Abstract
Ultraviolet (UV) A penetrates deeply into the skin and induces the generation of reactive oxygen species (ROS) causing damage to fibroblasts, which leads to aging of the skin. However, the body has developed an antioxidant defence system against the harmful effects of ROS. Enzymes such as superoxide dismutase (SOD) and catalase (CAT) play critical roles on the removal of excess ROS in living organisms. In this study, the antioxidant activities of anthocyanins (cyanidin 3-galactoside and cyanidin 3-lathyroside) from Acanthopanax divaricatus var. albeofructus (ADA) fruits were investigated by xylenol orange, thiobarbituric acid reactive substances (TBARS), and antioxidant enzyme assay. As a result, generation of H2O2 and lipid peroxide induced by UVA-irradiation in human dermal fibroblast (HDF-N) cells was reduced by treatment of anthocyanins. Also, augmented enzyme (SOD and CAT) activities were observed in UVA-irradiated cells when treated with anthocyanin. In conclusion, the results obtained show that anthocyanins from ADA fruits are potential candidates for the protection of fibroblast against the damaging effects of UVA irradiation. Furthermore, anthocyanin may be a good candidate for antioxidant agent development.
Keywords: UVA, Fibroblast, Acanthopanax, Anthocyanins, Antioxidant
INTRODUCTION
Exposure of human skin to ultraviolet A (UVA) radiation can induce various biological responses, ranging from erythema to photoaging (Matsumura and Ananthaswamy, 2004; Wlaschek et al., 2001), because the solar UV spectrum penetrates through the dermis to the subcutaneous tissue and affects both the epidermal and dermal components of skin (Tarozzi et al., 2005). At the cellular level, UVA radiation causes significant oxidative stress because it leads to the generation of ROS, exerting a variety of harmful effects including oxidation of nucleic acids, proteins and membrane lipids (Morita and Krutmann, 2000; Ichihashi et al., 2003; Urso and Clarkson, 2003).
Fundamentally, protection against solar UV-induced oxidative damage to human skin relies on avoiding excessive sunlight exposure and on the use of sunscreens. Topical and endogenous photoprotection by antioxidants could have the potential to complement these strategies (Chiu and Kimball, 2003; Tarozzi et al., 2005), since ROS are the main responsible for the biological effects caused by the photo-oxidative stress. The antioxidants may raise the levels of endogenous defense by up regulating the expression of genes encoding the enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and lipid peroxidase (Aruoma, 1994; McCord, 1994).
The use of natural ingredients in cosmetics is steadily increasing. High levels of phytochemicals, especially anti-oxidants are commonly found in many of these plants. Acanthopanax divaricatus var. albeofructus (ADA, Araliaceae), an indigenous variant of Acanthopanax species in Korea, is a medicinal herb with a long history of use. Several parts of this plant have been used for the treatment of various diseases such as rheumatism, hypertension, and hepatitis (Yi et al., 2001). The ripe berries of Acanthopanax species, Acanthopanacis fructus have been widely used as restorative beverage. Current studies revealed that the water extract of ADA leaves possesses anti-oxidative (Kim and Yang, 2004; Lyu et al., 2006) and immunomodulatory activities (Lyu et al., 2008), and the methanol extract of ADA exhibits anti-lipid peroxidation activity (Zu and Yang, 2004). Recently two anthocyanin compounds were isolated and identified from the fruits of ADA. Their structures were elucidated as cyanidin 3-lathyroside and cyanidin 3-galactoside (Hahn and Park, 2010).
Anthocyanins are classified as a flavonoid group of the phytochemicals (Cambie and Ferguson, 2003), which are usually found in teas, fruits, vegetables, and nuts (Wang et al., 1997; Zhang et al., 2005). Basically, anthocyanin contains the core polyphenolic ring structure, anthocyanidin. This anthocyanidin consists of an aromatic A ring bonded to an heterocyclic C ring that contains oxygen, which is in turn linked by a carbon–carbon bond to a third aromatic B ring (Wang et al., 1997; Escribano-Bailón et al., 2004). When anthocyanidin was found in their glycoside form, it is known as anthocyanin. Most of the natural anthocyanin glycosides are substituted at OH group of C ring by sugar moieties, such as arabinose, galactose, glucose, sophorose, rhamnose, and xylose (Escribano-Bailón et al., 2004). It has been known that anthocyanins possess antitumor, antiulcer and anti-inflammatory properties (Konczak-Islam et al., 2003; Hou et al., 2004; Stintzing and Carle, 2004). In earlier reports, a positive correlation was found between antioxidant activity and the content of anthocyanin (Heinonen et al., 1998; Kähkönen et al., 2001). Furthermore, some anthocyanins showed greater antioxidant effect than vitamins C and E (Bagchi et al., 1998).
In this study, we investigated the antioxidant activity of two anthocyanins (cyanidin 3-galactoside and cyanidin 3-lathy-roside) from ADA fruits in UV-irradiated human dermal fibroblasts (HDF-N) to evaluate the utility of ADA fruits as a potential source of antioxidants that can protect against UV-induced oxidative damage in human skin.
MATERIALS AND METHODS
Isolation of anthocyanins
Cyanidin 3-galactoside and cyanidin 3-lathyroside were isolated and identified as reported previously (Hahn and Park, 2010).
Cell culture and UVA irradiation
Human dermal fibroblast (HDF-N) cell line was obtained from Modern Tissue Technology (MTT, Korea) and maintained in DMEM (Dulbecco's modified Eagle's medium, GibcoBRL, Grand Island, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO BRL, Grand Island, USA), streptomycin (100 μg/ml; Sigma), and penicillin (100 U/ml; Sigma) at 37℃ in a humidified atmosphere containing 5% CO2.
HDF-N cells (1×106 cells/ml) were divided into 100 mm culture dish, incubated for 24 h, and washed with PBS (phosphate buffered saline, Sigma, St Louis, MI, USA). The culture medium of the whole plate was replaced by PBS and exposed to UVA (8 J/cm2) for 30 min. Immediately after UVA exposure, the cells were washed with PBS. The UV-irradiated cells were incubated with cyanidin 3-lathyroside and cyanidin 3-galactoside for 48 h. The cells in 300 μl of lysis buffer (20 mM HEPES, pH 7.0/1 mM EDTA, 2 mM phenylmethylsulfonal fluoride) were broken by sonication and centrifuged, and the supernatant was used for measurement of antioxidative activities.
MTT assay
The MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium bromide, a tetrazole)) assay is a colorimetric assay based on the ability of viable cells to reduce a soluble MTT to blue formazan crystals. Cells (1×104 cells/well) were seeded onto 96-well plates, grown for 24 h, treated with anthocyanin, and incubated for 48 h. After treatment, MTT dye (5 mg/ml; Sigma) was added and the plates were incubated for another 4 h at 37℃. The absorbance at λmax=595 nm was determined by dissolving the formazan crystals with dimethyl sulfoxide (DMSO), and the absorbance was measured with a microplate reader (Molecular Devices, Sunnylvale, CA, USA).
Detection of H2O2 in cells
The generation of H2O2 in cells was detected by addition of xylenol orange to the cell extract by measuring the absorbance at λmax=560 nm. To 50 μl of cell extract (2 mg/ml of protein), 950 μl of ferrous oxidation-xylenol orange (FOX) solution (0.1 mM xylenol orange, 0.25 mM ammonium ferrous sulfate, 100 mM sorbitol, 25 mM H2SO4) was added. The reaction was run for 30 min at room temperature, and the absorbance at λmax=560 nm was measured.
Lipid peroxidation activity
Thiobarbituric acid (TBA) reacts with malondialdehyde (MDA) to form a pink chromogen, which can be detected spectrophotometrically at 532 nm (Buege and Aust, 1978). In this reaction the thiobarbituric acid reactive substance (TBARS) was measured in terms of MDA and expressed as MDA equivalent. One milliliter of TBA-trichloroacetic acid-HCl solution (0.375% TBA/15% TCA in 0.25M HCl) was added to 20 μl of cell extract (5 mg/ml of protein), heated at 95℃ for 30 min in water bath, cooled, and centrifuged at 2,000 rpm for 10 min. The absorbance of supernatant was measured at λmax=532 nm and expressed as MDA level.
CAT assay
CAT assay was carried out by the method of Aebi (Aebi, 1984).
Twenty microliter of cell extract (5 mg/ml of protein) was added to 980 μl of 15 mM H2O2 solution (in 50 mM phosphate buffer, pH 7.0), and the decrease in optical density due to decomposition of hydrogen peroxide was measured. The units of catalase were expressed as the amount of H2O2 decomposed for 1 min by catalase in terms of 1 μM/min.
Estimation of SOD
The SOD assay was based on the inhibition of auto-oxidation of pyrogallol (Shin et al., 2007). Twenty microliter of cell extract (5 mg/ml of protein) and 10 μl of 0.2 mM pyrogallol were added to 970 μl of 50 mM Tris-HCl buffer (pH 8.2) containing 1 mM EDTA and incubated at 25℃ for 10 min. The reaction was stopped by adding 1 mM HCl and the absorbance at λmax=440 nm was measured. The units of SOD were expressed as the amount of SOD enzyme required to inhibit the rate of auto-oxidation of pyrogallol by 50%.
Statistical analysis
All values expressed as mean ± S.E.M. were obtained from at least three observations and were compared using Student’s t-test test. Differences were significant; probability values of <0.001, <0.01, or <0.05 were considered significant with 99.9%, 99% or 95% of confidence, respectively.
RESULTS
Effect of UV and anthocyanins on the HDF-N cells
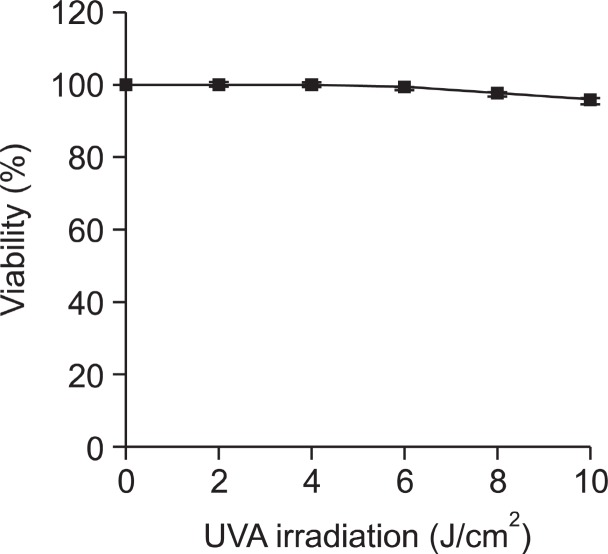
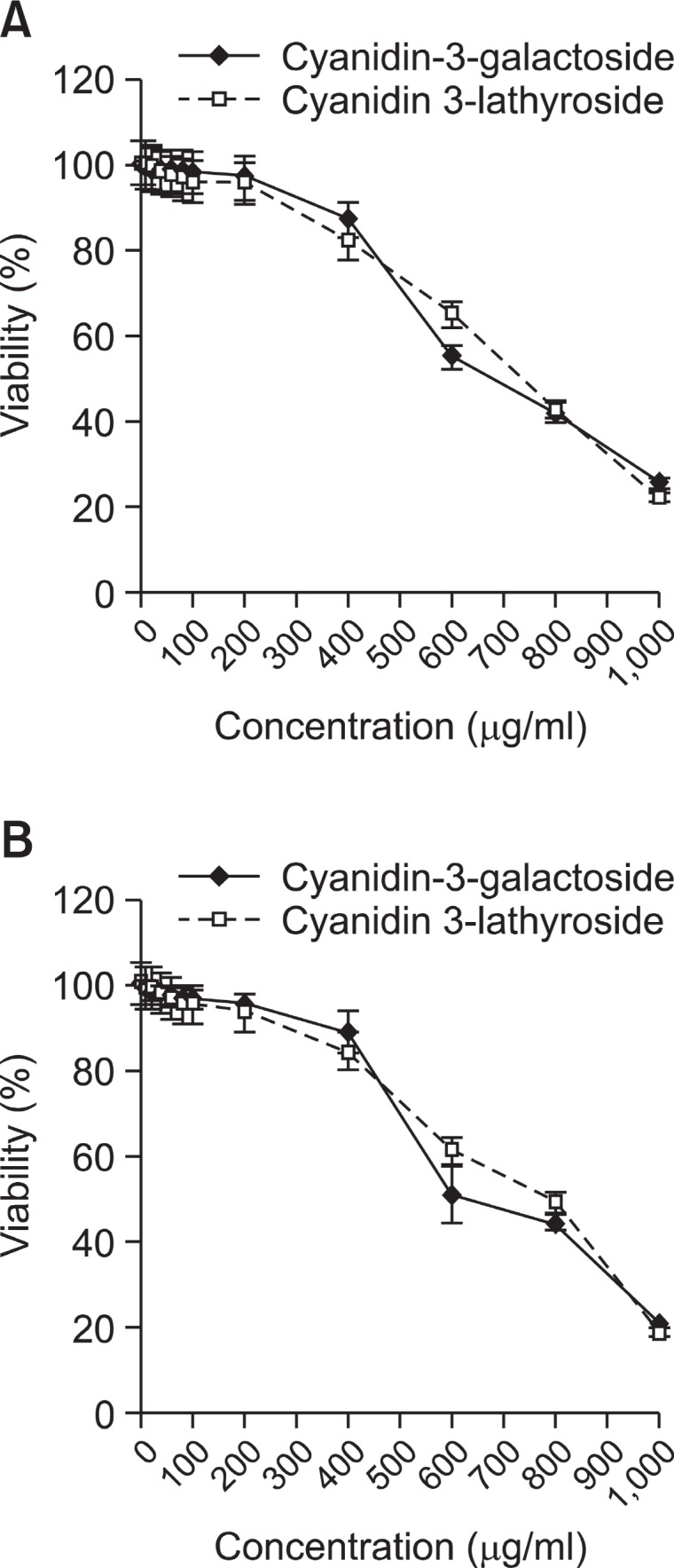
Prior to studying photoprotective activities of cyanidin 3-galactoside and cyanidin 3-lathyroside, cytotoxic effects of UV and the anthocyanins on HDF-N cells were investigated. Cells were irradiated with 0-10 J/cm2 of UVA, incubated for 48 h and the viability was determined by MTT assay. The HDF-N cells showed more than 95.6% survival rate when irradiated with 0-10 J/cm2 of UVA (Fig. 1). Next, cells treated with cyanidin 3-galactoside and cyanidin 3-lathyroside for 48 h showed more than 93.2% survival rate at concentrations lower than 100 μg/ml (Fig. 2A). In addition, UVA-irradiated cells treated with cyanidin 3-galactoside and cyanidin 3-lathyroside for 48 h showed more than 91.9% survival rate at the same concentration (Fig. 2B). The results showed that the selected UV intensity and concentration of cyanidin 3-galactoside and cyanidin 3-lathyroside used in this experiment had no major effect on the cells when compared to the controls.
Fig. 1. Viability of human dermal fibroblast (HDF)-N cells irradiated by UVA (0-8 J/cm2). The HDF-N cells were irradiated by UVA and the viability was determined by MTT assay. For all data, values were the mean S.E.M. of 3 separated experiments.
Fig. 2. Cell viability of HDF-N cells and UV (8 J/cm2)-irradiated HDF-N cells incubated with cyanidin-3-galactoside and cyanidin3-lathyroside. (A) HDF-N cells and (B) UVA (8 J/cm2)-irradiated HDF-N cells were incubated with cyanidin-3-galactoside and cyan-idin-3-lathyloside for 48 h and the viability was determined by MTT assay. For all data, values were the mean S.E.M. of 3 separated experiments.
Effect of cyanidin 3-galactoside and cyanidin 3-lathyroside on the generation of H2O2 in UVA -irradiated HDF-N cells
It has been reported that ROS, in particular superoxide anions, hydroxyl radicals, and hydrogen peroxides are the
key mediators of many of the UV light induced biological effects (Heck et al., 2004). UV light can stimulate donation of an electron to molecular oxygen (O2), present under normal conditions in cells, and produce the free radical superoxide anion, which can gain another electron from UV light to generate H2O2 (Poquet et al., 2008). The cytotoxicity of H2O2 results from its reaction with iron to form the hydroxyl radical, the most reactive compound of the ROS, which can react very rapidly with any type of molecule. Thus H2O2 is a good ROS indicator generated in cultured human fibroblasts by UV A radiation. In this study, it was observed that UVA-irradiation generated H2O2 1.64 times more than the control in HDF-N cells. However, the generation of H2O2 was reduced in the UVA-irradiated HDF-N cells incubated with cyanidin 3-galactoside and cyanidin 3-lathyroside in a dose-dependent manner. There was no significant difference between cyanidin 3-galactoside and cyanidin 3-lathyroside (Table 1).
Table 1.
Effect of cyanidin-3-galactoside and cyanidin 3-lathyroside on the generation of H2O2 in UVA (8 J/cm2)-irradiated HDF-N cells
| UVA (8 J/cm2) | Conc. (μg/ml) | Ratio of H2O2 generation | |
|---|---|---|---|
|
| |||
| Cyanidin-3-galactoside | Cyanidin 3-lathyroside | ||
|
| |||
| - | 0 | 1.00 | 1.00 |
| + | 0 | 1.64 ± 0.07 | 1.64 ± 0.07 |
| + | 5 | 1.67 ± 0.07 | 1.67 ± 0.06 |
| + | 10 | 1.62 ± 0.10 | 1.51 ± 0.04* |
| + | 50 | 1.46 ± 0.09* | 1.23 ± 0.10** |
| + | 100 | 1.18 ± 0.02*** | 1.13 ± 0.04*** |
UVA (8 J/cm2)-irradiated HDF-N cells were incubated with cyanidin-3-galactoside and cyanidin-3-lathyloside for 48 h. Cells in lysis buffer were broken by sonication and were centrifuged. The generation of H2O2 was detected by addition of xylenol orange to the cell extract by measuring the absorbance at λmax = 560 nm. Significant difference in comparison with control at *p<0.05, **p<0.01, and ***p<0.001.
Effect of cyanidin 3-galactoside and cyanidin 3-lathyroside on lipid peroxidation in UVA-irradiated HDF-N cells
The targets of reactive oxygen species in the skin are lipids, enzymes, collagen, carbohydrates, and DNA (Pugliese, 1995). In the present study, UVA-irradiated HDF-N cells were incubated with cyanidin 3-galactoside and cyanidin 3-lathyro-
side and the lipid peroxide in cells was measured by TBARS method. First, we measured the lipid peroxidation in UVA-irradiated cells. As a result, the lipid peroxidation was increased up to 1.85 times. The increase in MDA levels in cells suggests enhanced lipid peroxidation leading to tissue damage and failure of antioxidant defense mechanisms to prevent formation of excessive free radicals. However, the treatment of anthocyanins, cyanidin 3-galactoside and cyanidin 3-lathyroside, reversed these changes. The lipid peroxidation in cells was greatly reduced up to 1.19 times by the treatment of cyanidin 3-lathyroside, while the cyanidin 3-galactoside inhibited lipid peroxidation to a lesser extent (Table 2). This suggests that the protection of anthocyanins towards the lipid peroxidation which leads to damaging effects of UV in cells, may happen through an interference with the reactions initiated by reactive oxygen species such as H2O2.
Table 2.
Effect of cyanidin-3-galactoside and cyanidin 3-lathyroside on lipid peroxidation in UVA (8 J/cm2)-irradiated HDF-N cells
| UVA (8 J/cm2) | Conc. (μg/ml) | Ratio of lipid peroxidation | |
|---|---|---|---|
|
| |||
| Cyanidin-3-galactoside | Cyanidin 3-lathyroside | ||
|
| |||
| - | 0 | 1.00 | 1.00 |
| + | 0 | 1.85 ± 0.04 | 1.85 ± 0.04 |
| + | 5 | 1.72 ± 0.10 | 1.75 ± 0.11 |
| + | 10 | 1.63 ± 0.16* | 1.58 ± 0.02** |
| + | 50 | 1.51 ± 0.06** | 1.23 ± 0.09*** |
| + | 100 | 1.41 ± 0.05** | 1.19 ± 0.10*** |
UVA (8 J/cm2)-irradiated HDF-N cells were incubated with cyanidin-3-galactoside and cyanidin-3-lathyloside for 48 h. Cells in lysis buffer were broken by sonication and were centrifuged. The lipid peroxidation was determined by TBARS method. MDA levels were measured by the absorbance at 540 nm. Significant difference in comparison with control at *p<0.05, **p<0.01, and ***p<0.001.
Effect of cyanidin 3-galactoside and cyanidin 3-lathyroside on the activity of SOD and CAT in UVA-irradiated HDF-N cells
SOD, CAT and GPx enzymes are important scavengers of superoxide ion and hydrogen peroxide. These enzymes prevent generation of hydroxyl radical and protect the cellular constituents from oxidative damage (Dash et al., 2007). Therefore, modulation of antioxidant enzymes such as SOD and CAT may have the ability to protect against oxidative stress. SOD is found in all tissues and cells of the aerobic organism. SOD is considered fundamental in the process of eliminating ROS by reducing (adding an electron to) superoxide to form H2O2. The SOD that was demonstrated to have ROS-metabolizing activity can efficiently and specifically catalyze dismutation of O2·− to O2 and H2O2 (Waddington et al., 2000). CAT is also an enzymatic antioxidant widely distributed in all animal cells. CAT decomposes hydrogen peroxide and protects the tissue from highly reactive hydroxyl radicals. Therefore the reduction in the activity of the enzyme may result in a number of deleterious effects due to the accumulation of superoxide radicals and hydrogen peroxide. It has been reported that UV radiation of skin is thought to deplete antioxidant enzymes involved in the defence of the cells (Dash et al., 2007). In the present study, we detected the effect of anthocyanins on these enzymes in UVA ir-radiated HDF-N cells. It was observed that the UVA irradiation significantly reduced the enzyme activity in HDF-N cells. However, augmented enzyme activity was observed in cells treated with anthocyanins. Similar to our previous results on lipid peroxidation, cyanidin 3-lathyroside enhanced enzyme activity more significantly than cyanidin 3-galactoside (Table 3, 4). Our results suggests that the inhibitory effect of anthocyanins on lipid peroxidation induced by UVA exposure may be closely related to an interference with the reactions initiated by regenerating the enzymatic antioxidant defense system of cells.
Table 3.
Effect of cyanidin-3-galactoside and cyanidin 3-lathyroside on SOD activity (U/mg) in UVA (8 J/cm2)-irradiated HDF-N cells
| UVA (8 J/cm2) | Conc. (μg/ml) | SOD activity | |
|---|---|---|---|
|
| |||
| Cyanidin-3-galactoside | Cyanidin 3-lathyroside | ||
|
| |||
| - | 0 | 13.2 ± 1.5 | 14.3 ± 0.2 |
| + | 0 | 7.7 ± 1.1 | 8.5 ± 0.4 |
| + | 5 | 8.1 ± 1.1 | 9.0 ± 0.2 |
| + | 10 | 8.5 ± 1.2 | 9.8 ± 0.4 |
| + | 50 | 9.4 ± 0.9* | 10.6 ± 0.1* |
| + | 100 | 9.6 ± 0.9* | 11.2 ± 0.3** |
UVA (8 J/cm2)-irradiated HDF-N cells were incubated with cyanidin-3-galactoside and cyanidin-3-lathyloside for 48 h. Cells in lysis buffer were broken by sonication and were centrifuged. The activities of SOD (U/mg) were determined by measuring the absorbance at 420 nm of the solution of cell extract and pyrogallol. Significant difference in comparison with control at *p<0.05, **p<0.01, and ***p<0.001.
Table 4.
Effect of cyanidin-3-galactoside and cyanidin 3-lathyroside on the CAT activity (U/mg) in UVA (8 J/cm2)-irradiated HDF-N cells
| UVA (8 J/cm2) | Conc. (μg/ml) | Catalase activity | |
|---|---|---|---|
|
| |||
| Cyanidin-3-galactoside | Cyanidin 3-lathyroside | ||
|
| |||
| - | 0 | 55.4 ± 0.2 | 54.5 ± 0.7 |
| + | 0 | 27.8 ± 2.4 | 26.6 ± 0.4 |
| + | 5 | 28.9 ± 2.8 | 27.4 ± 0.4 |
| + | 10 | 30.0 ± 2.6 | 29.2 ± 1.3 |
| + | 50 | 30.3 ± 0.8 | 38.1 ± 1.1** |
| + | 100 | 34.9 ± 1.7* | 39.0 ± 1.2** |
UVA (8 J/cm2)-irradiated HDF-N cells were incubated with cyanidin-3-galactoside and cyanidin-3-lathyloside for 48 h. Cells in lysis buffer were broken by sonication and were centrifuged. The activities of catalase (U/mg) were determined by measuring the absorbance at 240 nm of the solution of cell extract and of H2O2. Significant difference in comparison with control at *p<0.05, **p<0.01, and ***p<0.001.
DISCUSSION
UVA penetrates deeply into the skin to the dermis and induces the generation of ROS causing damage to collagen and to fibroblasts and leading to aging and wrinkling of the skin (Matsumura and Ananthaswamy, 2004; Wlaschek et al., 2001). However, the body has developed an antioxidant defence system against these harmful effects of ROS. Anti-oxidant defence systems are classified as chain breaking anti-oxidants, preventative antioxidants, and antioxidant enzymes that catalyze the breakdown of ROS (Maxwell, 1995). There are several enzymes that play critical roles on the removal of excess ROS in living organism. Among them, SOD, CAT, GPx are the most crucial enzymes in the cellular antioxidant sys-tem. Anthocyanins are natural colorants belonging to the flavonoid family. In this study, we investigated whether anthocyanins (cyanidin 3-galactoside and cyanidin 3-lathyroside) from Acanthopanax divaricatus var. albeofructus fruits had antioxidative activity in human dermal fibroblasts irradiated by UVA. The UVA-irradiation of HDF-N cells increased the generation of H2O2 which was reduced by treatment of cyanidin 3-galactoside and cyanidin 3-lathyroside showing no significant difference between these two anthocyanins. The UVA-irradiation also increased lipid peroxidation in HDF-N cells. However, the treatment of cyanidin 3-galactoside and cyanidin 3-lathyroside protected the cells against lipid peroxidation induced by UVA exposure. In addition, the UVA irradiation significantly reduced the enzyme activity of SOD and CAT in HDF-N cells. However, augmented enzyme activities were observed in cells treated with anthocyanins. Similar to the results of lipid peroxidation, cyanidin 3-lathyroside enhanced enzyme activity more greatly than cyanidin 3-galactoside.
Our results suggested that the inhibitory effect of anthocyanins on lipid peroxidation may be closely related to an interference with the reactions initiated by regenerating the enzymatic antioxidant defense system of cells, rather than by an interference with the reactions initiated by H2O2. And higher activity of SOD and CAT could make the cells capable of tolerating high H2O2 concentrations.
In conclusion, the results obtained in the present work show that cyanidin 3-galactoside and cyanidin 3-lathyroside are potential candidates for the protection of fibroblast against the damaging effects of UVA irradiation. In addition, it is suggested that cyanidin 3-galactoside and cyanidin 3-lathyroside could be beneficial in the treatment of several diseases associated with oxidative stress through the enhanced antioxidant defense. This result reveals that anthocyanins from Acanthopanax divaricatus var. albeofructus fruits may be a good candidate for antioxidative agent development.
Acknowledgments
This work was supported by a special research grant from Seoul Women’s University (2012).
References
- 1.Aebi H. Catalase in vitro. Methods Enzymol. (1984);105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 2.Aruoma O. I. Nutrition and health aspects of free radicals and antioxidants. Food Chem. Toxicol. (1994);32:671–683. doi: 10.1016/0278-6915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 3.Bagchi D. Garg A. Krohn R. L. Bagchi M. Bagchi D. J. Balmoori J. Stohs S. J. Protective effects of grape seed pro-anthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen. Pharmacol. (1998);30:771–776. doi: 10.1016/S0306-3623(97)00332-7. [DOI] [PubMed] [Google Scholar]
- 4.Buege J. A. Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. (1978);52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 5.Cambie R. C. Ferguson L. R. Potential functional foods in the traditional Maori diet. Mutat. Res. (2003);523-524:109–117. doi: 10.1016/S0027-5107(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 6.Chiu A. Kimball A. B. Topical vitamins, minerals and botanical ingredients as modulators of environmental and chronological skin damage. Br. J. Dermatol. (2003);149:681–691. doi: 10.1046/j.1365-2133.2003.05540.x. [DOI] [PubMed] [Google Scholar]
- 7.Dash D. K. Yeligar V. C. Nayak S. S. Ghosh T. Rajalingam R. Sengupta P. Maiti B. C. Maity T. K. Evaluation of hepatoprotective and antioxidant activity of Ichnocarpus frutescens (Linn.) R.Br. on paracetamol-induced hepatotoxicity in rats. Trop. J. Pharm. (2007);6:755–765. [Google Scholar]
- 8.Escribano-Bailón M. T. Santos-Buelga C. Rivas-Gonzalo J. C. Anthocyanins in cereals. J. Chromatogr. A. (2004);1054:129–141. doi: 10.1016/j.chroma.2004.08.152. [DOI] [PubMed] [Google Scholar]
- 9.Hahn D. R. Park S. J. Two cyanidin compound from the fruits of Acanthopanax divaricatus var. albeofructus. Natural Product Sciences. (2010);16:198–201. [Google Scholar]
- 10.Heck D. E. Gerecke D. R. Vetrano A. M. Laskin J. D. Solar ultraviolet radiation as a trigger of cell signal transduction. Toxicol. Appl. Pharmacol. (2004);195:288–297. doi: 10.1016/j.taap.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Heinonen I. M. Meyer A. S. Frankel E. N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. (1998);46:4107–4112. doi: 10.1021/jf980181c. [DOI] [Google Scholar]
- 12.Hou D. X. Fujii M. Terahara N. Yoshimotom M. Molecular Mechanisms Behind the Chemopreventive Effects of Anthocyanidins. J. Biomed. Biotechnol. (2004);2004:321–325. doi: 10.1155/S1110724304403040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichihashi M. Ueda M. Budiyanto A. Bito T. Oka M. Fukunaga M. Tsuru K. Horikawa T. UV-induced skin damage. Toxicology. (2003);189:21–39. doi: 10.1016/S0300-483X(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 14.Kähkönen M. P. Hopia A. I. Heinonen M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. (2001);49:4076–4082. doi: 10.1021/jf010152t. [DOI] [PubMed] [Google Scholar]
- 15.Kim J. Y. Yang K. S. Antioxidantive activities of triterpenoids and lignans from Acanthopanax divaricatus var. albeofructus. Yakhak Hoeji. (2004);49:239–240. [Google Scholar]
- 16.Konczak-Islam I. Yoshimoto M. Hou D. X. Terahara N. Yamak-awa O. Potential chemopreventive properties of anthocyanin-rich aqueous extracts from in vitro produced tissue of sweet-potato (Ipomoea batatas L.) J. Agric. Food Chem. (2003);51:5916–5922. doi: 10.1021/jf030066o. [DOI] [PubMed] [Google Scholar]
- 17.Lyu S. Y. Kim J. Y. Noh B. Park W. B. Antioxidative activity of water extract of different parts of Acanthopanax divaricutus var. albeofructus. Yakhak Hoeji. (2006);50:191–198. [Google Scholar]
- 18.Lyu S. Y. Noh B. Park W. B. Modulation of Th1/Th2 cytokine secretion in a human peripheral blood mononuclear cell by water extract of Acanthopanax divaricatus var. albeofructus fruits. Yakhak Hoeji. (2008);52:27–32. [Google Scholar]
- 19.Matsumura Y. Ananthaswamy H. N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. (2004);195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell S. R. Prospects for the use of antioxidant therapies. Drugs. (1995);49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 21.McCord J. M. Free radicals and pro-oxidants in health and nutrition. Food Technol. (1994);48:106–111. [Google Scholar]
- 22.Morita A. Krutmann J. Ultraviolet A radiation-induced apoptosis. Methods Enzymol. (2000);319:302–309. doi: 10.1016/S0076-6879(00)19031-7. [DOI] [PubMed] [Google Scholar]
- 23.Poquet L. Clifford M. N. Williamson G. Effect of dihydrocaffeic acid on UV irradiation of human keratinocyte HaCaT cells. Arch. Biochem. Biophys. (2008);476:196–204. doi: 10.1016/j.abb.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Pugliese P. T. The skin, free radicals, and oxidative stress. Dermatol. Nurs. (1995);7:361–369. [PubMed] [Google Scholar]
- 25.Shin A. H. Noh B. Kim J. Kim O. K. Park W. B. Antioxidative activity of extracts of Acanthopanax divaricatus var. albeofructus leaves in human dermal fibroblast irradiated by UVA. Yakhak Hoeji. (2007);51:229–234. [Google Scholar]
- 26.Stintzing F. C. Carle R. Functional properties of antocyanins and betalain in plants, food and human nutrition. Trends. Food Sci. Technol. (2004);15:19–38. doi: 10.1016/j.tifs.2003.07.004. [DOI] [Google Scholar]
- 27.Tarozzi A. Marchesl A. Hrelia S. Angeloni C. Andrisano V. Fiori J. Cantelli-Forti G. Hrella P. Protective effects of cyanidin-3-O-beta-glucopyranoside against UVA-induced oxidative stress in human keratinocytes. Photochem. Photobiol. (2005);81:623–629. doi: 10.1562/2004-06-14-RA-200.1. [DOI] [PubMed] [Google Scholar]
- 28.Urso M. L. Clarkson P. M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. (2003);189:41–54. doi: 10.1016/S0300-483X(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 29.Waddington R. J. Moseley R. Embery G. Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis. (2000);6:138–151. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang R. J. Cao R. Prior G. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. (1997);45:304–309. doi: 10.1021/jf960421t. [DOI] [Google Scholar]
- 31.Wlaschek M. Tantcheva-Poór I. Naderi L. Ma W. Schneider L. A. Razi-Wolf Z. Schüller J. Scharffetter-Kochanek K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B. (2001);63:41–51. doi: 10.1016/S1011-1344(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 32.Yi M. Kim M. S. Seo S. W. Lee K. N. Yook C. S. Kim H. M. Acanthopanax senticosus root inhibits mast cell-dependent anaphylaxis. Clin. Chim. Acta. (2001);312:163–168. doi: 10.1016/S0009-8981(01)00613-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y. Vareed S. K. Nair M. G. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. (2005);76:1465–1472. doi: 10.1016/j.lfs.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Zu S. M. Yang K. S. Anti-lipid peroxidation activity of Acanthopanax divaricatus var. albeofructus. Yakhak Hoeji. (2004);48:99–103. [Google Scholar]