Abstract
Docosahexaenoic acid (DHA) is the major polyunsaturated fatty acid (PUFA) in the brain and a structural component of neuronal membranes. Changes in DHA content of neuronal membranes lead to functional changes in the activity of receptors and other proteins which might be associated with synaptic function. Accumulating evidence suggests the beneficial effects of dietary DHA supplementation on neurotransmission. This article reviews the beneficial effects of DHA on the brain; uptake, incorporation and release of DHA at synapses, effects of DHA on synapses, effects of DHA on neurotransmitters, DHA metabolites, and changes in DHA with age. Further studies to better understand the metabolome of DHA could result in more effective use of this molecule for treatment of neurodegenerative or neuropsychiatric diseases.
Keywords: Docosahexaenoic acid (DHA), Polyunsaturated fatty acid (PUFA), Neurodegeneration, Depression, Anti-nociception
BENEFICIAL EFFECTS OF DOCOSAHEXAENOIC ACID ON THE BRAIN
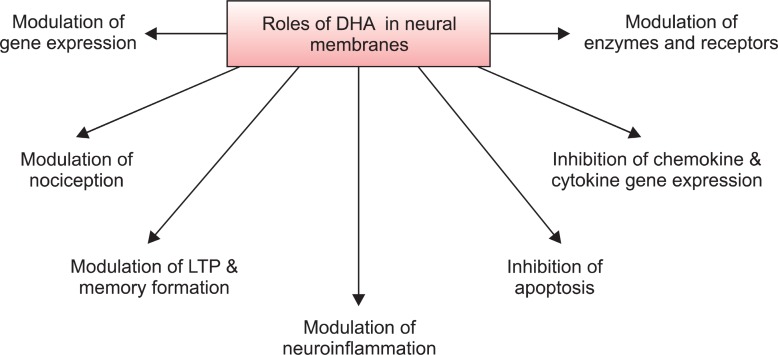
Docosahexaenoic acid (DHA) is an important component of neural membranes and is present in 30-40% of the phospholipids in the gray matter of cerebral cortex and photoreceptor cells in the retina. It mediates its molecular and cellular effects not only through regulation of physicochemical properties such as membrane fluidity, permeability and viscosity in synaptic membranes, but also via modulation of neurotransmission, gene expression, and activities of enzymes, receptors and ion channels (Fig.1 ) (Farooqui, 2009). These processes are closely associated with activation of signaling pathways that sustain synaptic function and neuronal survival (Oster and Pillot, 2010).
Fig. 1. Roles of DHA in the brain.
PUFAs including DHA have anti-oxidative stress, anti-inflammation and anti-apoptosis effects leading to neuroprotection in aged, damaged or Alzheimer’s disease brain (Fig.1 ) (Farooqui et al., 2007; Farooqui, 2009). Anti-oxidant and anti-inflammatory actions of DHA are associated with reduction in cellular levels of reactive species, pro-inflammatory mediators and nitrite levels, maintaining higher GSH levels and increasing anti-oxidant enzyme activities (Kim and Chung, 2007). DHA attenuates brain necrosis after hypoxic ischemic injury by modulating membrane biophysical properties and maintaining integrity in functions between presynaptic and postsynaptic areas, resulting in better stabilization of intracellular ions after hypoxic-ischemic insult (Mayurasakorn et al.., 2011). DHA also has a neuroprotective effect on glutamate-induced cytotoxicity in rat hippocampal cultures by inhibiting nitric oxide production and calcium influx, and increasing the activities of anti-oxidant enzymes glutathione peroxidase (GSH-Px) and glutathione reductase (Wang et al., 2003).
Many studies connect dietary consumption of DHA with antidepression. Areas with high consumption of seafood which is enriched in PUFAs such as DHA, have lower rates of bipolar and unipolar depression, post-partum depression and seasonal affective disorder compared to those where people consume less seafood (Logan, 2004). DHA interacts with transient receptor potential cation channel V1 (TRPV1), an ion channel expressed in nociceptive neurons and brain (Matta et al., 2007), and has an anti-nociceptive effect on pain from thermal or chemical stimulation (Nakamoto et al., 2010).
UPTAKE, INCORPORATION AND RELEASE OF DHA AT SYNAPSES
Uptake of DHA in the diet
DHA cannot be synthesized by the human body and must
be taken in the diet. Common sources of n-3 PUFA include fish oils and some plant oils such as flaxseed oil and algal oil, which contain precursors of DHA, namely alpha-linolenic acid (α-LNA). After uptake, n-3 PUFAs enter the brain and are incorporated at the sn-2 position of glycerol moiety of phospholipids including plasmalogens and phosphatidylserine. DHA is released through the action of plasmalogen-selective phospholipase A2 (PlsEtn-PLA2) and calcium-independent phospholipase A2 (iPLA2) (Farooqui et al., 2003; Strokin et al., 2003; Rosa and Rapoport, 2009; Farooqui, 2010a). Dietary supplementation with 10 and 40 mg/kg/day of DHA for 30 days results in significantly increased DHA serum levels of 123 and 175% over baseline respectively (Bailes and Mills, 2010). DHA concentrations of 17.6, 11.4, and 7.14 μmol/g are found in the brains of rats on diets with high DHA and α-LNA content, an n-3 PUFA adequate diet containing 4.6% α-LNA but no DHA,or an n-3 PUFA deficient diet containing 0.2% α-LNA and no DHA, respectively (Rapoport and Igarashi, 2009).
Incorporation of DHA at synapses
The transport of fatty acids such as DHA and arachidonic acid (AA) across the blood-brain barrier and other non-neural cellular membranes most likely occurs through passive diffusion. DHA and AA transport is facilitated by a number of membrane-associated and cytoplasmic proteins. These include membrane proteins fatty acid translocase (FAT/CD36), plasma membrane fatty acid-binding protein (FABP) and fatty acid-transport protein (FATP) (Utsunomiya et al., 1997). FABP binds to a broad range of saturated and unsaturated long chain free fatty acids, including DHA, eicosapentaenoic acid (EPA), and AA. Rat pheochromocytoma (PC12) cells exposed to nerve growth factor exhibit high FABP expression (Liu et al., 2008).
DHA is a major structural component of gray matter neuronal membranes. At the subcellular level, the highest concentration of DHA is found in synaptic membranes followed by mitochondria and microsomes (Scott and Bazan, 1989). DHA-containing ethanolamine phosphoglyceride species accumulate in response to n-3 fatty acid feeding, suggesting a causal relationship between two events (Kitajka et al., 2002). The developing brain or hippocampal neurons take up DHA and incorporates it into membrane phospholipids especially phosphatidylethanolamine, resulting in neurite outgrowth, synaptogenesis and neurogenesis. Exposure to n-3 PUFAs enhances synaptic plasticity by increasing synaptic protein expression, dendritic spine density, long-term potentiation and neurogenesis in the hippocampus for learning and memory processing (Fig.1 ).
DHA is mainly found in ethanolamine plasmalogen (PlsEtn) and phosphatidylserine (PtdSer), whereas the majority of AA is incorporated into phosphatidylcholine (PtdCho). From PlsEtn and PtdSer, DHA is released by the action of PlsEtn-PLA2 and PtdSer hydrolyzing PLA2, respectively (Farooqui, 2009; Farooqui, 2010a). DHA is necessary for the proper formation of phospholipids which are an essential component of the cell membrane. Most phospholipids contain glycerol esterified with saturated and unsaturated fatty acids at sn-1 and sn-2 position respectively. Brain phospholipid synthesis requires three circulating compounds: fatty acids (DHA or AA), uridine and choline (Wurtman et al., 2010). Animals given all three nutrients develop increased levels of brain phosphatides and proteins that are concentrated in synaptic membranes (e.g. PSD-95, synapsin-1), improved cognition and enhanced neurotransmitter release. These nutrients work by increasing the substrate-saturation of low-affinity enzymes that synthesize the phosphatides (Wurtman et al., 2009a). Nutritional studies indicate that the phospholipid content of synaptic plasma membrane is significantly increased in DHA-fed rats (Shahdat et al., 2004).
Release of DHA at synapses
PlsEtn-PLA2 releases DHA from PlsEtn (Farooqui et al., 2003; Farooqui, 2010a). In addition, iPLA2 releases DHA from the sn-2 position of glycerol moiety with the generation of lysophospholipid. Cellular studies suggest that AA and DHA release depend on Ca2+. The source of Ca2+ for activation of cPLA2 is largely extracellular, whereas Ca2+ released from the endoplasmic reticulum can activate iPLA2 by a number of mechanisms (Rosa and Rapoport, 2009). Imaging mass spectrometry shows not only a prominent increase of DHA-containing PtdCho in the gray matter but also insufficient membrane remodeling in iPLA2β knockout mice, a model of infantile neuroaxonal dystrophy (Beck et al., 2011). The released DHA is metabolized to resolvins and neuroprotectins by 15-lipoxygenase (Bazan, 2005). These mediators are collectively known as docosanoids. They have anti-inflammatory and anti-apoptotic properties and are involved in neuroprotection. In addition, docosanoids have anti-thrombotic, anti-arrhythmic, hypolipidemic, and vasodilatory effects (Farooqui, 2009).
EFFECTS OF DHA ON SYNAPSES
Neurite outgrowth
Treatment of PC12 cells for 3 days with n-3 PUFA significantly enhances neurite length without affecting the number of neurites (Shrivastava et al., 2005). DHA also induces neurite outgrowth in cells beginning as early as 2 h post-DHA supplementation and throughout differentiation. Transcripts of the neurogenesis markers Egr3 and PC3 are significantly elevated within 0.5 to 1 h of DHA supplementation (Dagai et al., 2009). DHA promotes differentiation and neurite outgrowth of neurons derived from mouse embryonic stem cells and increases proliferation of cells undergoing differentiation into neuronal lineages (He et al., 2009). Conversely, hippocampal neurons from DHA-depleted fetuses show reduced neurite growth and synaptogenesis (Cao et al., 2009). DHA is one of the most potent inducers of neurite outgrowth among fatty acids surveyed, on expression of a marker of axonal growth, growth-associated protein-43 (GAP-43). Exposure of human neuroblastoma SH-SY5Y cells to DHA increases the percentage of cells with longer neurites, associated with protein tyrosine phosphatase inhibition, mitogen-activated protein kinase/ERK kinase phosphorylation and sequentially ERK1/2 phosphorylation. In contrast, no effect was found after supplementation with AA, oleic acid or docosapentaenoic acid (Calderon and Kim, 2004; Wu et al., 2009).
Oral supplementation with DHA increases number of dendritic spines in the adult gerbil hippocampus, particularly when animals are co-supplemented with a uridine source, uridine-5'-monophosphate, which increases brain levels of the rate-limiting phosphatide precursor cytidine triphosphate (Sakamoto et al., 2007). Uridine and DHA supplementation in rodents also enhances dendritic spine levels and cognitive functions. Moroever, DHA treatment increases the expression of biochemical markers for neurites, neurofilament-M and neurofilament-70 (Wurtman et al., 2009b).
SNARE complex and other synaptic proteins
DHA facilitates the formation of v-SNARE/t-SNARE complex, necessary for fusion of synaptic vesicles and plasma membranes and neurite outgrowth-dependent plasticity (Walczewska et al., 2011). DHA affects the expression of the SNARE complex protein syntaxin-3 (Sharma et al., 2010). Treatment with DHA increases co-localization and interaction of SNAP-25 and syntaxin-3, and delivery of membrane and synaptic membrane biogenesis (Mazelova et al., 2003). Voluntary exercise potentiates the effects of a 12-day DHA dietary supplementation regimen on increasing the levels of syntaxin-3 and GAP-43 in the adult rat hippocampus (Chytrova et al., 2010).
Increased DHA (34%) and decreased AA (-23%) in brain fatty acid concentrations are detected after consumption of DHA-enriched diet. Compared to the n-3 PUFA-deficient diet, consumption of DHA results in increased spontaneous excitatory postsynaptic current frequency (Arsenault et al., 2011). Deficiency in DHA leads to reduced levels of the NR2B subunit of the NMDA receptor in rats (Sharma et al., 2010). Reduction of dietary n-3 PUFA in an Alzheimer’s mouse model results in 80-90% losses of the p85alpha subunit of phosphatidylinositol 3-kinase and the postsynaptic actin-regulating protein drebrin. The loss of postsynaptic proteins is associated with increased oxidation, without concomitant loss of neurons or presynaptic protein (Calon et al., 2004).
G-protein coupled receptor 40 (GPR40) is expressed ubiquitously in the human brain and pancreas, and is a seven-transmembrane receptor which is activated by DHA. DHA-induced neuronal differentiation, neurite growth and branching of adult rat stem cells are enhanced by increased GPR40 expression, suggesting that the DHA/GPR40 signaling pathway is related to adult neurogenesis in the hippocampus (Ma et al., 2010).
EFFECTS OF DHA ON NEUROTRANSMITTERS
Glutamate
DHA enhances glutamatergic synaptic activities with concomitant increases in synapsin and glutamate receptor subunit expression in hippocampal neurons (Kim et al., 2011b). Spontaneous synaptic activity is significantly increased due to enhanced glutamatergic activity in DHA-supplemented neurons. On the other hand, lack of DHA results in inhibition of synaptogenesis, decreases in synapsins and glutamate receptor subunits, and impairment of long-term potentiation in hippocampal neurons (Cao et al., 2009). DHA modulates the activities of glutamate transporters (GLT1, GLAST, and EAAC1). DHA stimulates GLT1 and EAAC1 through a mechanism that requires extracellular Ca2+, CaM kinase II, and protein kinase C but not protein kinase A. In contrast, the inhibitory effect of DHA on GLAST does not require extracellular Ca2+ and does not involve CaM kinase II (Berry et al., 2005; Farooqui, 2010b). DHA may also reduce uptake of the neurotransmitter d-[3H] aspartate by cultured astrocytes and cortical membrane suspensions, while AA does not. This effect is also found in astrocytes enriched with the anti-oxidant, α-tocopherol, suggesting that it is not due to oxidation products of DHA. Reduction of d-[3H] aspartate uptake by DHA does not involve any change in the expression of membrane-associated astroglial glutamate transporters (GLAST and GLT-1), indicating that DHA reduces the activities of the transporters. In contrast to inhibition induced by free-DHA, membrane-bound DHA has no effect on d-[3H] aspartate uptake (Grintal et al., 2009).
GABA
DHA inhibits GABA receptor-mediated responses in cultured neural cells in a concentration and time-dependent manner. It is shown that the γ subunit is essential for the potentiation of GABA induced Cl- channel activity and effect on desensitization kinetics of the GABAA receptor (Nabekura et al., 1998). DHA accelerates desensitization after the peak of the GABA-induced current, potentiates the peak amplitude of GABA response, and gradually suppresses the peak amplitude of GABA response (Nabekura et al., 1998). It is suggested that the effect of DHA on GABA receptor is due to the effect on the lipid microenvironment for the GABA receptors (Nabekura et al., 1998).
Dopamine
Chronic n-3 PUFA deficiency alters the internalization of dopamine (DA) in the storage pool of the frontal cortex (Zimmer et al., 1998). Diet-induced deficits in brain DHA lead to redistribution of DA vesicles in presynaptic terminals, greater basal extracellular DA concentrations and deficits in tyramine-induced elevations in extracellular DA concentrations in the prefrontal cortex and ventral striatum (Chalon, 2006). Moreover, mice on a DHA deficient diet show augmented amphetamine-induced locomotor sensitization, and this response is associated with alterations in the mesolimbic DA pathway (McNamara et al.,2008). These observations suggest that alterations in synaptic membrane DHA have an impact on DA synaptic neurotransmission and plasticity (Farooqui, 2010b).
Serotonin
N-3 PUFA deficiency results in changes in the vesicular pool of serotonin (5-HT) as well as DA, thus inducing several regulatory processes such as modification of cerebral receptors in specific brain areas (Chalon, 2006). Chronic n-3 PUFA dietary deficiency induces changes in synaptic levels of 5-HT under basal conditions and after stimulation with fenfluramine. Higher levels of basal 5-HT release and lower levels of fenfluramine stimulated 5-HT release are found in PUFA-deficient than control rats. These neurochemical modifications can be reversed by supply of balanced diet at birth or during the first 2 weeks of life, through maternal milk – however, they persist if the balanced diet is given after weaning (Kodas et al., 2004).
Noradrenaline
Both a high membrane DHA content and free DHA in the medium enhance the release of [3H]-noradrenaline in cultured SH-SY5Y cells (Mathieu et al., 2010).
Acetylcholine
Plasma [3H] DHA incorporation into synaptic membrane phospholipids of the rat brain is selectively increased after cholinergic activation (Jones et al., 1997). DHA deficiency produces a 10% decrease in muscarinic receptor binding, although acetylcholinesterase activity and the vesicular acetylcholine transporter are not affected (Aïd et al., 2003).
Endocannabinoids
The CB1 receptor which responds to endocannabinoids are uncoupled from their effector G (i/o) proteins in n-3 PUFA deficient mice (Lafourcade et al., 2011).
Other molecules
DHA supplementation elevates brain DHA content, normalizes levels of brain-derived neurotrophic factor (BDNF), synapsin I, cAMP responsive element-binding protein (CREB), and CaMKII and improves learning ability in rats after traumatic brain injury (Wu et al., 2011). DHA deficit induces down-regulation of brain glucose transporter protein expression, resulting in decreased glucose utilization in the cerebral cortex of DHA-deficient rats (Pifferi et al., 2005). DHA also interacts with the peroxisome proliferator activated receptors (PPAR), sterol regulatory element binding protein (SREBP), and carbohydrate response element binding protein (ChREBP) (de Urquiza et al., 2000; Lengqvist et al., 2004). These influence the activities of signal transduction processes during neurotransmission.
DHA limits amyloid- or oxidative stress-induced damage and cognitive deficits in an amyloid precursor protein (APP) transgenic mouse model of Alzheimer’s disease (Cole et al., 2005).
In addition, levels of the dendritic protein, drebrin are significantly increased in the hippocampus of APP transgenic mice on a DHA-rich diet. These findings support the notion that increased DHA consumption may play a role in reducing brain insults in Alzheimer’s disease patients (Perez et al., 2010).
DHA metabolites
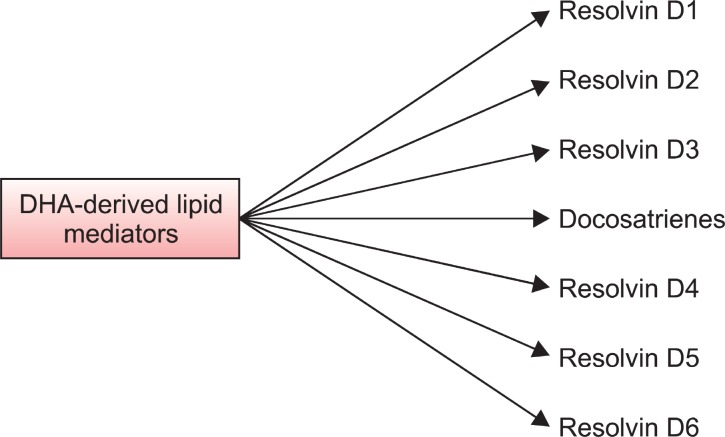
DHA metabolism to DEA leads to neurite growth, synaptogenesis, synaptic protein expression, and enhanced glutamatergic synaptic function (Kim et al., 2011a). DHA is converted to DEA by fetal mouse hippocampal neuronal cultures and hippocampal homogenates, and DEA is present endogenously in the mouse hippocampus. Furthermore, DEA stimulates neurite growth and synaptogenesis at substantially lower concentrations than DHA, and it enhances glutamatergic synaptic activities with concomitant increases in synapsin and glutamate receptor subunit expression in the hippocampal neurons (Kim et al., 2011b). The actions of DHA may also mediated by its metabolites, resolvins D1, D2, D3, D4, D5, D6, neuroprotectin D1 (NPD1), and N-docosahexaenoylethanolamide (DEA) (Fig.2 ). The resolvins D1-D6 induce anti-inflammatory activity. They reduce neutrophil infiltration; paw edema and proinflammatory cytokine expression. Similarly, NPD1 elicits potent anti-inflammatory actions and prohomeostatic bioactivity. It also produces anti-angiogenic effects and induces cell survival (Bazan et al., 2011). Calcium ionophore A-23187-induced synthesis of NPD1 in human ARPE-19 cells is enhanced by DHA. Under these conditions, there is a time-dependent release of endogenous free DHA followed by NPD1 formation. NPD1 potently counteracts H2O2/tumor necrosis factor alpha oxidative-stress-triggered apoptotic DNA damage (Mukherjee et al., 2004).
Fig. 2. DHA-derived lipid mediators in the brain. Enzymatic oxidation of DHA by 15-lipoxygenase-like enzyme followed by epoxidation and hydrolysis results in formation of resolvins D1-D6 and docosatrienes (neuroprotectin D1). By inhibiting the generation of eicosanoids, DHA-derived metabolites block neuroinflammation, prevent apoptosis, and suppress oxidative stress.
Changes in DHA with age
Aging not only reduces levels of n-3 PUFAs but also decreases neural membrane plasticity. Decline in n-3 PUFAs with age is accompanied by loss of learning and memory and ageing is also associated with decreases in the GluR2 and NR2B subunits of glutamate receptors in the prefrontal cortex, hippocampus and striatum. These decreases can be fully reversed by n-3 PUFA supplementation (Dyall et al., 2007). n-3 PUFA supplementation reverses age-related changes and maintains learning and memory performance in aged rats (Farooqui et al., 2007; Su, 2010). Multiple mechanisms of action can be associated with the neuroprotective effects of DHA, including anti-oxidant properties and activation of distinct cell signaling pathways. Dietary supplementation of DHA normalizes the age-related decrease in unsaturation index, reduces the levels of AA-containing phospholipids in both young and aged animals, and gives rise to production of new phosphatidylserine and phosphatidylinositol species (Little et al., 2007).
Further studies to better understand the metabolome of DHA could result in more effective use of this molecule for treatment of neurodegenerative or neuropsychiatric diseases.
Acknowledgments
The authors extend their appreciation to Deanship of Scientific Research at King Saud University for funding this work through the Visiting Professorship Program to WYO.
References
- 1.Aïd S. Vancassel S. Poumès-Ballihaut C. Chalon S. Guesnet P. Lavialle M. Effect of a diet-induced n-3 PUFA depletion on cholinergic parameters in the rat hippocampus. J. Lipid. Res. (2003);44:1545–1551. doi: 10.1194/jlr.M300079-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Arsenault. D. Julien C. Calon F. Chronic dietary intake of α-linolenic acid does not replicate the effects of DHA on passive properties of entorhinal cortex neurons. Br. J. Nutr. (2011);19:1–13. doi: 10.1017/S0007114511004089. [DOI] [PubMed] [Google Scholar]
- 3.Bailes J. E. Mills J. D. Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J. Neurotrauma. (2010);27:1617–1624. doi: 10.1089/neu.2009.1239. [DOI] [PubMed] [Google Scholar]
- 4.Bazan N. G. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. (2005);15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazan N. G. Molina M. F. Gordon W. C. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuro-inflammation, macular degeneration, Alzheimer's, and other neuro-degenerative diseases. Annu. Rev. Nutr. (2011);31:321–351. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck G. Sugiura Y. Shinzawa K. Kato S. Setou M. Tsujimoto Y. Sakoda S. Sumi-Akamaru H. Neuroaxonal dystrophy in calcium-independent phospholipase A2β deficiency results from insufficient remodeling and degeneration of mitochondrial and presynaptic membranes. J. Neurosci. (2011);31:11411–11420. doi: 10.1523/JNEUROSCI.0345-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry C. B. Hayes D. Murphy A. Wiessner M. Rauen T. McBean G. J. Differential modulation of the glutamate transporters GLT1, GLAST and EAAC1 by docosahexaenoic acid. Brain Res. (2005);1037:123–133. doi: 10.1016/j.brainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Calderon F. Kim H. Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. (2004);90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 9.Calon F. Lim G. P. Yang F. Morihara T. Teter B. Ubeda O. Rostaing P. Triller A. Salem N. Jr. Ashe K. H. Frautschy S. A. Cole G. M. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. (2004);43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao D. Kevala K. Kim J. Moon H. S. Jun S. B. Lovinger D. Kim H. Y. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J. Neurochem. (2009);111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins. Leukot. Essent. Fatty Acids. (2006);75:259–269. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Chytrova G. Ying Z. Gomez-Pinilla F. Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain Res. (2010);1341:32–40. doi: 10.1016/j.brainres.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole G. M. Lim G. P. Yang F. Teter B. Begum A. Ma Q. Harris-White M. E. Frautschy S. A. Prevention of Alzheimer's disease: Omega-3 fatty acid and phenolic anti-oxidant interventions. Neurobiol. Aging. (2005);26(Suppl 1):133–136. doi: 10.1016/j.neurobiolaging.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Dagai L. Peri-Naor R. Birk R. Z. Docosahexaenoic acid significantly stimulates immediate early response genes and neurite outgrowth. Neurochem. Res. (2009);34:867–875. doi: 10.1007/s11064-008-9845-z. [DOI] [PubMed] [Google Scholar]
- 15.de Urquiza A. M. Liu S. Sjöberg M. Zetterström R. H. Griffiths W. Sjövall J. Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. (2000);290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 16.Dyall S. C. Michael G. J. Whelpton R. Scott A. G. Michael-Titus A. T. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol. Aging. (2007);28:424–439. doi: 10.1016/j.neurobiolaging.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Farooqui A. A. Bebefi cial Effects of Fish Oil on Human Brain. Springer; New York.: (2009). [Google Scholar]
- 18.Farooqui A. A. Purification, properties and role of plasmalogen-selective PLA2 from pig brain. Mol. Neurobiol. (2010a);41:267–273. doi: 10.1007/s12035-009-8091-y. [DOI] [PubMed] [Google Scholar]
- 19.Farooqui A. A. Modulation of neurotransmission signaling by neural membrane polyunsaturated fatty acids in Biogenic Amines: Pharmacological, Neurochemical, and Molecular Aspects in CNS. Nova Science Publishers Inc; Hauppauge New York.: (2010b). pp. 219–246. [Google Scholar]
- 20.Farooqui A. A. Horrocks L. A. Farooqui T. Modulation of inflammation in brain: a matter of fat. J. Neurochem. (2007);101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 21.Farooqui A. A. Ong W. Y. Horrocks L. A. Plasmalogens, docosahexaenoic acid and neurological disorders. Adv. Exp. Med. Biol. (2003);544:335–354. doi: 10.1007/978-1-4419-9072-3_45. [DOI] [PubMed] [Google Scholar]
- 22.Grintal B. Champeil-Potokar G. Lavialle M. Vancassel S. Breton S. Denis I. Inhibition of astroglial glutamate transport by polyunsaturated fatty acids: evidence for a signalling role of docosahexaenoic acid. Neurochem. Int. (2009);54:535–543. doi: 10.1016/j.neuint.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 23.He C. Qu X. Cui L. Wang J. Kang J. X. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. USA. (2009);106:11370–11375. doi: 10.1073/pnas.0904835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones C. R. Arai T. Rapoportm S I. Evidence for the involvement of docosahexaenoic acid in cholinergic stimulated signal transduction at the synapse. Neurochem. Res. (1997);22:663–670. doi: 10.1023/A:1027341707837. [DOI] [PubMed] [Google Scholar]
- 25.Kim H. Y. Moon H. S. Cao D. Lee J. Kevala K. Jun S. B. Lovinger D. M. Akbar M. Huang B. X. N-Docosa-hexaenoylethanolamide promotes development of hippocampal neurons. Biochem. J. (2011a);435:327–336. doi: 10.1042/BJ20102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H. Y. Spector A. A. Xiong Z. M. A synaptogenic amide N-docosahexaenoylethanolamide promotes hippocampal development. Prostaglandins. Other. Lipid. Mediat. (2011b);96:114–120. doi: 10.1016/j.prostaglandins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y. J. Chung H. Y. Antioxidative and anti-inflammatory actions of docosahexaenoic acid and eicosapentaenoic acid in renal epithelial cells and macrophages. J. Med. Food. (2007);10:225–231. doi: 10.1089/jmf.2006.092. [DOI] [PubMed] [Google Scholar]
- 28.Kitajka K. Puskás L. G. Zvara A. Hackler L. Jr. Barceló-Coblijn G. Yeo Y. K. Farkas T. The role of n-3 polyunsaturated fatty acids in brain: modulation of rat brain gene expression by dietary n-3 fatty acids. Proc. Natl. Acad. Sci. USA. (2002);99:2619–2624. doi: 10.1073/pnas.042698699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kodas E. Galineau L. Bodard S. Vancassel S. Guilloteau D. Besnard J. C. Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J. Neurochem. (2004);89:695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- 30.Lafourcade M. Larrieu T. Mato S. Duffaud A. Sepers M. Matias I. De Smedt-Peyrusse V. Labrousse V. F. Bretillon L. Matute C. Rodriguez-Puertas R. Layé S. Manzoni O. J. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. (2011);14:345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- 31.Lengqvist J. Mata De Urquiza A. Bergman A. C. Willson T. M. Sjövall J. Perlmann T. Griffiths W. J. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol. Cell. Proteomics. (2004);3:692–703. doi: 10.1074/mcp.M400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Little S. J. Lynch M. A. Manku M. Nicolaou A. Docosahexaenoic acid-induced changes in phospholipids in cortex of young and aged rats: a lipidomic analysis. Prostaglandins. Leukot. Essent. Fatty. Acids. (2007);77:155–162. doi: 10.1016/j.plefa.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Liu J. W. Almaguel F. G. Bu L. De Leon D. D. De Leon M. Expression of E-FABP in PC12 cells increases neurite extension during differentiation: involvement of n-3 and n-6 fatty acids. J. Neurochem. (2008);106:2015–2029. doi: 10.1111/j.1471-4159.2008.05507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Logan A. C. Omega-3 fatty acids and major depression: a primer for the mental health professional. Lipids. Health Dis. (2004);3:25. doi: 10.1186/1476-511X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma D. Zhang M. Larsen C. P. Xu F. Hua W. Yamashima T. Mao Y. Zhou L. DHA promotes the neuronal differentiation of rat neural stem cells transfected with GPR40 gene. Brain Res. (2010);1330:1–8. doi: 10.1016/j.brainres.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Mathieu G. Denis S. Langelier B. Denis I. Lavialle M. Vancassel S. DHA enhances the noradrenaline release by SH-SY5Y cells. Neurochem. Int. (2010);56:94–100. doi: 10.1016/j.neuint.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Matta J. A. Miyares R. L. Ahern G. P. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J. Physiol. (2007);578:397–411. doi: 10.1113/jphysiol.2006.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayurasakorn. K. Williams J. J. Ten V. S. Deckelbaum R. J. Docosahexaenoic acid: brain accretion and roles in neuroprotection after brain hypoxia and ischemia. Curr. Opin. Clin. Nutr. Metab. Care. (2011);14:158–167. doi: 10.1113/jphysiol.2006.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazelova J. Ransom N. Astuto-Gribble L. Wilson M. C. omeg D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J. Cell Sci. (2003);122:2003–2013. doi: 10.1113/jphysiol.2006.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNamara R. K. Sullivan J. Richtand N. M. Jandacek R. Rider T. Tso P. Campbell N. Lipton J. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult DBA/2J mice: relationship with ventral striatum dopamine concentrations. Synapse. (2008);62:725–735. doi: 10.1002/syn.20542. [DOI] [PubMed] [Google Scholar]
- 41.Mukherjee P. K. Marcheselli V. L. Serhan C. N. Bazan N. G. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA. (2004);101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nabekura J. Noguchi K. Witt M. R. Nielsen M. Akaike N. Functional modulation of human recombinant gamma-aminobutyric acid type A receptor by docosahexaenoic acid. J. Biol. Chem. (1998);273:11056–11061. doi: 10.1074/jbc.273.18.11056. [DOI] [PubMed] [Google Scholar]
- 43.Nakamoto K. Nishinaka T. Mankura M. Fujita-Hamabe W. Tokuyama S. Antinociceptive effects of docosahexaenoic acid against various pain stimuli in mice. Biol. Pharm. Bull. (2010);33:1070–1072. doi: 10.1248/bpb.33.1070. [DOI] [PubMed] [Google Scholar]
- 44.Oster T. Pillot T. Docosahexaenoic acid and synaptic protection in Alzheimer's disease mice. Biochim. Biophys. Acta. (2010);1801:791–798. doi: 10.1016/j.bbalip.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Perez S. E. Berg B. M. Moore K .A. He B. Counts S. E. Fritz J. J. Hu Y. S. Lazarov O. Lah J. J. Mufson E. J. DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: possible gender effects. J. Neurosci. Res. (2010);88:1026–1040. doi: 10.1002/jnr.22266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pifferi F. Roux F. Langelier B. Alessandri J. M. Vancassel S. Jouin M. Lavialle M. Guesnet P. (n-3) polyunsaturated fatty acid deficiency reduces the expression of both isoforms of the brain glucose transporter GLUT1 in rats. J. Nutr. (2005);135:2241–2246. doi: 10.1093/jn/135.9.2241. [DOI] [PubMed] [Google Scholar]
- 47.Rapoport S. I. Igarashi M. Can the rat liver maintain normal brain DHA metabolism in the absence of dietary DHA? Prostaglandins. Leukot. Essent. Fatty. Acids. (2009);81:119–123. doi: 10.1016/j.plefa.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosa A. O. Rapoport S. I. Intracellular- and extracellular-derived Ca2+ influence phospholipase A(2)-mediated fatty acid release from brain phospholipids. Biochim. Biophys. Acta. (2009);1791:697–705. doi: 10.1016/j.bbalip.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakamoto T. Cansev M. Wurtman R. J. Oral supplementation with docosahexaenoic acid and uridine-5'-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. (2007);1182:50–59. doi: 10.1016/j.brainres.2007.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott B. L. Bazan N. G. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc. Natl. Acad. Sci. USA. (1989);86:2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shahdat H. Hashimoto M. Shimada T. Shido O. Synaptic plasma membrane-bound acetylcholinesterase activity is not affected by docosahexaenoic acid-induced decrease in membrane order. Life Sci. (2004);74:3009–3024. doi: 10.1073/pnas.86.8.2903. [DOI] [PubMed] [Google Scholar]
- 52.Sharma S. Ying Z. Gomez-Pinilla F. A pyrazole curcumin derivative restores membrane homeostasis disrupted after brain trauma. Exp. Neurol. (2010);226:191–199. doi: 10.1016/j.expneurol.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shrivastava R. Vincent B. Gobron S. Cucuat N. John G. W. Evidence for growth-promoting effects of omega n-3 fatty acids alone and in combination with a specific vitamin and mineral complex in rat neuroblastoma cells. Nutr. Neurosci. (2005);8:317–321. doi: 10.1080/10284150500510242. [DOI] [PubMed] [Google Scholar]
- 54.Strokin M. Sergeeva M. Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+. Br. J. Pharmacol. (2003);139:1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su H. M. Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance. J. Nutr. Biochem. (2010);21:364–373. doi: 10.1016/j.jnutbio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Utsunomiya A. Owada Y. Yoshimoto T. Kondo H. Localization of mRNA for fatty acid transport protein in developing and mature brain of rats. Brain Res. Mol. Brain Res. (1997);46:217–222. doi: 10.1016/S0169-328X(96)00303-8. [DOI] [PubMed] [Google Scholar]
- 57.Walczewska A. Stępień T. Bewicz-Binkowska D. Zgórzyńska E. The role of docosahexaenoic acid in neuronal function. Postepy. Hig. Med. Dosw (Online) (2011);65:314–327. doi: 10.5604/17322693.945763. [DOI] [PubMed] [Google Scholar]
- 58.Wang X. Zhao X. Mao Z. Y. Wang X. M. Liu Z. L. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. (2003);14:2457–2461. doi: 10.1097/00001756-200312190-00033. [DOI] [PubMed] [Google Scholar]
- 59.Wu A. Ying Z. Gomez-Pinilla F. The salutary effects of DHA dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J. Neurotrauma. (2011);28:2113–2122. doi: 10.1089/neu.2011.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu H. Ichikawa S. Tani C. Zhu B. Tada M. Shimoishi Y. Murata Y. Nakamura Y. Docosahexaenoic acid induces ERK1/2 activation and neuritogenesis via intracellular reactive oxygen species production in human neuroblastoma SH-SY5Y cells. Biochim. Biophys. Acta. (2009);1791:8–16. doi: 10.1016/j.bbalip.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Wurtman R. J. Cansev M. Sakamoto T. Ulus I. H. Use of phosphatide precursors to promote synaptogenesis. Annu. Rev. Nutr. (2009a);29:59–87. doi: 10.1146/annurev-nutr-080508-141059. [DOI] [PubMed] [Google Scholar]
- 62.Wurtman R. J. Cansev M. Ulus I. H. Synapse formation is enhanced by oral administration of uridine and DHA, the circulating precursors of brain phosphatides. J. Nutr. Health Aging. (2009b);13:189–197. doi: 10.1007/s12603-009-0056-3. [DOI] [PubMed] [Google Scholar]
- 63.Wurtman R. J. Cansev M. Sakamoto T. Ulus I. Nutritional modifiers of aging brain function: use of uridine and other phosphatide precursors to increase formation of brain synapses. Nutr. Rev. (2010);68(Suppl 2):S88–101. doi: 10.1111/j.1753-4887.2010.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmer L. Hembert S. Durand G. Breton P. Guilloteau D. Besnard J. C. Chalon S. Chronic n-3 polyunsaturated fatty acid diet-deficiency acts on dopamine metabolism in the rat frontal cortex: a microdialysis study. Neurosci. Lett. (1998);240:177–181. doi: 10.1016/S0304-3940(97)00938-5. [DOI] [PubMed] [Google Scholar]