Abstract
Ethanol exposure during gestational period is related to growth retardation, morphological abnormality, and even in neurological abnormalities including attention deficit/hyperactivity disorder (ADHD)-like behaviors on offspring. However, relatively little is known about the effects of maternal ethanol consumption prior to conception on their offspring. In this study, we investi-gated whether maternal ethanol administration during preconceptional phase produces ADHD-like behaviors in the rat offspring. Sprague-Dawley (SD) female rats were administrated ethanol via intragastric intubation with dosing regimen of 6 g/kg daily for 10 consecutive days and treated female rats then mated with non-treated male SD rats after 8 weeks. Another group subjected to the same procedure as those conducted on ethanol treated group except the saline administration instead of ethanol. Offspring was tested for their ADHD-like behaviors using open field test, Y maze test and impulsivity test that is performed in the aversive electronic foot shock paradigm. Offspring of preconceptional ethanol treated (EtOH) group showed hyperlocomotive activity, attention deficit and impulsivity. And reduction of striatal dopamine transporter (DAT) level was observed by Western blot in the EtOH group, compared to control (Con) group, while the immunohistochemical analysis exhibited increased expression of norepinephrine transporter (NET) in the frontal cortex. These results suggest that maternal ethanol consumption in the preconceptional phase induces ADHD-like behaviors in offspring that might be related to the abnormal expression of DAT and NET in rat.
Keywords: ADHD, Preconceptional ethanol consumption, Norepinephrine transporter, Dopamine transporter
INTRODUCTION
Exposure to ethanol during pregnancy may cause diverse array of adverse developmental effects, which are collectively called fetal alcohol spectrum disorder (FASD). Reported symptoms include prenatal and/or postnatal growth retardation, facial malformation and central nervous system (CNS) dysfunction (Riley and Mcgee, 2005). Previous studies showed that maternal ethanol consumption throughout pregnancy may induce mental disorders such as suicide attempts (Huggins et al., 2008), depression and anxiety (O’Leary et al., 2010) to their offspring. Because CNS dysfunction may happen without physical disabilities and the conditions may not improve sufficiently at the later period of life, maternal drinking during pregnancy may cause enormous socio-economical damages (Lupton et al., 2004) to patients that exposed ethanol prenatally as well as to their families. For these reasons, physiological, developmental, and behavioral effects on offspring induced by maternal ethanol consumption during pregnancy were of much interest to many investigators as well as general public. One of the symptoms recently attracting increased interest in FASD research is ADHD-like symptoms observed in FASD patients (Riley and McGee, 2005). It has been suggested that 14-25 % of parents of ADHD patients reportedly have alcohol abuse problem or drink more alcohol than normal (Barkley, 1990; Knopik et al., 2005). Recently, it has been also suggested that prenatally-ethanol exposed rats showed ADHD like behavioral phenotype, which was assessed using choice reaction time tests (Hausknecht et al., 2005). ADHD affects about 8.7% of children aged 8 to 15 in US (Froehlich et al., 2007), and worldwide-pooled prevalence is 5.29% (Polanczyk et al., 2007). Unlike previous studies that ADHD had been more common in males than females, recent study suggests that clinical correlates of ADHD are not influenced by gender (Biederman et al., 2005). Median age of onset is 8.1 years, and it is much earlier than other DSM-IV disorders (Kessler et al., 2005). Etiology of ADHD has not been fully identified even though investigators have made a great effort to confirm those pathophysiological, genetic, neurodevelopmental and toxicological hypotheses (Davids et al., 2003).
Core symptoms of ADHD are inattention and/or hyperactivity and impulsivity (American Psychiatric Association, 1994), and children suffer from ADHD demonstrate more problematic behaviors and less social skills than normal children (DuPaul et al., 2001) with learning disability, even though they have normal intellectual power including I.Q. score. Above mentioned features of ADHD make it a serious social and medical problem to tackle down by proving its etiology as well as pathophysiological mechanism. In addition to the FASD mediated by ethanol drinking during pregnancy, ethanol consumption prior to conception may also induce detrimental effects to offspring. These effects may or may not be same in relation to the effects induced by maternal ethanol intakes during pregnancy. In previous study performed using male and female C57BL/6J mice that were received chronic ethanol administration before pregnancy, fetuses that were born from dams treated with ethanol have lower fetal body weights compared to those treated with water (Livy et al., 2004). Moreover, maternal ethanol consumption before pregnancy exhibits a higher possible risk ratio for men-tal retardation in human offspring compared with the offspring born from mothers that consumed ethanol during pregnancy (Roeleveld et al., 1992). A recent opinion also suggested that the FASD might be induced by preconceptional parental ethanol consumption via epigenetic changes (Haycock, 2009). It has been reported half of all pregnant respondents consume ethanol prior to and early pregnancy (Floyd et al., 1999). Although maternal ethanol consumption prior to pregnancy had occurred frequently, very little is known about the potential risk of preconceptional ethanol consumption to progeny, which would be very important in terms of public welfare as well as expanding our understanding of the regulatory mechanisms governing neural development. In this study, we investigated whether preconceptional exposure of female rats to alcohol may affect ADHD-like behaviors in their offsprings.
MATERIALS AND METHODS
Subjects, treatment group, and drugs administration
Six female SD rats (250-300 g weight, typically 10 weeks old) were purchased from DBL (Seoul, Korea) at gestation day (GD) 2 and stabilized under environmental controlled rearing system maintained 12 hr light-dark cycle (light turns on 08:00) for 6 consecutive days. Stabilized rats were divided into two groups, and one group was treated with ethanol (Hayman, UK; 6 g/kg/day; 25 v/v %) diluted with normal saline from GD 7 to GD 16 via intragastric intubation. To control for intubation induced stress, the other group received the same volume of normal saline like ethanol treated group. Treatment was divided into two equal doses 6 hr apart, at 10:00 and 16:00. After parturition, treated dams were allowed to recover for 6 weeks and then were mated again with untreated male SD rats (300-350 g). Dams were checked vaginal plug every morning to confirm succeed at copulation. The dams that were observed a plug formation housed individually and were allowed to breed their pups freely. During this pregnancy, dams neither received ethanol nor normal saline treatment. The offspring born from ethanol treated dams before pregnancy was desig-nated as EtOH group, and the offspring born from normal saline treated dams was determined as Con group. The day after parturition was regarded postnatal day (PD) 1 and litters were culled on this day to a same number between groups. The number of allowed pups to a dam was 10. The offspring was weaned on PD 21, and weighed immediately after separated from mother. The offspring was accommodated in cage of 5 rats during the behavioral experiment period. All rats in this study were permitted to access rat chow and tap water ad libitum except in the case of particular experimental schedules. All behavioral tests were conducted after adaptation of subjects to test environment for 30 min. All animal experiments were approved either by the Konkuk University or Sahmyook University Animal Care and Experimentation Committee.
Open-field test
Open-field test experiment was conducted on PD 22 and 42.The open-field apparatus was made of Plexiglas (42×42×50 cm). Rats were placed in the center of the apparatus and allowed to move freely. Behavioral data in the open-field test was recorded for 20 min and analyzed using Noldus Etho-Vision software (Version 3.1; Noldus Information Technology, Wageningen, Netherlands). We quantified the following parameters:total distance moved, total movement duration time and rearing frequency. Those data were analyzed as indicator of hyperactive properties.
Y-maze test
Y-maze test experiment was conducted on PD 28. The standard Y-maze apparatus was made of Plexiglas and the size of each arm was 10 cm width, 50 cm length and 20 cm height. Rats were placed into the start arm of a standard Ymaze and allowed to move freely for 8 min. Behavioral data in the standard Y-maze were recorded and analyzed using Noldus EthoVision software. We quantified % of spontaneous alternation for attentive ability and total arm entries. Spontaneous alternation was calculated by the following equation:
Spontaneous alternation=(number of alternations/total arm entries-2)×100
Electro-foot shock aversive water drinking test
Several paradigms like extinction paradigms by punishment or reward-choice paradigms have been used to measure impulsive behavior in a laboratory setting (Moeller et al., 2001). With reference to those paradigms, water drinking test with aversive interference was developed. In brief, tendency to get a reward (fresh water for thirsty rats) in spite of a foreseeable punishment (electrofoot shock) was measured as an indicator of impulsive behavior. The constitution and execution of the electro-foot shock aversive water drinking test is as described below: Test apparatus basically comprises two compartments:one is start and the other is shock and water drinking area. Shock area was constructed with grid which is connected to electric stimulator. The impulsivity test was conducted on PD 39. Before training and testing sessions, rats were deprived of water for about 18 hr to reinforce the access to water. In training session, the rats were put in the start area of test ap-paratus and permitted to explore around the apparatus and to drink water freely for 5 min, which was conducted for two consecutive days to habituates the rat to the apparatus and to train them for the position of water. After two training sessions, rats were tested their impulsivity. Rats were put in the start area, and if a rat had took water for 5 sec, the electric current of 2 mA for 0.5 sec was given as a punishment. We quantified the following parameters: total distance moved and total movement duration time were used to prove hypertensive tendency, and the time required from the beginning of experiment to drink water (first drink latency), the frequency of drinking water, sum of drinking time and total number of entrance to the water area were analyzed to confirm impulsive property.
Tissue preparation
For Western-blot analysis, 7 weeks old rats were rapidly decapitated and forebrain samples, including the striatum and frontal cortex were taken swiftly. Forebrain regions including frontal cortex is involved in higher executive functions and have been implicated in the structural and functional abnor-malities in ADHD. These samples were then frozen and stored at -70℃ until the experiments. For immunohistochemical analysis, 7 weeks old rats were fixed with 4% paraformaldehyde solution (PFA; dissolved in PBS, Sigma St Louis, MO, USA) kept in ice. Fixed brain was put in cold PFA solution and one day later, brain was transferred to 30% sucrose solution (dissolved in phosphate buffered saline) for a few days in order to dehydrate. Dehydrated tissues were cut into 40 ㎛ thick coronal sections in a cryostat through the striatum, basal fore-brain and stored in stock solution which contained glycerol, ethylene glycol and phosphate buffer at 4℃.
Western-blot analysis
Equal amount of protein from homogenized samples, prepared in sucrose buffer (sucrose 0.32 M and Tris 4 mM), was electrophoresed on 10% SDS-polyacrylamide gel for 120 min and the proteins were electrically transferred to the nitrocellulose membranes (Whatman, Germany) for 90 min. The blot was blocked with 5% nonfat dried milk at room temperature and incubated overnight at 4℃ with anti-DAT antibody (Santa Cruz Biotechnology Inc., California, USA), which were diluted at 1:2,000 in 5% nonfat dried milk. After the membranes were incubated again with horseradish peroxidase-conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA) at room tem-perature for 2 hr, positive bands were detected with enhanced chemiluminescence detection system (Amersham Bioscienc-es, Piscataway, NJ, USA) and exposed to LAS-3000 image detection system (Fuji, Tokyo, Japan). Western blot against β-actin using a monoclonal antibody (Sigma) was used as a loading control.
Immunohistochemical analysis
Immunohistochemical analysis was conducted using free-floating method. Stored sections were dipped with 1.5% normal horse serum in PBS with 0.1% Triton X-100 solution (Sigma- Aldrich) and incubated overnight at 4℃ with anti-NET antibodies (Santa Cruz Biotechnology Inc.). After incubation, sections were washed three times for 5 min with PBS, followed by incubation for 1 hr with Alexa 594-labeled anti-rabbit secondary antibody (Invitrogen). Stained sections were washed three times again and then mounted on coated slides, and slides were investigated using Olympus BX61 fluorescence microscope system (Olympus, Tokyo, Japan).
Statistical analysis
For comparison of behavioral differences between EtOH group and Con group as well as for analysis of Western blot data, unpaired Student’s t-test were used. Data are presented as mean ± SEM. Statistical analyses and result presentation was done using Graphpad prism 4.01 software (GraphPad Software Inc, San Diego, CA, USA).
RESULTS
Postnatal growth
In this study, we treated pregnant rats with ethanol and mat-ed them again 6 weeks after parturition and all of them successfully got pregnant and delivered full term rat offsprings. No change in the number of live birth was observed (data not shown). These results suggest that preconceptional ethanol exposure used in this study did not affect reproductive behavior of rats. To check whether preconceptional ethanol exposure induces massive developmental problems to offspring, postnatal growth retardation was evaluated at PD 21. EtOH group gained significantly more weight than Con group (Table 1; Con group: 49.3 ± 0.5, EtOH group: 56.2 ± 0.9, p<0.001) suggesting that preconceptional ethanol did not adversely affect gross growth of rat offsprings.
Table 1.
The effects of preconceptional ethanol exposure on the weight of pups at postnatal day 21
| Total | Female | Male | |
|---|---|---|---|
|
| |||
| Con | 49.3 ± 0.5 | 48.6 ± 0.7 | 50.1 ± 0.5 |
| (n=30) | (n=16) | (n=14) | |
| EtOH | 56.2 ± 0.9*** | 55.3 ± 1.8*** | 56.8 ± 1.0*** |
| (n=20) | (n=8) | (n=12) | |
EtOH: offspring born from preconceptional ethanol exposed dams, Con: offspring born from normal saline exposed dams. Ethanol exposed pups weighted significantly more than control (***p<0.001. EtOH (n=20), Con (n=30)).
Increased locomotive activity
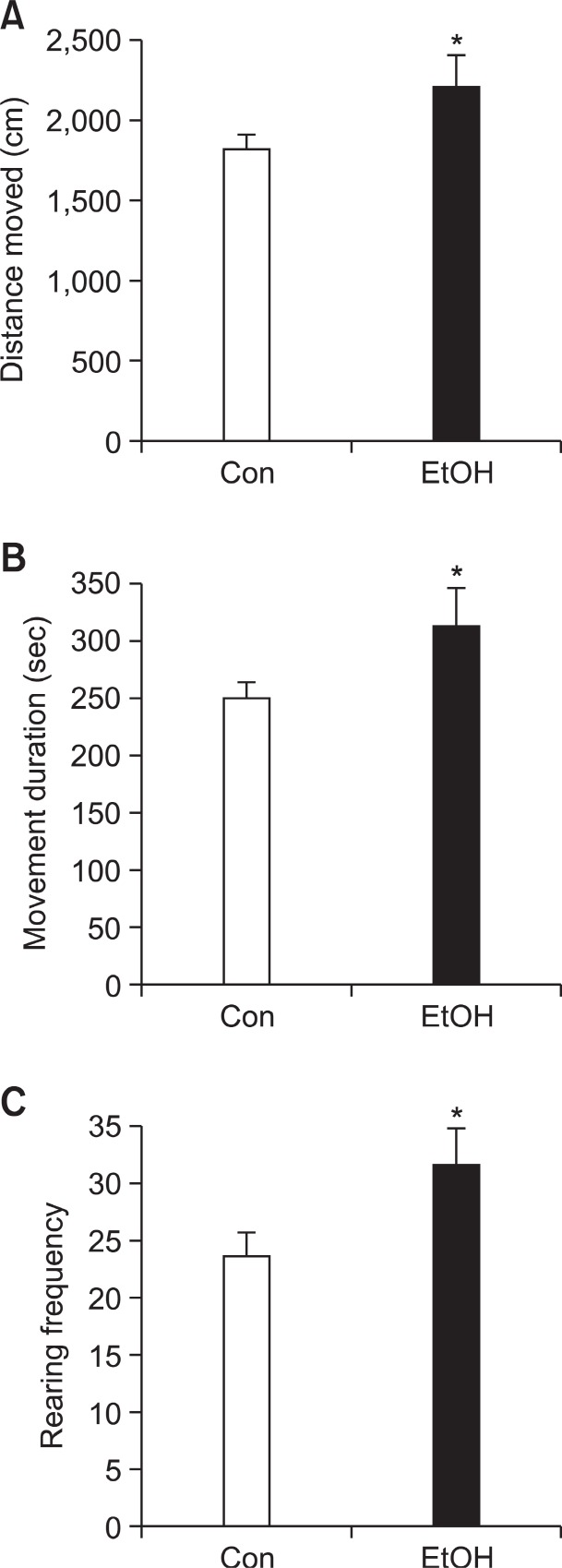
Hyperactivity is one of the core symptoms of ADHD and we investigated locomotive activity of EtOH group in open field test at PD 22. EtOH group significantly increased the distance moved (Fig. 1A; Con group: 1,823 cm ± 90.71, EtOH group: 2,214 cm ± 195.5, p<0.05), movement duration (Fig. 1B; Con group: 249.9 sec ± 13.78, EtOH group: 312.5 sec ± 30.82,p<0.05) and rearing frequency (Fig. 1C; Con group: 23.63 ±1.926, EtOH group: 31.61 ± 3.115, p<0.05) than Con group. These data indicate that preconceptional ethanol exposure induces hyperkinetic problem to offspring rats.
Fig. 1. Hyperactivity of preconceptional ethanol-exposed offspring at postnatal day 22. EtOH group showed significantly increased (A) distance moved (B), movement duration and (C), rearing frequency in the open-field test compared to the Con group. Data were expressed as the mean ± S.E.M. (*p<0.05, EtOH (n=16), Con (n=26)).
Decreased attention-related behaviors
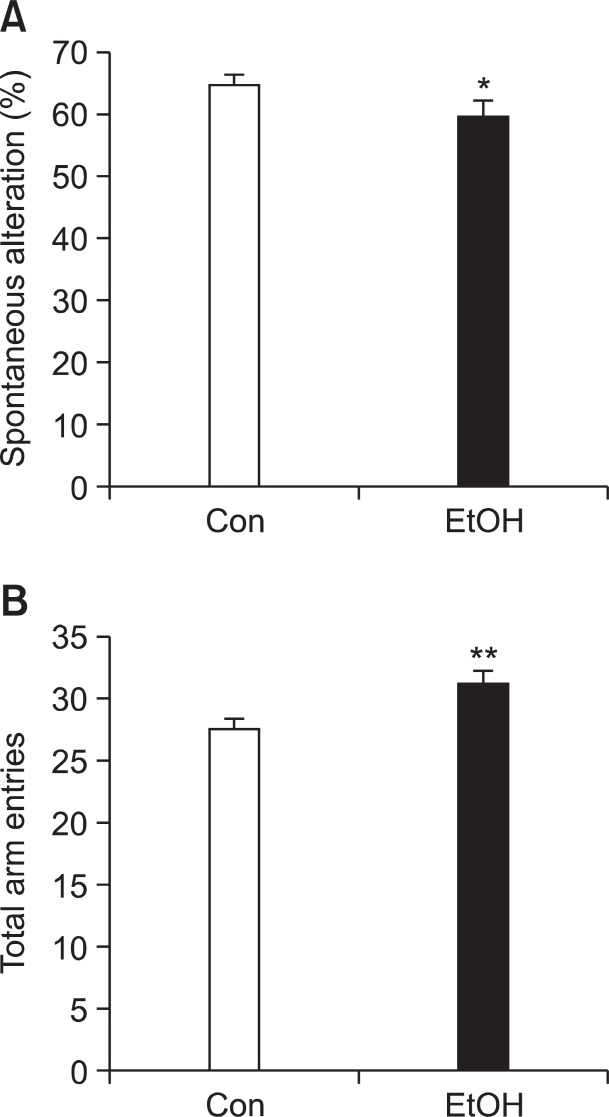
Spontaneous alternation behavior (SAB) in Y-maze test reflects not only spatial memory but also attention (Hughes, 2004). We measured SAB by Y maze test at PD 28 to determine whether EtOH group has the propensity of inattention. In this study, EtOH group exhibited decreased SAB (Fig. 2A; Con group: 65.08% ± 1.411, EtOH group: 59.65% ± 2.422, p<0.05), and more hyperactive properties as indicated by total arm entries (Fig. 2B; Con group: 27.52 ± 0.6822, EtOH group:31.21 ± 0.8904 p<0.01) compared with Con group. These re-
Fig. 2. Decreased attention and increased locomotive activity in the Y-maze test of the preconceptional ethanol-exposed litters at postnatal day 28. EtOH group (n=19) showed (A) significantly decreased spontaneous alternation (B) increased maximum alternation compared to the Con group (n=27). Data were expressed as the mean ± S.E.M. (*p<0.05, **p<0.01).
sults indicate that preconceptional ethanol consumption may induce inattention and deficits in spatial learning memory in offspring rats.
Increased impulsive choices
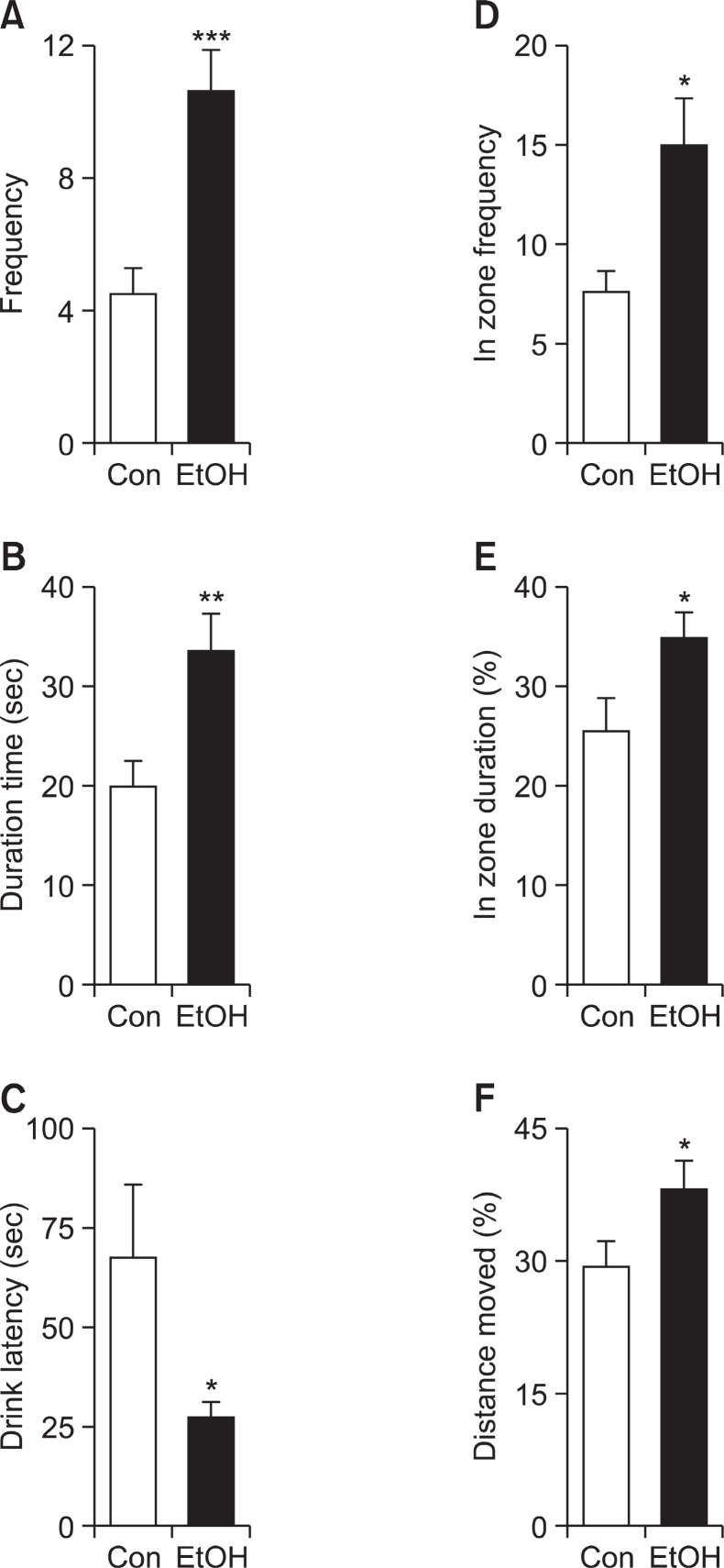
Several parameters obtained from electro-foot shock aver-sive water drinking test were used as evidences of impulsive tendencies. EtOH group displayed more impulsive tendency, as indicated by increased frequency of drinking water (Fig. 3A; Con group: 4.556 ± 0.6894, EtOH group: 10.67 ± 1.225, p<0.01) and total drinking duration (Fig. 3B; Con group: 19.98 sec ± 2.291, EtOH group: 33.53 ± 3.513, p<0.01). In addition, first drink latency, the time required from the beginning of experiment to arrive at water region, (Fig. 3C; Con group: 67.37 sec ± 18.26, EtOH group: 27.18 sec ± 3.420, p<0.05) was significantly decreased compared with Con group. EtOH group demonstrated strong preference for reinforcing reward regardless of painful stimulus, which is evidenced by the increased
Fig. 3. Enhanced impulsive behaviors of the E group at postnatal day 39. In the electro-foot shock aversive water drinking test, EtOH group exhibited increased (A) water drinking frequency, (B) total water drinking time, (D) frequency coming into the water zone, (E) percentile of duration time at shock area to total observation time and (F) percentile of distance moved at shock area to total distance moved. In (C), EtOH group showed attenuated latency to arrive at water zone from the beginning of experiment. EtOH: off-springborn from ethanol treated dams (n=10), Con: offspring born from normal saline treated dams (n=10). Data were expressed as the mean ± S.E.M. (*p<0.05; **p<0.01; ***p<0.001).
frequency of entering water zone (Fig. 3D; Con group: 7.444± 1.082, EtOH group: 15.00 ± 2.285, p<0.05). The increased movement in water and shock zone is not due to the general hyperactive phenotype of EtOH group as evidenced by the increased ratio of duration time in shock area (shock and water zone) to no-shock area (start zone, no shock zone; Fig. 3E; Con group: 25.46% ± 3.248, EtOH group: 34.92% ± 2.291, p<0.05). Similarly, the ratio of distance moved in shock area to no-shock area (Fig. 3F; Con group: 29.05% ± 3.093, EtOH group: 38.11% ± 2.875, p=0.0458) was increased in EtOH group.
Persistent hyper-locomotive activity of preconceptionally ethanol-exposed rats
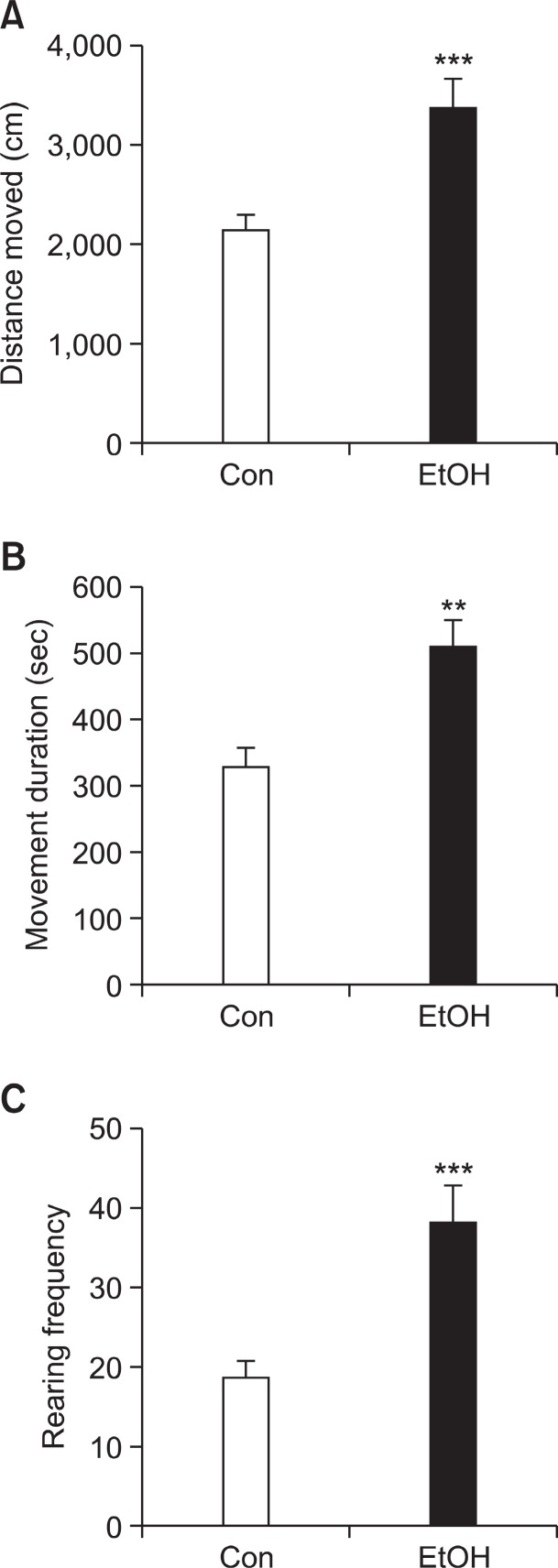
We investigated whether hyper-locomotive activity that was first appeared at PD 22 was maintained in preconceptional ethanol exposed offspring at PD 42. Rats were performed open-field test as described above at PD 42. Rats were per-formed open-field test at the same apparatuses previously used. In the open-field test, EtOH group exhibited significantly increased distance moved (Fig. 4A; Con group: 2,133 cm ± 130.6, EtOH group: 3,381 cm ± 260.0, p<0.001), movement duration (Fig. 4B; Con group: 328.9 sec ± 27.55, EtOH group: 510.5 sec ± 40.97, p<0.001) and rearing frequency (Fig. 4C; Con group: 18.68 ± 2.022, EtOH group: 38.13 ± 4.816, p<0.001) compared with Con group.
Fig. 4. Hyper-locomotive activity of EtOH group at PD 42 in the open-field test. EtOH group exhibited significantly increased (A) distance moved (B) movement duration and (C) rearing frequency compared with Con group. Generally, the hyperactive phenotype was greater in extent, compared with that observed at PD 22. EtOH: offspring born from ethanol treated dams (n=10), Con: off-springborn from normal saline treated dams (n=26). Data were expressed as the mean ± S.E.M. (**p<0.01, ***p<0.001).
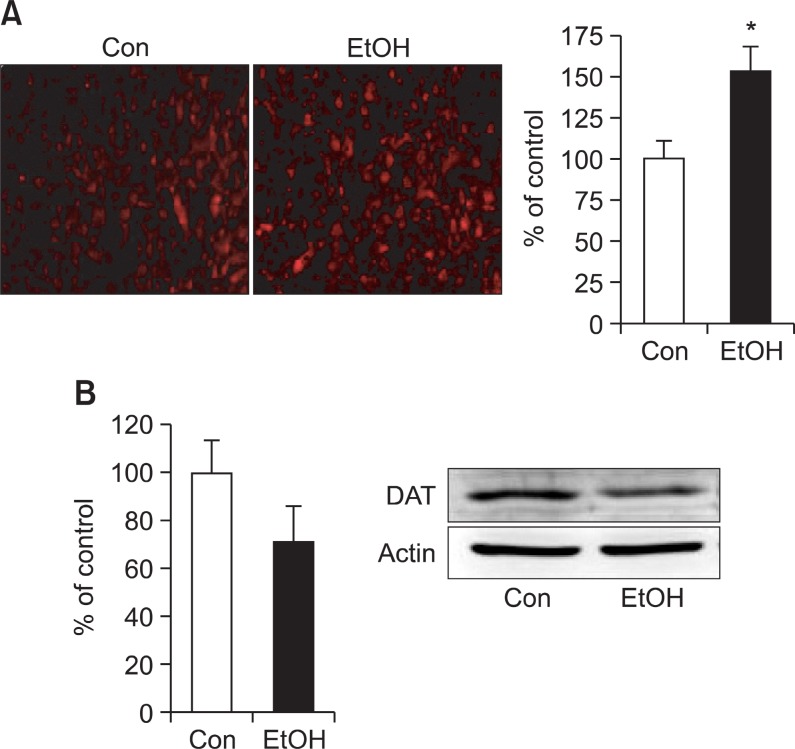
Changes of NET and DAT expression
Expression of NET in the rat brain was evaluated using immunohistochemistry analysis at 7 weeks. In immunohisto-
chemistry, EtOH group exhibited increased NET expression in frontal cortex (Fig. 5A). We next investigated the expression of DAT, which is highly implicated in ADHD, in striatum, one of the brain regions regulating motor coordination as well as executive functions. The expression of DAT in EtOH group, which was determined by Western blot, decreased (Fig. 5B) in striatum compared with Con group, although there was no statistical difference was observed.
Fig. 5. (A) Immunohistochemical analysis of the expression of norepinephrine transporter (NET) in the cortex. Data shown is representative of 3 independent studies. Magnification is ×200. Graph is the quantification of fluorescence intensities (*p<0.05). (B) Western blot analysis of the expression of dopamine transporter (DAT) in striatum. EtOH: offspring born from ethanol treated dams, Con: offspring born from normal saline treated dams. Data were expressed as the mean ± S.E.M.
DISCUSSION
Ethanol exerts detrimental effects on CNS such as in-creased risk for CNS infection, brain trauma and lasting dementia (Brust, 2010). In this study, we showed that maternal ethanol consumption before pregnancy induces behavioral changes such as hyperactivity, inattention and impulsivity, the three core symptoms of ADHD in rat offspring. These behavioral dysfunctions were observed from childhood (PD 22) to adolescent (PD 42) period. Hyper-locomotive activity of preconceptional ethanol exposed offspring is maintained from juvenile to adolescent periods. EtOH group exhibits impulsivity, a tendency of rapid reaction regardless of negative consequences of those reactions (Moeller et al., 2001). It should be emphasized that ethanol administration was conducted only to the mothers but not fathers and that offspring were not directly exposed to the ethanol. In addition, it should be stressed that maternal ethanol ingestion was conducted only for 10 days and there was no differences in the success rate of reproduction as well as the number of live birth. Notwithstanding, offspring born from ethanol treated dams was demonstrated changes of behaviors and catecholaminergic transporter expression. Although it is very hard to extrapolate the kinetics of preconceptional ethanol effects and also there may exist huge species differences between rat and human, it should be remembered that the quitting period of ethanol in rats were at least 7 weeks in this study, which suggests that the ethanol consumption may have prolonged effects on the neurological development in their offspring. At present, most of the concerns of maternal EtOH consumption are focused on the alcohol drinking during pregnancy, however, the results from the present study suggest that females are at risk of prenatal alcohol effects even before conception.
Preconceptionally maternal EtOH-exposed rat offspring may provide efficient model for ADHD compared with currently used ADHD models such as spontaneously hypertensive rats (SHR). EtOH group was more hyperkinetic compared with Con group in novel environment and this hyperactivity was more exacerbated when rats were exposed in same open field test again, similar with open field behavior of SHR (Knar-dahl and Sagvolden, 1979). A previous study suggests that the association between parental ethanol consumption and the manifestation of ADHD in offspring. In Coles’ study, prenatally ethanol affected children and clinical group that was diagnosed with ADHD showed equivalent intellectual abilities with poor performance. In another human longitudinal study, prenatally ethanol exposed children showed deficit in the ability to sustained attention, but changes of hyperactivity and impulsive behavior were not clear (Brown et al., 1991). In co-twin study that focalized parental ethanol consumption, paternal alcohol dependence was associated with increased possibility of risk for ADHD, but clear evidence for paternal genetic transmission could not be found in children with ADHD (Knopik et al., 2009). In animal study, offspring born from ethanol treated fathers had hyperactive tendency with problems in cholinergic nervous system (Abel, 1994). Altogether, these results suggest that parental ethanol consumption prior to conception also cause developmental and neurobehavioral defects in their offspring. In this study, EtOH exposure during short period of time prior to conception does not induce massive growth
retardation and morphological abnormalities in offspring but induced behavioral alterations. Epigenetic change is one of the possible mechanisms mediating the behavioral changes of offspring born from preconceptionally ethanol exposed parents. The epigenetic effects of maternal EtOH consumption have been observed by other investigators. In animal study using C57BL/6J mice, maternal ethanol consumption induced transcriptional silencing by hypermethylation at Agouti viable yellow (Avy) known as epigenetically-sensitive allele in offspring. These preconceptional effects are possibly transmittable to the next generation, but the mechanism related to the observed phenomena is not well known. Several mechanisms of epigenetic metamorphosis being caused by ethanol consumption include alteration of DNA methylation by changing the level of S-adenosylmethionine (SAM) known as methyl group donor of cytosine (Choi et al., 1999), abnormal change of DNA methyltransferase activity (Garro et al., 1991) and induction to post-translational histone modification (Kim and Shukla, 2006). Interestingly, it is relatively well known that the level of SAM is altered in alcohol-induced liver diseases (review in Fernández-Checa et al., 2002), as well as in perinatal and lactational exposure of ethanol to offspring.(Murillo-Fuentes M. L.et al., 2005) These results suggest that altered level of SAM by preconceptional exposure to ethanol may cause the epigenetic changes in offspring. However, not much data is available yet whether alcohol exposure affects the level of SAM chronically, at least 7 weeks after cessation of drinking in rats. It has also been also suggested that maternal ethanol consumption may cause detrimental effects on offspring by inducing chromosomal abnormality (Kaufman, 1997).
Alterations of dopamine and norepinephrine system in CNS are involved in behavioral symptoms of ADHD patients.NET and DAT play a central role in the regulation of extra-cellular catecholamine level in CNS and several medications that are applied to treat ADHD target both NET and DAT. In animal study using DAT knock out (DAT-K/O) mice, increased locomotive activity was observed in novel environment during both phases of light-dark cycle compared with wild type and heterozygote mice although hyperactivity was not observed in their home cage (Giros et al., 1996). In addition, DAT-K/O mice showed minimal habituation to the novel environment during observation time with paradoxical responses to psychostimulants. Although statistically insignificant, the decrease in DAT expression in striatum may be involved in the hyperactive phenotype in prenatally EtOH exposed rat off-springs. At present, there is huge species differences in the role of DAT in the regulation of hyperactive behavior and both hypodopaminergic and hyperdopaminergic function might be related with hyperlocomotive activity. Nevertheless, the data from the present study and previous reports from other researchers suggest the importance of dopaminergic system in the regulation of hyperactive behavior. Further study would ensure the understanding of the exact nature of the involvement of dopaminergic system in the regulation of hyperactive behavior and care should be given in the interpretation of the experimental data in terms of species difference, specific developmental time windows of the investigation as well as the interaction with other nervous systems including serotonergic nervous system.
NET has also been regarded as an important target of ADHD. Correlations between NET and behavior have been investigated by using NET knock out (NET-K/O) mice (Gainet-dinov and Caron, 2003). NET-K/O mice showed lower body weight and attenuated locomotor activity when exposed to a new environment. These results may suggest that hyper-locomotive activity in preconceptionally ethanol exposed off-springs observed in our study may also be closely connected with overexpression of NET in prefrontal cortex along with attenuated DAT density in striatum. In addition, the overex-pression of NET may be involved in impaired attention of EtOH group considering the important role of noradrenergic neurotransmitter system in improvement of attention (Barr et al., 2002). However, detailed kinetic experiments as well as pharmacological and molecular biological modulation of NET activity during the entire course of neural development might be needed to unequivocally demonstrate the causal relationship of NET expression and ADHD-like behaviors in preconceptionally
EtOH exposed rat offsprings.
Although the results from the present study implicates the caution to parents in child-bearing age against heavy drinking even before conception, care should be given before the full appreciation of the conclusion, especially considering the complex nature of the pathophysiology of ADHD as well as the limitations of animal study.
In summary, maternal ethanol consumption prior to pregnancy induces ADHD like-symptoms in rat offsprings without retardation of postnatal development. The increased expression of NET in frontal cortex and decreased expression of DAT in striatum, two well-known therapeutic targets of ADHD, may underlie the behavioral defects in offsprings born from preconceptionally EtOH exposed dams. Because of the high prevalence of maternal ethanol consumption prior to conception,the mechanisms underlying behavioral and neuropathological changes should be defined further in the future, which may improve our understanding of the pathological role of ethanol around pregnancy as well as the biological basis of ADHD phenotypes. In addition, it would again highlight the adverse effects of EtOH on the neural development of affected descendants.
Acknowledgments
This research was supported by research grants (09162KFDA566, 11182KFDA556) from the Korea Food & Drug Administration in 2009 and 2011.
Contributor Information
Jae Hoon Cheong, Phone: +82-2-3399-1605, FAX: +82-2-3399-1619.
Chan Young Shin, Phone: +82-2-2030-7834, FAX: +82-2-2030-7899.
References
- 1.Abel E. L. Effects of physostigmine on male offspring sired by alcohol-treated fathers. Alcohol. Clin. Exp. Res. (1994);18:648–652. doi: 10.1111/j.1530-0277.1994.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statisticalmanual of mental disorder. 4th Ed. American Psychiatry Press; Washington: (2000). [Google Scholar]
- 3.Barkley R. A. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. Guilford Press; New York: (1990). [Google Scholar]
- 4.Barr C. L. Kroft J. Feng Y. Wigg K. Roberts W. Malone M. Ickowicz A. Schachar R. Tannock R. Kennedy J. L. The norepinephrine transporter gene and attention-deficit hyperactivity disorder. Am. J. Med. Genet. (2002);114:255–259. doi: 10.1002/ajmg.10193. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J. Kwon A. Aleardi M. Chouinard V. A. Marino T. Cole H. Mick E. Faraone S. V. Absence of gender effects on attention deficit hyperactivity disorder: findings in nonreferred subjects. Am. J. Psychiatry. (2005);162:1083–1089. doi: 10.1176/appi.ajp.162.6.1083. [DOI] [PubMed] [Google Scholar]
- 6.Brown R. T. Coles C. D. Smith I. E. Platzman K. A. Silverstein J. Erickson S. Falek A. Effects of prenatal alcohol exposure at school age. II. Attention and behavior. Neurotoxicol. Teratol. (1991);13:369–376. doi: 10.1016/0892-0362(91)90085-B. [DOI] [PubMed] [Google Scholar]
- 7.Brust J. C. Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection: a review. Int. J. Environ. Res. Public. Health. (2010);7:1540–1557. doi: 10.3390/ijerph7041540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi S. W. Stickel F. Baik H. W. Kim Y. I. Seitz H. K. Mason J. B. Chronic alcohol consumption induces genomic but not p53-specific DNA hypomethylation in rat colon. J. Nutr. (1999);129:1945–1950. doi: 10.1093/jn/129.11.1945. [DOI] [PubMed] [Google Scholar]
- 9.Davids E. Zhang K. Tarazi F. I. Baldessarini R. J. Animal models of attention-deficit hyperactivity disorder. Brain Res. Brain Res. Rev. (2003);42:1–21. doi: 10.1016/S0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- 10.DuPaul G. J. McGoey K. E. Eckert T. L. VanBrakle J. Preschool children with attention-deficit/hyperactivity disorder: impairments in behavioral, social, and school functioning. J. Am. Acad. Child Adolesc. Psychiatry. (2001);40:508–515. doi: 10.1097/00004583-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Checa J. C. Colell A. Garcia-Ruiz C. S-Adenosyl-L-methionine and mitochondrial reduced glutathione depletion in alcoholic liver disease. Alcohol. (2002);27:179–183. doi: 10.1016/S0741-8329(02)00229-X. [DOI] [PubMed] [Google Scholar]
- 12.Floyd R. L. Decouflé P. Hungerford D. W. Alcohol use prior to pregnancy recognition. Am. J. Prev. Med. (1999);17:101–107. doi: 10.1016/S0749-3797(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 13.Froehlich T. E. Lanphear B. P. Epstein J. N. Barbaresi W. J. Katusic S. K. Kahn R. S. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch. Pediatr. Adolesc. Med. (2007);161:857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- 14.Gainetdinov R. R. Caron M. G. Monoamine transporters: from genes to behavior. Annu. Rev. Pharmacol. Toxicol. (2003);43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 15.Garro A. J. McBeth D. L. Lima V. Lieber C. S. Ethanol consumption inhibits fetal DNA methylation in mice: implications for the fetal alcohol syndrome. Alcohol Clin. Exp. Res. (1991);15:395–398. doi: 10.1111/j.1530-0277.1991.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 16.Giros B. Jaber M. Jones S. R. Wightman R. M. Caron M. G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. (1996);379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 17.Hausknecht K. A. Acheson A. Farrar A. M. Kieres A. K. Shen R. Y. Richards J. B. Sabol K. E. Prenatal alcohol exposure causes attention deficits in male rats. Behav. Neurosci. (2005);119:302–310. doi: 10.1037/0735-7044.119.1.302. [DOI] [PubMed] [Google Scholar]
- 18.Haycock P. C. Fetal alcohol spectrum disorders: the epigenetic perspective. Biol. Reprod. (2009);81:607–617. doi: 10.1095/biolreprod.108.074690. [DOI] [PubMed] [Google Scholar]
- 19.Huggins J. E. Grant T. O’Malley K. Suicide attempts among adults with fetal alcohol spectrum disorders: Clinical considerations. Ment. Health Aspects. Dev. Disabil. (2008);April-June:33–41. [Google Scholar]
- 20.Hughes R. N. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. (2004);28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman M. H. The teratogenic effects of alcohol following exposure during pregnancy, and its influence on the chromosome constitution of the pre-ovulatory egg. Alcohol. (1997);32:113–128. doi: 10.1016/j.alcohol.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Kessler R. C. Berglund P. Demler O. Jin R. Merikangas K. R. Walters E. E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. (2005);62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 23.Kim J. S. Shukla S. D. Acute in vivo effect of ethanol (binge drinking) on histone H3 modifications in rat tissues. Alcohol. (2006);41:126–132. doi: 10.1093/alcalc/agh248. [DOI] [PubMed] [Google Scholar]
- 24.Knardahl S. Sagvolden T. Open-field behavior of spontaneously hypertensive rats. Behav. Neural. Biol. (1979);27:187–200. doi: 10.1016/s0163-1047(79)91801-6. [DOI] [PubMed] [Google Scholar]
- 25.Knopik V. S. Jacob T. Haber J. R. Swenson L. P. Howell D. N. Paternal alcoholism and offspring ADHD problems: a children of twins design. Twin. Res. Hum. Genet. (2009);12:53–62. doi: 10.1016/S0163-1047(79)91801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knopik V. S. Sparrow E. P. Madden P. A. Bucholz K. K. Hudziak J. J. Reich W. Slutske W. S. Grant J. D. McLaughlin T. L. Todorov A. Todd R. D. Heath A. C. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol. Med. (2005);35:625–635. doi: 10.1017/S0033291704004155. [DOI] [PubMed] [Google Scholar]
- 27.Livy D. J. Maier S. E. West J. R. Long-term alcohol exposure prior to conception results in lower fetal body weights. Birth. Defects. Res. B. Dev. Reprod. Toxicol. (2004);71:135–141. doi: 10.1002/bdrb.20007. [DOI] [PubMed] [Google Scholar]
- 28.Lupton C. Burd L. Harwood R. Cost of fetal alcohol spectrum disorders. Am. J. Med. Genet. C. Semin. Med. Genet. (2004);127C:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- 29.Moeller F. G. Barratt E. S. Dougherty D. M. Schmitz J. M. Swann A. C. Psychiatric aspects of impulsivity. Am. J. Psychiatry. (2001);158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- 30.Murillo-Fuentes M. L. Artillo R. Ubeda N. Varela-Moreiras G. Mu-rillo M. L. Carreras O. Hepatic S-adenosylmethionine after maternal alcohol exposure on offspring rats. Addict. Biol. (2005);10:139–144. doi: 10.1080/13556210500123043. [DOI] [PubMed] [Google Scholar]
- 31.O'Leary C. M. Nassar N. Zubrick S. R. Kurinczuk J. J. Stanley F. Bower C. Evidence of a complex association between dose pattern and timing of prenatal alcohol exposure and child behaviour problems. Addiction. (2010);105:74–86. doi: 10.1111/j.1360-0443.2009.02756.x. [DOI] [PubMed] [Google Scholar]
- 32.Polanczyk G. de Lima M. S. Horta B. L. Biederman J. Rohde L. A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am. J. Psychiatry. (2007);164:942–948. doi: 10.1176/appi.ajp.164.6.942. [DOI] [PubMed] [Google Scholar]
- 33.Riley E. P. McGee C. L. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp. Biol. Med (Maywood). (2005);230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 34.Roeleveld N. Vingerhoets E. Zielhuis G. A. Gabreëls F. Mental retardation associated with parental smoking and alcohol consumption before, during, and after pregnancy. Prev. Med. (1992);21:110–119. doi: 10.1016/0091-7435(92)90010-F. [DOI] [PubMed] [Google Scholar]