Abstract
This research was designed to determine what components of Gardenia jasminoides play a major role in inhibiting the enzymes related antidepressant activity of this plant. In our previous research, the ethyl acetate fraction of G. jasminosides fruits inhibited the activities of both monoamine oxidase-A (MAO-A) and monoamine oxidase-B (MAO-B), and oral administration of the ethanolic extract slightly increased serotonin concentrations in the brain tissues of rats and decreased MAO-B activity. In addition, we found through in vitro screening test that the ethyl acetate fraction showed modest inhibitory activity on dopamine-β hydroxylase (DBH). The bioassay-guided fractionation led to the isolation of five bio-active compounds, protocatechuic acid (1), geniposide (2), 6'-O-trans-p-coumaroylgeniposide (3), 3,5-d-ihydroxy-1,7-bis (4-hydroxyphenyl) heptanes (4), and ursolic acid (5), from the ethyl acetate fraction of G. jasminoides fruits. The isolated compounds showed different inhibitory potentials against MAO-A, -B, and DBH. Protocatechuic acid showed potent inhibition against MAO-B (IC50 300 μmol/L) and DBH (334 μmol/L), exhibiting weak MAO-A inhibition (2.41 mmol/L). Two iridoid glycosides, geniposide (223 μmol/L) and 6'-O-trans-p-coumaroylgeniposide (127μmol/L), were selective MAO-B inhibitor. Especially, 6'-O-trans-p-coumaroylgeniposide exhibited more selective MAO-B inhibition than deprenyl, well-known MAO-B inhibitor for the treatment of early-stage Parkinson’s disease. The inhibitory activity of 3,5-di-hydroxy-1,7-bis (4-hydroxyphenyl) heptane was strong for MAO-B (196 μmol/L), modest for MAO-A (400 μmol/L), and weak for DBH (941 μmol/L). Ursolic acid exhibited significant inhibition of DBH (214 μmol/L), weak inhibition of MAO-B (780 μmol/L), and no inhibition against MAO-A. Consequently, G. jasminoides fruits are considerable for development of biofunctional food materials for the combination treatment of depression and neurodegenerative disorders.
Keywords: Gardenia jasminosides, Rubiaceae, Monoamine oxidase inhibitor, Dopamine β-hydroxylase inhibitor
INTRODUCTION
Gardenia is a popular ornamental shrub found worldwide. The fruits of Gardenia jasminoides (Rubiaceae) (Korean herbal name is Chi Za) have been used as a natural yellow colorant in foods and also in traditional medicine for the treatment of liver and bladder disorders and inflammatory disease. The major effective constituents of Gardenia fruits, iridoid glycosides, flavonoids, and carotenoids, are responsible for the biological activities such as hypoglycemic activity, anti-tumor effect, anti-angiogenic activity, antithrombotic effect, and anti-oxidant activity (Miura et al., 1996; Pham et al., 2000; Suzuki et al., 2001; Koo et al., 2004; Peng et al., 2005). In our previous research, cold drugs inhibited the activity of MAO (Hwang et al., 1999) and especially, the total methanolic extract of the fruit of G. jasminoides exhibited a significant inhibition on MAO activity (IC50 value of MAO-A is 1.23 mg/ml; MAO-B is 1.34 mg/ml) (Hwang, 2003). The ethyl acetate frac-tion of G. jasminosides fruits showed significant activities in in vitro assays on both MAO-A and MAO-B (IC50 value of MAO-A is 0.72 mg/ml; MAO-B is 0.77 mg/ml), and oral administration of the ethanolic extract slightly increased serotonin concentrations in the brain tissues of rats and decreased MAO-B activity (Hwang and Park, 2007). This tendency is similar to the activity of deprenyl which is a well-known MAO inhibitor having antidepressant effects. In addition, we found through in vitro screening test that the ethyl acetate fraction showed modest inhibitory activity on DBH. It is well known that major depression is related to the deficit of monoamine at critical synapses in the central neurous system whereas Parkinson’s disease (PD) is mainly due to a deficit of dopamine.
This research was designed to determine what components of G. jasminoides play a major role in inhibiting those enzymes.
MATERIALS AND METHODS
General experimental procedures
NMR experiments were performed on a Bruker/Ad-vance-500 (500 MHz), a Bruker/Advance-400 (400 MHz) or a Varian-Gemini-2000 (300 MHz) spectrometer. The chemical shifts are reported in ppm and the coupling constants (J values) are reported in hertz. Exact masses were measured using a Hewlett Packard 5890 Series II mass spectrometer. Column chromatography was carried out on silica gel 60 (0.063-0.200 mm; Merck 7734) and ODS gel (12 nm, S-150μm; YMC*GEL ODS-A AA12SA5). TLC analyses were carried out on silica gel 60 F254 (Merck 7734) and RP-18 F254s (Merck 15685) plates. Compounds on the TLC plates were detected using UV light and a 10% H2SO4/water spraying reagent. After spraying, the TLC plate was heated at 110℃ for 1-2 minutes. In the bioassay experiments, the UV absorbance was measured by a UVIKON XS UV/Vis spectrometer. Serotonin creatinine sulfate, tyramine-HCl, iproniazid (97% purity, Lot 80H0178), R-(-)-deprenyl hydrochloride (98% purity, Lot 087H4665) and Dowex 50 W ×8 and Amberlite CG50 were purchased from Sigma Co., USA, and benzylamine-HCl from Tokyo Kasei Co., Japan.
Plant material
The fruits of Gardenia jasminoides that were collected in Muju, Jeollabukdo Province, Korea were purchased from a store at Kyungdong Market in Seoul and authenticated by Dr. Hyung Jun Ji, an emeritus professor of Seoul National Univer-sity. A voucher specimen (NP20-017) has been deposited in the specimen room of Duksung Women’s University, Seoul, Korea.
Animals
Male Sprague-Dawley rats weighing 180-200 g were obtained from the Orient Animal Laboratory (Seoul, Korea) and were maintained on a 12 hour light-dark cycle (light phase: 06:30-18:30) in a temperature-controlled environment (22 ± 1℃) with free access to food and water. Experiment began after 10 day period of acclimatization. All procedures were approved by the KonKuk University Animal Care and Use Committee. They complied with the Guide for the Care and Use of Laboratory Animals, Bio - Food and Drug Research Center KonKuk University.
Extraction and bioassay-guided fractionation
The powdered sample of the fruits (10 kg) was extracted 3 times with 30 L of an 80% MeOH solution over one month at room temperature. The 80% MeOH extract (3.31 kg) was suspended in water and extracted with n-hexane and EtOAc, sequentially. The EtOAc layer was evaporated to yield the EtOAc residue (140.54 g).
The EtOAc residue (32.54 g) was submitted to a silica gel column (60-200 μm, 5×35 cm) using a step gradient of CHCl3-MeOH 50:1 (2 L), 10:1 (1 L), 8:1 (1 L), 6:1 (1 L), 5:1 (1 L), 3:1(1 L), and 1:1 (1 L) to yield a series of fractions (I-IV). Fraction II (2.8-4.5 L, 10.86 g) was subjected to a silica gel column (60-200 μm, 4×25 cm, eluent CHCl3). Fractions of 500 ml were collected. The fractions 2-8 (0.5-4 L, 2.1 g) were combined and identified as ursolic acid. Pure ursolic acid (5) (170 mg) was obtained by repeated recrystallization with CHCl3-MeOH solution; detection by TLC (RP-18, MeOH-water 95:5; 10% H2SO4/ water spraying reagent), Rf: 0.33. Fraction III (5-7 L, 11.67 g) was subjected to a silica gel column (60-200 μm, 5×40 cm) using a step gradient of CHCl3-MeOH 10:1 (2.5 L) and 3:1 (2 L) to yield fractions (1 and 2). Fraction 1 (0.96 g), the eluate with CHCl3-MeOH 10:1, was separated on a silica gel column (60-200 μm, 2×38 cm, eluent CHCl3-MeOH 10:1). The fraction (80-100 ml, 21 mg) was identified as 3,5-dihydroxy-1,7-bis (4-hydroxyphenyl) heptanes (4); detection by TLC (SiO2, CHCl3-MeOH 9:1, 10% H2SO4/water spraying reagent), Rf:0.27. Fraction 2 (4.02 g), the eluate with CHCl3-MeOH 3:1, was suspended in water and extracted with CHCl3. The water layer (3.54 g) was submitted to an ODS gel column (S-150μm, 2×25 cm, eluent MeOH-water 2:8). The fraction (75-150 ml, 250 mg) was identified as protocatechuic acid (1); detection by TLC (RP-18, 20% MeOH, UV light), Rf: 0.5.
The EtOAc residue (108 g) was submitted to a silica gel column (60-200 μm, 10×50 cm) using a step gradient of CHCl3-EtOAc-MeOH 25:250:0.6 (2 L), EtOAc-MeOH 10:1 (1.5 L), and EtOAc-MeOH-D.W. 30:7:8 upper layer (3 L) to yield a series of fractions (A-C). Fraction B, the eluate with EtOAc-MeOH 10:1, was subjected to a silica gel column (60-200 μm, 7.5×40 cm) using a step gradient of CHCl3-MeOH 10:1 (2 L), 9:1 (3 L), and 8:1 (1 L). The fraction (2.3-6 L) was separated on an ODS gel column (S-150 μm, 2×25 cm) using a step gradient of MeOH-water 40:60 (600 ml), 45:55 (200 ml), and 50:50 (200 ml). The eluate with MeOH-water 50:50 was subjected to a silica gel column (60-200 μm, 1×24 cm, eluent water-saturated EtOAc). The fraction (55-65 ml, 25 mg) was identified as 6'-O-trans-p-coumaroylgeniposide (3); detection by TLC (SiO2, water-saturated EtOAc, 10% H2SO4/water spraying reagent), Rf: 0.13.Faction C, eluate with EtOAc-MeOH-D.W. 30:7:8 upper layer,was submitted to a silica gel column (60-200 μm, 7.5×40 cm) using a step gradient of CHCl3-MeOH 100:0 (2 L), 25: 1 (1 L), 20:1 (1.5 L), and 10:1 (2 L). The fraction (4.7-6.5 L, 12.39 g) was identified as geniposide (2); detection by TLC (SiO2, EtO-Ac-MeOH-D.W. 30:7:8 upper layer, 10% H2SO4/water spraying reagent), Rf: 0.4.
Preparation of test samples for in vitro assays
Purely isolated compounds were dissolved in ethanol or DMSO and then suspended in water. The final concentration of ethanol or DMSO in the enzymatic reaction mixture was 1%. To compensate for the test solution’s own absorbance, the substrate was omitted in the compensate group.
MAO-A inhibition assay in vitro
Rat brain mitochondrial MAO was prepared by Zeller’s method (Zeller, 1951). The activity of MAO-A was measured according to Han et al., (2001), using serotonin as the substrate. A reaction mixture containing 0.5 ml of enzyme solution in 10 mM phosphate buffered saline (pH 7.1) and 1ml of the test solution was incubated at 37℃ for 15 min before the addition of 0.5 ml of a 1,000 μM solution of buffered serotonin creatinine sulfate. Following incubation at 37℃ while shaking for 90 min, the enzyme reaction was terminated by heating the reaction mixture for 3 min in a boiling water bath. After being centrifuged, 1.6 ml of the supernatant was applied to an Amberlite CG50 column (0.8 i.d. ×3 cm). The column was washed with 40 ml of distilled water and eluted with 3 ml of 4N acetic acid. The absorbance of the serotonin that remained after being reacted with MAO-A was measured at 277 nm using a UV/Vis spectrometer.
MAO-B inhibition assay in vitro
Rat liver mitochondrial MAO was prepared by Zeller’s method (Zeller, 1951). Activity of MAO-B was measured according to Han, (2001), using benzylamine as a substrate. A reaction mixture containing 0.5 ml of enzyme solution in 10 mM phosphate buffered saline (pH 7.1) and 1 ml of test solution was incubated at 37℃ for 15 min, before the addition of 0.5 ml of 4,000 μM benzylamine hydrochloride. After incubation at 37℃ for 90 min, the enzyme reaction was terminated by adding 0.2 ml of 60% perchloric acid solution to the reaction mixture. The reaction product, benzaldehyde, was extracted with 4 ml of cyclohexane and its absorbance was measured at 242 nm using a UV/Vis spectrometer.
DBH inhibition assay in vitro
The enzyme activity of bovine adrenal DBH was determined according to the method of Han et al. (1997). The following were sequentially added to 0.3 ml of enzyme solution in 0.25 M sucrose: 1 ml of test solution; 0.2 ml of 3 mg/ml catalase; 0.5 ml of 1 M acetate buffer (pH 5.0); and 0.5 ml of a reaction aid, prepared by dissolving fumaric acid, N - ethylmaleimide, iproniazide phosphate and ascorbic acid to concentrations of 0.06, 0.06, 0.006 and 0.06 M, respectively, in distilled water. The solution was allowed to stir at 37℃ for 15 min, and then 0.5 ml of a 120 mM tyramine hydrochloride solution was added and the resulting mixture was allowed to stir for 90 min. Next, 0.4 ml of a 3 M solution of trichloroacetic acid was added to the reaction mixture to terminate the enzyme reaction. Immediately thereafter, the solution was centrifuged and 3 ml of the supernatant was poured onto a Dowex 50 W ×8 column (0.8 i.d. ×3 cm, H+ form, 200-400 mesh) and the column was washed with 30 ml of distilled water. Three milliliters of a 4 N ammonia solution were then added to the column. The eluant was collected in a test tube and 0.2 ml of 4% sodium metaperiodate solution was added. The test tube was allowed to stand for 10 min and before 0.2 ml of 20% sodium metabisulfite solution was added. UV absorption of the resulting mixture was determined at 330 nm.
Activity was calculated as follows: MAO-A inhibition (%)=(sample−compensate-control)/(blank−control)×100, MAO-B and DBH inhibition (%)=(control−sample+compensate)/(control−blank)×100
Statistical analysis
Data were analyzed by using Duncana, Tukey HSDa in SPSS program and presented as the mean ± S.E.M. of 3-6 independent experiments. Inhibitory potency is obtained by Logic-log graph paper. p<0.05 was considered significant.
RESULTS
Structure identification of the isolated compounds
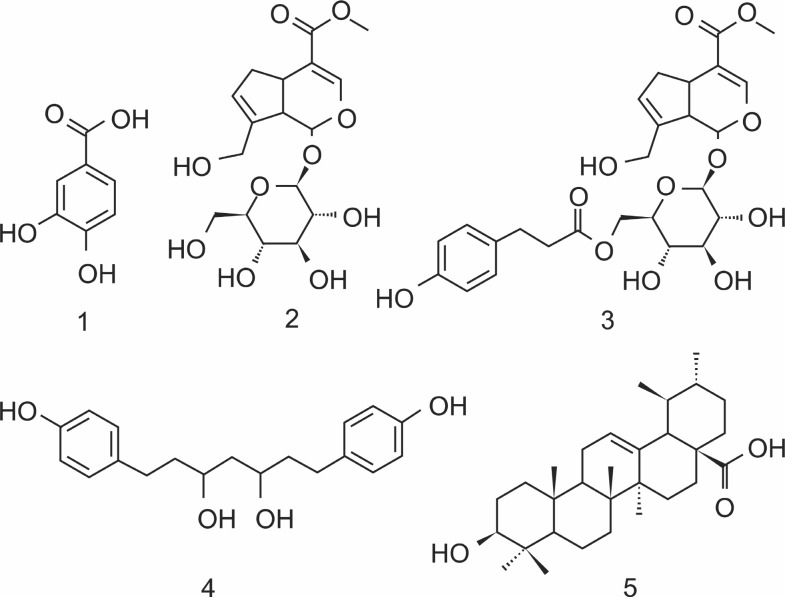
The bioassay-guided fractionation led to isolation of five bio-active compounds (Fig. 1) from the ethyl acetate fraction of G. jasminoides fruits. They were identified as protocatechuic acid (1) (Xu et al., 1994), geniposide (2) (Zhou et al., 2005), 6'-O-trans-p-coumaroylgeniposide (3) (Yu et al., 2009), 3,5-dihydroxy-1,7-bis(4-hydroxy-phenyl) heptanes (4) (Yokosuka et al., 2002) and ursolic acid (5) (Numata et al., 1989) by comparing the obtained spectral data (NMR, IR, Mass) with those in the literature data.
Fig. 1. Chemical structures of protocatechuic acid (1), geniposide(2), 6'-O-trans-p-coumaroylgeniposide (3), 3,5-dihydroxy-1,7-bis(4-hydroxyphenyl) heptanes (4) and ursolic acid (5).
Biological activity
To determine if the isolated compounds were inhibitors, the remaining concentration of serotonin was measured after reacting the compound with MAO-A, and for MAO-B and DBH, the reaction product was measured. 1 mM serotonin was used as the substrate for MAO-A inhibition assay and for MAO-B and DBH, 4 mM benzylamine and 120 mM tyramine were used, respectively. Inhibitory activities are expressed as the mean ± S.E.M. of 3-6 independent experiments. To explain the selectivities of the active compound on each enzyme, we tested two positive control compounds, simultaneously. Iproniazid and R-(-)-deprenyl were used as positive controls for MAO-A and MAO-B, respectively.
The isolated compounds showed different inhibitory potentials against MAO-A, -B, and DBH (Table 1). Protocatechuic acid showed weak MAO-A inhibition (IC50 2411 μmol/L), exhibiting significant inhibitions for MAO-B (IC50 300 μmol/L) and DBH (IC50 334 μmol/L) in a dose-dependent manner. Shown as Table 1, its inhibitory activities at the concentrations of 500, 100, and 50 μg/ml were as follows: 67.4 ± 5.2, 12.8 ± 4.8, and 7.3 ± 2.6% for MAO-A; 108.5 ± 1.3, 71.6 ± 9.3, and 50.4 ± 5.9% for MAO-B; 97.2 ± 2.0, 64.1 ± 6.8, and 49.2 ± 3.1% for DBH. Geniposide (IC50 223 μmol/L) and 6'-O-trans-p-coumaroylgeniposide (IC50 127 μmol/L) selectively inhibited the activity of MAO-B in a dose-dependent manner. Their inhibitory activities at 100, 50, 20, and 4 μg/ml were as follows: geniposide, 52.2 ± 1.5, 42.8 ± 2.2, 16.0 ± 0.6, and 9.2 ± 1.2%; 6'-Otrans-p-coumaroylgeniposide, 62.3 ± 2.9, 55.8 ± 0.7, 14.7 ±0.6, and 11.0 ± 0.6%. 3,5-dihydroxy-1,7-bis(4-hydroxyphenyl) heptanes showed modest MAO-A inhibition (IC50 400 μmol/L), significant MAO-B inhibition (IC50 196 μmol/L), and weak DBH inhibition (IC50 941 μmol/L) in a dose-dependent manner. Its inhibitory activities at 100 and 50 μg/ml were as follows: 33.2 ± 0.6 and 1.2 ± 2.6% for MAO-A; 67.4 ± 6.5 and 57.2 ± 3.6% for MAO-B; 25.6 ± 1.0 and 19.4 ± 3.7% for DBH. This compound in low doses (20 and 4 μg/ml) exhibited a bit inhibition (17.8 ±1.2 and 12.3 ± 0.6%) for only MAO-B. Compounds 1-4 inhibited more MAO-B activity than the other enzyme activities, but ursolic acid inhibited DBH more than MAO-B. Ursolic acid exhibited significant inhibition of DBH (IC50 98 μg/ml) and weak inhibition of MAO-B (IC50 780 μmol/L) in a doses-dependent manner. Its inhibitory activities were as follows: no inhibition for MAO-A; 28.7 ± 8.0% (100 μg/ml) and 17.1 ± 1.1% (50 μg/ml) for MAO-B; 51.2 ± 5.9% (100 μg/ml), 33.2 ± 6.9% (50 μg/ml), 20.8 ± 10.7% (10 μg/ml), and 18.2 ± 7.6% (2 μg/ml) for DBH.
Table 1.
Inhibitory potential of protocatechuic acid (1), geniposide (2), 6'-O-trans-p-coumaroylgeniposide (3), 3,5-dihydroxy-1,7-bis(4-hydroxyphenyl) heptanes (4) and ursolic acid (5)
| Compound | Yield (%) | IC50 valueb | Specific activityd | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| MAO-A μM | MAO-B μM | DBH μM | MAO-A | MAO-B Unitc/g | DBH | ||
|
| |||||||
| 1 | 0.01 | 2,411 | 300 | 334 | 2,695 | 21,739 | 19,608 |
| 2 | 0.16 | >12,887 | 223 | >12,887 | <200 | 11,628 | <200 |
| 3 | 0.0003 | >9,363 | 127 | >9,363 | <200 | 14,706 | <200 |
| 4 | 0.0009 | 400 | 196 | 941 | 7,937 | 16,129 | 3,367 |
| 5 | 0.007 | >10,965 | 780 | 214 | <200 | 2,809 | 10,204 |
| Iproniazida | - | 40 | 60 | - | |||
| Deprenyla | - | 3.3 | 0.05 | - | |||
aPositive control for MAO. bInhibitory potency is obtained by Logic-log graph paper. cOne unit is defined as a sample amount to give 50% inhibition against the enzymes. dSpecific activity means the amount of unit in 1 g of a test sample.
DISCUSSION
The major effective constituents of G. jasminoides fruits are monoterpenes and polyphenolic compounds such as iridoid glycosides and flavonoids. Iridoids are found in many medicinal plants and may be responsible for some of their pharmaceutical activities. They exhibited a wide range of bioactivities including cardiovascular, antihepatotoxic, choleretic, hypoglycemic, analgesic, anti-inflammatory, antimutagenic, antitumor, antiviral, immunomodulator, and purgative activities (Didna et al., 2007; Tundis et al., 2008). At present study, geniposide which was the well-known major iridoid glycosides of G. jasmi-noides fruits is considered as major MAO-B inhibitor because it was showed significantly selective MAO-B inhibition and
much higher yield than the other isolated compounds. 6'-Otrans-p-coumaroylgeniposide also exhibited selective MAO-B inhibition, and its inhibitory potential is stronger than geniposide. Especially, this compound was more selective MAO-B inhibitor than deprenyl (selegiline), well-known MAO-B in-hibitor for the treatment of early-stage Parkinson’s disease(PD) (Fig. 2). Protocatechuic acid, a polyphenol antioxidant, which exhibited significant inhibition on both MAO and DBH, is expected to elevate the level of released dopamine (DA) effectively, by preventing DBH from converting DA to norepinephrine and being destroyed by oxidative deamination effect of MAO. Moreover, this compound was reported to increase proliferation and inhibit apoptosis of neural stem cells (Guan et al., 2009). These results support that this compound has properties indicative of potential neuroptotective ability.
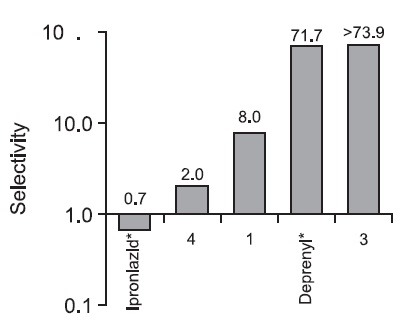
Fig. 2. Selectivity of protocatechuic acid (1), 6'-O-trans-p-couma-roylgeniposide (3), 3,5-dihydroxy-1,7-bis(4-hydroxyphenyl) heptanes (4), iproniazid and deprenyl. Selectivity for MAO is defined by the ratio of IC50 (MAO-A)/IC50 (MAO-B) for each compound. Increasing selectivity values indicate greater MAO-B inhibitory effects and smaller MAO-A inhibitory effects. When the value falls below 1, there is more inhibition of MAO-A. *Iproniazid and Deprenyl were used as positive controls for MAO-A and MAO-B, respectively.
Recently Sun group reported that the oral bioavailability of geniposide was dramatically enhanced when G. jasminoides was decocted with other Chinese medicinal plants (Sun et al., 2011). They argued that it can be deduced that herb-herb inter-action may increase the absorption, and significantly improve the oral bioavailability of geniposide in rat (Sun et al., 2011). In our previous research, oral administration of the ethanolic extract slightly increased serotonin concentrations in the brain tissues of rats and decreased MAO-B activity (Hwang and Park, 2007). This tendency is similar to the activity of deprenyl which is a well-known MAO inhibitor having antidepressant effects. It is well known that Selegiline itself is not that beneficial for the treatment of PD, but it becomes a good therapeutic agent with levodopa. In this study, we determined inhibitory effects of 5 compounds on both MAOs and DBH. In view of beneficial effects of the combination therapy on PD, it would be show the possible potency of 5 compounds as therapeutic molecules. Although the absorption percentage of the isolated geniposide is very low, the bioavailability of this compound is enhanced when G. jasminoides was used as a plant material itself (Hou et al., 2008; Lu et al., 2011). The extract of the G. jasminoides have been used as a food material and also in traditional medicine for the treatment of inflammatory disease in Korea from ancient.
The appropriate control of MAO-A inhibition is one of the most important elements in determining effective drug candidates for depression. Meyer et al. (2009) reported that MAO-A binding levels are significantly elevated in each brain region during major depressive episodes (MDEs) in major depressive disorder (MDD), even after serotonin reuptake inhibitor (SSRI) treatment. The elevated MAO-A binding after SSRI treatment indicates the persistence of a monoamine-lowering process that is not present in healthy individual (Meyer et al., 2009). In our research, compounds 1, 3, 4 and the positive controls exhibiting greater inhibition of MAO-A with no changes in MAO-B inhibition as selectivity values decreased (Fig. 2). Thus, according to the level of released serotonin, these compounds may be applied suitably as drug candidates. The different selectivity of the MAO inhibitors will play a major role in establishing a continuous treatment for depression to prevent the elevation of MAO-A binding that contributes to relapse while still avoiding serotonin syndrome. In addition, Kitaichi et al. (2010) suggested that the combined treatment with a MAOA inhibitor and a MAO-B inhibitor strengthens antidepressant effects because the combined treatment increases extracellular noradrenaline levels more than a MAO-A inhibitor alone through increases in β-phenylethylamine.
G. jasminoides has been used as a natural yellow colorant in foods and also in traditional medicine for the treatment of liver and bladder disorders and inflammatory disease. In general, the plant used as food materials showed moderate bio-activities, but these activities are also important in their activity evaluation, because the food materials are available on an ongoing basis without the side effects.
Consequently, our results showed that G. jasminoides fruits are considerable for development of biofunctional food mate-rials for the treatment of depression and neurodegenerative disorders.
A methoxy group and a hydroxyl group were regarded as an active site against MAO inhibition (Han et al., 1987). In our study, 3,5-dihydroxy-1,7-bis(4-hydroxyphenyl) heptanes, having only four hydroxyl groups as functional group, exhibited about 2 times more specific activity on MAO-B than that on MAO-A (Table 1). This result suggests that a hydroxyl group has approximately 2-folds more inhibitory potential on MAO-B over MAO-A. However, protocatechuic acid showed about 8 times more specific activity on MAO-B than that on MAO-A. This result seems to be because of the diminution of MAO-A inhibitory activity by an additional hydroxy group at the ortho-position. Ryu et al., (1988) also reported that there is a diminution in MAO-A inhibitory activity when an additional phenolic hydroxy group is present at the orthoposition on the A or B ring of resveratrol. Consequently, we suggest that a hydroxyl group has an approximately 2-fold greater inhibitory potential on MAO-B over MAO-A, and an additional hydroxyl group at the ortho-position alleviates the MAO-A inhibitory activity of a hydroxyl group approximately 4-fold. In addition, Ryu et al.,(1988) reported that resveratrol and rhapontigenin showed an inhibitory effect on MAO-A, but when the hydroxyl group was modified to an O-glucose moiety (compounds piceid and rhaponticin, respectively), the effects lessened. Han et al., (1990) also reported that masking the hydroxyl proton with methyl and glycosyl groups diminished antioxidant activity. Thus, it is possible that the O-glucose moiety caused 6'-O-trans-p-coumaroylgeniposide to lack an MAO-A inhibitory effect even though a hydroxy group was present.
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0029692).
References
- 1.Dinda B. Debnath S. Harigaya Y. Naturally occurring iridoids. A review, part 1. Chem. Pharm. Bull (Tokyo). (2007);55:159–222. doi: 10.1248/cpb.55.159. [DOI] [PubMed] [Google Scholar]
- 2.Guan S. Ge D. Liu T. Q. Ma X. H. Cui Z. F. Protocatechuic acid promotes cell proliferation and reduces basal apoptosis in cultured neural stem cells. Toxicol In Vitro. (2009);23:201–208. doi: 10.1016/j.tiv.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Han Y. N. Choo Y. Lee Y. C. Moon Y. I. Kim S. D. Choi J. W. Monoamine oxidase B inhibitors from the fruits of Opuntia ficus-indica var. saboten. Arch. Pharm. Res. (2001);24:51–54. doi: 10.1007/BF02976493. [DOI] [PubMed] [Google Scholar]
- 4.Han Y. N. Hwang K. H. Kim H. W. Lee S. J. Ahn S. K. Kim J. K. Compounds having dopamine beta-hydroxylase inhibitory activity. (1997) Patent WO 1997029076.
- 5.Han Y. N. Noh D. B. Han D. S. Studies on the monoamine oxidase inhibitors of medicinal plants I. isolation of MAO-B inhibitors from Chrysanthemum indicum. Arch. Pharm. Res. (1987);10:142–147. doi: 10.1007/BF02857780. [DOI] [Google Scholar]
- 6.Han Y. N. Ryu S. Y. Han B. H. Antioxidant activity of res-veratrol closely correlates with its monoamine oxidase-A inhibitory activity. Arch. Pharm. Res. (1990);13:132–135. doi: 10.1007/BF02857789. [DOI] [Google Scholar]
- 7.Hou Y. C. Tsai S. Y. Lai P. Y. Chen Y. S. Chao P. D. Metabolism and pharmacokinetics of genipin and geniposide in rats. Food Chem. Toxicol. (2008);46:2764–2769. doi: 10.1016/j.fct.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Hwang K. H. Kim I. R. Han Y. N. Effects of cold and hot drugs on the activity of monoamine oxidase. Korean J. Pharmacogn. (1999);30:145–150. [Google Scholar]
- 9.Hwang K. H. monoamine oxidase inhibitory activities of Korean medicinal plants classified to cold drugs by the theory of KIMI. Food Sci. Biotechnol. (2003);12:238–241. [Google Scholar]
- 10.Hwang K. H. Park T. K. Inhibitory activity of the fruit extract of Gardenia jasminoides on monoamine oxidase. Korean J. Pharmacogn. (2007);38:108–112. [Google Scholar]
- 11.Kitaichi Y. Inoue T. Nakagawa S. Boku S. Izumi T. Koyama T. Combined treatment with MAO-A inhibitor and MAO-B inhibitor increases extracellular noradrenaline levels more than MAO-A inhibitor alone through increases in beta-phenylethylamine. Eur. J. Pharmacol. (2010);637:77–82. doi: 10.1016/j.ejphar.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Koo H. J. Lee S. Shin K. H. Kim B. C. Lim C. J. Park E. H. Geniposide, an anti-angiogenic compound from the fruits of Gardenia jasminoides. Planta Med. (2004);70:467–469. doi: 10.1055/s-2004-818978. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y. Du S. Y. Chen X. L. Wu Q. Song X. Xu B. Zhai Y. S. Enhancing effect of natural borneol on the absorption of geniposide in rat via intranasal administration. J. Zhejiang. Univ. Sci. B. (2011);12:143–148. doi: 10.1631/jzus.B1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer J. H. Wilson A. A. Sagrati S. Miler L. Rusjan P. Bloomfield P. M. Clark M. Sacher J. Voineskos A. N. Houle S. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch. Gen. Psychiatry. (2009);66:1304–1312. doi: 10.1001/archgenpsychiatry.2009.156. [DOI] [PubMed] [Google Scholar]
- 15.Miura T. Nishiyama Y. Ichimaru M. Moriyasu M. Kato A. Hypoglycemic activity and structure-activity relationship of iridoidal glycosides. Biol. Pharm. Bull. (1996);19:160–161. doi: 10.1248/bpb.19.160. [DOI] [PubMed] [Google Scholar]
- 16.Numata A. Yang P. Takahashi C. Fujiki R. Nabae M. Fujita E. Cytotoxic triterpenes from a Chinese medicine, Goreishi. Chem. Pharm. Bull (Tokyo). (1989);37:648–651. doi: 10.1248/cpb.37.648. [DOI] [PubMed] [Google Scholar]
- 17.Peng C. H. Huang C. N. Wang C. J. The anti-tumor effect and mechanisms of action of penta-acetyl geniposide. Curr. Cancer Drug Targets. (2005);5:299–305. doi: 10.2174/1568009054064633. [DOI] [PubMed] [Google Scholar]
- 18.Pham T. Q. Cormier F. Farnworth E. Tong V. H. Van Calsteren M. R. Antioxidant properties of crocin from Gardenia jasminoides Ellis and study of the reactions of crocin with linoleic acid and crocin with oxygen. J. Agric. Food Chem. (2000);48:1455–1461. doi: 10.1021/jf991263j. [DOI] [PubMed] [Google Scholar]
- 19.Ryu S. Y. Han Y. N. Han B. H. Monoamine oxidase-A inhibitors from medicinal plants. Arch. Pharm. Res. (1988);11:230–239. doi: 10.1007/BF02861314. [DOI] [Google Scholar]
- 20.Sun Y. Feng F. Yu X. Pharmacokinetics of geniposide in Zhi-Zi-Hou-Pu decoction and in different combinations of its constituent herbs. Phytother. Res. (2011);26:67–72. doi: 10.1002/ptr.3516. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y. Kondo K. Ikeda Y. Umemura K. Antithrombotic effect of geniposide and genipin in the mouse thrombosis model. Planta Med. (2001);67:807–810. doi: 10.1055/s-2001-18842. [DOI] [PubMed] [Google Scholar]
- 22.Tundis R. Loizzo M. R. Menichini F. Statti G. A. Menichini F. Biological pharmacological activities of iridoids: recent developments. Mini-Rev. Med. Chem. (2008);8:399–420. doi: 10.2174/138955708783955926. [DOI] [PubMed] [Google Scholar]
- 23.Xu H. X. I. Kadota S. Wang H. U. A. Kurokawa M. Shiraki K. Matsumoto T. Namba T. A new hydrolyzable tannin from Geum japonicum and its antiviral activity. Heterocycles (Sen-dai) (1994);38:167–175. doi: 10.3987/COM-93-6550. [DOI] [Google Scholar]
- 24.Yokosuka A. Mimaki Y. Sakagami H. Sashida Y. New diarylheptanoids and diarylheptanoid glucosides from the rhizomes of Tacca chantrieri and their cytotoxic activity. J. Nat. Prod. (2002);65:283–289. doi: 10.1021/np010470m. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y. Xie Z. L. Gao H. Ma W. W. Dai Y. Wang Y. Zhong Y. Yao X. S. Bioactive iridoid glucosides from the fruit of Gardenia jasminoides. J. Nat. Prod. (2009);72:1459–1464. doi: 10.1021/np900176q. [DOI] [PubMed] [Google Scholar]
- 26.Zeller E. A. Oxidation of amines. In J. B. Sumner and K. Myrbäck, Eds. The enzymes. Academic Press; New York: (1951). pp. 536–558. [Google Scholar]
- 27.Zhou T. Fan G. Hong Z. Chai Y. Wu Y. Large-scale isolation and purification of geniposide from the fruit of Gardenia jasminoides Ellis by high-speed counter-current chromatography. J. Chromatogr. A. (2005);1100:76–80. doi: 10.1016/j.chroma.2005.09.026. [DOI] [PubMed] [Google Scholar]