Abstract
Background
The clinical utility of nondiagnostic core needle biopsies is not fully understood. Understanding the clinical and radiologic factors associated with nondiagnostic core needle biopsies may help determine the utility of these nondiagnostic biopsies and guide clinical decision making.
Questions/purposes
We asked (1) whether benign or malignant bone and soft tissue lesions have a higher rate of nondiagnostic core needle biopsy results, and which diagnoses have the lowest diagnostic yield; (2) how often nondiagnostic results affected clinical decision-making; and (3) what clinical factors are associated with nondiagnostic but useful core needle biopsies.
Methods
A retrospective study was performed of 778 consecutive image-guided core needle biopsies of bone and soft tissue lesions referred to the musculoskeletal radiology department at a single institution. The reference standard was (1) the final diagnosis at surgery or (2) clinical followup. Diagnostic yield was calculated for the most common diagnoses. Clinical and imaging features related to each nondiagnostic core needle biopsy were assessed for their association with clinical usefulness. Useful nondiagnostic biopsies were defined as those that help guide treatment. Each lesion was assessed before biopsy by the orthopaedic oncologist as (1) “likely to be benign” or (2) “suspicious for malignancy.” The overall diagnostic yield was 74%.
Results
Malignant lesions had higher diagnostic yield than benign lesions: 94% (323 of 345) versus 58% (252 of 433), yielding a relative risk (RR) of 1.61 and 95% CI of 1.48 to 1.75. Soft tissue lesions had a higher diagnostic yield than bone lesions: 82% (291 of 355) versus 67% (284 of 423); RR, 1.22; 95% CI, 1.22 (1.12–1.33). Ganglion cyst (36%, four of 11), myositis ossificans (40%, two of five), Langerhans cell histiocytosis (0%, 0 of four), and simple bone cyst 0%, 0 of six) had the lowest diagnostic yield. Of the nondiagnostic biopsies assessed for clinical usefulness by the orthopaedic oncologist, 60% (85 of 142) of the biopsies were useful in guiding clinical decision making. Useful nondiagnostic core needle biopsy results occurred more often in painless, nonaggressive lesions, assessed as “likely to be benign” before biopsy.
Conclusions
Nondiagnostic core needle biopsy results in musculoskeletal lesions are not entirely useless. At times, they can be supportive of benign processes and can help avert unnecessary surgical procedures.
Level of Evidence
Level II, diagnostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Accurate diagnosis of a musculoskeletal lesion is dependent on the clinical, imaging, and pathologic findings. For the latter, open surgical biopsies are considered the reference standard in obtaining tissue for diagnosis; however, an image-guided percutaneous core needle biopsy has become the initial step in acquiring tissue for the diagnosis of musculoskeletal lesions at many institutions [8, 16, 19, 25, 26]. The shift from open biopsies to image-guided core needle biopsies can be attributed to the less invasive nature, decreased time of sedation, lower cost, shorter recovery time, and lower complication rates [7, 8, 12, 13, 16]. Image-guided core needle biopsies often are performed using ultrasound or CT guidance and the diagnostic accuracy is high (66% to 98%) [2–11, 14, 18, 21, 22, 24, 26].
However, 5% to 31% of image-guided core needle biopsies can be nondiagnostic [2, 4, 5, 7–9, 11, 16–18, 20, 21, 23, 24, 26]. A nondiagnostic biopsy result can necessitate the need for a repeat core needle biopsy or an open biopsy, which can delay final diagnosis and treatment, leading to increased cost, pain, and patient anxiety [8, 26]. Studies have shown that diagnostic yield is related to the final histology of the lesion (benign or malignant), imaging characteristics, size of the lesion, anatomic location, modality used, and number of cores obtained [2, 4–12, 18, 20, 22, 24–26]. However, a nondiagnostic biopsy may not be useless, and the clinical utility of these nondiagnostic biopsies has not been fully studied. At times, core needle biopsies are performed in lesions with low likelihood for malignancy and/or low possibility of obtaining a true pathologic diagnosis. For instance, aggressive cystic changes and marrow edema in the clavicle at the sternoclavicular joint, while likely attributable to osteoarthritis, could raise the concern for osteomyelitis or tumor necessitating biopsy. However, a definitive diagnosis of osteoarthritis would be difficult for a pathologist to conclude based on tissue from a core needle biopsy of the clavicle. Understanding which lesions are likely to have low diagnostic yield on core needle biopsy can aid in patient education and the formulation of an appropriate treatment plan. In these cases, a nondiagnostic result can be reassuring to the physician and the patient.
We therefore sought to answer (1) whether benign or malignant bone and soft tissue lesions have a higher rate of nondiagnostic core needle biopsy results, and which diagnoses have the lowest diagnostic yield; (2) how often nondiagnostic results affect clinical decision-making; and (3) what clinical factors are associated with nondiagnostic but useful core needle biopsies.
Patients and Methods
After institutional review board approval, this Health Insurance Portability and Accountability Act (HIPAA)-compliant retrospective study was performed on 778 consecutive image-guided core needle biopsies of bone (n = 423) and soft tissue (n = 355) lesions in patients referred to the musculoskeletal radiology department at one institution from July 2004 to August 2011. These biopsies were performed on musculoskeletal lesions that were of indeterminate etiology or suspicious for malignancy requiring tissue diagnosis for clinical management. Of the 778 biopsies, 308 were in males and 470 were in females. The patients’ ages ranged from 17 to 95 years (mean age, 54 years). The core needle biopsies were performed either under CT guidance (n = 574) or ultrasound (US) guidance (n = 204) by 11 musculoskeletal radiologists with 2 to 10 years of experience. No spine biopsies or core needle biopsies in children were included because these are not performed by the musculoskeletal radiologists at our institution.
Biopsy Technique and Analysis
In general, US guidance was used for soft tissue lesions and CT guidance for intraosseous lesions. However, CT was used for some soft tissue lesions, especially if the lesion was difficult to see under US. Conversely, US guidance was used for some bone lesions with a large soft tissue component, highly vascular lesions, or lesions adjacent to large vessels. The anatomic location, size, and type of the lesion also contributed to the choice of modality. The biopsy approach for all cases was determined by the orthopaedic oncologist and radiologist so that the biopsy tract could be resected at surgery to prevent recurrence along the biopsy track. A Bonopty 15-gauge bone biopsy system (RADI Medical Systems, Uppsala, Sweden) was used for core needle biopsies of intraosseous lesions. The Achieve 14-, 16-, or 18-gauge coaxial automated biopsy systems (Cardinal Health, Dublin, OH, USA) were used for core needle biopsies of soft tissue lesions and bone lesions with soft tissue components. A minimum of three and a maximum of six cores were obtained for each biopsy. The pathology samples and pathology reports were reviewed by an experienced musculoskeletal pathologist (JG) at the same institution. The histologic reports were rereviewed with the same pathologist and a biopsy was considered nondiagnostic if a distinct pathologic diagnosis could not be rendered from the biopsy tissue that explained the lesion clinically and by imaging. The final diagnosis was determined by (1) the final diagnosis at surgery (biopsy or definitive treatment); or (2) clinical followup (mean, 20 months). Diagnostic yield, defined as the number of diagnostic core needle biopsies divided by the total number of core needle biopsies, was calculated for the most common diagnoses. Additional core needle biopsy parameters reviewed were final pathology (benign or malignant), lesion type (soft tissue or bone), and imaging guidance modality (CT or US).
Assessment of Clinical Utility of Nondiagnostic Biopsies
Of the 203 nondiagnostic core needle biopsies, 142 (70%) were ordered by two orthopaedic oncologists (MEA, MGG). The clinical and imaging data for these cases were rereviewed with these two orthopaedic oncologists to determine how the nondiagnostic core needle biopsy result affected clinical decision-making. For each case, several clinical and imaging features related to each biopsy were rereviewed by the orthopaedic oncologists and musculoskeletal radiologists. The clinical features included (1) pain resulting from the lesion; (2) history of malignancy; and (3) signs of infection (fever, white blood cell count ≥ 11 × 103/μL, erythrocyte sedimentation rate ≥ 20 mm/hour, C-reactive protein ≥ 100 mg/L). The imaging features included (1) lesion aggressiveness (aggressive or nonaggressive); and (2) whether the lesion presented as an incidental finding. Aggressive bone lesions had at least one of the following features: moth-eaten or permeative pattern of bone destruction, indistinct margins or wide zone of transition, cortical breakthrough, aggressive periosteal reaction, and/or an associated soft tissue mass. Nonaggressive bone lesions had the following features: a geographic pattern of bone destruction, a narrow zone of transition, well-defined or sclerotic margins, intact cortex, nonaggressive pattern of periosteal reaction, and no soft tissue component. Aggressive soft tissue lesions showed solid areas of enhancement on MRI, heterogeneity, perilesional edema, and noncystic areas on US, whereas nonaggressive soft tissue lesions did not contain any of those features.
For the initial review, based on these findings, before biopsy and blinded to the final diagnosis, the orthopaedic surgeons recorded the lesion as (1) “likely to be benign” or (2) “suspicious for malignancy.” Lesions that were “likely to be benign” had the following features: minimal or no pain, nonaggressive imaging features, small size (< 5 cm), improvement with time, and were seen in patients without a known malignancy. Lesions that were “suspicious for malignancy” had the following features: pain, aggressive imaging features, large size (≥ 5 cm), worsening clinical and imaging features with time, and were seen in patients with a history of malignancy.
In the second review, after viewing the pathology results and followup clinical and imaging data, the orthopaedic oncologist determined whether the nondiagnostic core needle biopsy had been (1) useful or (2) not useful in guiding the treatment plan. This determination was based on all the prebiopsy and postbiopsy data and the overall clinical assessment of the patient by the surgeon. Nondiagnostic biopsies were characterized as “useful” if they occurred in lesions that were “likely to be benign” or in lesions that were “suspicious for malignancy” but showed stability or improvement clinically and on imaging. For instance, a nondiagnostic biopsy of a 1 cm painless rib lesion in a woman with treated breast cancer would be deemed “useful” if subsequent imaging showed stability. Conversely, nondiagnostic biopsies were characterized as “not useful” if they occurred in “likely to be benign” lesions that worsened on followup or in lesions that were “suspicious for malignancy”. Finally, we documented whether the patient progressed to surgery or underwent clinical followup after the nondiagnostic core needle biopsy.
Overall Diagnostic Yield
Of the 778 lesions biopsied, 56% (433 of 778) proved benign and 44% (345 of 778) proved malignant. The overall diagnostic yield with image-guided core needle biopsy was 74% (575 of 778).
Statistical Analysis
A power analysis suggested that a sample size of 600 core needle biopsies (assuming 450 diagnostic and 150 nondiagnostic lesions) would have greater than 80% power to detect a statistically significant difference between benign and malignant lesions with an alpha error of 5%. Statistical analyses were conducted using SAS Version 8.02 (SAS Institute Inc, Cary, NC, USA). We performed chi square or Fisher’s exact test for categorical variables.
Results
Malignant lesions had higher diagnostic yield than benign lesions: 94% (323 of 345) versus 58% (252 of 433), yielding a relative risk (RR) of 1.61 and 95% CI of 1.48 to 1.75 (Fig. 1). Soft tissue lesions had a higher diagnostic yield than bone lesions: 82% (291 of 355) versus 67% (284 of 423) with an RR of 1.22 and 95% CI of 1.22 (1.12–1.33) (Table 1). The benign soft tissue lesions associated with the highest rate of nondiagnostic results included ganglion cyst, myositis ossificans, and tenosynovitis. Of the malignant soft tissue lesions, spindle cell carcinoma was mostly likely to be nondiagnostic (Table 2). The benign bone lesions associated with the highest rate of nondiagnostic results included Langerhans cell histiocytosis, unicameral (simple) bone cyst, healing fractures, degenerative changes, red marrow, and osteomyelitis (Fig. 2; Table 3). In the 203 lesions with nondiagnostic core needle biopsy results, surgery was performed on 43% (87 of 203) and the remaining 57% (116 of 203) were followed clinically. In 91% (79 of 87) of cases the surgery was performed within 3 months from the initial biopsy. In the remaining eight cases, the surgery was performed after 6 months. In most of these delayed cases, the lesion was stable to slightly larger in size and surgery was performed because of pain.
Fig. 1A–C.
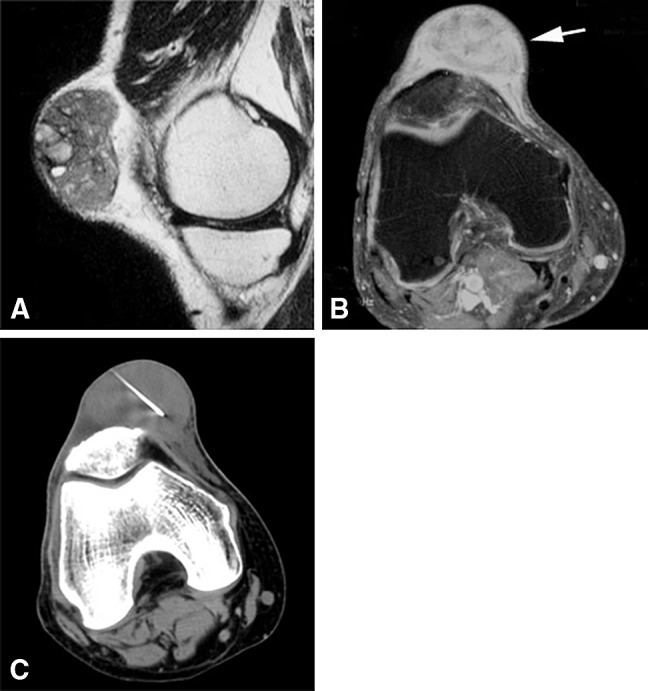
A 56-year-old man had a melanoma soft tissue metastasis, which was diagnostic at core needle biopsy. Malignant soft tissue lesions had the highest diagnostic yield (94%) in our study. Sagittal (A) T1-weighted and (B) fat-saturated T1-weighted postcontrast MR images show a soft tissue anterior knee mass with solid enhancement (white arrow). (C) A CT-guided core needle biopsy image shows the biopsy needle in the lesion.
Table 1.
Diagnostic yield: lesion-related and technical factors
| Factor | Diagnostic | Nondiagnostic | Total | Diagnostic yield (%) | p value |
|---|---|---|---|---|---|
| Diagnosis | |||||
| Benign | 252 | 181 | 433 | 58 | |
| Malignant | 323 | 22 | 345 | 94 | |
| Total | 575 | 203 | 778 | 74 | < 0.001 |
| Lesion type | |||||
| Bone | 284 | 139 | 423 | 67 | |
| Soft tissue | 291 | 64 | 355 | 82 | |
| Total | 575 | 203 | 778 | 74 | < 0.001 |
Table 2.
Diagnostic yield: soft tissue lesions
| Diagnosis* | Percent diagnostic | Total | Diagnostic | Nondiagnostic |
|---|---|---|---|---|
| Benign | 77 | 252 | 194 | 58 |
| Giant cell tumor of tendon sheath | 100 | 22 | 22 | 0 |
| Myxoma | 100 | 19 | 19 | 0 |
| Desmoid | 100 | 17 | 17 | 0 |
| Desmoplastic fibroblastoma | 100 | 4 | 4 | 0 |
| Foreign body granuloma | 100 | 4 | 4 | 0 |
| Lipoma | 100 | 4 | 4 | 0 |
| Peripheral nerve sheath tumor | 96 | 27 | 26 | 1 |
| Intramuscular hemangioma | 76 | 17 | 13 | 4 |
| Gout | 75 | 4 | 3 | 1 |
| Nodular fasciitis | 75 | 4 | 3 | 1 |
| Rheumatoid arthritis nodule | 60 | 5 | 3 | 2 |
| Scar/fibrosis | 60 | 5 | 3 | 2 |
| Tenosynovitis | 50 | 4 | 2 | 2 |
| Myositis ossificans | 40 | 5 | 2 | 3 |
| Ganglion cyst | 36 | 11 | 4 | 7 |
| Malignant | 94 | 103 | 97 | 6 |
| Lymphoma | 100 | 11 | 11 | 0 |
| Metastasis | 100 | 25 | 25 | 0 |
| Myxofibrosarcoma | 100 | 5 | 5 | 0 |
| Synovial sarcoma | 100 | 6 | 6 | 0 |
| Liposarcoma | 92 | 26 | 24 | 2 |
| Sarcoma not otherwise specified | 92 | 24 | 22 | 2 |
| Spindle cell carcinoma | 75 | 4 | 3 | 1 |
* Diagnoses with three or fewer total cases were excluded.
Fig. 2A–D.
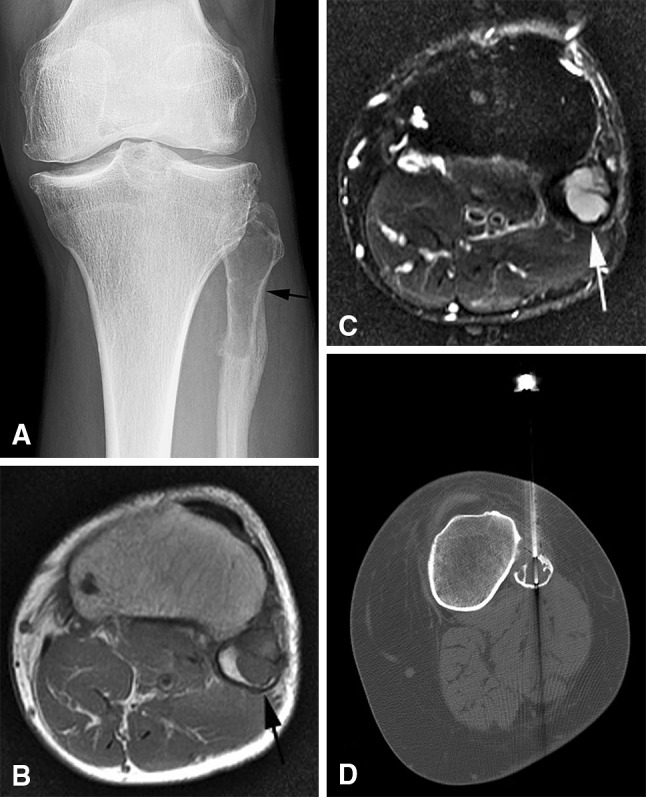
A 23-year-old man had a left proximal fibular simple (unicameral) bone cyst, which was nondiagnostic at core needle biopsy. Benign bone lesions had the lowest diagnostic yield (32%) in our study. (A) An AP radiograph shows a well-circumscribed lytic lesion in the proximal fibula (black arrow). A healing fracture is seen at the inferior margin of the lesion. The lesion (arrows) is low signal on the (B) axial T1-weighted and high signal on the (C) axial T2-weighted MR images. (D) A CT-guided core needle biopsy image shows a biopsy needle in the lesion.
Table 3.
Diagnostic yield: bone lesions
| Diagnosis* | Percent diagnostic | Total | Diagnostic | Nondiagnostic |
|---|---|---|---|---|
| Benign | 32 | 181 | 58 | 123 |
| Synovial (osteo)chondromatosis | 100 | 6 | 6 | 0 |
| Benign cartilage | 100 | 4 | 4 | 0 |
| Giant cell tumor of bone | 79 | 19 | 15 | 4 |
| Benign bone forming lesion | 75 | 4 | 3 | 1 |
| Fibrous dysplasia | 62 | 13 | 8 | 5 |
| Aneurysmal bone cyst | 60 | 5 | 3 | 2 |
| Osteonecrosis | 60 | 5 | 3 | 2 |
| Paget disease | 60 | 5 | 3 | 2 |
| Reactive changes | 50 | 6 | 3 | 3 |
| Osteomyelitis | 33 | 18 | 6 | 12 |
| Red marrow | 25 | 4 | 1 | 3 |
| Degenerative changes | 19 | 16 | 3 | 13 |
| Fracture | 13 | 8 | 1 | 7 |
| Simple bone cyst | 0 | 6 | 0 | 6 |
| Langerhans cell histiocytosis | 0 | 4 | 0 | 4 |
| Malignant | 93 | 242 | 226 | 16 |
| Chondrosarcoma | 100 | 9 | 9 | 0 |
| Leiomyosarcoma | 100 | 4 | 4 | 0 |
| Metastasis | 97 | 153 | 148 | 5 |
| Plasmacytoma | 94 | 17 | 16 | 1 |
| Lymphoma | 91 | 33 | 30 | 3 |
| Multiple myeloma | 83 | 6 | 5 | 1 |
| Ewing sarcoma | 80 | 5 | 4 | 1 |
| Osteosarcoma | 70 | 10 | 7 | 3 |
* Diagnoses with three or fewer total cases were excluded.
Of the 203 nondiagnostic core needle biopsies, 142 were ordered by two orthopaedic oncologists (MEA, MGG) with nine and 30 years of experience. The orthopaedic oncologists deemed 60% (85 of 142) of the nondiagnostic biopsies useful in guiding clinical decision-making (Table 4).
Table 4.
Nondiagnostic core needle biopsy: clinical utility
| Factor | Useful | Not useful | Total | Percent useful | p value |
|---|---|---|---|---|---|
| Symptoms | |||||
| Pain | 64 | 53 | 117 | 55 | |
| No pain | 21 | 4 | 25 | 84 | |
| Total | 85 | 57 | 142 | 60 | 0.007 |
| Malignancy history | |||||
| Prior malignancy | 18 | 13 | 31 | 58 | |
| No malignancy | 67 | 44 | 111 | 60 | |
| Total | 85 | 57 | 142 | 60 | 0.82 |
| Infection | |||||
| Infection | 12 | 12 | 24 | 50 | |
| No infection | 73 | 45 | 118 | 62 | |
| Total | 85 | 57 | 142 | 60 | 0.28 |
| Imaging factors | |||||
| Lesion aggressiveness | |||||
| Aggressive | 29 | 35 | 64 | 45 | |
| Nonaggressive | 56 | 22 | 78 | 72 | |
| Total | 85 | 57 | 142 | 60 | 0.004 |
| Incidental | |||||
| Incidental | 9 | 3 | 12 | 75 | |
| Not incidental | 76 | 54 | 130 | 59 | |
| Total | 85 | 57 | 142 | 60 | 0.26 |
| Other | |||||
| Prebiopsy assessment | |||||
| Suspicious for malignancy | 23 | 35 | 58 | 40 | |
| Likely benign | 62 | 22 | 84 | 74 | |
| Total | 85 | 57 | 142 | 60 | < 0.001 |
| Followup | |||||
| Surgery | 25 | 45 | 70 | 36 | |
| No surgery | 60 | 12 | 72 | 83 | |
| Total | 85 | 57 | 142 | 60 | < 0.001 |
Useful nondiagnostic core needle biopsy results occurred significantly more often in asymptomatic versus painful lesions: 84% (21 of 25) versus 55% (64 of 117; p = 0.007), nonaggressive versus aggressive lesions: 72% (56 of 78) versus 45% (29 of 64; p = 0.004), and in lesions assessed before biopsy as “likely to be benign” instead of “suspicious for malignancy”: 74% (62 of 84) versus 40% (23 of 58; p < 0.001) (Table 4). History of prior malignancy, suspicion for infection, or whether the lesion was an incidental finding had no association with whether the nondiagnostic core needle biopsy result was or was not clinically useful (Table 4). As expected, nondiagnostic biopsy results in patients that continued to surgical biopsy or excision were less useful than in patients that did not undergo surgery (36% [25 of 70] versus 83% [60 of 72]; p < 0.001) (Table 4).
Discussion
Nondiagnostic results from image-guided core needle biopsies of musculoskeletal lesions can delay patient care, create anxiety for the physician and patient, and lead to a repeat biopsy or surgical procedure. Although sampling error is an important consideration, nondiagnostic results can occur even when the lesion is adequately sampled. Thus, it is important to understand the factors associated with nondiagnostic results and whether nondiagnostic core needle biopsies have any clinical utility. In this study, we asked whether (1) benign or malignant bone and soft tissue lesions have a higher rate of nondiagnostic core needle biopsy results, and which diagnoses have the lowest diagnostic yield; (2) how often nondiagnostic results affected clinical decision making; and (3) what clinical factors are associated with nondiagnostic but useful core needle biopsies?
A few limitations of this study deserve mention. First, no pediatric or spine cases were assessed, because children are not treated at our hospital and our musculoskeletal interventional service does not perform the biopsies of spine lesions. Inclusion of these cases, especially tumors common to children, could change our results. Second, surgery was performed in slightly less than ½ of the lesions with a nondiagnostic core needle biopsy, and the remainder was followed clinically. This limits the accuracy of the final diagnosis; however, the average clinical followup was 20 months, which is likely sufficient time to assess worsening clinical symptoms or imaging features to allow for a change in the final diagnosis. Finally, the criteria for determining the usefulness of a nondiagnostic core needle biopsy is subjective. Determining whether the lesion is “likely to be benign” or “suspicious for malignancy” can vary among orthopaedic oncologists; however, we believe that this study can provide comparative data for future studies assessing the clinical use of a nondiagnostic core needle biopsy.
Benign lesions had a significantly lower diagnostic yield than malignant lesions: 58% versus 94%; and bone lesions had a significantly lower diagnostic yield than soft tissue lesions: 67% versus 82%. These findings are similar to those of other studies [4, 8–11, 14, 18, 21, 25, 26]; however, our study further investigates the diagnoses that are most likely to be nondiagnostic. Many of these diagnoses are not true neoplasms and would be difficult to accurately diagnose on a core needle biopsy. The most common nondiagnostic diagnoses in our study include ganglia, tenosynovitis, and degenerative changes which often require large biopsy specimens with intact tissue architecture to confirm the diagnosis. Core needle biopsy samples can suggest these diagnoses, but it is difficult for the pathologist to be conclusive in these cases. Moreover, several of the neoplasms with the lowest diagnostic yield are often cystic bone lesions (simple bone cysts and Langerhans cell histiocytosis), which makes acquiring solid material for analysis more difficult than soft tissue lesions and can be associated with low diagnostic yield [11, 15]. Understanding which entities have low diagnostic yield on core needle biopsy can be helpful to the clinician in determining which lesions should have core needle biopsy and how to interpret and act on the results of a nondiagnostic biopsy. Moreover, the majority of nondiagnostic core needle biopsies (91%) occurred in benign processes, which can be clinically useful, especially if the prebiopsy clinical and radiologic features suggest a benign entity. If the clinical scenario is supportive, then a nondiagnostic biopsy may indicate that these lesions potentially can be left alone or treated as benign processes without additional biopsy [1].
To our knowledge, only two previous studies have investigated the usefulness of core needle biopsy of musculoskeletal lesions in guiding clinical treatment [13, 26]. However, both evaluated the clinical usefulness of diagnostic core needle biopsies rather than nondiagnostic core needle biopsies [13, 26]. Lack et al. [13] reported a clinical usefulness of 70% from 69 musculoskeletal core needle biopsies, and usefulness was greater in patients with suspected bone metastasis as opposed to suspected primary bone lesions and soft tissue lesions. Yang et al. [26] reported a diagnostic accuracy and clinical usefulness of 89%; however, nondiagnostic or inconclusive core needle biopsies were treated as not clinically useful. However, this assumption that a nondiagnostic core needle biopsy cannot be clinically useful is not completely valid and is one of our main study questions. The orthopaedic oncologists in our study categorized 60% of the nondiagnostic core needle biopsies as useful in guiding clinical decision-making indicating that a nondiagnostic core needle biopsy can have good clinical utility.
Lesions that were painless, had nonaggressive features on imaging, and assessed as “likely to be benign” before the biopsy were more likely to have a clinically useful nondiagnostic core needle biopsy result compared with lesions that were painful, had aggressive imaging features, or were “suspicious for malignancy” before biopsy. In the cases in which the nondiagnostic biopsy was clinically useful (Fig. 3), the suspected prebiopsy diagnoses were typically benign processes with unusual features; therefore, the absence of malignancy in the core needle biopsy was viewed as reassuring to the clinician that a benign lesion was likely. These lesions could then be left alone, undergo imaging followup, or curative surgical excision. Conversely, in painful lesions or lesions suspicious for malignancy (Fig. 4), the orthopaedists were more pressed to arrive at a diagnosis and provide definitive treatment. Thus, in these cases, nondiagnostic core needle biopsy results generally were not useful because the diagnosis and the treatment plan were still in question. In the majority of these cases, surgical biopsy is necessary. Surprisingly, the presence or absence of prior malignancy was not associated with the usefulness of a nondiagnostic core needle biopsy. One would hypothesize that lesions in patients without known malignancy would be more likely to have a clinically useful nondiagnostic core needle biopsy result because there would be less suspicion for a metastasis. The reasons for this result are unclear but may relate to the fact that most metastases were diagnostic on core needle biopsy. Therefore, although these patients had a known malignancy, the lesion undergoing core needle biopsy was most likely unrelated to the primary malignancy. In the cases in which doubt remained, an open biopsy was performed.
Fig. 3A–C.
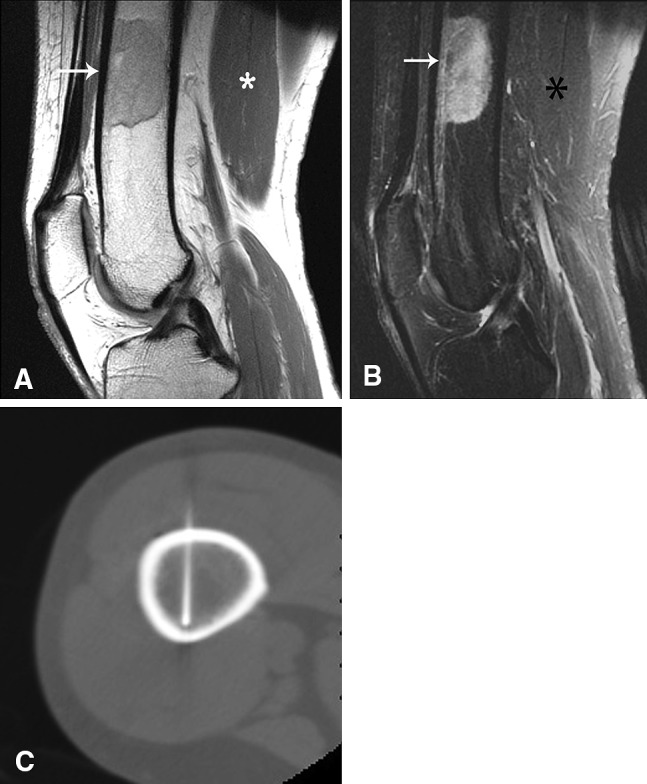
A 54-year-old woman has a focus of red marrow in the distal femur, which was nondiagnostic at core needle biopsy. This lesion was painless and seen incidentally on routine knee MR images. Given the lack of pain and the MRI features suggestive of red marrow, no additional intervention was performed. Followup MRI studies over 2 years showed no change. (A) The sagittal T1-weighted MR image shows a well-circumscribed lesion with intermediate T1 signal intensity (white arrow) that is higher than muscle (asterisk), suggesting red marrow. On the (B) sagittal T2-weighted MR image, the lesion is high signal (white arrow) relative to muscle (white asterisk). (C) A CT-guided core needle biopsy image shows the biopsy needle in the lesion.
Fig. 4A–C.
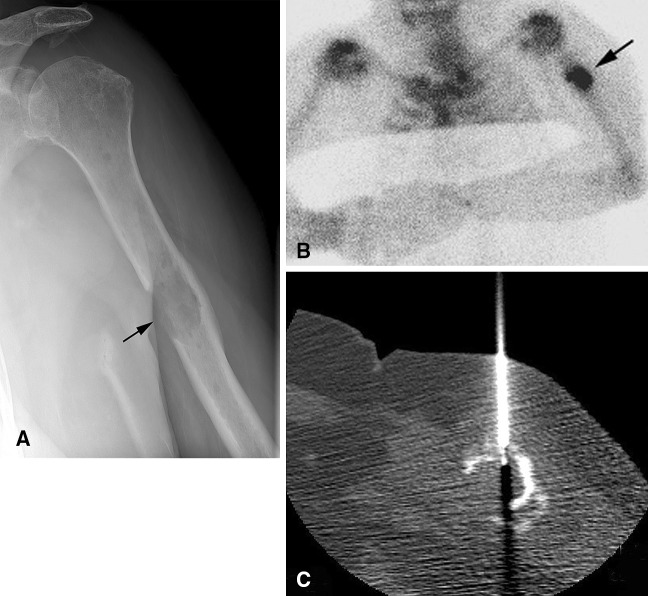
A 67-year-old woman had a history of breast cancer and a painful left humerus lesion, which was nondiagnostic at core needle biopsy. Given the pain, history of malignancy, and aggressive radiographic features, surgical biopsy was performed revealing a plasmacytoma. (A) An AP radiograph shows a lytic lesion with a wide transition zone and surrounding periostitis (black arrow) in the midhumeral shaft. The (B) bone scan shows focal radiotracer uptake in the lesion (black arrow). (C) A CT-guided core needle biopsy image shows the biopsy needle in the lesion.
We found that nondiagnostic core needle biopsies are more likely to occur in benign than malignant lesions, and in bone than soft tissue lesions. Thus, if prebiopsy clinical and radiologic features suggest a benign entity, a nondiagnostic core needle biopsy could be interpreted as supportive of a benign process. Moreover, 60% of the nondiagnostic core needle biopsies were helpful in guiding clinical decision making from the perspective of orthopaedic oncologists, especially in lesions that were painless with a nonaggressive imaging appearance and assessed before biopsy as “likely to be benign.” Nondiagnostic core needle biopsy results in musculoskeletal lesions need not be considered failures. At times, they can be supportive of benign processes and can help avert unnecessary surgical procedures.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
This study was approved by the institutional review board (IRB) at our institution. The study has been performed in accordance with the ethical standards in the 1964 Declaration of Helsinki. The study was performed in accordance with relevant regulations of the US Health Insurance Portability and Accountability Act (HIPAA).
References
- 1.Adams SC, Potter BK, Pitcher DJ, Temple HT. Office-based core needle biopsy of bone and soft tissue malignancies: an accurate alternative to open biopsy with infrequent complications. Clin Orthop Relat Res. 2010;468:2774–2780. doi: 10.1007/s11999-010-1422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altuntas AO, Slavin J, Smith PJ, Schlict SM, Powell GJ, Ngan S, Toner G, Choong PF. Accuracy of computed tomography guided core needle biopsy of musculoskeletal tumours. ANZ J Surg. 2005;75:187–191. doi: 10.1111/j.1445-2197.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 3.Cronin CG, Cashell T, Mhuircheartaigh JN, Swords R, Murray M, O’Sullivan GJ, O’Keeffe D. Bone biopsy of new suspicious bone lesions in patients with primary carcinoma: prevalence and probability of an alternative diagnosis. AJR Am J Roentgenol. 2009;193:W407–W410. doi: 10.2214/AJR.08.1882. [DOI] [PubMed] [Google Scholar]
- 4.Datir A, Pechon P, Saifuddin A. Imaging-guided percutaneous biopsy of pathologic fractures: a retrospective analysis of 129 cases. AJR Am J Roentgenol. 2009;193:504–508. doi: 10.2214/AJR.08.1823. [DOI] [PubMed] [Google Scholar]
- 5.Dupuy DE, Rosenberg AE, Punyaratabandhu T, Tan MH, Mankin HJ. Accuracy of CT-guided needle biopsy of musculoskeletal neoplasms. AJR Am J Roentgenol. 1998;171:759–762. doi: 10.2214/ajr.171.3.ajronline_171_3_001. [DOI] [PubMed] [Google Scholar]
- 6.Espinosa LA, Jamadar DA, Jacobson JA, DeMaeseneer MO, Ebrahim FS, Sabb BJ, Kretschmer MT, Biermann JS, Kim SM. CT-guided biopsy of bone: a radiologist’s perspective. AJR Am J Roentgenol. 2008;190:W283–W289. doi: 10.2214/AJR.07.3138. [DOI] [PubMed] [Google Scholar]
- 7.Fraser-Hill MA, Renfrew DL. Percutaneous needle biopsy of musculoskeletal lesions: 1. Effective accuracy and diagnostic utility. AJR Am J Roentgenol. 1992;158:809–812. doi: 10.2214/ajr.158.4.1546597. [DOI] [PubMed] [Google Scholar]
- 8.Hau A, Kim I, Kattapuram S, Hornicek FJ, Rosenberg AE, Gebhardt MC, Mankin HJ. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skeletal Radiol. 2002;31:349–353. doi: 10.1007/s00256-002-0474-3. [DOI] [PubMed] [Google Scholar]
- 9.Hwang S, Lefkowitz RA, Landa J, Zheng J, Moskowitz CS, Maybody M, Hameed M, Panicek DM. Percutaneous CT-guided bone biopsy: diagnosis of malignancy in lesions with initially indeterminate biopsy results and CT features associated with diagnostic or indeterminate results. AJR Am J Roentgenol. 2011;197:1417–1425. doi: 10.2214/AJR.11.6820. [DOI] [PubMed] [Google Scholar]
- 10.Issakov J, Flusser G, Kollender Y, Merimsky O, Lifschitz-Mercer B, Meller I. Computed tomography-guided core needle biopsy for bone and soft tissue tumors. Isr Med Assoc J. 2003;5:28–30. [PubMed] [Google Scholar]
- 11.Jelinek JS, Murphey MD, Welker JA, Henshaw RM, Kransdorf MJ, Shmookler BM, Malawer MM. Diagnosis of primary bone tumors with image-guided percutaneous biopsy: experience with 110 tumors. Radiology. 2002;223:731–737. doi: 10.1148/radiol.2233011050. [DOI] [PubMed] [Google Scholar]
- 12.Kattapuram SV, Rosenthal DI. Percutaneous biopsy of skeletal lesions. AJR Am J Roentgenol. 1991;157:935–942. doi: 10.2214/ajr.157.5.1927811. [DOI] [PubMed] [Google Scholar]
- 13.Lack W, Donigan JA, Morcuende J, Buckwalter J, El-Khoury GY. Clinical utility of CT-guided biopsies in orthopaedic oncology. Iowa Orthop J. 2010;30:76–79. [PMC free article] [PubMed] [Google Scholar]
- 14.Lis E, Bilsky MH, Pisinski L, Boland P, Healey JH, O’Malley B, Krol G. Percutaneous CT-guided biopsy of osseous lesion of the spine in patients with known or suspected malignancy. AJNR Am J Neuroradiol. 2004;25:1583–1588. [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy EF. CT-guided needle biopsies of bone and soft tissue tumors: a pathologist’s perspective. Skeletal Radiol. 2007;36:181–182. doi: 10.1007/s00256-006-0244-8. [DOI] [PubMed] [Google Scholar]
- 16.Mitsuyoshi G, Naito N, Kawai A, Kunisada T, Yoshida A, Yanai H, Dendo S, Yoshino T, Kanazawa S, Ozaki T. Accurate diagnosis of musculoskeletal lesions by core needle biopsy. J Surg Oncol. 2006;94:21–27. doi: 10.1002/jso.20504. [DOI] [PubMed] [Google Scholar]
- 17.Ogilvie CM, Torbert JT, Finstein JL, Fox EJ, Lackman RD. Clinical utility of percutaneous biopsies of musculoskeletal tumors. Clin Orthop Relat Res. 2006;450:95–100. doi: 10.1097/01.blo.0000229302.52147.c7. [DOI] [PubMed] [Google Scholar]
- 18.Omura MC, Motamedi K, UyBico S, Nelson SD, Seeger LL. Revisiting CT-guided percutaneous core needle biopsy of musculoskeletal lesions: contributors to biopsy success. AJR Am J Roentgenol. 2011;197:457–461. doi: 10.2214/AJR.10.6145. [DOI] [PubMed] [Google Scholar]
- 19.Pohlig F, Kirchhoff C, Lenze U, Schauwecker J, Burgkart R, Rechl H, von Eisenhart-Rothe R. Percutaneous core needle biopsy versus open biopsy in diagnostics of bone and soft tissue sarcoma: a retrospective study. Eur J Med Res. 2012;17:29. doi: 10.1186/2047-783X-17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puri A, Shingade VU, Agarwal MG, Anchan C, Juvekar S, Desai S, Jambhekar NA. CT-guided percutaneous core needle biopsy in deep seated musculoskeletal lesions: a prospective study of 128 cases. Skeletal Radiol. 2006;35:138–143. doi: 10.1007/s00256-005-0038-4. [DOI] [PubMed] [Google Scholar]
- 21.Rimondi E, Rossi G, Bartalena T, Ciminari R, Alberghini M, Ruggieri P, Errani C, Angelini A, Calabro T, Abati CN, Balladelli A, Tranfaglia C, Mavrogenis AF, Vanel D, Mercuri M. Percutaneous CT-guided biopsy of the musculoskeletal system: results of 2027 cases. Eur J Radiol. 2011;77:34–42. doi: 10.1016/j.ejrad.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 22.Saifuddin A, Mitchell R, Burnett SJ, Sandison A, Pringle JA. Ultrasound-guided needle biopsy of primary bone tumours. J Bone Joint Surg Br. 2000;82:50–54. doi: 10.1302/0301-620X.82B1.10141. [DOI] [PubMed] [Google Scholar]
- 23.Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78:644–649. doi: 10.2106/00004623-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Sung KS, Seo SW, Shon MS. The diagnostic value of needle biopsy for musculoskeletal lesions. Int Orthop. 2009;33:1701–1706. doi: 10.1007/s00264-009-0835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JS, Goldsmith JD, Horwich PJ, Shetty SK, Hochman MG. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology. 2008;248:962–970. doi: 10.1148/radiol.2483071742. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Frassica FJ, Fayad L, Clark DP, Weber KL. Analysis of nondiagnostic results after image-guided needle biopsies of musculoskeletal lesions. Clin Orthop Relat Res. 2010;468:3103–3111. doi: 10.1007/s11999-010-1337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]