead to therapeutic interventions for various brain disorders.
References
- 1.Agrawal S. Agrawal P. Durbeej M. van Rooijen N. Ivars F. Opdenakker G. Sorokin L. M. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. (2006);203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal S. M. Lau L. Yong V. W. MMPs in the central nervous system: where the good guys go bad. Semin. Cell Dev. Biol. (2008);19:42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Aldonyte R. Brantly M. Block E. Patel J. Zhang J. Nuclear localization of active matrix metalloproteinase-2 in cigarette smoke-exposed apoptotic endothelial cells. Exp. Lung Res. (2009);35:59–75. doi: 10.1080/01902140802406059. [DOI] [PubMed] [Google Scholar]
- 4.Allan J. A. Docherty A. J. Barker P. J. Huskisson N. S. Reynolds J. J. Murphy G. Binding of gelatinases A and B to type-I collagen and other matrix components. Biochem. J. (1995);309:299–306. doi: 10.1042/bj3090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asahi M. Asahi K. Jung J. C. del Zoppo G. J. Fini M. E. Lo E. H. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J. Cereb. Blood Flow Metab. (2000);20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Asahi M. Sumii T. Fini M. E. Itohara S. Lo E. H. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport. (2001);12:3003–3007. doi: 10.1097/00001756-200109170-00050. [DOI] [PubMed] [Google Scholar]
- 7.Auble D. T. Brinckerhoff C. E. The AP-1 sequence is necessary but not sufficient for phorbol induction of collagenase in fibroblasts. Biochemistry. (1991);30:4629–4635. doi: 10.1021/bi00232a039. [DOI] [PubMed] [Google Scholar]
- 8.Avolio C. Ruggieri M. Giuliani F. Liuzzi G. M. Leante R. Riccio P. Livrea P. Trojano M. Serum MMP-2 and MMP-9 are elevated in different multiple sclerosis subtypes. J. Neuroimmunol. (2003);136:46–53. doi: 10.1016/S0165-5728(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 9.Baba M. Nakajo S. Tu P. H. Tomita T. Nakaya K. Lee V. M. Trojanowski J. Q. Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am. J. Pathol. (1998);152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 10.Backstrom J. R. Miller C. A. Tökés Z. A. Characterizationof neutral proteinases from Alzheimer-affected and control brainspecimens: identifi cation of calcium-dependent metalloproteinasesfrom the hippocampus. J. Neurochem. (1992);58:983–992. doi: 10.1111/j.1471-4159.1992.tb09352.x. [DOI] [PubMed] [Google Scholar]
- 11.Bannikov G. A. Karelina T. V. Collier I. E. Marmer B. L. Goldberg G. I. Substrate binding of gelatinase B induces its enzymatic activity in the presence of intact propeptide. J. Biol. Chem. (2002);277:16022–16027. doi: 10.1074/jbc.M110931200. [DOI] [PubMed] [Google Scholar]
- 12.Becker J. W. Marcy A. I. Rokosz L. L. Axel M. G. Burbaum J. J. Fitzgerald P. M. Cameron P. M. Esser C. K. Hagmann W. K. Hermes J. D. Springer J. P. Stromelysin-1: three-dimensionalstructure of the inhibited catalytic domain and of the C-truncated proenzyme. Protein Sci. (1995);4:1966–1976. doi: 10.1002/pro.5560041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benesová Y. Vasku A. Novotná H. Litzman J. Stourac P. Beránek M. Kadanka Z. Bednarik J. Matrix metalloproteinase-9 and matrix metalloproteinase-2 as biomarkers of various courses in multiple sclerosis. Mult. Scler. (2009);15:316–322. doi: 10.1177/1352458508099482. [DOI] [PubMed] [Google Scholar]
- 14.Bode W. A helping hand for collagenases: the haemopexin-like domain. Structure. (1995);3:527–530. doi: 10.1016/S0969-2126(01)00185-X. [DOI] [PubMed] [Google Scholar]
- 15.Bode W. Gomis-Rüth F. X. Stöckler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the 'metzincins'. FEBS Lett. (1993);331:134–140. doi: 10.1016/0014-5793(93)80312-I. [DOI] [PubMed] [Google Scholar]
- 16.Bonifati V. Rizzu P. van Baren M. J. Schaap O. Breedveld G. J. Krieger E. Dekker M. C. Squitieri F. Ibanez P. Joosse M. van Dongen J. W. Vanacore N. van Swieten J. C. Brice A. Meco G. van Duijn C. M. Oostra B. A. Heutink P. Mutationsin the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. (2003);229:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 17.Bozdagi O. Nagy V. Kwei K. T. Huntley G. W. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol. (2007);98:334–344. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell B. C. McLean C. A. Culvenor J. G. Gai W. P. Blumbergs P. C. Jäkälä P. Beyreuther K. Masters C. L. Li Q. X. The solubility of alpha-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson's disease. J. Neurochem. (2001);76:87–96. doi: 10.1046/j.1471-4159.2001.00021.x. [DOI] [PubMed] [Google Scholar]
- 19.Candelario-Jalil E. Yang Y. Rosenberg G. A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinfl ammation and cerebral ischemia. Neuroscience. (2009);158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cañete Soler R. Gui Y. H. Linask K. K. Muschel R. J. MMP-9 (gelatinase B) mRNA is expressed during mouse neurogenesis and may be associated with vascularization. Brain Res. Dev. Brain Res. (1995);88:37–52. doi: 10.1016/0165-3806(95)00079-S. [DOI] [PubMed] [Google Scholar]
- 21.Catrina A. I. Lampa J. Ernestam S. af Klint E. Bratt J. Klareskog L. Ulfgren A. K. Anti-tumour necrosis factor (TNF)-alpha therapy (etanercept) down-regulates serum matrix metalloproteinase(MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology (Oxford). (2002);41:484–489. doi: 10.1093/rheumatology/41.5.484. [DOI] [PubMed] [Google Scholar]
- 22.Chandler S. Miller K. M. Clements J. M. Lury J. Corkill D. Anthony D. C. Adams S. E. Gearing A. J. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J. Neuroimmunol. (1997);72:155–161. doi: 10.1016/S0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 23.Chang D. I. Hosomi N. Lucero J. Heo J. H. Abumiya T. Mazar A. P. del Zoppo G. J. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J. Cereb. Blood Flow Metab. (2003);23:1408–1419. doi: 10.1097/01.WCB.0000091765.61714.30. [DOI] [PubMed] [Google Scholar]
- 24.Chin J. R. Murphy G. Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis isolation characterization, and substrates. J. Biol. Chem. (1985);260:12367–12376. [PubMed] [Google Scholar]
- 25.Cho K. O. La H. O. Cho Y. J. Sung K. W. Kim S. Y. Minocycline attenuates white matter damage in a rat model of chronic cerebral hypoperfusion. J. Neurosci. Res. (2006);83:285–291. doi: 10.1002/jnr.20727. [DOI] [PubMed] [Google Scholar]
- 26.Choi D. H. Hwang O. Lee K. H. Lee J. Beal M. F. Kim Y. S. DJ-1 cleavage by matrix metalloproteinase 3 mediates oxidative stress-induced dopaminergic cell death. Antioxid. Redox. Signal. (2011a);14:2137–2150. doi: 10.1089/ars.2009.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi D. H. Kim Y. J. Kim Y. G. Joh T. H. Beal M. F. Kim Y. S. Role of matrix metalloproteinase 3-mediated alpha-synuclein cleavage in dopaminergic cell death. J. Biol. Chem. (2011b);286:14168–14177. doi: 10.1074/jbc.M111.222430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi D. H. Kim E. M. Son H. J. Joh T. H. Kim Y. S. Kim D. Flint Beal M. Hwang O. A novel intracellular role of matrix metalloproteinase-3 during apoptosis of dopaminergic cells. J. Neurochem. (2008);106:405–415. doi: 10.1111/j.1471-4159.2008.05399.x. [DOI] [PubMed] [Google Scholar]
- 29.Choi D. H. Kim E. M. Son H. J. Joh T. H. Kim Y. S. Kim D. Beal M. Hwang O. A novel intracellular role of matrix metalloproteinase-3 during apoptosis of dopaminergic cells. J. Neurochem. (2008);106:405–415. doi: 10.1111/j.1471-4159.2008.05399.x. [DOI] [PubMed] [Google Scholar]
- 30.Cossins J. A. Clements J. M. Ford J. Miller K. M. Pigott R. Vos W. Van der Valk P. De Groot C. J. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta. Neuropathol. (1997);94:590–598. doi: 10.1007/s004010050754. [DOI] [PubMed] [Google Scholar]
- 31.Cuadrado E. Rosell A. Borrell-Pagès M. García-Bonilla L. Hernández-Guillamon M. Ortega-Aznar A. Montaner J. Matrix metalloproteinase-13 is activated and is found in the nucleus of neural cells after cerebral ischemia. J. Cereb. Blood Flow Metab. (2009);29:398–410. doi: 10.1038/jcbfm.2008.130. [DOI] [PubMed] [Google Scholar]
- 32.Cuzner M. L. Gveric D. Strand C. Loughlin A. J. Paemen L. Opdenakker G. Newcombe J. The expression of tissuetype plasminogen activator, matrix metalloproteases and endogenous inhibitors in the central nervous system in multiple sclerosis: comparison of stages in lesion evolution. J. Neuropathol. Exp. Neurol. (1996);55:1194–1204. doi: 10.1097/00005072-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Edwards D. R. Murphy G. Reynolds J. J. Whitham S. E. Docherty A. J. Angel P. Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. (1987);6:1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eguchi T. Kubota S. Kawata K. Mukudai Y. Uehara J. Ohgawara T. Ibaragi S. Sasaki A. Kuboki T. Takigawa M. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Mol. Cell Biol. (2008);28:2391–2413. doi: 10.1128/MCB.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fainardi E. Castellazzi M. Tamborino C. Trentini A. Manfrinato M. C. Baldi E. Tola M. R. Dallocchio F. Granieri E. Bellini T. Potential relevance of cerebrospinal fl uid and serum levels and intrathecal synthesis of active matrix metalloproteinase-2 (MMP-2) as markers of disease remission in patients with multiple sclerosis. Mult. Scler. (2009);15:547–554. doi: 10.1177/1352458509102372. [DOI] [PubMed] [Google Scholar]
- 36.Forsyth P. A. Wong H. Laing T. D. Rewcastle N. B. Morris D. G. Muzik H. Leco K. J. Johnston R. N. Brasher P. M. Sutherland G. Edwards D. R. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br. J. Cancer. (1999);79:1828–1835. doi: 10.1038/sj.bjc.6690291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freise C. Erben U. Muche M. Farndale R. Zeitz M. Somasundaram R. Ruehl M. The alpha 2 chain of collagen type VI sequesters latent proforms of matrix-metalloproteinases and modulates their activation and activity. Matrix Biol. (2009);28:480–489. doi: 10.1016/j.matbio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Gasch Y. Copin J. C. Sugawara T. Fujimura M. Chan P. H. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. (2001);21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Gearing A. J. Beckett P. Christodoulou M. Churchill M. Clements J. Davidson A. H. Drummond A. H. Galloway W. A. Gilbert R. Gordon J. L. Gordon J .L. Leber T. M. Mangan M. Miller K. Nayee P. Owen K. Patel S. Thomas W. Wells G. Wood L. M. Woolley K. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. (1994);370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 40.Giuliani F. Fu S. A. Metz L. M. Yong V. W. Effective combination of minocycline and interferon-beta in a model of multiple sclerosis. J. Neuroimmunol. (2005);165:83–91. doi: 10.1016/j.jneuroim.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 41.Gross J. Lapiere C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc. Natl. Acad. Sci. USA. (1962);48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu Z. Cui J. Brown S. Fridman R. Mobashery S. Strongin A. Y. Lipton S. A. A highly specifi c inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J. Neurosci. (2005);25:6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu Z. Kaul M. Yan B. Kridel S. J. Cui J. Strongin A. Smith J. W. Liddington R. C. Lipton S. A. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. (2002);297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 44.Hewson A. K. Smith T. Leonard J. P. Cuzner M. L. Suppression of experimental allergic encephalomyelitis in the Lewis rat by the matrix metalloproteinase inhibitor Ro31-9790. Inflamm. Res. (1995);44:345–349. doi: 10.1007/BF01796266. [DOI] [PubMed] [Google Scholar]
- 45.Horstmann S. Budig L. Gardner H. Koziol J. Deuschle M. Schilling C. Wagner S. Matrix metalloproteinases in peripheral blood and cerebrospinal fluid in patients with Alzheimer's disease. International psychogeriatrics. (2010);IPA 22:966–972. doi: 10.1017/S1041610210000827. [DOI] [PubMed] [Google Scholar]
- 46.Hu J. Van den Steen P. E. Sang Q. X. Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. (2007);6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 47.Ip Y. C. Cheung S. T. Fan S. T. Atypical localization of membrane type 1-matrix metalloproteinase in the nucleus is associated with aggressive features of hepatocellular carcinoma. Mol. Carcinog. (2007);46:225–230. doi: 10.1002/mc.20270. [DOI] [PubMed] [Google Scholar]
- 48.Ishiguro N. Ito T. Obata K. Fujimoto N. Iwata H. Determination of stromelysin-1, 72 and 92 kDa type IV collagenase, tissue inhibitor of metalloproteinase-1 (TIMP-1), and TIMP-2 in synovial fluid and serum from patients with rheumatoid arthritis. J. Rheumatol. (1996);23:1599–1604. [PubMed] [Google Scholar]
- 49.Ito S. Kimura K. Haneda M. Ishida Y. Sawada M. Isobe K. Induction of matrix metalloproteinases (MMP3, MMP12 and MMP13) expression in the microglia by amyloid-beta stimulation via the PI3K/Akt pathway. Exp. Gerontol. (2007);42:532–537. doi: 10.1016/j.exger.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Joo S. H. Kwon K. J. Kim J. W. Kim J. W. Hasan M. R. Lee H. J. Han S. H. Shin C. Y. Regulation of matrix metalloproteinase-9 and tissue plasminogen activator activity by alpha-synuclein in rat primary glial cells. Neurosci. Lett. (2010);469:352–356. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- 51.Kheradmand F. Werner E. Tremble P. Symons M. Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. (1998);280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- 52.Kim E. M. Hwang O. Role of matrix metalloproteinase-3 in neurodegeneration. J. Neurochem. (2011);116:22–32. doi: 10.1111/j.1471-4159.2010.07082.x. [DOI] [PubMed] [Google Scholar]
- 53.Kim K. S. Kim H. Y. Joe E. H. Jou I. Matrix metalloproteinase-3 induction in rat brain astrocytes: focus on the role of two AP-1 elements. Biochem. J. (2008);410:605–611. doi: 10.1042/BJ20071207. [DOI] [PubMed] [Google Scholar]
- 54.Kim Y. S. Choi D. H. Block M. L. Lorenzl S. Yang L. Kim Y. J. Sugama S. Cho B. P. Hwang O. Browne S. E. Kim S. Y. Hong J. S. Beal M. F. Joh T. H. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. (2007);21:179–187. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- 55.Kim Y. S. Kim S. S. Cho J. J. Choi D. H. Hwang O. Shin D. H. Chun H. S. Beal M. F. Joh T. H. Matrix metalloproteinase-3: a novel signaling proteinase from apoptotic neuronal cells that activates microglia. J. Neurosci. (2005);25:3701–3711. doi: 10.1523/JNEUROSCI.4346-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korzus E. Nagase H. Rydell R. Travis J. The mitogen-activated protein kinase and JAK-STAT signaling pathways are required for an oncostatin M-responsive element-mediated activation of matrix metalloproteinase 1 gene expression. J. Biol. Chem. (1997);272:1188–1196. doi: 10.1074/jbc.272.2.1188. [DOI] [PubMed] [Google Scholar]
- 57.Kwan J. A. Schulze C. J. Wang W. Leon H. Sariahmetoglu M. Sung M. Sawicka J. Sims D. E. Sawicki G. Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose)polymerase (PARP) in vitro. FASEB J. (2004);18:690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 58.Larsen P. H. DaSilva A. G. Conant K. Yong V. W. Myelin formation during development of the CNS is delayed in matrix metalloproteinase-9 and -12 null mice. J. Neurosci. (2006);26:2207–2214. doi: 10.1523/JNEUROSCI.1880-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsen P. H. Wells J. E. Stallcup W. B. Opdenakker G. Yong V. W. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J. Neurosci. (2003);23:11127–11135. doi: 10.1523/JNEUROSCI.23-35-11127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee M. K. Stirling W. Xu Y. Xu X. Qui D. Mandir A. S. Dawson T. M. Copeland N. G. Jenkins N. A. Price D. L. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. USA. (2002);99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee R. Kermani P. Teng K. K. Hempstead B. L. Regulation of cell survival by secreted proneurotrophins. Science. (2001);294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 62.Lee S. R. Kim H. Y. Rogowska J. Zhao B. Q. Bhide P. Parent J. M. Lo E. H. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J. Neurosci. (2006);26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li W. Chang L. Rong Z. Liu W. Retinoic aacid diminished the expression of MMP-2 in hyperoxia-exposed premature rat lung fibroblasts through regulating mitogen-activated protein kinases. J. Huazhong. Univ. Sci. Technolog. Med. Sci. (2011);31:251–257. doi: 10.1007/s11596-011-0262-1. [DOI] [PubMed] [Google Scholar]
- 64.Li W. West N. Colla E. Pletnikova O. Troncoso J. C. Marsh L. Dawson T. M. Jäkälä P. Hartmann T. Price D. L. Lee M. K. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proc. Natl. Acad. Sci. USA. (2005);102:2162–Limb. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Limb G. A. Matter K. Murphy G. Cambrey A. D. Bishop P. N. Morris G. E. Khaw P. T. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am. J. Pathol. (2005);166:1555–1563. doi: 10.1016/S0002-9440(10)62371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu C. W. Giasson B. I. Lewis K. A. Lee V. M. Demartino G. N. Thomas P. J. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J. Biol. Chem. (2005);280:22670–22678. doi: 10.1074/jbc.M501508200. [DOI] [PubMed] [Google Scholar]
- 67.Liu X. Manzano G. Lovett D. H. Kim H. T. Role of AP-1 and RE-1 binding sites in matrix metalloproteinase-2 transcriptional regulation in skeletal muscle atrophy. Biochem. Biophys. Res. Commun. (2010);396:219–223. doi: 10.1016/j.bbrc.2010.04.067. [DOI] [PubMed] [Google Scholar]
- 68.Lorenzl S. Albers D. S. Narr S. Chirichigno J. Beal M. F. Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson's disease. Exp. Neurol. (2002);178:13–20. doi: 10.1006/exnr.2002.8019. [DOI] [PubMed] [Google Scholar]
- 69.Lorenzl S. Albers D. S. Relkin N. Ngyuen T. Hilgenberg S. L. Chirichigno J. Cudkowicz M. E. Beal M. F. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer's disease. Neurochem. Int. (2003);43:191–196. doi: 10.1016/S0197-0186(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 70.Maeda A. Sobel R. A. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. (1996);55:300–309. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Marchenko N. D. Marchenko G. N. Weinreb R. N. Lindsey J. D. Kyshtoobayeva A. Crawford H. C. Strongin A. Y. Beta-catenin regulates the gene of MMP-26, a novel metalloproteinase expressed both in carcinomas and normal epithelial cells. Int. J. Biochem. Cell. Biol. (2004);36:942–956. doi: 10.1016/j.biocel.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 72.Matrisian L. M. Glaichenhaus N. Gesnel M. C. Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. (1985);4:1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsuno H. Yudoh K. Watanabe Y. Nakazawa F. Aono H. Kimura T. Stromelysin-1 (MMP-3) in synovial fl uid of patients with rheumatoid arthritis has potential to cleave membrane bound Fas ligand. J. Rheumatol. (2001);28:22–28. [PubMed] [Google Scholar]
- 74.McCarthy S. M. Bove P. F. Matthews D. E. Akaike T. an derVliet A. Nitric oxide regulation of MMP-9 activation and its relationship to modifications of the cysteine switch. Biochemistry. (2008);47:5832–5840. doi: 10.1021/bi702496v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McClain J. A. Phillips L. L. Fillmore H. L. Increased MMP-3 and CTGF expression during lipopolysaccharide-induced dopaminergic neurodegeneration. Neurosci. Lett. (2009);460:27–31. doi: 10.1016/j.neulet.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 76.Meighan S. E. Meighan P. C. Choudhury P. Davis C. J. Olson M. L. Zornes P. A. Wright J. W. Harding J. W. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem. (2006);96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- 77.Metz L. M. Zhang Y. Yeung M. Patry D. G. Bell R. B. Stoian C. A. Yong V. W. Patten S. B. Duquette P. Antel J. P. Mitchell J. R. Minocycline reduces gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann. Neurol. (2004);55:756. doi: 10.1002/ana.20111. [DOI] [PubMed] [Google Scholar]
- 78.Miller D. W. Ahmad R. Hague S. Baptista M. J. Canet-Aviles R. McLendon C. Carter D. M. Zhu P. P. Stadler J. Chandran J. Klinefelter G. R. Blackstone C. Cookson M. R. L166P mutant DJ-1, causative for recessive Parkinson's disease, is degraded through the ubiquitin-proteasome system. J. Biol. Chem. (2003);278:36588–36595. doi: 10.1074/jbc.M304272200. [DOI] [PubMed] [Google Scholar]
- 79.Miners J. S. Baig S. Palmer J. Palmer L. E. Kehoe P. G. Love S. Abeta-degrading enzymes in Alzheimer's disease. Brain Pathol. (2008);18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohanam S. Wang S. W. Rayford A. Yamamoto M. Sawaya R. Nakajima M. Liotta L. A. Nicolson G. L. Stetler-Stevenson W. G. Rao J. S. Expression of tissue inhibitors of metalloproteinases:negative regulators of human glioblastoma invasion in vivo. Clin. Exp. Metastasis. (1995);13:57–62. doi: 10.1007/BF00144019. [DOI] [PubMed] [Google Scholar]
- 81.Ogier C. Bernard A. Chollet A. M. LE Diguardher T. Hanessian S. Charton G. Khrestchatisky M. Rivera S. Matrix metalloproteinase-2 (MMP-2) regulates astrocyte motility in connection with the actin cytoskeleton and integrins. Glia. (2006);54:272–284. doi: 10.1002/glia.20349. [DOI] [PubMed] [Google Scholar]
- 82.Ohnishi S. Takano K. Amyloid fibrils from the viewpoint of protein folding. Cell Mol. Life Sci. (2004);61:511–524. doi: 10.1007/s00018-003-3264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okamoto T. Akaike T. Sawa T. Miyamoto Y. van der Vliet A. Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfi de S-oxide formation. J. Biol. Chem. (2001);276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 84.Opdenakker G. Dillen C. Fiten P. Martens E. Van Aelst I. Vanden Steen P. E. Nelissen I. Starckx S. Descamps F. J. Hu J. Piccard H. Van Damme J. Wormald M. R. Rudd P. M. Dwek R. A. Remnant epitopes autoimmunity and glycosylation. Biochim. Biophys. Acta. (2006);1760:610–615. doi: 10.1016/j.bbagen.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 85.Osteen K. G. Bruner K. L. Sharpe-Timms K. L. Steroid and growth factor regulation of matrix metalloproteinase expression and endometriosis. Semin. Reprod. Endocrinol. (1996);14:247–255. doi: 10.1055/s-2007-1016334. [DOI] [PubMed] [Google Scholar]
- 86.Polymeropoulos M. H. Lavedan C. Leroy E. Ide E. Dehejia A. Dutra A. Pike B. Root H. Rubenstein J. Boyer R. Stenroos E. Chandrasekharappa S. Athanassiadou A. Papapetropoulos T. Johnson W. G. Lazzarini A. M. Duvoisin R. C. Di Iorio G. Golbe L. I. Nussbaum R. L. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. (1997);276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 87.Rao J. S. Yamamoto M. Mohaman S. Gokaslan Z. L. Fuller G. N. Stetler-Stevenson W. G. Rao V. H. Liotta L. A. Nicolson G. L. Sawaya R. E. Expression and localization of 92 kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin. Exp. Metastasis. (1996);14:12–18. doi: 10.1007/BF00157681. [DOI] [PubMed] [Google Scholar]
- 88.Reunanen N. Westermarck J. Häkkinen L. Holmström T. H. Elo I. Eriksson J. E. Kähäri V. M. Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J. Biol. Chem. (1998);273:5137–5145. doi: 10.1074/jbc.273.9.5137. [DOI] [PubMed] [Google Scholar]
- 89.Ries C. Petrides P. E. Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol. Chem. Hoppe. Seyler. (1995);376:345–355. [PubMed] [Google Scholar]
- 90.Roher A. E. Kasunic T. C. Woods A. S. Cotter R. J. Ball M. J. Fridman R. Proteolysis of A beta peptide from Alzheimer disease brain by gelatinase A. Biochem Biophys Res Commun. (1994);205:1755–1761. doi: 10.1006/bbrc.1994.2872. [DOI] [PubMed] [Google Scholar]
- 91.Romanic A. M. White R. F. Arleth A. J. Ohlstein E. H. Barone F. C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. (1998);29:1020–1030. doi: 10.1161/01.STR.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 92.Rosenberg G. A. Cunningham L. A. Wallace J. Alexander S. Estrada E. Y. Grossetete M. Razhagi A. Miller K. Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. (2001);893:104–112. doi: 10.1016/S0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 93.Sang Q. X. Jin Y. Newcomer R. G. Monroe S. C. Fang X. Hurst D. R. Lee S. Cao Q. Schwartz M. A. Matrix metalloproteinase inhibitors as prospective agents for the prevention and treatment of cardiovascular and neoplastic diseases. Curr. Top. Med. Chem. (2006);6:289–316. doi: 10.2174/156802606776287045. [DOI] [PubMed] [Google Scholar]
- 94.Sapolsky A. I. Howell D. S. Woessner JF Jr. Neutral proteases and cathepsin D in human articular cartilage. The Journal ofclinical investigation. (1974);53:1044–1053. doi: 10.1172/JCI107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sellebjerg F. Sørensen T. L. Chemokines and matrix metalloproteinase-9 in leukocyte recruitment to the central nervous system. Brain Res. Bull. (2003);61:347–355. doi: 10.1016/S0361-9230(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 96.Shin E. J. Kim E. M. Lee J. A. Rhim H. Hwang O. Matrix metalloproteinase-3 is activated by HtrA2/Omi in dopaminergic cells: Relevance to Parkinson's disease. Neurochem. Int. (2012);60:249–256. doi: 10.1016/j.neuint.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 97.Singh N. K. Quyen D. V. Kundumani-Sridharan V. Brooks P. C. Ra G. N. AP-1 (Fra-1/c-Jun)-mediated induction of expression of matrix metalloproteinase-2 is required for 15S-hy-droxyeicosatetraenoic acid-induced angiogenesis. J. Biol. Chem. (2010);285:16830–16843. doi: 10.1074/jbc.M110.106187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Si-Tayeb K. Monvoisin A. Mazzocco C. Lepreux S. Decossas M. Cubel G. Taras D. Blanc J. F. Robinson D. R. Rosenbaum J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am. J. Pathol. (2006);169:1390–1401. doi: 10.2353/ajpath.2006.060005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spillantini M. G. Schmidt M. L. Lee V. M. Trojanowski J. Q. Jakes R. Goedert M. Alpha-synuclein in Lewy bodies. Nature. (1997);388:893–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 100.Starckx S. Van den Steen P. E. Verbeek R. van Noort J. M. Opdenakker G. A novel rationale for inhibition of gelatinase B in multiple sclerosis: MMP-9 destroys alpha B-crystallin and generates a promiscuous T cell epitope. J. Neuroimmunol. (2003);141:47–57. doi: 10.1016/S0165-5728(03)00217-0. [DOI] [PubMed] [Google Scholar]
- 101.Steffensen B. Wallon U. M. Overall C. M. Extracellular matrix binding properties of recombinant fibronectin type II-like modules of human 72-kDa gelatinase/type IV collagenase. High affinity binding to native type I collagen but not native type IV collagen. J. Biol. Chem. (1995);270:11555–11566. doi: 10.1074/jbc.270.19.11555. [DOI] [PubMed] [Google Scholar]
- 102.Stojic J. Hagemann C. Haas S. Herbold C. Kühnel S. Gerngras S. Roggendorf W. Roosen K. Vince G. H. Expression of matrix metalloproteinases MMP-1, MMP-11 and MMP-19 is correlated with the WHO-grading of human malignant gliomas. Neurosci. Res. (2008);60:40–49. doi: 10.1016/j.neures.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 103.Sung J. Y. Park S. M. Lee C. H. Um J. W. Lee H. J. Kim J. Oh Y. J. Lee S. T. Paik S. R. Chung K. C. Proteolytic cleavage of extracellular secreted {alpha}-synuclein via matrix metalloproteinases. J. Biol. Chem. (2005);280:25216–25224. doi: 10.1074/jbc.M503341200. [DOI] [PubMed] [Google Scholar]
- 104.Sung M. M. Schulz C. G. Wang W. Sawicki G. Bautista-López N. L. Schulz R. Matrix metalloproteinase-2 degrades the cytoskeletal protein alpha-actinin in peroxynitrite mediated myocardial injury. J. Mol. Cell Cardiol. (2007);43:429–436. doi: 10.1016/j.yjmcc.2007.07.055. [DOI] [PubMed] [Google Scholar]
- 105.Szklarczyk A. Lapinska J. Rylski M. McKay R. D. Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J. Neurosci. (2002);22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tiraboschi P. Hansen L. A. Thal L. J. Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. (2004);62:1984–1989. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- 107.Treadwell B. V. Neidel J. Pavia M. Towle C. A. Trice M. E. Mankin H. J. Purification and characterization of collagenase activator protein synthesized by articular cartilage. Arch. Biochem. Biophys. (1986);251:715–723. doi: 10.1016/0003-9861(86)90381-4. [DOI] [PubMed] [Google Scholar]
- 108.Uhm J. H. Dooley N. P. Villemure J. G. Yong V. W. Glioma invasion in vitro: regulation by matrix metalloprotease-2 and protein kinase C. Clin. Exp. Metastasis. (1996);14:421–433. doi: 10.1007/BF00128958. [DOI] [PubMed] [Google Scholar]
- 109.Ulisse S. Farina A. R. Piersanti D. Tiberio A. Cappabianca L. D'Orazi G. Jannini E. A. Malykh O. Stetler-Stevenson W. G. D'Armiento M. Follicle-stimulating hormone increases the expression of tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2 and induces TIMP-1 AP-1 site binding complex(es) in prepubertal rat Sertoli cells. Endocrinology. (1994);135:2479–2487. doi: 10.1210/en.135.6.2479. [DOI] [PubMed] [Google Scholar]
- 110.Vaillant C. Meissirel C. Mutin M. Belin M. F. Lund L. R. Thomasset N. MMP-9 deficiency affects axonal outgrowth, migration, and apoptosis in the developing cerebellum. Mol. Cell Neurosci. (2003);24:395–408. doi: 10.1016/S1044-7431(03)00196-9. [DOI] [PubMed] [Google Scholar]
- 111.Van den Steen P. E. Van Aelst I. Hvidberg V. Piccard H. Fiten P. Jacobsen C. Moestrup S. K. Fry S. Royle L. Wormald M. R. Wallis R. Rudd P. M. Dwek R. A. Opdenakker G. The hemopexin and O-glycosylated domains tune gelatinase B/MMP-9 bioavailability via inhibition and binding to cargo receptors. J. Biol. Chem. (2006);281:18626–18637. doi: 10.1074/jbc.M512308200. [DOI] [PubMed] [Google Scholar]
- 112.Van Lint P. Libert C. Chemokine and cytokine processing by matrix metalloprteinases and its effect on leukocyte migrationand inflammation. J. Leukoc. Biol. (2007);82:1375–1381. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 113.Van Wart H. E. Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA. (1990);87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vincenti M. P. The matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) genes. (2001) Transcriptional and posttranscriptional regulation, signal transduction and celltype-specific expression. Methods Mol. Biol. (2001);151:121–148. doi: 10.1385/1-59259-046-2:121. [DOI] [PubMed] [Google Scholar]
- 115.Vincenti M. P. Brinckerhoff C. E. Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J. Cell Physiol. (2007);213:355–364. doi: 10.1002/jcp.21208. [DOI] [PubMed] [Google Scholar]
- 116.Walker E. J. Rosenberg G. A. Divergent role for MMP-2 in myelin breakdown and oligodendrocyte death following transient global ischemia. J. Neurosci. Res. (2010);88:764–773. doi: 10.1002/jnr.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang W. Sawicki G. Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc. Res. (2002a);53:165–174. doi: 10.1016/S0008-6363(01)00445-X. [DOI] [PubMed] [Google Scholar]
- 118.Wang W. Schulze C. J. Suarez-Pinzon W. L. Dyck J. R. Sawicki G. Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. (2002b);106:1543–1549. doi: 10.1161/01.CIR.0000028818.33488.7B. [DOI] [PubMed] [Google Scholar]
- 119.Waubant E. Goodkin D. E. Gee L. Bacchetti P. Sloan R. Stewart T. Andersson P. B. Stabler G. Miller K. Serum MMP-9 and TIMP-1 levels are related to MRI activity in relapsing multiple sclerosis. Neurology. (1999);53:1397–1401. doi: 10.1212/wnl.53.7.1397. [DOI] [PubMed] [Google Scholar]
- 120.Wenk G. L. Neuropathologic changes in Alzheimer's disease. J. Clin. Psychiatry. (2003);64(Suppl 9):7–10. [PubMed] [Google Scholar]
- 121.Werb Z. Reynolds J. J. Stimulation by endocytosis of the secretion of collagenase and neutral proteinase from rabbit synovial fibroblasts. J. Exp. Med. (1974);140:1482–1497. doi: 10.1084/jem.140.6.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Woo M. S. Park J. S. Choi I. Y. Kim W. K. Kim H. S. Inhibition of MMP-3 or -9 suppresses lipopolysaccharide-induced expression of proinflammatory cytokines and iNOS in microglia. J. Neurochem. (2008);106:770–780. doi: 10.1111/j.1471-4159.2008.05430.x. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto M. Hirayama R. Naruse K. Yoshino K. Shimada A. Inoue S. Kayagaki N. Yagita H. Okumura K. Ikeda S. Structure-activity relationship of hydroxamate-based inhibitors on membrane-bound Fas ligand and TNF-alpha processing. Drug Des. Discov. (1999);16:119–130. [PubMed] [Google Scholar]
- 124.Yamamoto M. Mohanam S. Sawaya R. Fuller G. N. Seiki M. Sato H. Gokaslan Z. L. Liotta L. A. Nicolson G. L. Rao J. S. Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase A activation in human malignant brain tumors in vivo and in vitro. Cancer Res. (1996);56:384–392. [PubMed] [Google Scholar]
- 125.Yan C. Boyd D. D. Regulation of matrix metalloproteinase gene expression. J. Cell Physiol. (2007);211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]
- 126.Yan P. Hu X. Song H. Yin K. Bateman R. J. Cirrito J. R. Xiao Q. Hsu F. F. Turk J. W. Xu J. Hsu C. Y. Holtzman D. M. Lee J. M. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J. Biol. Chem. (2006);281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- 127.Yan W. Zhang W. Sun L. Liu Y. You G. Wang Y. Kang C. You Y. Jiang T. Identifi cation of MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. Brain Res. (2011);1411:108–115. doi: 10.1016/j.brainres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 128.Yang Y. Candelario-Jalil E. Thompson J. F. Cuadrado E. Estrada E. Y. Rosell A. Montaner J. Rosenberg G. A. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J. Neurochem. (2010);112:134–149. doi: 10.1111/j.1471-4159.2009.06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang Y. Estrada E. Y. Thompson J. F. Liu W. Rosenberg G. A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. (2007);27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 130.Ye H. Cai P. C. Zhou Q. Ma W. L. Transforming growth factor-β1 suppresses the up-regulation of matrix metalloproteinase-2 by lung fibroblasts in response to tumor necrosis factor-α. Wound Repair Regen. (2011);19:392–399. doi: 10.1111/j.1524-475X.2011.00680.x. [DOI] [PubMed] [Google Scholar]
- 131.Yong V. W. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat. Rev. Neurosci. (2005);6:931–944. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- 132.Yong V. W. Krekoski C. A. Forsyth P. A. Bell R. Edwards D. R. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. (1998);21:75–80. doi: 10.1016/S0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- 133.Yong V. W. Zabad R. K. Agrawal S. Goncalves Dasilva A. Metz L. M. Elevation of matrix metalloproteinases (MMPs) in multiple sclerosis and impact of immunomodulators. J. Neurol. Sci. (2007);259:79–84. doi: 10.1016/j.jns.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 134.Yoshida D. Watanabe K. Takahashi H. Sugisaki Y. Teramoto A. Apoptotic induction by BE16627B on human malignant glioma cell lines by an anti-matrix metalloproteinase agent. Brain Tumor Pathol. (2003);20:13–19. doi: 10.1007/BF02478942. [DOI] [PubMed] [Google Scholar]
- 135.Yoshiyama Y. Asahina M. Hattori T. Selective distribution of matrix metalloproteinase-3 (MMP-3) in Alzheimer's disease brain. Acta. Neuropathol. (2000);99:91–95. doi: 10.1007/PL00007428. [DOI] [PubMed] [Google Scholar]
- 136.Yushchenko M. Weber F. Mäder M. Schöll U. Maliszewska M. Tumani H. Felgenhauer K. Beuche W. Matrix metalloproteinase-9 (MMP-9) in human cerebrospinal fluid (CSF): elevated levels are primarily related to CSF cell count. J. Neuroimmunol. (2000);110:244–251. doi: 10.1016/S0165-5728(00)00339-8. [DOI] [PubMed] [Google Scholar]
- 137.Zucker S. Lysik R. M. Zarrabi M. H. Greenwald R. A. Gruber B. Tickle S. P. Baker T. S. Docherty A. J. Elevated plasma stromelysin levels in arthritis. J. Rheumatol. (1994);21:2329–2333. [PubMed] [Google Scholar]


