Abstract
Tetrazolium violet is a tetrazolium salt and has been proposed as an antitumor agent. In this study, we reported for the first time that tetrazolium violet not only inhibited human lung cancer A549 cell proliferation but also induced apoptosis and blocked cell cycle progression in the G1 phase. The results showed that tetrazolium violet significantly decreased the viability of A549 cells at 5-15 μM. Tetrazolium violet -induced apoptosis in A549 cells was confirmed by H33258 staining assay. In A549, tetrazolium violet blocked the progression of the cell cycle at G1 phase by inducing p53 expression and further up-regulating p21/WAF1 expression. In addition, an enhancement in Fas/APO-1 and its two forms of ligands, membrane-bound Fas ligand (mFasL) and soluble Fas ligand (sFasL), as well as caspase, were responsible for the apoptotic effect induced by tetrazolium violet. The conclusion of this study is that tetrazolium violet induced p53 expression which caused cell cycle arrest and apoptosis. These findings suggest that tetrazolium violet has strong potential for development as an agent for treatment lung cancer.
Keywords: A549 cell apoptosis, Fas, Fas ligand, p21, p53, Tetrazolium violet
INTRODUCTION
Lung cancer is the leading cause of cancer death in the world. Non-small cell lung carcinoma (NSCLC) accounts for approximately 75-85% of lung cancers. Non-small cell lung cancers commonly develop resistance to radiation and chemotherapy, and they often present at stages beyond surgical respectability. Since current treatment modalities are inadequate, novel therapies are necessary to reduce the effects of the increasing incidence in pulmonary neoplasm (Cheng et al., 2003; Kim et al., 2003). Tetrazolium violet is a tetrazolium salt and has been proposed as an antitumor agent. Studies in our laboratory demonstrated that tetrazolium violet (TV) was effective in the inhibition of the growth of tumors of various kinds (Kong et al., 2003; Kong et al., 2004). In addition, tetrazolium violet induced apoptosis in Rat C6 glioma cells via a caspase-3-dependent mechanism (Zhao et al., 2006; Cai et al., 2009). However, the underlying mechanism of the action of tetrazolium violet in Human lung cancer cells has remained largely unknown.
Apoptosis, a mode of cell death, is a physiologic event that regulates cell numbers and eliminates damaged cells (Wang et al., 2005; Saretzki, 2010), and is characterized by a number of unique distinguishing features (Brüne, 2005). These events are the result of complex cellular biochemical pathways (Igney and Krammer, 2002; Saretzki, 2010). Emerging evidence has demonstrated that the anticancer activities of chemotherapeutic agents are involved in the induction of apoptosis, which is regarded as the preferred way to manage cancer (Brown and Wouters, 1999; Hengartner, 2000). Thus, manipulation of apoptotic pathways is a promising approach for the treatment of various cancers. Apoptosis and cell-cycle arrest are frequently regulated by the transcription factor p53, which is able to elicit the expression of cyclin-dependent kinase inhibitors, Fas/FasL, and Caspase.
In this study, we determined the antiproliferative activity of TV, and examined its effect on cell cycle distribution and apoptosis in the human lung cancer cell line, A549. Furthermore, to establish the anticancer mechanism of TV we assayed the levels of p53, p21/WAF1, Fas/FasL, and caspases, which are strongly associated with the signal transduction pathway of apoptosis and affect the chemosensitivity of tumor cells to anticancer agents.
MATERIALS AND METHODS
Chemicals
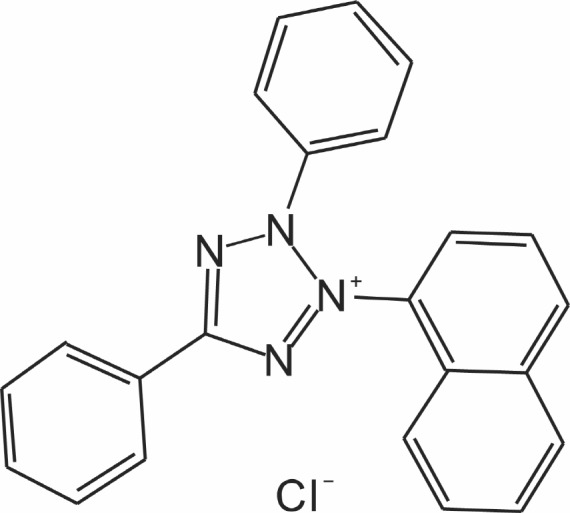
Tetrazolium violet (TV; Fig.1 ) was synthesized by us in our lab (Lanjin Biotech Co., Jinan, China), and was structurally confirmed. It was dissolved in PBS as stock solution. Unless otherwise stated, PBS was used as the control in all studies.
Fig. 1. The structural formulae of tetrazolium violet (TV).
Except for those indicated intentionally, all the materials were purchased from commercial sources, including: MTT, DMSO, propidium iodide, RNase A, and trypan (Sigma Aldrich Chemical Co., St. Louis, MO); BD ApoAlertTM Caspase-3, -8 Colorimetric Assay Kits and ApoAlert Caspase9/6 Fluorescence Assay Kits (Clontech Laboratories, Inc., Palo Alto, CA); PBS, HEPES, DMEM (Dulbecco’s modified Eagle’s medium), fetal bovine serum, and culture media (Life Technologies, Inc., Grand Island, NY); Bromodeoxyuridine (BrdU) Cell Proliferation Assay Kit (Oncogene Research, Cambridge, MA); Lactate Dehydrogenase (LDH) Kit (ZhongSheng Co., Beijing, China); p53 pan ELISA Kit (Roche Diagnostics GmbH, Germany); WAF1 ELISA, Fas ligand and Fas ELISA Kits (Calbiochem, Cambridge, MA).
Cell line and cell culture
Human lung cancer cell lines A549 were obtained from American Type Culture Collection (Manassas, VA). Cells were grown in RPMI 1640, supplemented with 10% fetal bovine serum, L-glutamine (2 mM), penicillin (100 units/ml), streptomycin(100 units/ml), and HEPES (25 mM). Normal human lung cells (Wi-38) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with the same supplements. All cells were maintained in the presence of 5% CO2 at 37℃.
Cell proliferation assay
Inhibition of cell proliferation by TV was measured by MTT assay. Briefly, cells were plated in 96-well plates (4×103 cells per well), and treated with TV at 0, 5, 10, and 15 μM for 24, 48 and 72 h, respectively. MTT dissolved in PBS (0.5 mg/ml) was added to each well (100 μl/well) of the 96-well tissue culture plates containing the test cells and further incubated at 37℃ for 4 h. MTT is reduced by mitochondrial dehydrogenases of viable cells to purple formazan product. The MTT-formazan product dissolved in DMSO was estimated by measuring absorbance at 560 nm using a SpectraMAX 190 microplate spectrophotometer (GMI co., USA).
BrdU incorporation assay
A549 cells were seeded into 96-well plates (4×103 cells per well), and treated with TV at 0, 15 μM for 24, 48 and 72 h, respectively. Cell proliferation in A549 cells was quantified by measuring the amount of BrdU incorporated into nuclear DNA, using the BrdU Cell Proliferation Assay (QIA58), according to the manufacturer’s protocol. The absorbance was measured using a plate reader at 450 nm (540 nm reference wavelength). Cell proliferation was expressed as a percentage of the value of control cells.
Cell cycle analysis
To determine cell cycle distribution, A549 cells were plated in 100-mm dishes, treated with TV (0, 15 μM) for 12-48 h. After treatment, the cells were collected by trypsinization and then fixed in 70% ethanol. They were washed with phosphate-buffered saline (PBS) and resuspended in 1 ml of PBS contain
ing 1 mg/ml RNase and 10 mg/ml propidium iodide. Following incubation in the dark for 30 min at room temperature, the cells were analyzed with a flow cytometer (Becton Dickinson FACScan).
H33258 stained assay
Cells grown on 4-chamber slides were treated with or without TV (15 μM) for 24-48 h. After incubation, the supernatant was discarded, and A549 cells were fixed with 1% glutaraldehyde in PBS for 30 minutes at room temperature, washed three times with PBS, exposed to H 33258 at 15 μM for 30 minutes at room temperature, and visualized using a fluorescence microscope (Olympus, Japan).
LDH release assay
A549 cells were treated with TV for 24-72 h, at 0, 5, 10, and 15 μM, respectively. The detection was performed as described previously (Zhao et al., 2006).
Caspases-3, 8 activities assay
The activation of Caspase-3/-8 proteolytic cascade was assessed by caspase-3 and caspase-8 colorimetric assay kit according to the manufacturer’s protocol. Whole-cell pellets obtained from 2×106 cells were re-suspended in 50 μl of chilled lyses buffer and incubated on ice for 10 min. The cell lysate was centrifuged at 12,000 rpm at 4℃ for 5 min, with the supernatant being collected for later use. Then, the supernatants were mixed with 2×Reaction Buffer containing DTT (10 mM) and caspase-specific substrate (DEVD-pNA for caspase-3 activity assay and IETD-pNA for that of caspase-8), and incubated for 1 h at 37℃. The release of p-nitroaniline was monitored using SpectraMAX 190 microplate spectrophotometer (GMI co., USA) at 405 nm.
Caspases 9/6 activity assay
Caspases 9/6 activity in A549 cells was assessed according to the instructions provided by BD Bioscience. The assay was based on the ability of the active enzyme to cleave the fluorophore from the caspase-9/6 substrate, LEHD-AMC. The cell lysates were incubated with or without a caspase-9 inhibitor in 2×Reaction Buffer /DTT mix for half an hour on ice, and then incubated with the substrate for 3 h at 37℃. The release of 7-amino-4-methyl coumarin (AMC) was monitored using a 380-nm excitation filter and 460-nm emission filter in a FP-6500 Spectrofluorometer (Jasco, Tokyo, Japan).
p53, p21, Fas and Fas ligand (mFasL and sFasL) ELISA assay
p53 pan ELISA, WAF1 ELISA, Fas ligand and Fas ELISA kits were used to detect p53, p21, Fas ligand and Fas receptor. Briefly, cells were treated with 0 and 15 μM of TV for 6, 12, 24, 36, and 48 h. The samples of cell lysate were placed in 96 well (1×106 cells/well) microtiter plates coated with monoclonal detective antibodies, and were incubated for 1 h (Fas), 2 h (p53 or p21/WAF1) or 3 h (FasL) at room temperature. After removing the unbound material by washing with PBS, horseradish peroxidase conjugated streptavidin was added to bind to the antibodies. Horseradish peroxidase catalyzed the conversion of a chromogenic substrate (tetramethylbenzidine) to a colored solution with color intensity proportional to the amount of protein present in the sample. The absorbance of each well was measured at 450 nm. Concentrations of p53, p21/WAF1, Fas and FasL were determined by interpolating from standard curves obtained with known concentrations of standard proteins (Mediavilla et al., 1999; Castaneda and Kinne, 2001).
Data presentation
All experiments were repeated three times. Data were expressed as means ± S.D. Statistical significance was compared between various treatment groups and controls using analysis of variance (ANOVA). Data were considered statistically significant when p-values were <0.05.
RESULTS
Effects of TV on cells growth and cell cycle distribution
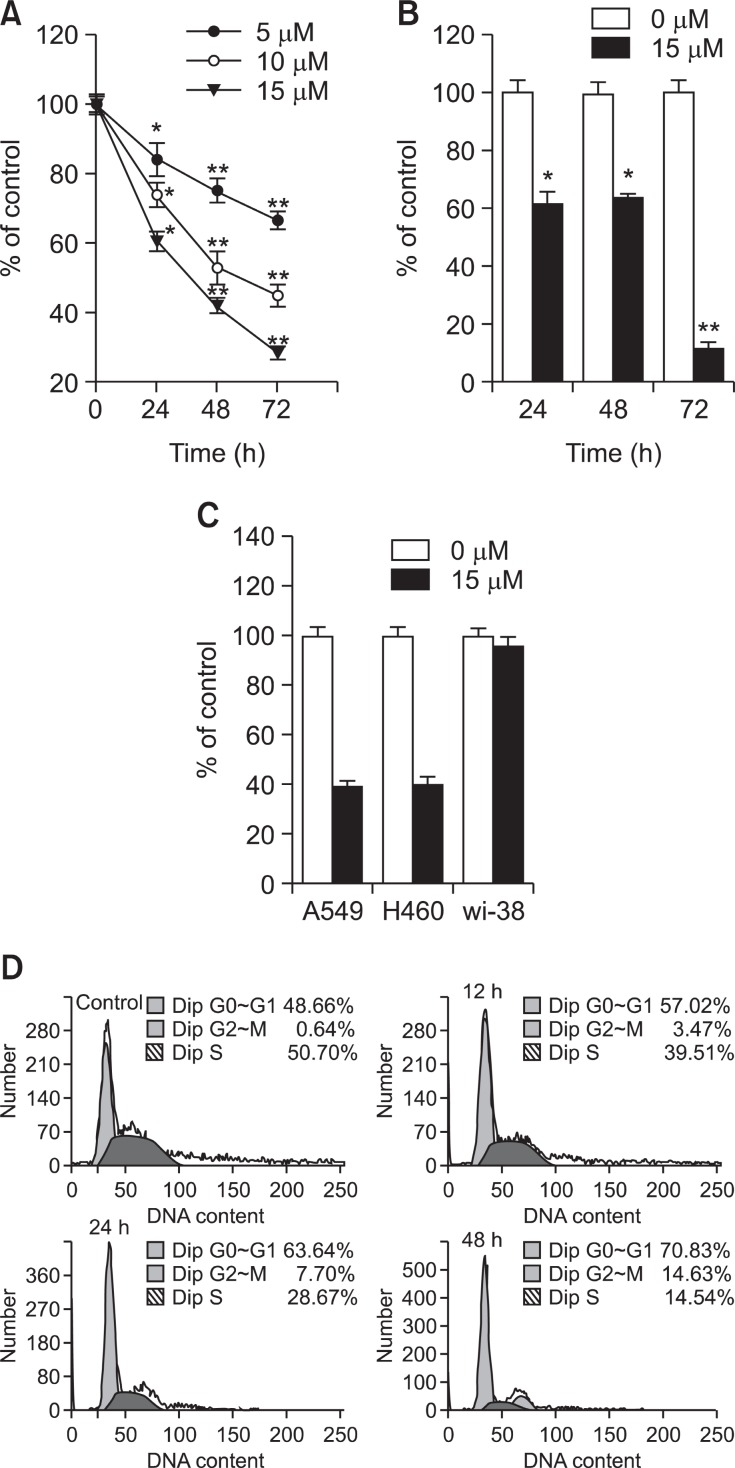
In the initial serial experiments, escalating doses of TV were tested on A549 cells. At the concentrations of 2.5 mΜ or less, TV produced no apparent effects on the cell’s viability or growth (data not shown). At higher concentrations, however, a statistically significant reduction in cell growth (p<0.001, n=3) was observed, which is consistent with that seen with 9L and CNS-1 tumors reported previously (Kong et al., 2004). As quantified by MTT assay (Fig.2 A), three concentrations of TV were tested, with the growth inhibition on the cells being analyzed at three time points. TV showed a dose and time dependent inhibitory effect on the growth of A549 cells. To confirm the results, a BrdU incorporation assay was carried out. As shown in Fig.2 B, cell proliferation was signifi cantly inhibited after being treated with 15 mM TV for 24, 48 and 72 h, respectively. Based on these results, we demonstrated that TV could significantly inhibit the growth of A549 cells in a time and dose dependent manner. After three rounds of screening, TV was found to kill both A549 and H460 cells but not Wi-38 at the concentration of about 15 μM (Fig.2 C).
Fig. 2. Effect of TV on cell growth inhibition in A549 cells. A549 cells were treated with TV at 0, 5, 10, and 15 μM for 24, 48 and 72h, respectively. (A) Cell viability was assessed by the MTT assay. (B) Cell proliferation was assessed by the BrdU incorporation assay. Each data point is a mean of three independent experiments with six wells/concentration and is given as mean ± S.D. *p<0.05, **p<0.01 vs. controls. (C) Effect of TV on cell growth inhibition in A549, H460 and Wi-38 cells. A549 H460 and Wi-38 cells were treated with TV at 0, 15 μM for 48 h, respectively. Cell viability was assessed by the MTT assay. (D) Effect of TV on cell cycle distribution in A549 cells. A549 cells were treated with or without TV 15μM for 12 h, 24 h, and 48 h, harvested, fixed and stained with PI. The DNA content of the stained cells was analyzed by flow cytometry: Data are representative of three independent experiments with similar results.
To determine whether cell growth inhibition observed in response to TV treatment was associated with cell cycle arrest, the distribution of cells in different phases of cell cycle was assessed after 12-48 h of treatment with 15 μM TV. Fig.2 C shows a time-dependent accumulation of cells in G0/G1 (from 48.66% to 70.83%), and in G2/M (from 0.64% to 14.63%), whilst the percentage of cells in S phase decreased sharply. The results showed that within 12-48 h, TV completely locked A549 cells at the G1 phase and partly at G2/M, which did not allow the cells to progress through the check point or to re-enter the G1 phase of a second cell cycle. Then, cells were on their way to apoptosis.
The forms of cell death induced by TV on A549 cells
Concomitant with cell growth inhibition induced by TV, cell morphological changes were observed using a phase contrast microscope. The cells shrinkage, vacuoles and membrane blebbing were observed following TV treatment at 15 μM for 24-48 h (data not shown). To confirm the apoptosis induced by TV, an H33258 assay was conducted. Fig. 3 shows the time course of nucleus condensation and nucleus fragmentation in
Fig. 3. TV-induced morphological changes of A549 cells. Morphological changes of A549 cell nuclei were observed by fluorescence microscopy. The cells were stained with Hoechst 33258 to identify apoptotic cells. Some of the condensed and fragmented nuclei are indicated by arrows. Data are representative of three independent experiments with similar results.
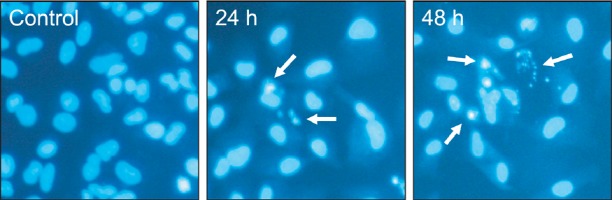
continuous treatment with 15 μM of TV. Nucleus condensation and nucleus fragmentation of A549 was found at 12 h (data not shown) and maximized at 48 h after the addition of TV.
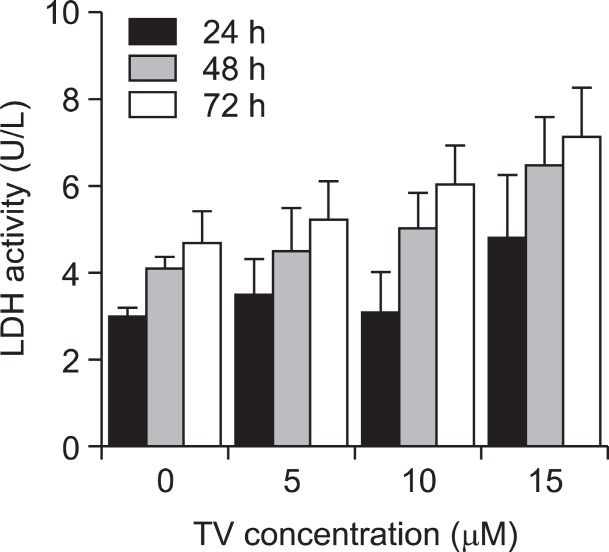
Since apoptosis is an energy-dependent process and necrosis is a passive act, differentiation between these two forms of cell death could have a significant impact on accessing the outcome of anticancer drug therapy in the clinic (Lockshin and Zakeri, 2004). To examine the drug toxicity and to confirm the mode of cell death induced by TV, an LDH activity-based cytotoxicity assay was performed. As shown in Fig. 4 , there was no significant difference (p>0.05, n=3) in LDH release between the cells in the control group and in the tested group. Therefore, TV inhibited A549 cell growth mainly through inducing apoptosis rather than necrosis.
Fig. 4. LDH release in A549 cells after treatment with TV. The cellculture medium from the test group and the control group was collected after 24-72 h of treatment with TV at 0, 5, 10, and 15 μM. These data are presented as the mean ± S.D. of the results for three independent experiments. *p<0.05 vs. controls.
Changes of the caspases activity by TV therapy
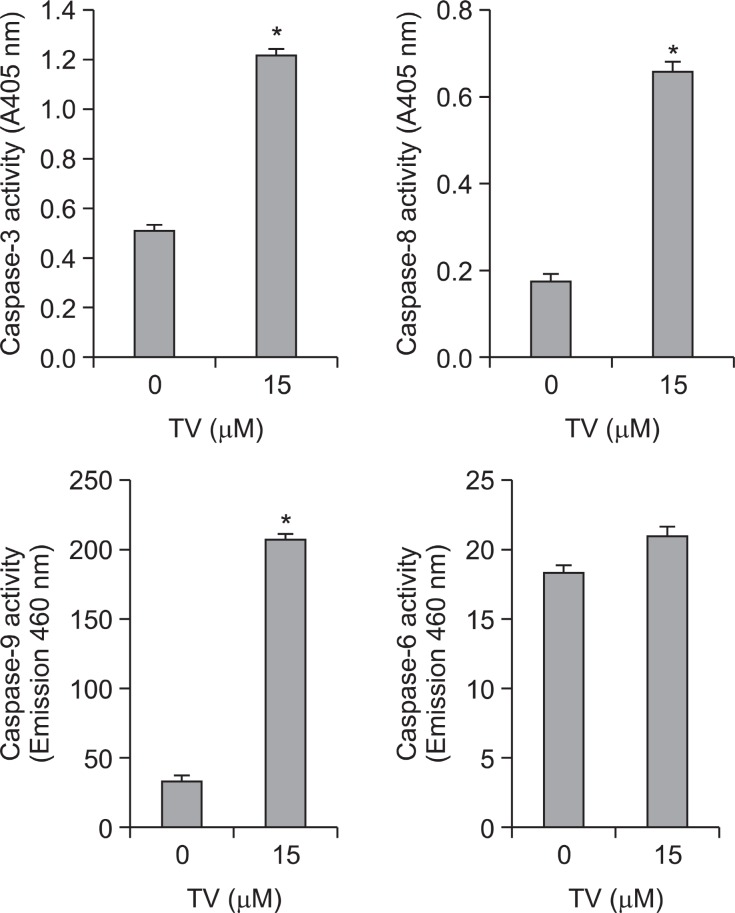
The caspase family is at the heart of apoptotic machinery, where these enzymes play key roles in the execution of apoptosis (Philchenkov et al., 2004; Riedl and Shi, 2004). Morphological assay of apoptosis led us to hypothesize that TV might activate the caspase-dependent cell death pathway. Thus, the activation of caspase induced by TV was further evaluated in A549 cells. To identify whether caspase was involved in the mechanism, we measured the catalytic activity of caspase-8 and caspase-3 using BD ApoAlertTM Caspase-3 and caspase-8 Colorimetric Assay Kit, and caspase-9 and caspase-6 using BD ApoAlertTM Caspase 9/6 Fluorescent Assay Kit. Compared to the control, treatment with TV for the indicated time of A549
cells resulted in an increase in the activities of caspase-8, -9, and -3, but not of caspase-6 (Fig.5 ). These data indicated that caspase-3, caspaes-8 and caspase-9 are involved in the TV-induced apoptosis.
Fig. 5. Activities of caspase proteases. A549 cells (2×106 cells) were treated with or without 15 μM and caspase-3, -6, -8, and -9 activities were measured. The data are presented as the mean ± S.D. of the results for three independent experiments.
TV increases the expression of p53 and p21/WAF1 proteins in A549 cells
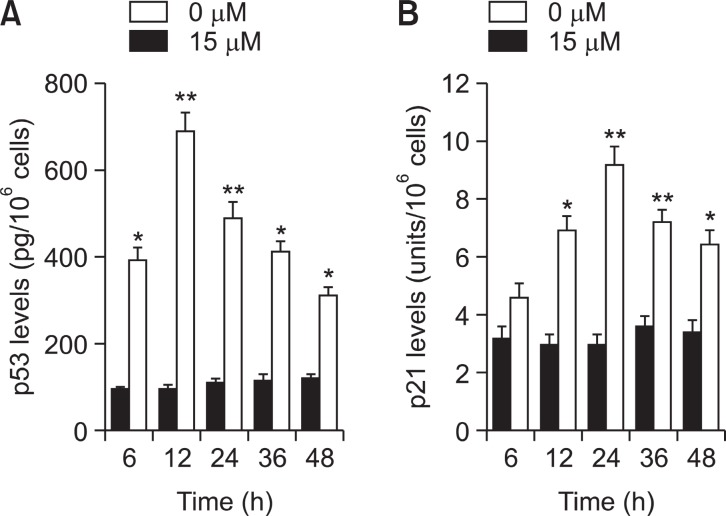
To determine whether tumor suppression factor p53 and its downstream molecule p21/WAF1 were involved in the TV-mediated antiproliferative effect of A549 cells, the levels of these proteins were assayed by ELISA. Fig.6 A shows that an increase in p53 protein was apparent at 6 h and reached maximum induction at 24 h in TV treated A549 cells. As observed in the induction of p53, the accumulation of p21 was observed at 12 h after TV treatment, and progressively increased up to 24 h (Fig.6 B).
Fig. 6. Expression of p53 and p21/WAF1 tested by ELISA in A549cells. A549 cells were incubated with or without TV (15 μM) for 3, 6, 12, 24, and 48 h. (A) The level of p53 in A549 cells, (B) the amount of p21/WAF1 in A549 cells. Each value is the mean ± S.D. of three independent experiments. *p<0.05, **p<0.01 vs. controls.
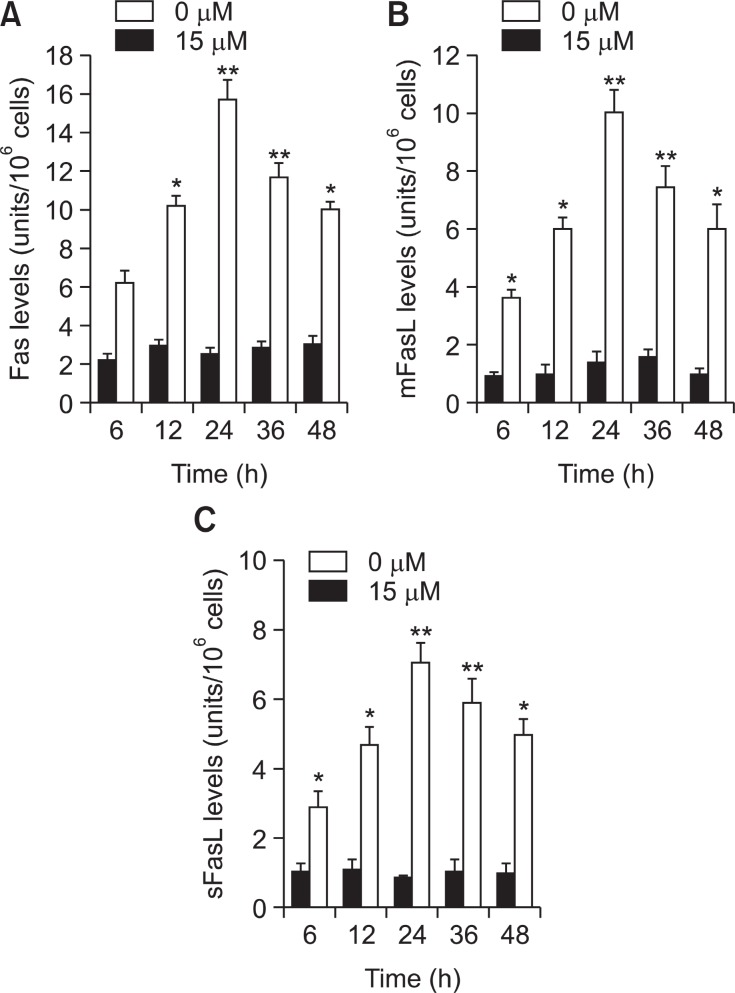
Fas/FasL apoptotic system might be a possible pathway of TV-induced apoptosis
To determine whether Fas and FasL system were involved in the TV-induced apoptosis of A549 cells, the levels of these proteins were assayed by ELISA. Fig.7 A shows that an increase in Fas protein was apparent at 6 h and reached maximum induction at 24 h in TV treated A549 cells.
Fig. 7. Fas/FasL apoptotic system was involved in TV-mediated apoptosis. A549 cells were incubated with 0, 5, 10 and 15 μM of TV for 6, 12, 24 and 48 h. (A) The level of Fas receptor in A549cells, (B) the amount of mFasL in A549 cells, (C) the amount of sFasL in A549 cells. Each value is the mean ± S.D .of three inde-pendent experiments. Statistical significance: *p<0.01 vs. control.
As observed in the induction of Fas, the accumulation of mFasL was observed at 6 h after TV treatment, and progressively increased up to 24 h (Fig.7 B). A similar result was observed for sFasL (Fig.7 C).
DISCUSSION
In this study, we have demonstrated that TV inhibited cell growth, arrested the cell cycle and induced apoptosis in A549 cells. These biochemical events were possibly associated with the p53 tumor suppressor gene. Under normal conditions, inactive p53 protein is maintained at low levels, primarily in the cytoplasm. High levels of activated and stabilized p53 accumulate in the nucleus in response to various forms of cell stress, including DNA damage (Haupt et al., 2003; Saretzki, 2010). Stabilized p53 can initiate the transcription of genes in cell cycle checkpoints and in both death pathways (Ryan et al., 2001; Maddika et al., 2007). Recently, more and more compounds are found to increase the expression of p53, and then lead to p53-dependent cell cycle arrest and apoptosis in many cancer cells (Chen et al., 2005; Goel et al., 2006; Chiu et al., 2009; Vidya et al., 2010; Saretzki, 2010). Our results demonstrated that p53 plays an important role in TV induced antiproliferative activity in A549 cells. Induction of p53 by TV not only caused A549 cell cycle arrest, but also triggered apoptosis. This finding is supported by the following results: First, flow cytometry assay indicated that TV can induce A549 cell cycle arrest in the G1 phase, which was attributed to the enhancement of p21/WAF1 protein that was induced by p53. Second, TV increased the expression of p53’s downstream molecules, Fas, which restores apoptosis sensitivity of A549 cells.
The Fas/FasL system is a key signaling transduction pathway of apoptosis in cells and tissues (Nagata and Golstein, 1995). The binding of Fas ligand to Fas induces receptor oligomerization and formation of death-inducing signaling complex (DISC), followed by the activation of a series caspase cascade resulting in cell apoptotic death (Peter and Krammer, 2003; Philchenkov, 2004); Philchenkov et al., 2004). Fas, also known as CD95/Apo-1, is constitutively expressed by human alveolar epithelial cells, including A549 cells (Hamann et al., 1998), and plays a critical role in the pathophysiology of various pulmonary disorders (Fujita et al., 2002). FasL is a TNF related type II membrane protein. Cleavage of membrane-bound Fas ligand (mFasL) by a metalloproteinase-like enzyme results in the formation of soluble Fas ligand (sFasL) (Kayagaki et al., 1995).
Our data showed that the expression of Fas significantly increased in TV-treated A549 cells. Thus, we suggest that the enhancement of Fas by TV is beneficial in restoring apoptotic sensitivity of A549 cells to the natural immune defense system or chemotherapy.
It is well established that activation of caspases is a critical component of the execution phase of cell death in most forms of apoptosis. Therefore, factors affecting caspase activation might be important determinants of drug sensitivity (Tsagara-kis et al., 2011; Zhao et al., 2011). Up to now, several reagents, such as interferon-γ (Kurdi and Booz, 2007), proinflammatory cytokine IL-4 (Shankaranarayanan and Nigam, 2003), and gossypol (Chang et al., 2004), have been reported to initiate apoptosis in A549 cells by activating Fas and caspases. Interferon-γ, proinflammatory cytokine IL-4 and gossypol can trigger the activation of caspase-8, but not caspase-9. In contrast, in addition to activation of caspase-8, TV also elevated the activity of caspase-9. The present study showed that activation of caspase -8, -9, and -3 contributed to apoptosis in response to TV. Multiple kinds of caspase activation greatly enhance the effects of TV on the apoptosis of A549 cells. Theoretically, activation of caspases in lung cancer cells would be a novel strategy for brain cancer therapy.
In summary, TV could significantly inhibit A549 cell growth in vitro, which effect might be performed by up-regulation of p53 protein, blocking the cell cycle partly at the G1 phase, and activation of caspase-3, -8, -9. In addition, it further induced apoptotic cell death by initiation of the Fas/FasL death receptor system in A549 cells. Activation of caspases is a critical component of the execution phase of cell death in most forms of apoptosis. Therefore, factors affecting caspase activation might be important determinants of drug sensitivity. Our study has clearly demonstrated that TV may be a promising chemopreventive agent for treating lung cancer.
Acknowledgments
We thank Dr. Bradford J.A. Franklin for the careful English revision. This project was supported by the Foundation of the Education Department of Shandong Province (J07YD13), Natural Science Foundation of Shandong Province (Y2008D05).
References
- 1.Brown J. M. Wouters B. G. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. (1999);59:1391–1399. [PubMed] [Google Scholar]
- 2.Brüne B. The intimate relation between nitric oxide and superoxide in apoptosis and cell survival. Antioxid. Redox. Signal. (2005);7:497–507. doi: 10.1089/ars.2005.7.497. [DOI] [PubMed] [Google Scholar]
- 3.Cai C. F. Feng L. Wang L. Kong Q. Z. Zhao Y. F. Tetrazolium violet induces apoptosis via caspases-8, -9 activation and Fas/FasL up-regulation in rat C6 glioma cells. Arch. Pharm. Res. (2009);32:575–581. doi: 10.1007/s12272-009-1414-8. [DOI] [PubMed] [Google Scholar]
- 4.Castañeda F. Kinne R. K. Apoptosis induced in HepG2 cells by short exposure to millimolar concentrations of ethanol involves the Fas-receptor pathway. J. Cancer Res. Clin. Oncol. (2001);127:418–424. doi: 10.1007/s004320000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang J. S. Hsu Y. L. Kuo P. L. Chiang L. C. Lin C. C. Upregulation of Fas/Fas ligand-mediated apoptosis by gossypol in an immortalized human alveolar lung cancer cell line. Clin. Exp. Pharmacol. Physiol. (2004);31:716–722. doi: 10.1111/j.1440-1681.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen S. Gao J. Halicka H. D. Huang X. Traganos F. Darzynkiewicz Z. The cytostatic and cytotoxic effects of oridonin (Rubescenin), a diterpenoid from Rabdosia rubescens, on tumor cells of different lineage. Int. J. Oncol. (2005);26:579–588. [PubMed] [Google Scholar]
- 7.Cheng Y. L. Chang W. L. Lee S. C. Liu Y. G. Lin H. C. Chen C. J. Yen C. Y. Yu D. S. Lin S. Z. Harn H. J. Acetone extract of Bupleurum scorzonerifolium inhibits proliferation of A549 human lung cancer cells via inducing apoptosis and suppressing telomerase activity. Life Sci. (2003);73:2383–2394. doi: 10.1016/S0024-3205(03)00648-9. [DOI] [PubMed] [Google Scholar]
- 8.Chiu T. H. Lai W. W. Hsia T. C. Yang J. S. Lai T. Y. Wu P. P. Ma C. Y. Yeh C. C. Ho C. C. Lu H. F. Wood W. G. Chung J. G. Aloe-emodin induces cell death through S-phase arrest and caspase-dependent pathways in human tongue squamous cancer SCC-4 cells. Anticancer Res. (2009);29:4503–4511. [PubMed] [Google Scholar]
- 9.Fujita T. Maruyama M. Araya J. Sassa K. Kawagishi Y. Hayashi R. Matsui S. Kashii T. Yamashita N. Sugiyama E. Kobayashi M. Hydrogen peroxide induces upregulation of Fas in human airway epithelial cells via the activation of PARP-p53 pathway. Am. J. Respir. Cell Mol. Biol. (2002);27:542–552. doi: 10.1165/rcmb.4775. [DOI] [PubMed] [Google Scholar]
- 10.Goel A. Fuerst F. Hotchkiss E. Boland C. R. Selenomethionine induces p53 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cancer Biol. Ther. (2006);5:529–535. doi: 10.4161/cbt.5.5.2654. [DOI] [PubMed] [Google Scholar]
- 11.Hamann K. J. Dorscheid D. R. Ko F. D. Conforti A. E. Sperling A. I. Rabe K. F. White S. R. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am. J. Respir. Cell Mol. Biol. (1998);19:537–542. doi: 10.1165/ajrcmb.19.4.3100. [DOI] [PubMed] [Google Scholar]
- 12.Haupt S. Berger M. Goldberg Z. Haupt Y. Apoptosis -the p53 network. J. Cell Sci. (2003);116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 13.Hengartner M. O. The biochemistry of apoptosis. Nature. (2000);407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 14.Igney F. H. Krammer P. H. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer. (2002);2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 15.Kayagaki N. Kawasaki A. Ebata T. Ohmoto H. Ikeda S. Inoue S. Yoshino K. Okumura K. Yagita H. Metalloproteinase-mediated release of human Fas ligand. J. Exp. Med. (1995);182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim P. K. Park S. Y. Koty P. P. Hua Y. Luketich J. D. Billiar T. R. Fas-associating death domain protein overexpression induces apoptosis in lung cancer cells. J. Thorac. Cardiovasc. Surg. (2003);125:1336–1342. doi: 10.1016/S0022-5223(02)73227-3. [DOI] [PubMed] [Google Scholar]
- 17.Kong Q. Zhang N. Yang X. Q. Chen L. W. Treating cancer using tetrazolium compounds. Proc. Am. Assoc. Cancer Res. (2003):A154. [Google Scholar]
- 18.Kong Q. Zhang N. Zhao Y. F. Tetrazolium violet sensitizes tumor cells to chemotherapeutic agents. Proc. Am. Assoc. Cancer Res. (2004):A556. [Google Scholar]
- 19.Kurdi M. Booz G. W. Jak inhibition, but not Stat1 knockdown, blocks the synergistic effect of IFN-gamma on Fas-induced apoptosis of A549 human non-small cell lung cancer cells. J. Interferon Cytokine Res. (2007);27:23–31. doi: 10.1089/jir.2007.0074. [DOI] [PubMed] [Google Scholar]
- 20.Lockshin R. A. Zakeri Z. Apoptosis, autophagy, and more. Int. J. Biochem. Cell Biol. (2004);36:2405–2419. doi: 10.1016/j.biocel.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Maddika S. Ande S. R. Panigrahi S. Paranjothy T. Weglarczyk K. Zuse A. Eshraghi M. Manda K. D. Wiechec E. Los M. Cell survival cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist. Updat. (2007);10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Mediavilla M. D. Cos S. Sánchez-Barceló E. J. Melatonin increases p53 and p21WAF1 expression in MCF-7 human breast cancer cells in vitro. Life Sci. (1999);65:415–420. doi: 10.1016/S0024-3205(99)00262-3. [DOI] [PubMed] [Google Scholar]
- 23.Nagata S. Golstein P. The Fas death factor. Science. (1995);267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 24.Peter M. E. Krammer P. H. The CD95 (APO-1/Fas) DISC and beyond. Cell Death Differ. (2003);10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 25.Philchenkov A. Caspases: potential targets for regulating cell death. J. Cell Mol. Med. (2004);8:432–444. doi: 10.1111/j.1582-4934.2004.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philchenkov A. Zavelevich M. Kroczak T. J. Los M. Caspases and cancer: mechanisms of inactivation and new treatment modalities. Exp. Oncol. (2004);26:82–97. [PubMed] [Google Scholar]
- 27.Riedl S. J. Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. (2004);5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 28.Ryan K. M. Phillips A. C. Vousden K. H. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. (2001);13:332–337. doi: 10.1016/S0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 29.Saretzki G. Cellular senescence in the development and treatment of cancer. Curr. Pharm. Des. (2010);16:79–100. doi: 10.2174/138161210789941874. [DOI] [PubMed] [Google Scholar]
- 30.Shankaranarayanan P. Nigam S. IL-4 induces apoptosis in A549 lung adenocarcinoma cells: evidence for the pivotal role of 15-hydroxyeicosatetraenoic acid binding to activated peroxisome proliferator-activated receptor gamma transcription factor. J. Immunol. (2003);170:887–894. doi: 10.4049/jimmunol.170.2.887. [DOI] [PubMed] [Google Scholar]
- 31.Tsagarakis N. J. Drygiannakis I. Batistakis A. G. Kolios G. Kouroumalis E. A. Octreotide induces caspase activation and apoptosis in human hepatoma HepG2 cells. World J. Gastroenterol. (2011);17:313–321. doi: 10.3748/wjg.v17.i3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidya Priyadarsini R. Senthil Murugan R. Maitreyi S. Ramalingam K. Karunagaran D. Nagini S. The fl avonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. (2010);649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z. B. Liu Y. Q. Cui Y. F. Pathways to caspase activation. Cell Biol. Int. (2005);29:489–496. doi: 10.1016/j.cellbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y. Wu S. Wu J. Jia P. Gao S. Yan X. Wang Y. Introduction of hypoxia-targeting p53 fusion protein for the selective therapy of non-small cell lung cancer. Cancer Biol. Ther. (2011);11:95–107. doi: 10.4161/cbt.11.1.13960. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y. Zhang N. Kong Q. Tetrazolium violet induces G0/G1 arrest and apoptosis in brain tumor cells. J. Neurooncol. (2006);77:109–115. doi: 10.1007/s11060-005-9012-1. [DOI] [PubMed] [Google Scholar]