Abstract
Background
The Oxford knee is a unicompartmental implant featuring a mobile-bearing polyethylene component with excellent long-term survivorship results reported by the implant developers and early adopters. By contrast, other studies have reported higher revision rates in large academic practices and in national registries. Registry data have shown increased failure with this implant especially by lower-volume surgeons and institutions.
Questions/purposes
In the setting of a high-volume knee arthroplasty practice, we sought to determine (1) the failure rate of the Oxford unicompartmental knee implant using a failure definition for aseptic loosening that combined clinical features, plain radiographs, and scintigraphy, and (2) whether increased experience with this implant would decrease failure rate, if there is a learning curve effect.
Methods
Eighty-three Oxford knee prostheses were implanted between September 2005 and July 2008 by the principal investigator. Radiographic and clinical data were available for review for all cases. A failed knee was defined as having recurrent pain after an earlier period of recovery from surgery, progressive radiolucent lines compared with initial postoperative radiographs, and a bone scan showing an isolated area of uptake limited to the area of the replaced compartment.
Results
Eleven knees in this series failed (13%); Kaplan-Meier survivorship was 86.5% (95% CI, 78.0%–95.0%) at 5 years. Failure occurrences were distributed evenly over the course of the study period. No learning curve effect was identified.
Conclusions
Based on these findings, including a high failure rate of the Oxford knee implant and the absence of any discernible learning curve effect, the principal investigator no longer uses this implant.
Level of Evidence
Level III, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
The Oxford unicompartmental knee implant (Oxford, Biomet, IN, USA) uses a fully congruent mobile bearing designed to minimize wear and increase implant longevity [14, 22]. Initial reports documented more than 97% implant survival at 7 years [16]. Implant proponents report minimal wear [14] with survivorship greater than 90% at 20 years [22]. Early US adopters of the Oxford knee implant have reported similar excellent results [3]. These excellent results, however, are not universal. A much higher rate of revision has been reported in national registries and large academic practices [1, 4–6, 11, 15, 17, 21, 28].
Why might there be a difference between unicompartmental knee arthroplasty (UKA) proponents and registry reports? First, Oxford and unicompartmental proponents in general argue that surgeons have an increased willingness to revise UKAs because they are relatively easy to revise and frequently are revised for “no obvious reason” [8]. In addition, radiographic changes suggesting loosening have been shown to be overread in unicompartmental knees, which may increase the revision of implants that are not truly failing [9, 23]. Second, a UKA is a technically demanding procedure which has been shown to have a significant learning curve [10]. Registry data for the UKA with an Oxford implant shows a higher revision rate for surgeons who perform fewer than 13 procedures per year [1], supporting this concept of a learning curve effect during which surgeons develop expertise with the surgical technique [19, 24].
The purpose of this study was first to find the failure rate of Oxford unicompartmental knee implants in a high-volume knee arthroplasty practice. Rather than looking at revision for all reasons, the concern expressed by Oxford proponents, we evaluated Oxford knee implant failure with strict criteria using a standardized workup of any painful postoperative knee arthroplasty.
Specifically, we sought to determine (1) the failure rate of the Oxford unicompartmental knee implant using a failure definition for aseptic loosening that combined clinical features, plain radiographs, and scintigraphy, and (2) whether increased experience with this implant would decrease the failure rate, if there is a learning curve effect.
Patients and Methods
The Oxford unicompartmental knee prosthesis was implanted in 83 knees (77 patients) between September 2005 and July 2008 (Table 1). This represented 7% (83/1224) of the principal investigator’s (WCS) primary knee arthroplasty practice during this time. All UKAs were performed for isolated anteromedial knee osteoarthritis confirmed with stress radiographs as per Oxford surgical technique guidelines. In general, indications for this procedure during this period were conservative. Concern regarding ACL stability or presence of inflammatory arthritis, arthritis in the lateral compartment, or patellofemoral arthritis disqualified the patient from a UKA. We restrict elective knee arthroplasty to patients with a BMI less than 45 kg/m2. These surgical indications remained constant throughout the study period. No patient in this study was involved in either work-related or personal injury claims. All participated in a standardized recovery program focused on early return of motion and activities. Patients were seen postoperatively at 3, 6, and 12 weeks, 1 year, and every 2 years thereafter for routine followup and clinical evaluation. Radiographs, The Knee Society pain and function scores, Oxford Knee Score, and patient-rated satisfaction results were obtained at annual followups (Table 2) as part of ongoing institutional review board-approved knee arthroplasty data collection.
Table 1.
Patient demographics
| Parameter | Oxford knee implants |
|---|---|
| Number of patients | 77 |
| Number of knees | 83 |
| Number of patients with bilateral procedures | 6 |
| Patient age: mean (range) years | 57 (40–76) |
| Proportion of right knees | 37% |
| Proportion of male patients | 59% |
| BMI: mean (range) kg/m2 | 32 (21–46) |
| Followup: mean (range) years | 3.6 (0.3–7.1) |
Table 2.
Clinical scores and patient satisfaction for surviving knees
| Parameter | Oxford knee implants |
|---|---|
| Clinical score | |
| Knee Society—pain: mean (range) | 87 (41–100) |
| Knee Society—function: mean (range) | 77 (10–100) |
| Oxford Knee Score: mean (range) | 20 (12–51) |
| Satisfaction | |
| Excellent | 51% |
| Very good | 7% |
| Good | 29% |
| Fair | 9% |
| Poor | 4% |
Patients with Oxford knee implants who had pain develop at 6 or more months after surgery were evaluated through a standardized protocol. History included antecedent trauma, location of pain, association of pain with activities, and determination if the knee pain initially had abated after surgery and now was increasing or had never improved after surgery. Physical examination criteria included isolated swelling, joint effusion, decreased ROM, or increased varus/valgus instability. Nonoperative management with NSAIDs and physical therapy was initiated. Plain radiographs were obtained, and if they were suspicious for changes compared with the 6-week postoperative radiographs and if it was more than 1 year after the index procedure, a technetium bone scan was obtained. At the time of the bone scan, screening for infection was done with blood work including C-reactive protein and erythrocyte sedimentation rate [2].
Our surgical technique followed the manufacturer’s protocol and we used Oxford Phase II instrumentation. The surgical leg was draped free on a thigh support. These procedures were performed under a single tourniquet inflation, through a medial parapatellar incision and arthrotomy. The medial meniscus was removed while the ACL and MCL were protected. The extramedullary tibial alignment guide was placed parallel to the tibial shaft in an effort to create a 7° posterior tibial slope. Feeler gauges ensured adequate bone removal, and then intramedullary alignment guides were used for the femur. The femoral drill guide was placed in three planes as described in the manufacturer’s Oxford Surgical Technique, and alignment holes were made in the distal femur. The posterior femoral condyle was cut, followed by initial milling of the distal femur. Feeler gauges were used to determine further milling of the distal femur to ensure equal flexion and extension gaps and final balance was verified. Holes were drilled in sclerotic bone on the femur and tibia, and the tibial keel hole was made. Bone surfaces were irrigated and dried and the tibial and femoral components were cemented with a single batch of cement. A trial polyethylene component was inserted and the knee held at 40° flexion while the cement cured. Motion and stability then were confirmed and a final polyethylene component was inserted. The arthrotomy was closed with a running reabsorbable suture and the skin was closed in layers with staples. The surgical technique did not change during the period of study.
For the purposes of this study, a failed result was defined as a knee that (1) initially had recovered well from the index procedure; (2) displayed new increasing pain in the medial joint line of the knee that did not respond to conservative management; (3) was negative for sepsis; (4) had radiographic changes with concerning features for loosening; and (5) had a positive bone scan after 1 year, with asymmetric increased uptake adjacent to either Oxford component. This failure definition contrasts with those of previous studies reporting a primary outcome of revision for any reason which may increase significantly the published implant failure rate [1, 4–6, 11, 15, 17, 21, 28] First, no knee was classified as a failure for pain alone. Second, radiographic analysis alone was deemed inadequate to determine knee failure. Radiolucencies around the Oxford implants have been an ongoing source of concern. While some authors suggest that these lucencies are not associated with loosening [22], others argue that lucent lines around Oxford implants were 64% sensitive and 94% specific for loosening and should not be ignored [12]. In the current study, bone scans were obtained to supplement radiographic analysis in patients with knee pain and progressive radiolucencies. Finally, knees with Oxford implants that had further surgery for arthritis progression or instability of the mobile bearing were not included as failed knees. We focused simply on the aseptic loosening rate of the Oxford knee prostheses implanted by the principal investigator (WCS). Kaplan-Meier survivorship was determined [13].
All knees were evaluated chronologically to determine if a greater number of knee failures occurred early during the learning curve. The 83 knees were divided into sequential thirds designating 28 cases for early-stage, 28 for middle-stage, and 27 for late-stage procedural groups (Fig. 1). Failures were subcategorized in each stage. In addition, an estimated annual volume was determined for each procedural group to determine not only the surgeon’s experience, but also the relative volume at the time of the procedures. The first group of 28 knee arthroplasties was performed over 15 months (rate, 22.4 procedures annually), the second group over 6 months (rate, 56 procedures annually), and the third group over 14 months (rate, 23.1 procedures annually).
Fig. 1.
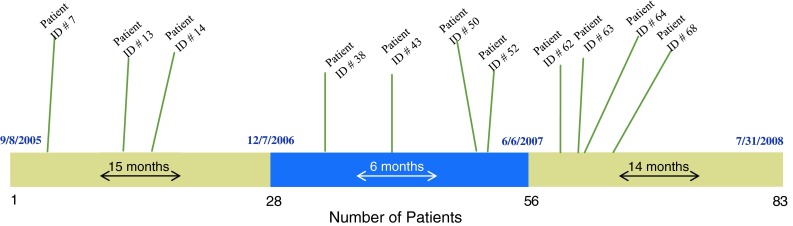
The Oxford knee failures were distributed evenly across the study period.
Results
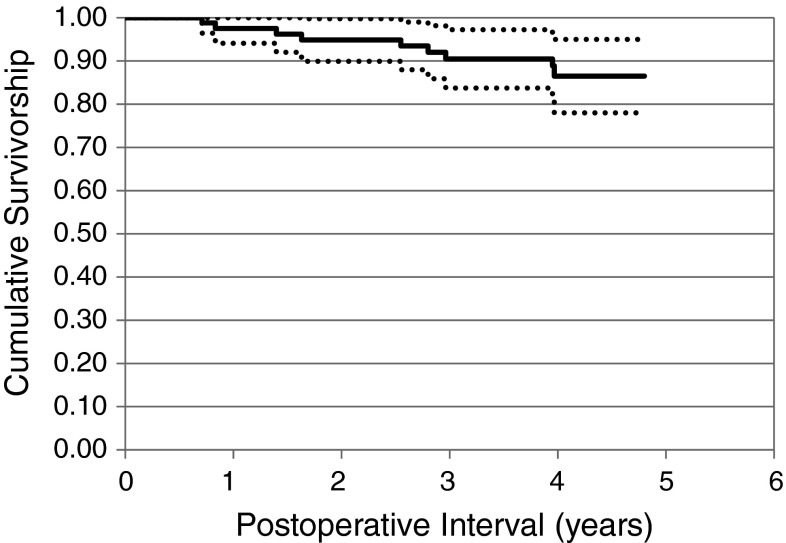
Eleven (13%) of the 83 knees with Oxford implants met failure criteria (Table 3). Kaplan-Meier survivorship was 86.5% (95% CI, 78.0%–95.0%) at 5 years (Fig. 2).
Table 3.
Failures with Oxford knee implants
| Patient identification number | Gender | Age (years) | BMI | Time to failure (years) | Method of failure | Satisfied after revision |
|---|---|---|---|---|---|---|
| 7 | M | 50 | 31 | 5.0 | Tibial loosening | Yes |
| 13 | M | 61 | 36 | 1.3 | Tibial loosening | Yes |
| 14 | F | 52 | 21 | 4.0 | Femoral and tibial loosening | Yes |
| 38 | M | 54 | 35 | 1.0 | Tibial loosening | Yes |
| 43 | M | 51 | 32 | 2.5 | Tibial loosening | Yes |
| 50 | M | 61 | 46 | 5.0 | Femoral loosening | Revision pending |
| 52 | F | 50 | 37 | 2.8 | Femoral and tibial loosening | Yes |
| 62 | M | 65 | 30 | 1.6 | Tibial loosening | Yes |
| 63 | F | 55 | 30 | 1.0 | Tibial loosening | Yes |
| 64 | M | 55 | 33 | 2.9 | Tibial loosening | Yes |
| 68 | M | 50 | 33 | 4.0 | Tibial loosening | Revision pending |
| Revisions not included as failures | ||||||
| 10 | F | 55 | 27 | 4.9 | Lateral degenerative joint disease | Yes |
| 45 | M | 64 | 30 | 4.0 | Polyethylene dissociation | Yes |
Fig. 2.
Kaplan-Meier survivorship analysis for Oxford knee implants from September 2005 through July 2008 indicates 86.5% (95% CI, 78.0%–95.0%) survivorship at 4.8 years. The dotted lines represent the 95% CI.
No learning-curve effect was seen in this series. The 11 failures occurred fairly evenly during the study period: three in the early-stage group, four in the middle-stage group, and four in the late-stage group (Fig. 1). To date, nine of 11 failures have been revised. At the time of surgery, tibial or femoral component loosening was determined for each knee (Table 3). Patient satisfaction was noted to improve after all revisions. Clinical scores and patient satisfaction were obtained for the nonfailed Oxford knee implants (Table 2). At the most recent followup, 51% of patients rated their knees with Oxford implants as excellent, 7% as very good, 29% as good, 9% as fair, and 4% as poor. Two additional knees with Oxford implants that were not considered failures by study criteria were revised for alternative diagnoses, one for lateral disease progression and one for an isolated polyethylene exchange for insert instability (Table 3).
Discussion
The Oxford unicompartmental knee replacement uses a fully congruent mobile bearing designed to minimize wear and increase implant longevity [14, 22]. This implant has achieved worldwide use, and currently is the most commonly implanted UKA design. Since its introduction, reported results with the Oxford unicompartmental knee have been mixed. Initial results by the implant designers in 1998 indicated greater than 97% survivorship at 10 years [20]. More recent studies by the implant designers and early adopters have continued to report excellent results with greater than 97% implant survival at 7 years [3, 20] and greater than 90% survival at 20 years [22] with minimal wear seen [14]. However, not all investigators report similarly good results with this implant. The Swedish Knee Arthroplasty Study reported 50 revisions (7%) in 699 Oxford knees within 6 years of implantation, 20 of which were revised for technique concerns (loose implants or unstable knees), which led to a recommendation that the Oxford knee implant only be used in long-term comparative studies [16]. Several centers around the world have reported significantly higher revision rates with the Oxford knee implant than the initial excellent reports [4–6, 11, 15, 17, 21, 28]. In our current study of a single high-volume surgeon’s experience with the Oxford implant, 11 (13%) of the 83 Oxford implants met failure criteria representing a 5-year survivorship of 86.5% (Fig. 2). During the course of this review, no benefit was determined with increased experience using the Oxford implant. No learning curve effect was seen.
This study is limited because it involves a series of procedures performed by one surgeon. However, the surgeon is a high-volume knee arthroplasty subspecialist [26], was an experienced UKA surgeon at the start of the study, and performed what is considered by Baker et al. [1] as a high volume of Oxford knee implants throughout the study period. Although more than 22 of these procedures were performed annually throughout the study, the Oxford knee implant was selected only for patients meeting strict eligibility criteria, representing only 7% of the surgeon’s knee arthroplasty practice. This rate of selection is low compared with the rate reported by some UKA enthusiasts and may reflect a lack of commitment to learn and incorporate this procedure into this busy practice [3]. Although bone scan evaluation of painful knees with Oxford implants was obtained only more than 1 year postoperatively, the false-positive scan rate in asymptomatic knees and the false-negative rate in symptomatic knees are unknown. To our knowledge, our study is the first regarding Oxford knee implant failure using the criteria of pain, plain radiographs, and bone scintigraphy. The sensitivity and specificity of these failure criteria are unknown, although each failed knee undergoing revision was found to have a loose Oxford component. Finally, and importantly, our survivorship analysis presents what we believe to be a “best-case” analysis, as it excludes reoperations for disease progression and for problems related to the mobile bearing. We sought instead to focus only on aseptic loosening, which means that the percentage of failures we report, if anything, will be lower than an estimate that considers failures from all causes.
Eleven knees in the current series were considered failures according to our strict criteria (Table 3). The failure rate determined in the current study is in line with multiple national registries and single institution reviews reporting a revision rate of knees with Oxford implants between 7% to 31% within 5 years of implantation [1, 4–6, 11, 15, 17, 21, 27, 28]. It has been suggested that revision is a poor outcome measure for evaluating results of Oxford implants because UKAs are easier than TKAs to revise such that surgeons are more likely to revise a UKA for “no obvious reason” [8]. UKAs are three times more likely to be revised than TKAs for the same low clinical score [8]. Conversely, this argument asserts that UKAs are revised for higher clinical scores than TKAs. Yet as defined by their indication for arthritic changes involving only a single compartment, UKAs initially are performed on knees with higher clinical scores, have been shown to have higher clinical scores when successful [18], and therefore would be expected to have higher clinical scores when failing. A failed arthroplasty in a single compartment generally is not as problematic as a failed arthroplasty involving two or more compartments. In contrast to studies in which “revision for any reason” was used as the end point, we used a more narrow definition of failure. No knee was revised for pain without positive findings on plain films and bone scans. Several patients in our series have poor clinical scores and satisfaction but do not meet failure criteria and have not had revision surgery (Table 2). Finally, failed knees that were revised were found to have loose components at the time of surgery and improved patient satisfaction at the most recent followup (Table 3). If a patient had persistent knee pain, concerning radiographs, a positive bone scan, and clinical improvement with a revision, it is hard to argue that the revision was done “for no obvious reason.”
In the current study, failures occurred in an even distribution over the 83 cases performed over 3 years by the surgeon. The benefit of a learning curve was not observed. A learning curve was reported for UKA during which time a surgeon develops competency with a procedure [10]. However, learning a new skill requires not only the experience of a sufficient number of cases, but requires a high enough volume of cases during a distinct learning period [7, 25]. Registry data show a volume threshold of 14 cases per year as an indicator of success for Oxford implants [1]. During the current study, the principal investigator, a high-volume surgeon [26], performed 83 cases with a minimum annual volume of 22 Oxford implantations during the 3 years of the study without a learning curve effect. Although a learning curve may exist beyond the first 100 cases, the majority of surgeons performing UKA would require years of “learning” and simply will not reach this level in time to master this surgical technique. It cannot be assumed that all surgeons, with a few days of training, will experience a short learning curve and quickly attain the same clinical results as the expert developers of this implant. This will lead to a higher failure and revision rate with the Oxford knee implant.
We suggest that the contrast in Oxford knee implant failure rates may be attributable primarily to the inability of surgeons to develop the expertise required with the Oxford implant to obtain similar results to the designers and early adopters. Not all surgeons can obtain the same high level of expertise with all surgical procedures. Previous studies have suggested that a learning curve exists with the Oxford implant, inferring that surgeons may expect, after a period of learning, to obtain similar success as the developers of the implant [19, 24]. Alternatively, it may be that the key skill set needed to obtain excellent results is innate and a sufficient level of expertise is not attainable for all surgeons. This implication is especially disconcerting in the current study when a high-volume knee arthroplasty surgeon performing more than 500 TKAs per year, who would seem to have the requisite skill set to adopt this surgical technique, cannot obtain comparable outcomes. Importantly, a UKA may be a less forgiving procedure than a TKA [10]. The unacceptable rate of failure with the Oxford knee implant has led the principal investigator to discontinue its use in his practice.
Footnotes
One of the authors (WCS), or a member of his or her immediate family, certifies that he has received or will receive payments or benefits, during the study period an amount less than USD 10,000 from Biomet, Inc, (Warsaw, IN, USA). One of the authors (CLB), or a member of his or her immediate family, certifies that he has received or will receive payments or benefits, during the study period, an amount less than USD 10,000 from Wright Medical (Arlington, TN, USA); an amount less than USD 10,000 from DJO/Conformis (Vista, CA, USA); and an amount less than USD 10,000 from J & J/Stryker (Kalamazoo, MI, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed at the St Louis Joint Replacement Institute, St Louis, MO.
References
- 1.Baker P, Jameson S, Critchley R, Gregg P, Deehan D. Center and surgeon volume influence the revision rate following unicondylar knee replacement: an analysis of 23,400 medial cemented unicondylar knee replacements. J Bone Joint Surg Am. 2013;95:702–709. doi: 10.2106/JBJS.L.00520. [DOI] [PubMed] [Google Scholar]
- 2.Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, Steckelberg J, Osmon D. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010;92:2102–2109. doi: 10.2106/JBJS.I.01199. [DOI] [PubMed] [Google Scholar]
- 3.Berend KR, Lombardi AV., Jr Liberal indications for minimally invasive Oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193–197. [PubMed] [Google Scholar]
- 4.Chou DT, Swamy GN, Lewis JR, Badhe NP. Revision of failed unicompartmental knee replacement to total knee replacement. Knee. 2012;19:356–359. doi: 10.1016/j.knee.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Choy WS, Kim KJ, Lee SK, Yang DS, Lee NK. Mid-term results of Oxford medial unicompartmental knee arthroplasty. Clin Orthop Surg. 2011;3:178–183. doi: 10.4055/cios.2011.3.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dervin GF, Carruthers C, Freibel RJ, Gianchino AA, Kim PR, Thurston PR. Initial experience with the Oxford unicompartmental knee arthroplasty. J Arthroplasty. 2011;26:192–197. doi: 10.1016/j.arth.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100:363–406. doi: 10.1037/0033-295X.100.3.363. [DOI] [Google Scholar]
- 8.Goodfellow JW, O’Connor JJ, Murray DW. A critique of revision rate as an outcome measure: re-interpretation of knee joint registry data. J Bone Joint Surg Br. 2010;92:1628–1631. doi: 10.1302/0301-620X.92B12.25193. [DOI] [PubMed] [Google Scholar]
- 9.Gulati A, Chau R, Pandit HG, Gray H, Price AJ, Dodd CA, Murray DW. The incidence of physiological radiolucency following Oxford unicompartmental knee replacement and its relationship to outcome. J Bone Joint Surg Br. 2009;91:896–902. doi: 10.1302/0301-620X.91B7.21914. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton WG, Ammeen D, Engh CA, Jr, Engh GA. Learning curve with minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2010;25:735–740. doi: 10.1016/j.arth.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Heller S, Fenichel I, Salai M, Luria T, Velkes S. The Oxford unicompartmental knee prosthesis for the treatment of medial compartment knee disease: 2 to 5 year follow-up. Isr Med Assoc J. 2009;11:266–268. [PubMed] [Google Scholar]
- 12.Kalra S, Smith TO, Berko B, Walton NP. Assessment of radiolucent lines around the Oxford unicompartmental knee replacement: sensitivity and specificity for loosening. J Bone Joint Surg Br. 2011;93:777–781. doi: 10.1302/0301-620X.93B6.26062. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 14.Kendrick BJ, Simpson DJ, Kapstein BL, Valstar ER, Gill HS, Murray DW, Price AJ. Polyethylene wear of mobile-bearing unicompartmental knee replacement at 20 years. J Bone Joint Surg Br. 2011;93:470–475. doi: 10.1302/0301-620X.93B4.25605. [DOI] [PubMed] [Google Scholar]
- 15.Kuipers BM, Kollen BJ, Bots PC, Burger BJ, Van Raay JJ, Tulp NJ, Verheyen CC. Factors associated with reduced early survival in the Oxford phase III medial unicompartmental knee replacement. Knee. 2010;17:48–52. doi: 10.1016/j.knee.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Lewold S, Goodman S, Knutson K, Robertson O, Lidgren L. Oxford meniscal bearing knee versus the Marmor knee in unicompartmental arthroplasty for arthrosis: a Swedish multicenter survival study. J Arthroplasty. 1995;10:722–731. doi: 10.1016/S0883-5403(05)80066-X. [DOI] [PubMed] [Google Scholar]
- 17.Mercier N, Wimsey S, Saragaglia D. Long-term clinical results of the Oxford medial unicompartmental knee arthroplasty. Int Orthop. 2010;34:1137–1143. doi: 10.1007/s00264-009-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray DW, Goodfellow JW, O’Connor JJ. The Oxford medial unicompartmental arthroplasty: a ten-year survival study. J Bone Joint Surg Br. 1998;80:983–989. doi: 10.1302/0301-620X.80B6.8177. [DOI] [PubMed] [Google Scholar]
- 19.New Zealand Orthopaedic Association. The New Zealand Joint Registry. Thirteen year report: January 1999–December 2012. Available at: www.nzoa.org.nz/nz-joint-registry. Accessed July 3, 2013.
- 20.Pandit H, Jenkins C, Barker K, Dodd CA, Murray DW. The Oxford medial unicompartmental knee replacement using a minimally-invasive approach. J Bone Joint Surg Br. 2006;88:54–60. [DOI] [PubMed]
- 21.Pearse AJ, Hooper GJ, Rothwell A, Frampton C. Survival and functional outcome after revision of a unicompartmental to a total knee replacement: the New Zealand National Joint Registry. J Bone Joint Surg Br. 2010;92:508–512. doi: 10.2106/JBJS.I.00530. [DOI] [PubMed] [Google Scholar]
- 22.Price AJ, Svard U. A second decade lifetable survival analysis of the Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2011;469:174–179. doi: 10.1007/s11999-010-1506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rea P, Short A, Pandit H, Price AJ, Kyberd P, Beard DJ, Gill HS, Murray DW. Radiolucency and migration after Oxford unicompartmental knee arthroplasty. Orthopedics. 2007;30(5 suppl):24–27. [PubMed] [Google Scholar]
- 24.Robertsson O, Knutson K, Lewold S, Lidgren L. The routine of surgical management reduces failure after unicompartmental knee arthroplasty. J Bone Joint Surg Br. 2001;83:45–49. doi: 10.1302/0301-620X.83B1.10871. [DOI] [PubMed] [Google Scholar]
- 25.Schroer WC, Calvert GT, Diesfeld PJ, Reedy ME, LeMarr AR. Effects of increased surgical volume on total knee arthroplasty complications. J Arthroplasty. 2008;23(6 suppl 1):61–67. doi: 10.1016/j.arth.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Schroer WC, Stormont DM, Pietrzak WS. Seven-year survivorship and functional outcomes of the high-flexion Vanguard complete knee system. J Arthroplasty. 2013 May 20. pii: S0883-5403(13)00311-2 [Epub ahead of print]. [DOI] [PubMed]
- 27.Vorlat P, Putzeys G, Cottenie D, Van Isacker T, Pouliart N, Handelberg F, Casteleyn PP, Gheysen F, Verdonk R. The Oxford unicompartmental knee prosthesis: an independent 10-year survival analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14:40–45. doi: 10.1007/s00167-005-0621-1. [DOI] [PubMed] [Google Scholar]
- 28.Zermatten P, Munzinger U. The Oxford II medial unicompartmental knee arthroplasty: an independent 10-year survival study. Acta Orthop Belg. 2012;78:203–209. [PubMed] [Google Scholar]