Abstract
In this study, we examined the estrogenic activity of bavachin, a component of Psoralea corylifolia that has been used as a traditional medicine in Asia. Bavachin was purified from ethanolic extract of Psoralea corylifolia and characterized its estrogenic activity by ligand binding, reporter gene activation, and endogenous estrogen receptor (ER) target gene regulation. Bavachin showed ER ligand binding activity in competitive displacement of [3H] E2 from recombinant ER. The estrogenic activity of bavachin was characterized in a transient transfection system using ERα or ERβ and estrogen-responsive luciferase plasmids in CV-1 cells with an EC50 of 320 nM and 680 nM, respectively. Bavachin increased the mRNA levels of estrogen-responsive genes such as pS2 and PR, and decreased the protein level of ERα by proteasomal pathway. However, bavachin failed to activate the androgen receptor in CV-1 cells transiently transfected with the corresponding receptor and hormone responsive reporter plasmid. These data indicate that bavachin acts as a weak phytoestrogen by binding and activating the ER.
Keywords: Psoralea corylifolia, Bavachin, Estrogen receptor, Phytoestrogen
INTRODUCTION
Phytoestrogens are plant-derived compounds that share structural similarities with and exert similar molecular effects to 17β-estradiol (E2) (Setchell, 1998). Despite the fact that they are less potent than genuine estrogens, it is supposed that high concentrations can compensate for their comparatively lower binding affinity for the estrogen receptor (ER) in exerting their effects. The most commonly used alternative herbal medicines for estrogen replacement are soy, black cohosh, ginseng, pomegranate, evening primrose oil, and flax seed (Kronenberg and Fugh-Berman, 2002; Newton et al., 2005). Accumulating studies have suggested important potential for phytoestrogens to improve health. Soy origin phytoestrogens possess cancer preventive effects and lowers the incidence of menopausal symptoms (Cederroth and Nef, 2009). However, not all reports present the beneficial effects of phytoestrogen. A recent study warned that black cohosh increases metastatic cancer in transgenic mice (Davis et al., 2008). Thus, intensive research is required to provide a scientific rationale for traditional uses despite the many beneficial effects of phytoestrogen.
The molecular target of phytoestrogen is the ER. The ER is a ligand-activated transcription factor that modulates the transcription of target genes on ligand binding with the aid of many accompanying coregulator proteins. There are two isoforms of ER: ERα and ERβ. The tissue distribution and biological functions of these isoforms are distinct and overlapping. In contrast to ERα, which is an activator of cancer cell growth, ERβ inhibits cancer cell proliferation through the modulation of key regulators of the cell cycle (Ström et al., 2004). In addition, ERβ can modulate the function of ERα itself. ERβ expression attenuates the growth-promoting activity of ERα (Chang et al., 2006). Phytoestrogens can bind to the ER isoforms with different binding affinities. For example, genistein shows a 20-fold higher binding affinity toward ERβ. Hence, phytoestrogen activity, which can be modulated by the proportion of the ER subtypes and selective estrogen receptor modulators from phytoestrogenic sources, has considerable therapeutic potential for breast cancer (Chang et al., 2008).
Psoralea coryfolia is a commonly used Chinese medicine in Asia. Traditionally, it has been applied to a variety of conditions such as osteoporosis, bone fractures, and bacterial infections; it has also been used as a mood stimulant (Haraguchi et al., 2002). Studies have proven that the extracts and the components of P. coryfolia contain antibacterial, tumor-suppressive, estrogen-like, and cholesterol-lowering activities (Latha et al., 2000; Yin et al., 2004; Choi et al., 2008). In the course of
our study, Xin et al. reported that compounds isolated from P. coryfolia showed phytoestrogenic activity as examined by reporter gene assays (Xin et al., 2010). In this study, we examined the ER-mediated activities of bavachin (Fig.1 ), one of the compounds from P. coryfolia, in human breast cancer MCF-7 cells (Park et al., 2011).
Fig. 1. The chemical structure of bavachin isolated from Psoralea coryfolia.

MATERIALS AND METHODS
Materials
E2, tamoxifen, and dihydrotestosterone (DHT) were purchased from Sigma (St. Louis, MO, USA) and dissolved in 100% ethanol. ICI 182, 780 (ICI) was obtained from ZENECA Pharmaceuticals (Tocris, UK). MG132 was purchased from Sigma (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide. All of the compounds were added to the medium such that the total solvent concentration was lower than 0.1%. An untreated group served as a control. Bavachin was purified from the alcoholic extract of Psoralea coryfolia. In brief, the dried seeds of Psoralea coryfolia (8.8 kg) was extracted with n-hexane to remove lipid and extracted with methanol. The methanol extracts were suspended in water and successively partitioned with n-hexane and ethylacetate. The ethylacetate soluble fraction (153 g) was subjected to silica gel column chromatography eluting with n-hexane: ethylacetate gradient system (4:1 → 1:1) and 19 fractions were collected. Fraction 12 was further fractionated by silica gel column chromatography with n-hexane: ethylacetate (contained 10% isopropanol) gradient system (20:1 → 1:3) and by reverse phase column chromatography with a gradient elution of methanol (50% →100%) to yield pure bavachin (39.7 mg). The chemical structure was confirmed by the spectroscopic analysis and comparison with the reported data (Wang et al., 2001).
Cell culture and treatment
MCF-7 (ATCC HTB-22), CV-1 (ATCC CCL-70), and HEK293 (ATCC CRL-1573) cells were grown at 37℃ in a humidified atmosphere of 95% air/5% CO2 in phenol red-free RPMI supplemented with 10% fetal bovine serum (FBS; WelGENE, Seoul, Korea). Before treatment, the cells were washed with phosphate-buffered saline (PBS) and cultured in RPMI/5% charcoal-dextran stripped FBS (ST-FBS) for 1 day to eliminate any estrogenic source before treatment. We used 10 nM E2 unless otherwise noted.
Ligand binding assay
Ligand binding assay to full-length human ER was assessed as described previously (Obourn et al., 1993; Jung et al., 2010). The hERα and hERβ (Invitrogen Corp., Carlsbad, CA) were diluted to 5 nM with the binding buffer [10 mM Tris (pH 7.5), 10% glycerol, 1 mM DTT, and 1 mg/ml BSA] and incubated with 5 nM [3H]-E2 and test compounds. For the measurement of nonspecific binding, 10−6 M unlabelled estradiol was used. After 2 h incubation at 25℃, the reactions were terminated by rapid filtration through glass fiber filters presoaked with ice-cold 0.05% polyethylenimine using a Per-kin Elmer Filter Mate Harvester. The filters were washed five times with 1 ml of the ice-cold buffer, dried, and then placed in scintillation vials containing scintillation cocktail. After shaking and overnight equilibration of the vials, the radioactivity trapped on each filter was measured with a Packard 2000CA liquid scintillation counter. The specific binding (%) to each ER was determined as follows: [(dpmsample – dpmnonspecific)/(dpmveh –dpmnonspecific)] ×100.
Transient transfection and luciferase assay
CV-1 cells were seeded at a density of 4.5×106 cells in phenol red-free media with 10% ST-FBS for 24 h. The cells were transiently transfected with estrogen response element (ERE)-luciferase (Luc) and either hERα or hERβ for 6 h, and then treated with samples for 24 h. For androgen response element (ARE)-Luc assays, HEK293 cells were transfected with human androgen receptor (hAR) and ARE-Luc. To normalize transfection efficiency, β-galactosidase plasmid was cotransfected. The luciferase activities in cell lysates were measured using luciferase assay system (Promega Corp., Madison, WI) and the β-gal activities were measured as the absorbance at 410 nm by using an ELISA plate reader. Data are reported as relative luciferase activity divided by the β-galactosidase activity.
Quantitative Real-time PCR
RNA from MCF-7 cells was extracted using TRIzol reagent (Invitrogen Corp.) according to the manufacture's instruction. The yield of RNA was quantified by absorbance at 260 nm. To synthesize first strand cDNA, 3 μg total RNA was incubated at 70℃ for 5 min with 0.5 μg of random primer (Promega Corp., Madison, WI) and diethylpyrocarbonate-treated water. The reverse transcription reaction was performed using 40 unit of MMLV reverse transcriptase (Promega Corp., Madison, WI) in 5X reaction buffer, and 2.5 mM dNTP mixtures at 37℃ for 60 min. The reaction was terminated by heating at 70℃ for 8 min, followed by cooling at 4℃. qPCR was perfomed using iQTM SYBR Green Supermix (Bio-Rad). The β-actin primers were sense (5'-CAAATGCTTCTAGGCGGACTATG-3') and antisense (5'-TGCGCAAGTTAGGTTTTGTCA-3'). The PR primers were sense (5'-CGCGCTCTACCCTGCACTC-3') and antisense (5'-TGAATCCGGCCTCAGGTAGTT-3'). The pS2 primers were sense (5'-CATCGACGTCCCTCCAGAAGAG-3') and antisense (5'-CTCTGGGACTAATCACCGTGCTG-3'). A final volume was 25 μl, and an iCycler iQ Real-time PCR De-tection System (Bio-Rad, Hercules, CA) was used for qPCR.
Western blot analysis
Protein was separated using a radioimmune precipitation buffer (including 150 mM NaCl, 50 mM Tris-HCl, 1% NP-40, 0.5% deoxycholic acid and 0.1% SDS, with a protease inhibitor cocktail) on ice for 1 h and then centrifuged for 25 min at 13,000 rpm. Protein concentrations were determined using the Bradford method (Bio-Rad). For immunoblotting, same amounts of proteins were separated by SDS-PAGE. After SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane. Membranes were blocked with 5% non-fat dry milk in Tris-buffered saline/0.05% Tween (TBST), and incubated with anti-rabbit polyclonal antibody to ERα (0.4 mg/ml: Santa Cruz Biotechnology Inc., Santa Cruz, CA) or anti-mouse polyclonal antibody to β-actin (Sigma). After washing with TBST, blots were incubated with goat anti-rabbit or antimouse horseradish peroxidase-conjugated secondary antibody and visualized with enhanced chemiluminescence ECL kits (Amersham Bioscience, Little Chalfont, UK).
Cell proliferation assay
MCF-7 BOS cells were kindly provided by Prof. Ana M Soto at Tufts University School of Medicine. MCF-7 BOS cells were seeded at 4,000 cells/well in 96-well plates for 24 h after cultured in phenol red-free DMEM containing 5% ST-FBS for 2 days. Cells were treated with 10 nM E2, 1 μM tamoxifen and bavachin as indicated for 6 days. The cell growth was determined by using MTT based colorimetric assay (Haraguchi et al., 2002). After adding 100 μl of MTT solution (final concentration, 0.5 mg/ml) to each well, the plates were incubated for 5 h at 37℃. Extraction buffer (10% SDS in 0.01 M HCl) was added for the dissolution of formazan crystals. The absorbance was measured at 570 nm by using an ELISA plate reader.
Statistical analysis
Values shown represent the mean ± SD. Statistical analysis was performed by Student’s t-test, and a p-value<0.05 was considered significant.
RESULTS
Bavachin binds to ERα and ERβ
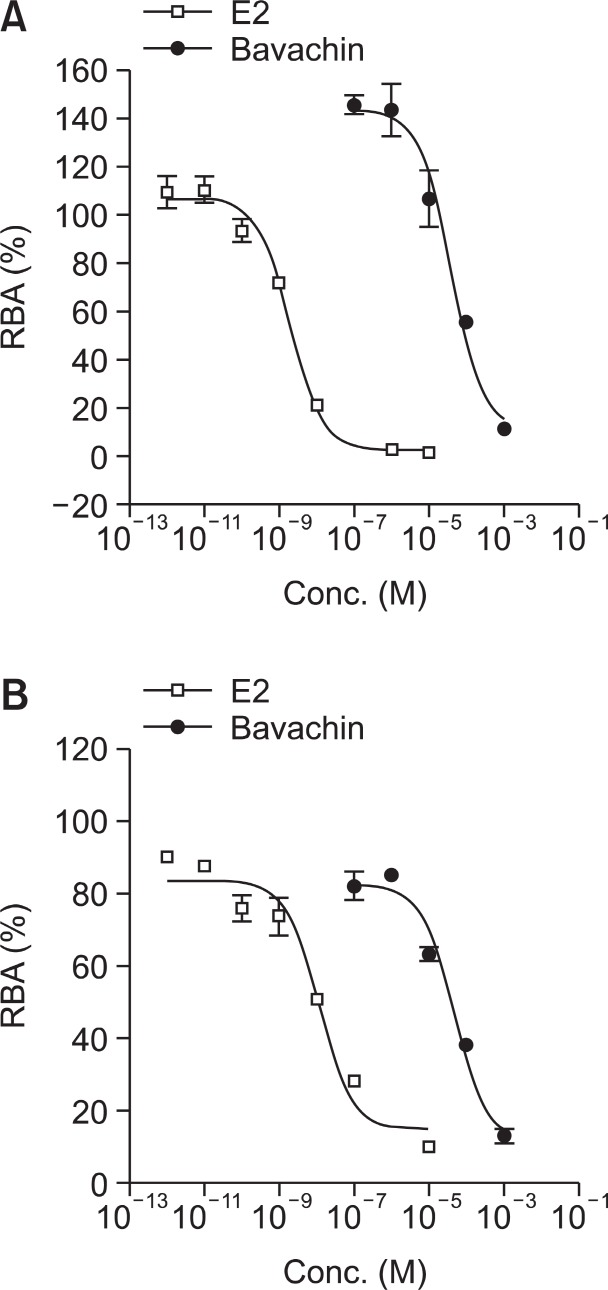
The biological activities of estrogen are mediated by two distinct receptors: ERα and ERβ. The activation of ER is initiated by ligand binding to ERs followed by dimerization of receptors and binding to DNA. The binding ability of bavachin to ERs was determined by the competitive displacement of [3H] E2 from full-length recombinant ERα and ERβ (Obourn et al., 1993; Wang et al., 2001; Jung et al., 2010). A competitive binding assay showed that bavachin displaced the [3H] E2 in a dose-dependent manner (Fig.2 ) with IC50 values of 6.57×10−5 M for hERα and 3.8×10−5 M for hERβ. The IC50 of E2 for hERα was 1.4×10−9 M measured simultaneously, which agrees with the reported Kd value of E2 (Lee and Gorski, 1996).
Fig. 2. Competitive inhibition of [3H] E2 binding to hERα (A) and hERβ (B) by bavachin. The hERα and hERβ are incubated with 5 nM [3H]-E2 and test compounds for 2 h and the receptor-bound radioactivities were determined by scintillation counting. The relative specific binding activity (RBA) was calculated as described in experimental section and the IC50 values were determined from the concentration-binding curve (n=4).
ER-mediated transcriptional activation in CV-1 cells
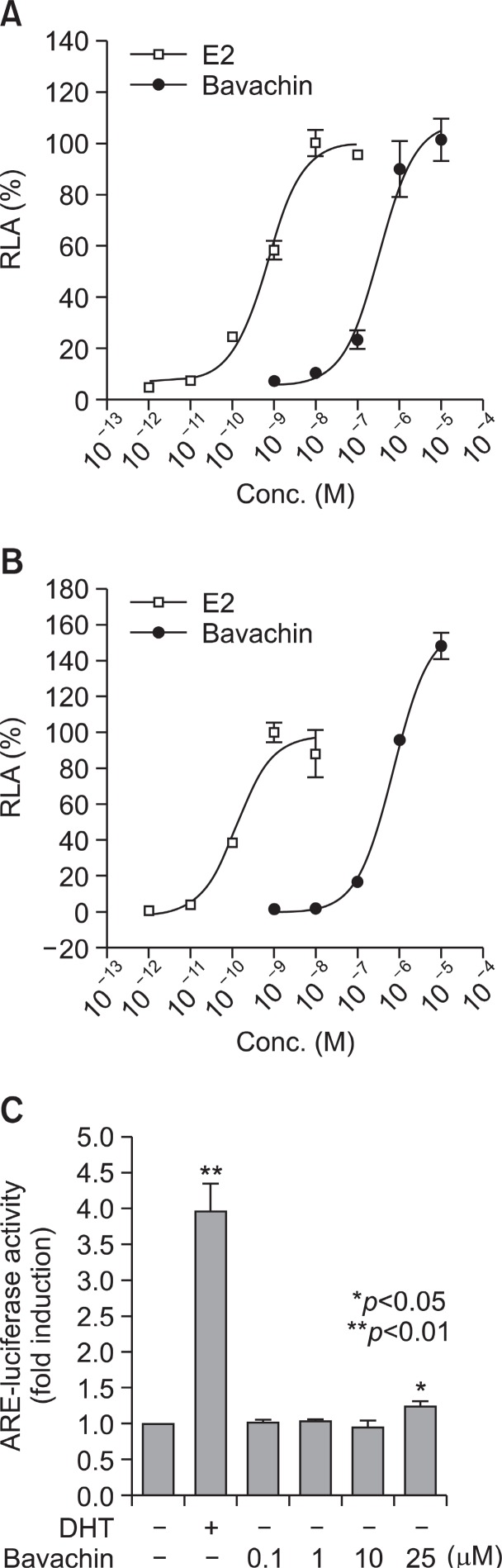
Proper ligand binding to the ER initiates transcriptional activation through the specific ERE in certain target genes (Gehm et al., 2000). We examined whether bavachin activates the transcription of an ERE containing a reporter plasmid in CV-1 cells. Bavachin induced luciferase activity in CV-1 cells transiently transfected with plasmids containing the ERE gene and ERα or ERβ expression plasmids (Lee et al., 2003). The EC50 values of 3.2×10−7 M for ERα (Fig.3 A) and 6.8×10−7 M for ERβ (Fig.3 B) were determined. It is estimated from these luciferase data that bavachin is approximately 500-fold weaker an ER ligand than E2 (Fig.3 ). To further examine whether the estrogenic activity of bavachin is ER-specific, we examined the androgen activity of bavachin. HEK293 cells were transfected with ARE-luc and an expression vector for AR to evaluate the effects of bavachin on AR-mediated transcription. DHT, which
Fig. 3. Dose-dependent ERE-luciferase reporer activity by bavachin in CV-1 cells. Cells were transiently transfected with ERE-luciferase reporter plasmid and either hERα (A) or hERβ (B) expression plasmid. Cells were treated with E2 or bavachin for 24h followed by transient transfection. Cell lysates were prepared and analyzed by luciferase assays. The data are representative of at least three independent experiments performed in triplicate and presented as relative luciferase activity (RLA). (C) HEK293 cells were transiently transfected with ARE-luc and expression vector for AR and treated for 24 h with 10 nM DHT or varying molar concentrations of bavachin as indicated. An untreated group served as a control. These experiments were repeated at least three times.* and ** indicate significant differences from the control at the p<0.05 and 0.001 levels, respectively.
was used as a positive control, significantly increased ARE-luciferase activity after 24 h of treatment. However, bavachin was not effective on the ARE-luciferase activity up to 25 μM (Fig.3 C).
Induction of an endogenous estrogen-responsive PR and pS2 mRNA and ERα protein levels in MCF-7 cells
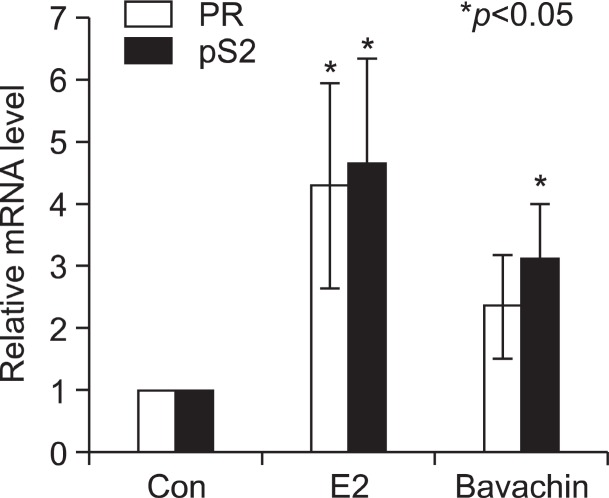
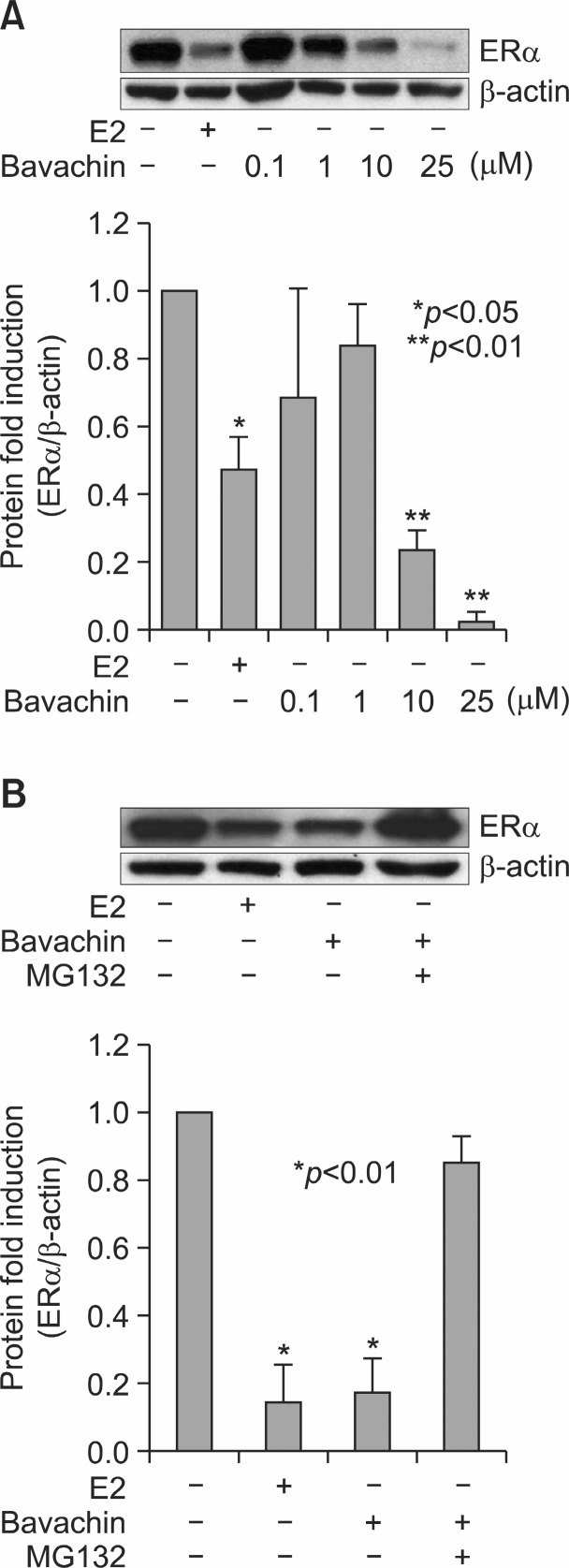
To evaluate the potential of bavachin as an activator of estrogen-responsive genes, we examined pS2 and PR mRNA induction in MCF-7 cells after treatment with bavachin (Park et al., 2011). Steady-state pS2 and PR mRNA levels were measured by carrying out qRT-PCR assays on total RNA prepared from treated cells. As an internal control, constitutively expressed human β-actin mRNA was used. Bavachin activated transcription of the pS2 and PR genes by the treatment for 24 h as E2 (Fig.4 ). These data confirm that bavachin can act as a weak estrogen agonist. It is well known that E2 induces downregulation of ERs as early as 30 min in E2-responsive cells. ERα protein levels were downregulated by the treatment of 10 nM E2 or 10 μM bavachin as compared with the vehicle control. But 0.1 μM and 1 μM of bavachin did not affect ERα protein levels (Fig.5 A). These data indicate that more than 10 μM of bavachin is capable of inducing ERα degradation as effectively as E2 in MCF-7 cells.
Fig. 4. Effects of bavachin on endogenous estrogen-responsive PR and pS2 mRNA levels were examined. Cells were exposed to E2 and bavachin for 24 h. The qRT-PCR results for PR, pS2 in MCF-7 cells are shown (n=3).
Fig. 5. Effects of bavachin on ERα protein levels. (A) MCF-7 cells were treated for 24 h with 10 nM E2 or increasing concentrations of bavachin as shown. An untreated group served as a control. After the incubation, the cells were lysed and total protein extracts were resolved by SDS-PAGE and immunoblotted using an anti-ER antibody or an anti-β-actin antibody. ER densitometry values are expressed as a percentage of the control (down). These experiments were repeated at least three times. * and ** indicate statistically significant differences from the control. (B) MCF-7 cells were treated for 12 h with or without 10 μM bavachin and 10 μM MG132. After the incubation, the cells were lysed and total protein extracts were resolved by SDS-PAGE and immunoblotted using an anti-ERα antibody or an anti-β-actin antibody. ER densitometry valuesare expressed as a percentage of the control (down). These experiments were repeated at least three times. *Indicate statistically significant differences from control.
ERα is degradaded at the post-translational steps upon E2
binding via ubiquitin–proteasome pathway (Lim et al., 2011). To evaluate the involvement of the proteasomal pathway in bavachin-mediated degradation of ERα, MCF-7 cells were treated with or without 10 μM bavachin and 1 μM of the proteasome inhibitor MG132 for 12 h. As shown in Fig.5 B, ERα degradation was blocked by MG132, suggesting that bavachin
caused proteasomal-mediated ERα degradation.
Effects of bavachin on the proliferation of MCF-7 BOS cell
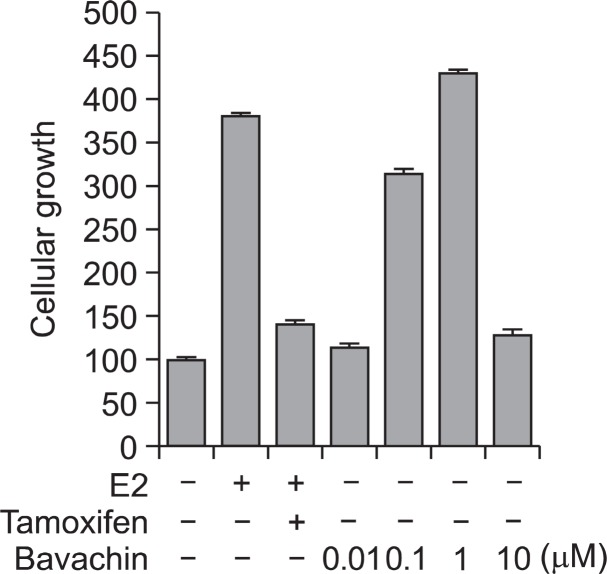
The estrogenic activities of bavachin were confirmed by the proliferation assay of MCF-7 BOS cells that make up an ER positive human breast adenocarcinoma cell line (Bae et al., 2011). The maximum cell proliferative effect was observed by the treatment of 10 nM E2. The proliferation effect of bavachin was expressed as the relative proliferative effect to that of media treated control. Bavachin significantly stimulated the proliferation of MFC-7 BOS cells in a dose-dependent manner by 6 days of treatment (Fig.6 ).
Fig. 6. Effects of bavachin on the proliferation of MCF-7 BOS cells. MCF-7 BOS cells were plated in 96 well plates and grown in phenol red-free DMEM containing 5% CD-FBS for 2 days. Cells were treated as indicated and cultured for 6 days. The cell growth was determined by MTT method and expressed as the relative proliferative effect to E2. Data show mean ± SD (n=4).
DISCUSSION
About 25 million women worldwide experience menopause each year. It has been estimated that by the year 2030, the world population of menopausal and postmenopausal women will be 1.2 billion, with 47 million new entrants each year (Borrelli and Ernst, 2008). Hormone replacement therapy (HRT) using synthetic female steroid hormones is the treatment of choice to alleviate menopausal symptoms, but because of the potential for serious adverse effects of long-term HRT, there has been an increased interest in complementary and alternative medicine, including phytoestrogen (Borrelli and Ernst, 2010). For example, pomegranate was recently shown to contain estrogenic activity, and sales of pomegranate fruit juice reached up to 63 million dollars in 2005 in the US (Sturgeon and Ronnenberg, 2010). Regarding the increase in the population of menopausal women and the interest in alternative medicine to relieve menopausal symptoms, two scientific approaches should be considered. One is to reveal the clinical efficacies and cellular mechanisms of the actions of phytoestrogenic herbs, and the other is to search for new and improved phytoestrogen sources from natural products. The Gao group recently reported that 7 compounds from P. corylifolia activated ER-responsive reporter gene activity (Xin et al., 2010). In this study, we evaluated the estrogenic activity of bavachin, a compound purified from P. coryfolia in further detail. We examined the induction of pS2 and PR mRNA and degradation of ERα by bavachin treatment. In accordance with both the luciferase assay and the ligand binding data, bavachin effectively regulated ER targets through binding both isoforms of ER. In addition, our data showed that bavachin ef
fectively downregulated ERα, which can lead to differential expression of ER isoforms depending on the stability and duration of bavachin in vivo. Bavachin activated both ERα and ERβleading to modulation of ER target genes. However, the EC50 values of receptor binding as examined by receptor competition assays were higher than those of reporter gene activation. This implies that bavachin activates ERs both by direct binding and by an indirect mechanism other than that of classical hormone-mediated activation. ERs can be activated by modu
lation of phosphorylation pathways in a ligand-independent pathway (O’Malley et al., 1995). It is possible that bavachin modulates cellular targets other than ERs, which can in turn influence ERs. A recent report showed that bavachin decreases the IL-1β-induced activation of IκB kinase-IkBα-NF-κB signaling pathway in human chondrocytes (Cheng et al., 2010). This implies that bavachin can be chondroprotective like E2 through estrogenic potential. One of the most extensively studied phytoestrogen is soy derived isoflavone genistein. Genistein was studied both in vitro and in vivo. In in vitro studies, genistein has a 20-to 30- higher binding affinity toward ERβ but only a slight preference to ERβ in transactivation efficacy (Mueller et al., 2004; Henley and Korach, 2010). This indicates that genistein binding to ERβ cannot fully activate ERβ. The potential therapeutic benefits of genistein is still unconfirmed because although some studies show beneficial effects on breast cancer but others demonstrated contradictory results that genistein intake increase mammary cancer rates (Henley and Korach, 2010; Virk-Baker et al., 2010). Bavachin showed approximately two-fold preference toward ERβ over ERα in both binding and reporter assays which implies that no inhibiting mechanism exists in ERβ activation. Since accumulating studies are showing that ERβ has tumor suppressing activity bavachin may elicit less tumor promoting activity than genistein. Continued studies are required to demonstrate the characteristics of bavachin as an applicable phytoestrogen.
In this study, we showed that bavachin can effectively modulate cellular ER targets through ERs. Further studies will investigate the detailed cellular mechanism and in vivo efficacy of bavachin.
Acknowledgments
This work was supported by Basic Science Research Program (KRF-2008-313-E00769) and MRC (2011-0030699) grant of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) and Technology Development Program for Agriculture and Forestry, Ministry for Agriculture, Forestry and Fisheries (109127-03-1-HD110).
Contributor Information
Young Joo Lee, Phone: +82-2-3408-3640, FAX: +82-2-3408-4334.
Jae-Ha Ryu, Phone: +82-2-710-9568, FAX: +82-2-714-0745.
References
- 1.Bae U. J. Lee D. Y. Song M. Y. Lee S. M. Park J. W. Ryu J. H. Park B. H. A prenylated flavan from Broussonetia kazinoki prevents cytokine-induced β-cell death through suppression of nuclear factor-κB activity. Biol. Pharm. Bull. (2011);34:1026–1031. doi: 10.1248/bpb.34.1026. [DOI] [PubMed] [Google Scholar]
- 2.Borrelli F. Ernst E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms: a systematic review of its efficacy. Pharmacol. Res. (2008);58:8–14. doi: 10.1016/j.phrs.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Borrelli F. Ernst E. Alternative and complementary therapies for the menopause. Maturitas. (2010);66:333–343. doi: 10.1016/j.maturitas.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Cederroth C. R. Nef S. Soy, phytoestrogens and metabolism: A review. Mol. Cell Endocrinol. (2009);304:30–42. doi: 10.1016/j.mce.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Chang E. C. Charn T. H. Park S. H. Helferich W. G. Komm B. Katzenellenbogen J. A. Katzenellenbogen B. S. Estrogen Receptors alpha and beta as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol. Endocrinol. (2008);22:1032–1043. doi: 10.1210/me.2007-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang E. C. Frasor J. Komm B. Katzenellenbogen B. S. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. (2006);147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 7.Cheng C. C. Chen Y. H. Chang W. L. Yang S. P. Chang D. M. Lai J. H. Ho L. J. Phytoestrogen bavachin mediates anti-inflammation targeting Ikappa B kinase-I kappaB alpha-NF-kappaB signaling pathway in chondrocytes in vitro. Eur. J. Pharmacol. (2010);636:181–188. doi: 10.1016/j.ejphar.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Choi J. H. Rho M. C. Lee S. W. Choi J. N. Kim K. Song G. Y. Kim Y. K. Bavachin and isobavachalcone acyl-coenzyme A: cholesterol acyltransferase inhibitors from Psoralea corylifolia. Arch. Pharm. Res. (2008);31:1419–1423. doi: 10.1007/s12272-001-2126-x. [DOI] [PubMed] [Google Scholar]
- 9.Davis V. L. Jayo M. J. Ho A. Kotlarczyk M. P. Hardy M. L. Foster W. G. Hughes C. L. Black cohosh increases metastatic mammary cancer in transgenic mice expressing c-erbB2. Cancer Res. (2008);68:8377–8383. doi: 10.1158/0008-5472.CAN-08-1812. [DOI] [PubMed] [Google Scholar]
- 10.Gehm B. D. McAndrews J. M. Jordan V. C. Jameson J. L. EGF activates highly selective estrogen-responsive reporter plasmids by an ER-independent pathway. Mol. Cell Endocrinol. (2000);159:53–62. doi: 10.1016/S0303-7207(99)00195-1. [DOI] [PubMed] [Google Scholar]
- 11.Haraguchi H. Inoue J. Tamura Y. Mizutani K. Antioxidative components of Psoralea corylifolia (Leguminosae). Phytother. Res. (2002);16:539–544. doi: 10.1002/ptr.972. [DOI] [PubMed] [Google Scholar]
- 12.Henley D. V. Korach K. S. Physiological effects and mechanisms of action of endocrine disrupting chemicals that alter estrogen signaling. Hormones (Athens). (2010);9:191–205. doi: 10.14310/horm.2002.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung B. I. Kim M. S. Kim H. A. Kim D. Yang J. Her S. Song Y. S. Caffeic acid phenethyl ester, a component of beehive propolis, is a novel selective estrogen receptor modulator. Phytother. Res. (2010);24:295–300. doi: 10.1002/ptr.2966. [DOI] [PubMed] [Google Scholar]
- 14.Kronenberg F. Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann. Intern. Med. (2002);137:805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 15.Latha P. G. Evans D. A. Panikkar K. R. Jayavardhanan K. K. Immunomodulatory and antitumour properties of Psoralea corylifolia seeds. Fitoterapia. (2000);71:223–231. doi: 10.1016/S0367-326X(99)00151-3. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y. Jin Y. Lim W. Ji S. Choi S. Jang S. Lee S. A ginsenoside-Rh1, a component of ginseng saponin, activates estrogen receptor in human breast carcinoma MCF-7 cells. J. Steroid. Biochem. Mol. Biol. (2003);84:463–468. doi: 10.1016/S0960-0760(03)00067-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y. J. Gorski J. Estrogen-induced transcription of the progesterone receptor gene does not parallel estrogen receptor occupancy. Proc. Natl. Acad. Sci. USA. (1996);93:15180–15184. doi: 10.1073/pnas.93.26.15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim W. Park Y. Cho J. Park C. Park J. Park Y. K. Park H. Lee Y. Estrogen receptor beta inhibits transcriptional activity of hypoxia inducible factor-1 through the downregulation of arylhydrocarbon receptor nuclear translocator. Breast Cancer Res. (2011);13:R32. doi: 10.1186/bcr2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller S. O. Simon S. Chae K. Metzler M. Korach K. S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol. Sci. (2004);80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 20.Newton K. M. Reed S. D. Grothaus L. Ehrlich K. Guiltinan J. Ludman E. Lacroix A. Z. The Herbal Alternatives for Menopause (HALT) Study: background and study design. Maturitas. (2005);52:134–146. doi: 10.1016/j.maturitas.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Obourn J. D. Koszewski N. J. Notides A. C. Hormone-and DNA-binding mechanisms of the recombinant human estrogen receptor. Biochemistry. (1993);32:6229–6236. doi: 10.1021/bi00075a016. [DOI] [PubMed] [Google Scholar]
- 22.O'Malley B. W. Schrader W. T. Mani S. Smith C. Weigel N. L. Conneely O. M. Clark J. H. An alternative ligand-independent pathway for activation of steroid receptors. Recent Prog. Horm. Res. (1995);50:333–347. doi: 10.1016/b978-0-12-571150-0.50020-2. [DOI] [PubMed] [Google Scholar]
- 23.Park Y. Park J. Lee Y. Lim W. Oh B. C. Shin C. Kim W. Lee Y. Mammalian MST2 kinase and human Salvador activate and reduce estrogen receptor alpha in the absence of ligand. J. Mol. Med. (Berl). (2011);89:181–191. doi: 10.1007/s00109-010-0698-y. [DOI] [PubMed] [Google Scholar]
- 24.Setchell K. D. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. (1998);68((6 Suppl)):1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 25.Ström A. Hartman J. Foster J. S. Kietz S. Wimalasena J. Gustafsson J. A. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc. Natl. Acad. Sci. USA. (2004);101:1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sturgeon S. R. Ronnenberg A. G. Pomegranate and breast cancer: possible mechanisms of prevention. Nutr. Rev. (2010);68:122–128. doi: 10.1111/j.1753-4887.2009.00268.x. [DOI] [PubMed] [Google Scholar]
- 27.Virk-Baker M. K. Nagy T. R. Barnes S. Role of phytoestrogens in cancer therapy. Planta. Med. (2010);76:1132–1142. doi: 10.1055/s-0030-1250074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D. Li F. Jiang Z. Osteoblastic proliferation stimulating activity of Psoralea corylifolia extracts and two of its flavonoids. Planta Med. (2001);67:748–749. doi: 10.1055/s-2001-18343. [DOI] [PubMed] [Google Scholar]
- 29.Xin D. Wang H. Yang J. Su Y. F. Fan G. W. Wang Y. F. Zhu Y. Gao X. M. Phytoestrogens from Psoralea corylifolia reveal estrogen receptor-subtype selectivity. Phytomedicine. (2010);17:126–131. doi: 10.1016/j.phymed.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Yin S. Fan C. Q. Wang Y. Dong L. Yue J. M. Anti-bacterial prenylflavone derivatives from Psoralea corylifolia, and their structure-activity relationship study. Bioorg. Med. Chem. (2004);12:4387–4392. doi: 10.1016/j.bmc.2004.06.014. [DOI] [PubMed] [Google Scholar]