Abstract
The present study examined the effects of krill-derived phosphatidylserine (Krill-PS) on the learning and memory function and the neural activity in rats with trimethyltin (TMT)-induced memory deficits. The rats were administered vehicle (medium-chain triglyceride: MCT) or Krill-PS (50, 100 mg/kg, p.o.) daily for 21 days. The cognitive improving efficacy of Krill-PS in TMT-induced amnesic rats was investigated by assessing the Morris water maze test and by performing choline acetyltransferase (ChAT), acetylcholinesterase (AChE) and cAMP responsive element binding protein (CREB) immunohistochemistry. The rats with TMT injection showed impaired learning and memory of the tasks and treatment with Krill-PS produced a significant improvement of the escape latency to find the platform in the Morris water maze at the 2nd and 4th day compared to that of the MCT group (p<0.05). In the retention test, the Krill-PS+MCT groups showed increased time spent around the platform compared to that of the MCT group. Consistent with the behavioral data, Krill-PS 50+MCT group significantly alleviated the loss of acetylcholinergic neurons in the hippocampus and medial septum compared to that of the MCT group. Treatment with Krill-PS significantly increased the CREB positive neurons in the hippocampal CA1 area as compared to that of the MCT group. These results suggest that Krill-PS may be useful for improving the cognitive function via regulation of cholinergic marker enzyme activity and neural activity.
Keywords: Trimethyltin (TMT), Choline acetyltransferase (ChAT), Acetylcholinesterase (AChE), cAMP responsive element binding protein (CREB), Krill-derived phosphatidylserine (Krill-PS), Learning and memory
INTRODUCTION
Phosphatidylserine (PS) is the main acid phospholipid in the inner leaflet of mammalian plasma membranes (Freyz and Vincendon, 1982). Because of its abundant presence in the brain and Vincendon, PS has been shown to play a key role in the functioning of central nervous system (Vakhapova et al., 2010). The attenuating effects of PS on memory impairment associated with Alzheimer’s disease (AD) or aging have been demonstrated in several clinical study (Cenacchi et al., 1993; Kataoka-Kato et al., 2005). Early observations associated the administration of PS extracted from bovine cortex (BC-PS) with positive effects on brain function (Crook et al., 1992; Cenacchi et al., 1993). However, the use of BC-PS in medicine or dietary supplements is now discouraged because of the risk of bovine spongiform encephalopathy (BSE) (Prusiner, 1991). In addition, only about 3 grams of PS can be obtained from one bovine cortex, which is too small for inexpensive supply (Kato-Kataoka et al., 2010). For this reason, PS which originated from other organisms has been considered a possible alternative of BC-PS.
Trimethyltin (TMT) is an organotin compound with potent neurotoxicant effects. This substance is regarded as being particularly useful for studying the response to injury on account of the distinct pattern of degeneration it causes in rodent brain. In particular, the rat hippocampus constitutes the most suitable model for TMT-induced brain injury (Cannon et al., 1994a, b; O'Connell et al., 1994a, b). Intoxication with TMT leads to profound behavioral and cognitive deficits in both humans and experimental animals (Dyer, 1982). In rats, TMT induces the degeneration of pyramidal neurons in the hippocampus and the cortical areas connected to the hippocampus, but there is also neuronal loss in the association areas (Chang et al., 1983; Balaban et al., 1988). TMT intoxication impairs the performance of learning acquisition of water maze and Biel maze tasks as well as the performance of Hebb-Williams maze, radial arm maze tasks and passive avoidance retention (Walsh et al., 1982a; Ishida et al., 1997). These findings have made TMT-intoxicated rats an attractive model for degenerative diseases such as AD (Earley et al., 1990).
Some of previous studies researched increase of acetylcoline (ACh) and choline (Ch) by treatment of PS. Chronic treatment of PS improve ACh release in aging rats by increasing the availability of Ch for ACh synthesis (Casamenti et al.,1991; Lee et al., 2010). ACh is a neurotransmitter in the brain that is related to learning and memory. Damage to the cholinergic system in the brain is known to be closely associated with the memory deficits (Piovesan et al., 1995). The consistent findings of AD patients are impairment in cognitive performances, such as attention, learning and memory, and loss of cholinergic markers, including levels of acetylcholinesterase (AChE) and choline acetyltransferase (ChAT) (Perry et al., 1977; Giacobini, 1998).
The cAMP responsive element binding protein (CREB), transcription factor of gene, is a component of intracellular signaling events that regulate a wide range of biological functions, from spermatogenesis to circadian rhythms and memory. In the mammalian brain, CREB is phosphorylated and CREB-dependent transcription is induced in glutamatergic neurons after training in hippocampus-dependent and amygdala-dependent memory tasks (Taubenfeld et al., 1999; Porte et al., 2008). A large number of behavioral studies have explored the learning and memory phenotype of CREB mutant strains and CREB virally transduced animals (Bourtchuladze et al., 1994; Guzowski and McGaugh, 1997).
The present study was undertaken to evaluate the neuro-protective effect of krill-derived phosphatidylserine (Krill-PS) on the TMT-induced learning and memory deficits in rats. Rats were tested on a Morris water maze for spatial learning and memory. The analyzed parameters included the expression of cholinergic neurons and CREB in the brain.
METERIALS AND METHODS
Animals
Male Sprague-Dawley rats weighting 250-280 g each were purchased from Samtaco Animal Corp. (Kyungki-do, Korea). The animals were allowed to acclimatize themselves for at least 7 days prior to the experimentation. The animals were housed in individual cages under light-controlled conditions (12/12-hr light/dark cycle) and at 23℃ room temperature. Food and water were made available ad libitum. All the experiments were approved by the Kyung Hee University institutional animal care and use committee. Also, this experimental protocol was approved by an Institutional Review Committee for the use of Human or Animal Subjects or that procedures are in compliance with at least the Declaration of Helsinki for human subjects, or the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985), the UK Animals Scientific Procedures Act 1986 or the European Communities Council Directive of 24 Novem-ber 1986 (86/609/EEC).
Experimental groups
The rats were randomly divided into four groups each as follows: non-treated, naïve normal group (Normal, n=10); TMT injection with vehicle administered group (MCT, n=7); TMT in-jection with 50 mg/kg-1 Krill-PS administered group (Krill-PS 50 + MCT, n=10); TMT injection with 100 mg/kg-1 Krill-PS ad-ministered group (Krill-PS 100+MCT, n=5) used in this study.The rats were injected intraperitoneally (i.p.) with TMT (8.0 mg/kg, body weight) dissolved in 0.9% saline.
The applied Krill-PS formula obtained from 50 kg of krill base material, the overall yield was 91.6%. The obtained Krill-PS contains 92% phosphatidylserine (PS), 1% phospha-tidylcholine (PC), and 5% phosphatidic acid (PA). The compositions of Krill-PS are palmitic (26.9%), palmitoleic (1.8%), stearic (5.5%), oleic (6.9%), linoleic (2.1%), linolenic (1.8%), EPA (31.2%), DHA (14.4%) and others (9.4%). The Krill-PS was manufactured and kindly provided by Doosan Co. Glonet BU (Youngin, Korea). The rats were orally administrated with Krill-PS, daily for 21 days. From the 16th after the injection of TMT, the Morris water maze test was performed for 5 days.
Morris water maze test
The swimming pool of the Morris water maze was a circular
water tank 200 cm in diameter and 35 cm deep. It was filled to a depth of 21 cm with water at 23 ± 2℃. A platform 15 cm in diameter and 20 cm in height was placed inside the tank with its top surface being 1.5 cm below the surface of the water. The pool was surrounded by many cues that were external to the maze (D'Hooge and De Deyn, 2001). A CCD camera was equipped with a personal computer for the behavioral analysis. Each rat received four daily trials. For 4 consecutive days, the rats were tested with three acquisition tests. They also received retention tests on the 5th day. For the acquisition test, the rat was allowed to search for the hidden platform for 180s and the latency to escape onto the platform was recorded. The animals were trained to find the platform that was in a fixed position during 4 days for the acquisition test, and then for the retention test (at the 5th day), they received a 1 min probe trial in which the platform was removed from the pool. The intertrial interval time was 1 min. The performance of the test animals in each water maze trial was assessed by a personal computer for the behavioral analysis (S-mart program, Spain).
Immunohistochemistry
Briefly, the rats were anesthetized (sodium pentobarbital,100 mg/kg, i.p.) then perfused transcardially with phosphate-buffered saline (PBS; pH 7.4) for 30 s followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 10-15 min. The brains were postfixed in the same fixative overnight, cryoprotected in 30% sucrose solution in PBS, embedded and serially sectioned on a cryostat (Leica, Germany) at 30 ㎛ thickness in the coronal plane and they were collected in PBS. The primary antibodies against the following specific antigen were used: Cholinacetyl transferase (sheep polyclonal ChAT, concentration 1:2,000; Cambridge Research Biochemicals, Wilm-ington, D.E.), acetylcholine esterase (rabbit polyclonal AChE, concentration 1:200; Santacruz biotechnology, Delaware Avenue Santa Cruz, CA, USA) and cAMP responsive element binding protein (rabbit polyclonal CREB, concentration 1:250; Cell Signaling, Boston, USA). The primary antibody was prepared and diluted in 0.2% PBST, 2% blocking serum and 0.001% kehole limpit hemocyanin (Sigma, USA). The sections were incubated in the primary antiserum for 72 h at 4℃. After three more rinses in PBST, the sections were placed in Vectastain Elite ABC reagent (Vector laboratories, Burlingame, CA) for 2 h at room temperature. Following a further rinsing in PBS, the tissue was developed using diaminobenzadine (Sigma, USA) as the chromogen. The images were captured using a DP2-BSW imaging system (Olympus, CA, USA) and they were processed using Adobe Photoshop. For measuring the cells that were positive for ChAT, AChE and CREB, the grid was placed on CA1 and CA3 in the hippocampus and medial septum area according to the method of Paxinos et al (1985). The number of cells was counted at 100× magnification using a microscope rectangle grid that measured 200×200 ㎛2. The cells were counted in three sections per rat within the hippocampus and medial septum.
Statistical analysis
Statistical comparisons were done for the behavioral and histochemical studies using one-way ANOVA and repeated measures of ANOVA, respectively and LSD test was done. All of the results are presented as means ± S.E.M., and we used SPSS 15.0 for Windows for analysis of the statistics. The significance level was set at p<0.05.
RESULTS
Effect of Krill-PS on the water maze test
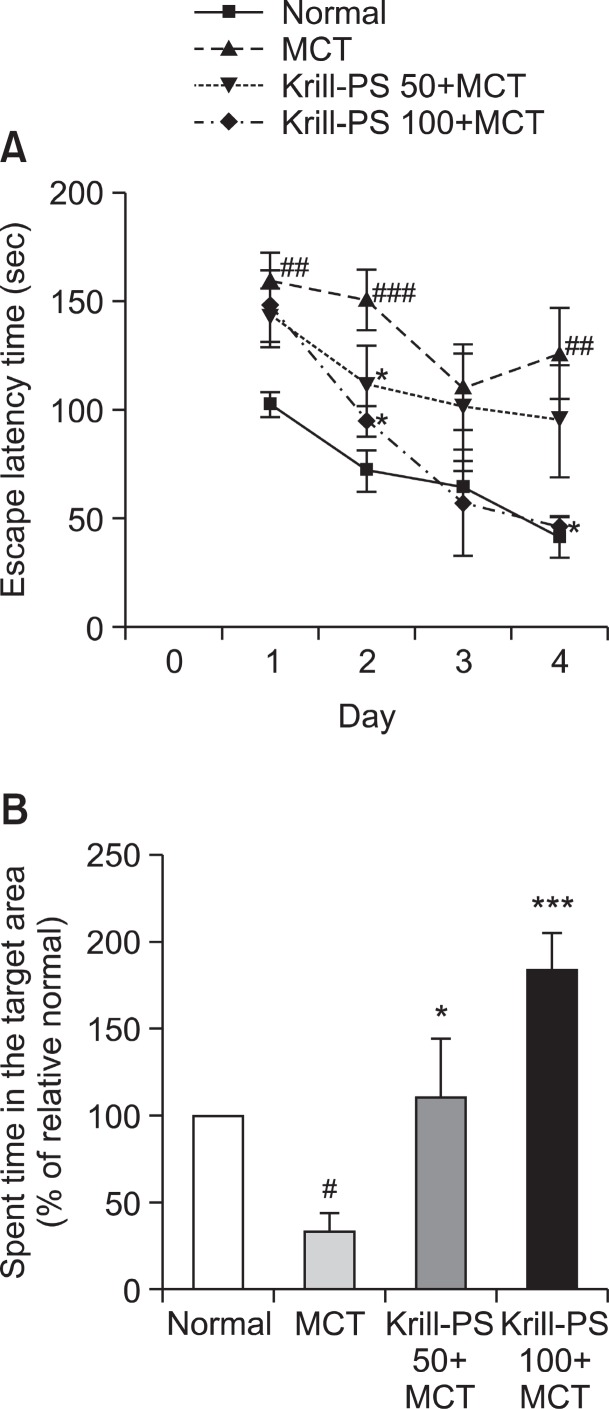
The effect of Krill-PS (50, 100 mg/kg) on spatial learning was evaluated on the Morris water maze test. As shown in Fig. 1A, the escape latency of the MCT group was longer by means of memory impairment than that of the Normal group during all the trial sessions, and Fig. 1A shows the mean time latencies to reach to hidden platform in the MWM for all the groups for 4 days. The escape latency differed among the groups when the results were averaged over all the session. The MCT group showed a worse performance than did the normal group (p<0.01, at the Day 1, 2 and 4). There were significant main effects, and treatment with Krill-PS 50 mg/kg had a significant interaction effect on the distance traveled to reach the platform from the 2nd day (p<0.05). Also, Krill-PS 100+MCT group significantly decreased escape latency time at the Day 2 and 4 (p<0.05).
Fig. 1. (A) The latency to escape onto the hidden platform during the Morris water maze. The task was performed with 3 trials per day during 4 days for the acquisition test. The values are presented as means ± S.E.M. ##p<0.01 and ###p<0.001 vs. Normal group; *p<0.05 vs. MCT group, respectively. (B) Retention performance was tested on 5th day. The rats received a 1 min probe trial in which the platform was removed from the pool for retention testing. The values are presented as means ± S.E.M. #p<0.05 vs. Normal group; *p<0.05 and ***p<0.001 vs. MCT group, respectively.
To examine the spatial memory of rats, the time spent swimming to the platform was compared and the analysis is illustrated in Fig. 1B. The times spent on the platform (% of relative normal) were significantly different among the groups (F3,31=5.476, p<0.01) the MCT group spent less time around the platform than the Normal group (p<0.05). However, treatment with Krill-PS (50 and 100 mg/kg) had a significant effect on the time spent around the platform compared to that of the
MCT group (p<0.05, p<0.001) (Fig. 1B).
ChAT immunoreactive neurons of the hippocampus and medial septum
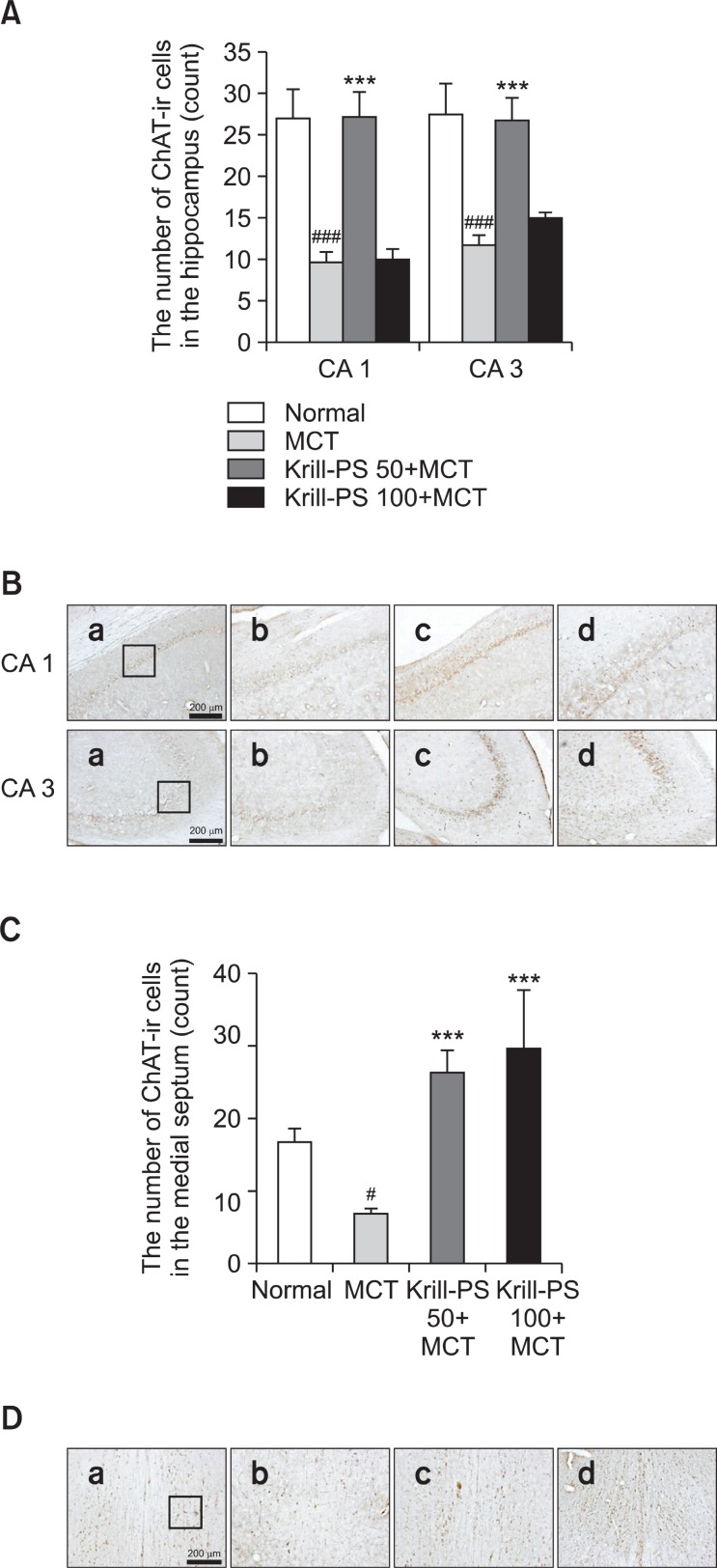
The results of the evaluations of the ChAT immunoreactive cells per section from the different hippocampal formations are shown in Fig. 2A and B. Post-hoc comparisons indicated that the ChAT activity in the hippocampus of the MCT group was significantly lower than that of the Normal group (p<0.001). In particular, there were significant differences in both CA1 (F3,20=17.73, p<0.001) and CA3 (F3,22=12.02, p<0.001). However, the ChAT reactivity in the Krill-PS 50+MCT group was higher than that of the MCT group, and particularly in CA1 and CA3. However, the ChAT reactivity in the hippocampus of the Krill-PS 100+MCT group showed no statistically significantly differences among the groups.
Fig. 2. (A) The number of choline acetyltransferase (ChAT) immunostained nuclei in different hippocampal CA1 and CA3 of the experimental groups. The values are presented as means ± S.E.M. ###p<0.001 vs. Normal group; ***p<0.001 vs. MCT group, respectively. (B) Photographs showing the distribution of ChAT-immunoreactive cells in the hippocampus of Normal group (a), MCT group (b), Krill-PS 50+MCT group (c) and Krill-PS 100+MCT group (d). Sections were cut coronally at 30 ㎛ and the scale bar represents 200 ㎛. (C) The number of choline acetyltransferase (ChAT) immunostained nuclei in the medial septum of the experimental groups. The values are presented as means ± S.E.M. #p<0.05 vs. Normal group; ***p<0.001 vs. MCT group, respectively. (D) Photographs showing the distribution of ChAT-immunoreactive cells in the medial septum of Normal group (a), MCT group (b), Krill-PS 50+MCT group (c) and Krill-PS 100+MCT group (d). Sections were cut coronally at 30 ㎛ and the scale bar represents 200 ㎛.
Also, the results of the evaluations of the ChAT immunoreactive cells per section from the medial septum are shown in Fig. 2C and D. Post-hoc comparisons indicated that the ChAT activity in the medial septum of the MCT group was significantly lower than that of the Normal group (p<0.05). In particular, there were significant differences in the medial septum (F3,18=9.57, p<0.01). However, the ChAT reactivity in both Krill-PS 50 +MCT and Krill-PS 100+MCT groups was higher than that of the MCT group (p<0.001).
AChE immunoreactive neurons of the hippocampus
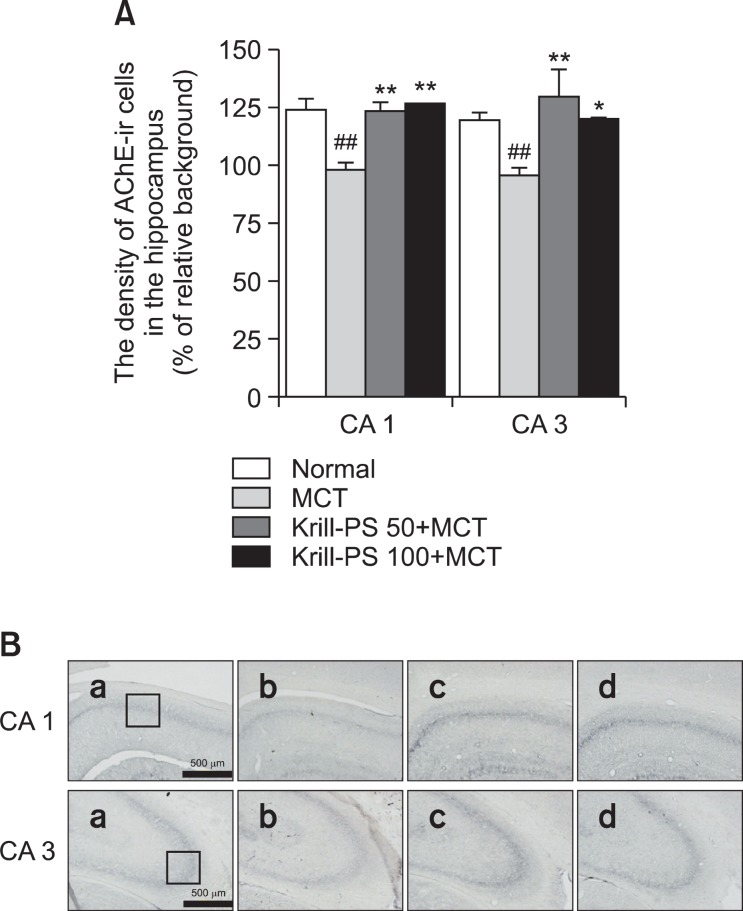
The results of the evaluations of the AChE immunoreactive cells per section from the different hippocampal formations are shown in Fig. 3. Post-hoc comparisons indicated that the AChE activity in the hippocampus of the MCT group was significantly lower than that of the Normal group (p<0.01). In particular, there were significant differences in both CA1 (F3,18=7.47, p<0.01) and CA3 (F3,18=5.35, p<0.01). However, the AChE reactivity in the Krill-PS+MCT groups was higher than that of the MCT group, and particularly in CA1 and CA3.
Fig. 3. (A) The density of acetylcholine esterase (AChE) immunostained nuclei in different hippocampal CA1 and CA3 of the experimental groups. The values are presented as means ± S.E.M. ##p<0.01 vs. Normal group; *p<0.05 and **p<0.01 vs. MCT group, respectively. (B) Photographs showing the distribution of AChE-immunoreactive cells in the hippocampus of Normal group (a), MCT group (b), Krill-PS 50+MCT group (c) and Krill-PS 100+MCT group (d). Sections were cut coronally at 30 ㎛ and the scale bar represents 500 ㎛.
CREB immunoreactive neurons of the hippocampus
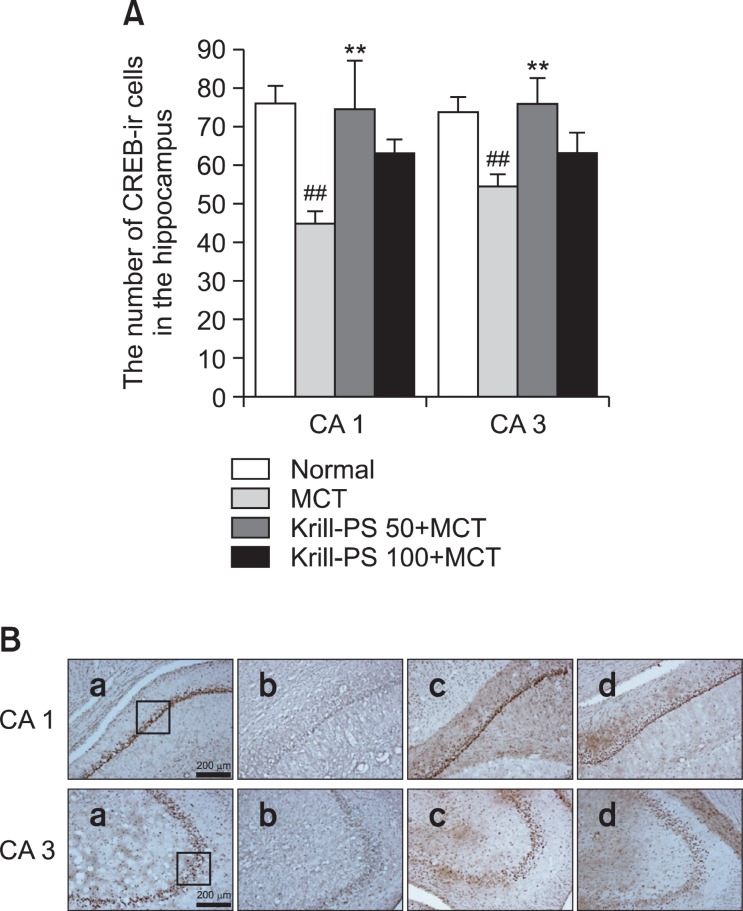
The results of the evaluations of the CREB immunoreactive cells per section from the different hippocampal formations are shown in Fig. 4. Post-hoc comparisons indicated that the CREB activity in the hippocampus of the MCT group was significantly lower than that of the Normal group. In particular, there were significant differences in the hippocampal CA1 (F3,19=7.20, p<0.01) and CA3 (F3,18=5.56, p<0.01). The CREB reactivity in the Krill-PS 50+MCT group was higher than that of the MCT group and particularly in hippocampus, the number of CREB positive neurons in the Krill-PS 50+MCT group was significantly increased by 60.21% compared to that of the MCT group (p<0.01).
Fig. 4. (A) The number of cAMP responsive element binding protein (CREB) immunostained nuclei in different hippocampal CA1 and CA3 of the experimental groups. The values are presented as means S.E.M. ##p<0.01 vs. Normal group; **p<0.01 vs. MCT group, respectively. (B) Photographs showing the distribution of CREB-immunoreactive cells in the hippocampus of Normal group (a), MCT group (b), Krill-PS 50+MCT group (c) and Krill-PS 100+MCT group (d). Sections were cut coronally at 30 ㎛ and the scale bar represents 200 ㎛.
DISCUSSION
The present study demonstrated that TMT injections produced severe memory deficits in a Morris water maze along with signs of neuro-degeneration, including decreased cholinergic neurons and cAMP responsive element binding protein (CREB) activity in the hippocampus. Repeated treatment with Krill-PS attenuated the TMT-induced learning and memory deficits in the water maze test and it had a protective effect against the TMT-induced decrease in cholinergic and CREB positive neurons.
Intoxication with TMT leads to profound behavioral and cognitive deficits in both humans (Fortemps et al., 1978) and experimental animals (Dyer, 1982; Ishida et al., 1997). In one reported case, postmortem examination revealed generalized chromatolysis of the neurons in the brain, spinal cord and spinal ganglia and neuronal necrosis in the Fascia Dentate and in the pyramidal cell layer of the hippocampus, cerebral cortex, basal ganglia and Purkinje cell layer of the cerebellum, findings similar to those described in experimental TMT intoxication (Kreyberg et al., 1992). Furthermore, behavioral studies have shown increased disruption in memory and learning deficits in TMT-intoxicated rats (Swartzwelder et al., 1982; Andersson et al., 1995). The Morris water maze is well-established paradigm for evaluating deficits in hippocampal-dependent memory and the MWM spatial learning task has been used in the validation of rodent models for neurocognitive disorders and for the evaluation of possible neurocognitive treatments (D'Hooge and De Deyn, 2001). The impairment in spatial learning produced by TMT in the current studies is consistent with previous reports of spatial learning impairments (Walsh et al., 1982a, b; Hagan et al., 1988; Earley et al., 1992; Ales-sandri et al., 1994). It is likely that performance in the MWM by the TMT-injected rats in our study was influenced by memory impairment. However, this study proved that spatial memory continued to improve in Krill-PS treated groups during the training days compared to the MCT group. Also, the data of spatial probe trial demonstrated that Krill-derived PS protects against the TMT-induced decrease of the spatial retention, especially long-term memory.
The neuroprotective effects of natural drugs on the central acetylcholine system were also examined by performing immunohistochemistry of the hippocampal neurons. The degeneration of the cholinergic innervations from the basal forebrain to the hippocampal formation in the temporal lobe is thought to be one of the factors determining the progression of memory decay, both during normal aging and AD (Sun et al., 2003). The best available marker for cholinergic neurons in the basal forebrain is ChAT activity. ChAT synthesizes the neurotransmitter acetylcholine in the basal forebrain, cortex, hippocampus and amygdala. A significant reduction in ChAT activity in the postmortem brains of demented patients has been reported. In addition, there was a 20-50% decrease in ChAT activity in the hippocampus and medial septum of the TMT-induced rats in this current study. However, the present results show the Krill-PS beneficial effects on cholinergic neurotransmission in the brain by increasing the ChAT activities.
In AChE immunohistochemistry, the Krill-PS+MCT groups showed higher AChE reactivity in both hippocampal CA1 and CA3. This study demonstrated that the cholinergic system might be affected by exposure to TMT (Christ et al., 1989). These results are consistent with previous reports showing that the cholinergic neurons in the brain are involved in learning and memory in humans (Safer and Allen, 1971) and animals (Mizoguchi et al., 2001). In particular, the hippocampus cholinergic neurons are involved in the formation and maintenance of short-term working memory or retention and retrieval processes in long-term reference memory (Pope et al.,1987; Murai et al., 1995; Izquierdo et al., 1998; Mizoguchi et al., 2002). Based on a previous study, this result suggests that the treatment of Krill-PS can promote the memory function.
CREB is critical for activating the transcription of genes controlled by the cAMP-response element, and many of these genes may be involved in neuronal growth and plasticity and they may take part in neuronal survival (Wu et al., 1999; Kim et al., 2007). Many studies have indicated that disruption or deficiency of the CREB gene leads to neurodegeneration (Sala et al., 2000; Hardingham and Bading, 2003). CREB is also a molecular marker of long term potentiation and memory formation. Previous studies have proved that the CREB mutation affected learning and memory, and the mutant gene disrupted long term memory and hippocampus-dependent tasks (Lonze and Ginty, 2002; Mantamadiotis et al., 2002). Genetic and pharmacological studies have provided strong evidence that the CREB signaling pathway is crucial for learning and memory across species (Bourtchuladze et al., 1994; Kogan et al., 1997). Consistent with the previous studies, our results showed that the levels of CREB in the hippocampus showed significant differences among the groups. The TMT treated group showed a reduction, by approximately 30-40%, of the CREB activity. Thus, we may draw a conclusion that the CREB loss after TMT exposure might be at least partially responsible for the TMT-induced cell death. It has been reported that CREB could be inactivated by stressful stimuli such as zinc deficiency or hypoxia (Hardingham and Bading, 2003; Jackson and Ramaswami, 2003). Our results indicated that TMT played a role as stressful stimulation on the CREB gene. However, the CREB expression was significantly up-regulated after PS-Krill treatment in this experiment. Perhaps the activation of CREB was related to a neuroprotective effect such as a defense mechanism.
In summary, treatment with Krill-PS attenuated the TMT-induced learning and memory deficits in the Morris water maze, and Krill-PS treatment had a protective effect against a TMT-induced decrease of the cholinergic neurons and CREB activation. Thus, Krill-PS is a good candidate for further investigations that may ultimately result in its clinical use. Further studies that will examine the effects of Krill-PS activation on additional behavioral tasks will help to elucidate whether increasing the CREB signaling may also improve other types of memory.
Acknowledgments
This research was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Wel-fare& Family Affairs (grant no. A091037) and the Cognitive Neuroscience Program (M10644000017-06N4400-01710) of the Korea Ministry of Science and Technology and Republic of Korea.
References
- 1.Alessandri B. FitzGerald R. E. Schaeppi U. Krinke G. J. Clas-sen W. The use of an unbaited tunnel maze in neurotoxicology: I. Trimethyltin-induced brain lesions. Neurotoxicology. (1994);15:349–357. [PubMed] [Google Scholar]
- 2.Andersson H. Luthman J. Lindqvist E. Olson L. Time-course of trimethyltin effects on the monoaminergic systems of the rat brain. Neurotoxicology. (1995);16:201–210. [PubMed] [Google Scholar]
- 3.Balaban C. D. O'Callaghan J. P. Billingsley M. L. Trimethyltin-induced neuronal damage in the rat brain: comparative studies using silver degeneration stains, immunocytochemistry and immunoassay for neuronotypic and gliotypic proteins. Neuroscience. (1988);26:337–361. doi: 10.1016/0306-4522(88)90150-9. [DOI] [PubMed] [Google Scholar]
- 4.Bourtchuladze R. Frenguelli B. Blendy J. Cioffi D. Schutz G. Silva A. J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. (1994);79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 5.Cannon R. L. Hoover D. B. Baisden R. H. Woodruff M. L. Effects of trimethyltin (TMT) on choline acetyltransferase activity in the rat hippocampus. Influence of dose and time following exposure. Mol. Chem. Neuropathol. (1994a);23:27–45. doi: 10.1007/BF02858505. [DOI] [PubMed] [Google Scholar]
- 6.Cannon R. L. Hoover D. B. Baisden R. H. Woodruff M. L. The effect of time following exposure to trimethyltin (TMT) on cholinergic muscarinic receptor binding in rat hippocampus. Mol. Chem. Neuropathol. (1994b);23:47–62. doi: 10.1007/BF02858506. [DOI] [PubMed] [Google Scholar]
- 7.Casamenti F. Scali C. Pepeu G. Phosphatidylserine reverses the age-dependent decrease in cortical acetylcholine release: a microdialysis study. Eur. J. Pharmacol. (1991);194:11–16. doi: 10.1016/0014-2999(91)90117-9. [DOI] [PubMed] [Google Scholar]
- 8.Cenacchi T. Bertoldin T. Farina C. Fiori M. G. Crepaldi G. Cognitive decline in the elderly: a double-blind, placebo-controlled multicenter study on efficacy of phosphatidylserine administration. Aging (Milano) (1993);5:123–133. doi: 10.1007/BF03324139. [DOI] [PubMed] [Google Scholar]
- 9.Chang L. W. Tiemeyer T. M. Wenger G. R. McMillan D. E. Neuropathology of trimethyltin intoxication. III. Changes in the brain stem neurons. Environ. Res. (1983);30:399–411. doi: 10.1016/0013-9351(83)90226-8. [DOI] [PubMed] [Google Scholar]
- 10.Christ D. Chang L. W. McMillan D. E. Neurotoxicological effects of trimethyltin on the stellate ganglion. Neurotoxicol. Teratol. (1989);11:453–460. doi: 10.1016/0892-0362(89)90023-8. [DOI] [PubMed] [Google Scholar]
- 11.Crook T. Petrie W. Wells C. Massari D. C. Effects of phosphatidylserine in Alzheimer's disease. Psychopharmacol. Bull. (1992);28:61–66. [PubMed] [Google Scholar]
- 12.D'Hooge R. De Deyn P. P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. (2001);36:60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 13.Dyer R. S. Physiological methods for assessment of Trimethyltin exposure. Neurobehav. Toxicol. Teratol. (1982);4:659–664. [PubMed] [Google Scholar]
- 14.Earley B. Burke M. Leonard B. E. Behavioural, biochemical and histological effects of trimethyltin (TMT) induced brain damage in the rat. Neurochem. Int. (!992);21:351–366. doi: 10.1016/0197-0186(92)90186-U. [DOI] [PubMed] [Google Scholar]
- 15.Earley B. Burke M. Leonard B. E. Gouret C. J. Junien J. L. A comparison of the psychopharmacological profiles of phencyclidine, ketamine and (+) SKF 10,047 in the trimethyltin rat model. Neuropharmacology. (1990);29:695–703. doi: 10.1016/0028-3908(90)90121-7. [DOI] [PubMed] [Google Scholar]
- 16.Fortemps E. Amand G. Bomboir A. Lauwerys R. Laterre E. C. Trimethyltin poisoning. Report of two cases. Int. Arch. Occup. Environ Health. (1978);41:1–6. doi: 10.1007/BF00377794. [DOI] [PubMed] [Google Scholar]
- 17.Freyz L D. J. Vincendon C. Asymmetry of brain micro-somal membrances. In Phospholipids in thenervous system (L. Horrocks Ed.) Vol. 1 Raven Press; NewYork.: (1982). pp. 37–47. [Google Scholar]
- 18.Giacobini E. Cholinergic foundations of Alzheimer's disease therapy. J. Physiol. Paris. (1998);92:283–287. doi: 10.1016/S0928-4257(98)80034-X. [DOI] [PubMed] [Google Scholar]
- 19.Guzowski J. F. McGaugh J. L. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc. Natl. Acad. Sci. USA. (1997);94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagan J. J. Jansen J. H. Broekkamp C. L. Selective behavioural impairment after acute intoxication with trimethyltin (TMT) in rats. Neurotoxicology. (1988);9:53–74. [PubMed] [Google Scholar]
- 21.Hardingham G. E. Bading H. The Yin and Yang of NMDA receptor signalling. Trends. Neurosci. (2003);26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 22.Ishida N. Akaike M. Tsutsumi S. Kanai H. Masui A. Sadamatsu M. Kuroda Y. Watanabe Y. McEwen B. S. Kato N. Trimethyltin syndrome as a hippocampal degeneration model: temporal changes and neurochemical features of seizure susceptibility and learning impairment. Neuroscience. (1997);81:1183–1191. doi: 10.1016/S0306-4522(97)00220-0. [DOI] [PubMed] [Google Scholar]
- 23.Izquierdo I. Izquierdo L. A. Barros D. M. Mello e Souza T. de Sou-za M. M. Quevedo J. Rodrigues C. Sant'Anna M. K. Madruga M. Medina J. H. Differential involvement of cortical receptor mechanisms in working short-term and long-term memory. Behav. Pharmacol. (1998);9:421–427. doi: 10.1097/00008877-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Jackson T. Ramaswami M. Prospects of memory-modifying drugs that target the CREB pathway. Curr. Opin. Drug Discov. Devel. (2003);6:712–719. [PubMed] [Google Scholar]
- 25.Kataoka-Kato A. Ukai M. Sakai M. Kudo S. Kameyama T. Enhanced learning of normal adult rodents by repeated oral administration of soybean transphosphatidylated phosphatidylserine. J. Pharmacol. Sci. 2005);98:307–314. doi: 10.1254/jphs.FP0050366. [DOI] [PubMed] [Google Scholar]
- 26.Kato-Kataoka A. Sakai M. Ebina R. Nonaka C. Asano T. Mi-yamori T. Soybean-derived phosphatidylserine improves memory function of the elderly Japanese subjects with memory complaints. J. Clin. Biochem. Nutr. (2010);47:246–255. doi: 10.3164/jcbn.10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J. H. Ha H. C. Lee M. S. Kang J. I. Kim H. S. Lee S. Y. Pyun K. H. Shim I. Effect of Tremella fuciformis on the neurite outgrowth of PC12h cells and the improvement of memory in rats. Biol. Pharm. Bull. (2007);30:708–714. doi: 10.1248/bpb.30.708. [DOI] [PubMed] [Google Scholar]
- 28.Kogan J. H. Frankland P. W. Blendy J. A. Coblentz J. Marowitz Z. Schütz G. Silva A. J. Spaced training induces normal long-term memory in CREB mutant mice. Curr. Biol. (1997);7:1–11. doi: 10.1016/S0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- 29.Kreyberg S. Torvik A. Bjørneboe A. Wiik-Larsen W. Jacobsen D. Trimethyltin poisoning: report of a case with postmortem examination. Clin. Neuropathol. (1992);11:256–259. [PubMed] [Google Scholar]
- 30.Lee B. Sur B. J. Han J. J. Shim I. Her S. Lee H. J. Hahm D. H. Krill phosphatidylserine improves learning and memory in Morris water maze in aged rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. (2010);34:1085–1093. doi: 10.1016/j.pnpbp.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Lonze B. E. Ginty D. D. Function and regulation of CREB family transcription factors in the nervous system. Neuron. (2002);35:605–623. doi: 10.1016/S0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 32.Mantamadiotis T. Lemberger T. Bleckmann S. C. Kern H. Kretz O. Martin Villalba A. Tronche F. Kellendonk C. Gau D. Kapf-hammer J. Otto C. Schmid W. Schütz G. Disruption of CREB function in brain leads to neurodegeneration. Nat. Genet. (2002);31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- 33.Mizoguchi K. Yuzurihara M. Ishige A. Sasaki H. Tabira T. Effect of chronic stress on cholinergic transmission in rat hippocampus. Brain Res. (2001);915:108–111. doi: 10.1016/S0006-8993(01)02834-7. [DOI] [PubMed] [Google Scholar]
- 34.Mizoguchi K. Yuzurihara M. Ishige A. Sasaki H. Tabira T. Chronic stress impairs rotarod performance in rats: implications for depressive state. Pharmacol. Biochem. Behav. (2002);71:79–84. doi: 10.1016/S0091-3057(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 35.Murai S. Saito H. Masuda Y. Odashima J. Itoh T. AF64A disrupts retrieval processes in long-term memory of mice. Neuroreport. (1995);6:349–352. [PubMed] [Google Scholar]
- 36.O'Connell A. Earley B. Leonard B. E. Changes in muscarinic (M1 and M2 subtypes) and phencyclidine receptor density in the rat brain following trimethyltin intoxication. Neurochem. Int. (1994a);25:243–252. doi: 10.1016/0197-0186(94)90068-X. [DOI] [PubMed] [Google Scholar]
- 37.O'Connell A. Earley B. Leonard B. E. The neuroprotective effect of tacrine on trimethyltin induced memory and muscarinic receptor dysfunction in the rat. Neurochem. Int. (1994b);25:555–566. doi: 10.1016/0197-0186(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G. Watson C. Pennisi M. Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J. Neurosci. Methods. (1985);13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 39.Perry E. K. Gibson P. H. Blessed G. Perry R. H. Tomlinson B. E. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase ac-tivities in necropsy brain tissue. J. Neurol. Sci. (1977);34:247–265. doi: 10.1016/0022-510X(77)90073-9. [DOI] [PubMed] [Google Scholar]
- 40.Piovesan P. Quatrini G. Pacifici L. Taglialatela G. Angelucci L. Acetyl-L-carnitine restores choline acetyltransferase activity in the hippocampus of rats with partial unilateral fimbria-fornix transection. International Journal of Developmental Neuroscience: the Official Journal of the International Society for Developmental Neuroscience. (1995);13:13–19. doi: 10.1016/0736-5748(94)00070-j. [DOI] [PubMed] [Google Scholar]
- 41.Pope C. N. Ho B. T. Wright A. A. Neurochemical and behavioral effects of N-ethyl-acetylcholine aziridinium chloride in mice. Pharmacol. Biochem. Behav. (1987);26:365–371. doi: 10.1016/0091-3057(87)90131-6. [DOI] [PubMed] [Google Scholar]
- 42.Porte Y. Buhot M. C. Mons N. E. Spatial memory in the Morris water maze and activation of cyclic AMP response element-binding (CREB) protein within the mouse hippocampus. Learn. Mem. (2008);15:885–894. doi: 10.1101/lm.1094208. [DOI] [PubMed] [Google Scholar]
- 43.Prusiner S. B. Molecular biology of prion diseases. Science. (1991);252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 44.Safer D. J. Allen R. P. The central effects of scopolamine in man. Biol. Psychiatry. (1971);3:347–355. [PubMed] [Google Scholar]
- 45.Sala C. Rudolph-Correia S. Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J. Neurosci. (2000);20:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun H. Hu Y. Zhang J. M. Li S. Y. Li W. Effects of one Chinese herbs on improving cognitive function and memory of Alzheimer's disease mouse models. Zhongguo Zhong Yao Za Zhi. (2003);28:751–754. [PubMed] [Google Scholar]
- 47.Swartzwelder H. S. Hepler J. Holahan W. King S. E. Leverenz H. A. Miller P. A. Myers R. D. Imparied maze performance in the rat caused by trimethyltin treatment: problem-solving deficits and perseveration. Neurobehav. Toxicol. Teratol. (1982);4:169–176. [PubMed] [Google Scholar]
- 48.Taubenfeld S. M. Wiig K. A. Bear M. F. Alberini C. M. A molecular correlate of memory and amnesia in the hippocampus. Nat. Neurosci. (1999);2:309–310. doi: 10.1038/7217. [DOI] [PubMed] [Google Scholar]
- 49.Vakhapova V. Cohen T. Richter Y. Herzog Y. Korczyn A. D. Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: a double-blind placebo-controlled trial. Dement. Geriatr. Cogn. Disord. (2010);29:467–474. doi: 10.1159/000310330. [DOI] [PubMed] [Google Scholar]
- 50.Walsh T. J. Gallagher D. B. Bostock E. Dyer R. S. Trimethyltinimpairs retention of a passive avoidance task. Neurobehav. Toxicol. Teratol. (1982a);4:163–167. [PubMed] [Google Scholar]
- 51.Walsh T. J. Miller D. B. Dyer R. S. Trimethyltin, a selective limbic system neurotoxicant, impairs radial-arm maze performance. Neurobehav. Toxicol. Teratol. (1982b);4:177–183. [PubMed] [Google Scholar]
- 52.Wu X. Glinn M. A. Ostrowski N. L. Su Y. Ni B. Cole H. W. Bry-ant H. U. Paul S. M. Raloxifene and estradiol benzoate both fully restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res. (1999);847:98–104. doi: 10.1016/S0006-8993(99)02062-4. [DOI] [PubMed] [Google Scholar]