Abstract
Background
The outcome of revision surgery depends on accurate determination of the cause of prosthesis failure because treatment differs profoundly among aseptic loosening, mechanical failure, and prosthetic joint infections (PJI).
Questions/purposes
We sought to determine (1) the predictive role of the interval from primary to revision surgery in determining the reason for prosthesis failure of a hip, knee, shoulder, or elbow arthroplasty, and (2) whether positive cultures during revision surgery for aseptic loosening were associated with shorter event-free survival of the prosthesis.
Methods
All patients undergoing revision surgery between July 2010 and January 2012 were included in a prospective cohort of 112 patients, and were classified as having had failure from aseptic loosening (56%), mechanical failure (15%), or PJI (29%). To make the diagnosis of PJI, at surgery we used a standardized enhanced diagnostic approach in all patients including sampling of five periprosthetic tissue specimens, sonication of removed prosthetic components, prolonged incubation of aerobic and anaerobic cultures, and multiplex PCR of sonication fluid in aseptic loosening cases. Kaplan-Meier survival and Cox proportional hazards regression analysis were performed.
Results
The median time from primary to revision surgery was (p < 0.001) longer for patients with aseptic loosening (7.8 years) than for patients with mechanical failure (1.6 years) or PJI (2 years). No difference in the time to revision was observed for patients with aseptic loosening with positive or negative microbiological cultures (p = 0.594). Propionibacterium acnes was cultured below the established microbiological criteria for positivity in 12 (19%) procedures that had been presumed to have been revisions for aseptic loosening.
Conclusions
PJI should be considered in all revisions performed within 2 years of implantation even in the absence of clinical or laboratory findings suggestive for infection. However, the growth of low-virulence microorganisms below the cut-off in revisions for apparent aseptic loosening is not associated with early prosthesis failure.
Level of Evidence
Level II, diagnostic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
The number of prosthesis revision surgeries is increasing worldwide as a result of an increase in the number of primary arthroplasties and longer prosthesis implantation times [13]. Despite improvements in implant design and biocompatibility, surgical technique, and perioperative prophylaxis, a considerable number of prostheses fail within 10 to 15 years of implantation [7, 15]. The reasons for prosthesis failure can be broadly divided into aseptic loosening, mechanical failure, or prosthetic joint infection (PJI) [1, 14]. Accurate diagnosis of the reason for prosthesis failure is important because treatment differs profoundly among these clinical entities [3]. In particular, the correct diagnosis of PJI is important because a missed diagnosis of infection often leads to early prosthesis failure as a result of relapse of the untreated infection [4].
The diagnosis of PJI can be challenging in low-grade infections, which mimic aseptic loosening and often manifest with only scarce or nonspecific clinical signs and symptoms. Data on the probability of PJI come principally from large arthroplasty registries and case series [8, 11, 18, 26, 31]. In these studies, PJI was not actively identified in a systematic manner and some low-grade infections may have been missed. Misclassification of low-grade PJI as aseptic loosening is further supported by the American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines [5], which suggest that PJI is unlikely (and joint aspiration is not recommended) if C-reactive protein and erythrocyte sedimentation rate are normal. Normal results of systemic markers of inflammation do not exclude PJI caused by low-virulence microorganisms [6, 16, 19, 30, 32]. Furthermore, these guidelines do not take into account the time elapsed since the primary arthroplasty.
We therefore performed a prospective cohort study on patients undergoing hip, knee, shoulder, or elbow revision surgery for any cause to evaluate the probability of PJI based on the occurrence of prosthesis failure after implantation and to compare microbiologic findings among patients with PJI, mechanical failure, and aseptic loosening. We sought to minimize the probability of PJI misclassification as aseptic loosening by a systematic search for criteria defining PJI by applying an enhanced diagnostic algorithm to all included patients independent of clinical or laboratory findings.
Specifically, we sought to determine (1) whether the interval from primary to revision surgery was associated with the reason for failure of a hip, knee, shoulder, or elbow arthroplasty; and (2) whether positive cultures during revision surgery of aseptic loosening cases were associated with shorter event-free survival of the prosthesis.
Patients and Methods
A cohort study was conducted in two tertiary medical care centers, Hospital del Mar (approximately 400 beds) and Hospital de l’Esperança (approximately 200 beds) in Barcelona, Spain. Approximately 900 orthopaedic surgical procedures are performed annually in both institutions, including primary and revision arthroplasties. The study protocol was approved by both institutional review boards.
We prospectively included all patients 18 years or older who were hospitalized from July 2010 through January 2012 undergoing revision of a knee, hip, shoulder, or elbow prosthesis. An enhanced diagnostic algorithm was applied to all patients to accurately determine the cause of prosthesis failure. This algorithm included standardized sampling of five periprosthetic tissue specimens, sonication of removed prosthetic components (including UHMWPE components), prolonged incubation of synovial, periprosthetic tissues, and sonication fluid cultures, and multiplex PCR of sonication fluid in aseptic loosening cases. We recorded demographic, clinical, laboratory, and microbiologic data.
An initial total of 116 patients undergoing revision surgery were included in the study. Four patients subsequently were excluded because of obvious contamination of the implant during handling in the laboratory.
PJI was defined as the presence of at least one of the following: (1) visible purulence of the preoperative joint aspirate or in the surgical site (as determined by the surgeon); (2) presence of a cutaneous sinus tract communicating with the prosthesis; (3) acute inflammation in permanent histopathology sections of periprosthetic tissue (as determined by a pathologist); (4) acute inflammation in preoperative joint aspirate (ie, leukocyte count > 1.7 g/L or > 65% neutrophils in a knee prosthesis [28], or leukocyte count > 4.2 g/L or > 80% neutrophils in a hip prosthesis [25]); and (5) positive microbiology defined as growth of microorganisms in preoperative aspirate, periprosthetic tissue culture, or sonication fluid culture. Positive sonication fluid culture was considered when the same organism grew 50 or more colony-forming units (CFU)/mL [1, 29]. However, when the patient had previously received antibiotics, any growth in sonication fluid culture was considered positive [22]. Previous antimicrobial therapy was defined as receiving an antibiotic for 24 hours or more in the 14 days before surgery. Low-virulence microorganisms such as coagulase-negative staphylococci, Corynebacterium species, Bacillus species, or Propionibacterium species were considered pathogens if the same organism was isolated in at least two samples or in one sample if at least one clinical criterion for PJI also was present (see previously). In the absence of these criteria for PJI, the prosthesis failure was further classified as aseptic loosening or mechanical failure. Aseptic loosening was defined when mechanical or radiologic loosening with or without polyethylene wear of the implant was the primary indication for the revision. Mechanical failure was defined when periprosthetic fracture, joint instability, prosthesis malalignment, or malposition was the primary indication for the revision.
Synovial fluid was aspirated at the discretion of the surgeon and was sent for determination of leukocyte count and differential (in an EDTA-containing vial) and for culture (in a plain vial). Five periprosthetic tissue specimens, showing macroscopically the most inflammatory changes, were collected from independent surgical sites. Tissue specimens were crushed, 0.5 mL of homogenate was plated on agar plates, and the remaining volume was inoculated in thioglycollate broth. All cultures were incubated at 37º C for 7 days (aerobically) or 14 days (anaerobically) and inspected daily for microbial growth.
The removed prosthesis components were transported to the microbiology laboratory in air-tight containers. Sonication was performed for 5 minutes at a frequency of 40 ± 5 kHz, as previously described (Model SM25E-MT, Branson Ultrasonics Corporation, Geneva, Switzerland) [29], except that sonication was performed in thioglycollate broth (covering 80% of the prosthesis in the container) rather than in Ringer’s salt solution. The container subsequently was vortexed for an additional 1 minute and aliquots of 0.5 mL sonication fluid were plated within 4 hours after sonication onto aerobic and anaerobic agar plates and 0.5 mL was inoculated into thioglycollate broth. Cultures were incubated at 37º C for 7 days (aerobically) or 14 days (anaerobically) and inspected daily for microbial growth. Real-time multiplex PCR (SeptiFast©; Roche Diagnostics, San Cugat del Vallès, Barcelona) was performed in sonication fluid of aseptic failure cases, as previously described [21, 22].
Data from patients were stratified by cause of prosthesis failure (aseptic loosening, mechanical failure, and PJI). We compared groups using chi-square or Fisher’s exact tests for clinical characteristics of the patients and the Mann-Whitney U-test for time between implantation and revision surgery. A Kaplan-Meier survival method was calculated to evaluate the probability of event-free survival and 95% CI, and the survival probabilities between groups were compared by the Cox proportional hazards regression analysis. Differences were considered significant when the p value was less than 0.05. For statistical analysis and graphics, Prism software (Version 6.01; GraphPad, La Jolla, CA, USA) was used.
Results
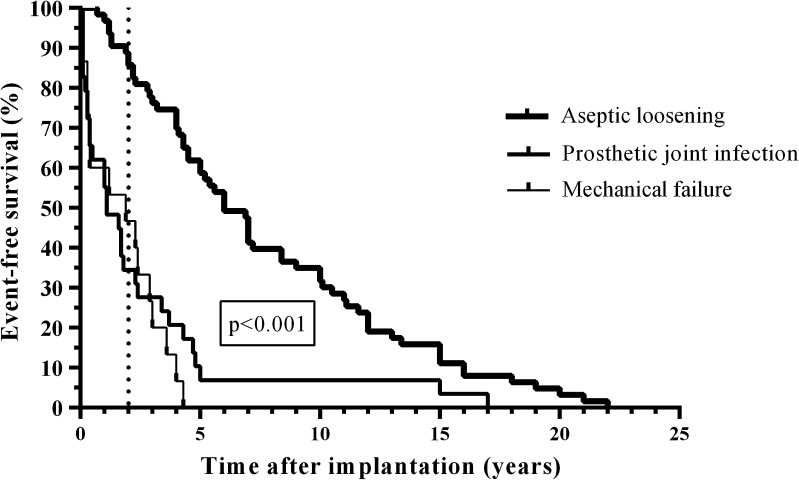
The median duration between primary implantation and surgical procedure was longer for patients with aseptic loosening than mechanical failure or PJI (7.8 versus 1.6 years and 2 years, respectively [p < 0.001]; Fig. 1). In the first 2 years after implantation, revisions were performed more for PJI (69%) and for mechanical failure (53%) than for aseptic loosening (16%) (p < 0.001). For PJI, debridement and implant retention were performed in 44% of patients, in whom polyethylene inserts and liners were exchanged and subjected to sonication. Among the 32 PJI, 10 (31%) were early postoperative (infections occurring within the 3 first months after implantation), 12 (38%) were delayed postoperative (low-grade) (infections occurring between the third month and second year after implantation), and 10 (31%) were late hematogenous (infections occurring after the second year after implantation caused by hematogenous seeding from remote infections). Among the 32 patients with PJI, 18 (56%) had received antimicrobial treatment before surgery. For the 112 patients included in this study (Table 1), aseptic loosening was diagnosed in 63 (56%), mechanical failure in 17 (15%), and PJI in 32 (29%).
Fig. 1.
The Kaplan-Meier survival analysis is shown for the 112 patients (112 prostheses) stratified to aseptic loosening (n = 63), mechanical failure (n = 17), and PJI (n = 32). The vertical spikes denote censored cases. The survival time of the prostheses was longer in patients with aseptic loosening compared with mechanical failure and PJI (p < 0.001).The vertical line indicates the survival probability 2 years after prosthesis implantation.
Table 1.
Characteristics of 112 patients in whom prosthesis revision was performed
| Patient characteristic | Aseptic loosening (n = 63) | Mechanical failure (n = 17) | Prosthetic joint infection (n = 32) |
|---|---|---|---|
| Patient age, years, median (range) | 72 (27–86) | 76 (48–89) | 74 (51–87) |
| Male sex | 24 (38%) | 6 (35%) | 14 (44%) |
| Type of joint prosthesis | |||
| Knee (n = 73) | 44 (70%) | 6 (35%) | 23 (72%) |
| Hip (n = 31) | 19 (30%) | 8 (47%) | 4 (16%) |
| Shoulder (n = 4) | 0 | 1 (6%) | 3 (9%) |
| Elbow (n = 4) | 0 | 2 (12%) | 2 (6%) |
| Time from prosthesis implantation to explantation, median (range) | 7.8 years (252 days to 22 years) | 1.6 years (7 days to 4.7 years) | 2 years (15 days to 17 years) |
| Type of revision surgery | |||
| Débridement and prosthesis retention | 0 | 0 | 14 (44%) |
| One-stage exchange | 63 (100%) | 15 (88%) | 1 (3%) |
| Two-stage exchange* | 0 | 2 (12%) | 17 (53%) |
| Clinical signs of infection | |||
| Sinus tract (fistula) | 0 | 0 | 7 (22%) |
| Visible pus/soft tissue abscess around the prosthesis | 0 | 0 | 22 (69%) |
| Synovial fluid cell count, mean (range) | |||
| Leukocyte count, g/L | 0.3 (0.06–0.8) | ND | 45 (1.4–76) |
| Neutrophils, % | 19 (2–44) | ND | 90 (54–98) |
| Inflammation in histopathology† | 0 (0%) | 0 (0%) | 30 (88%) |
| Received previous antibiotics | 0 | 0 | 18 (56%) |
Values represent numbers (%) if not indicated otherwise; * the median interval between explantation and reimplantation of a new prosthesis was 118 days (range, 19–357 days); ND = not determined; †acute inflammation (> 1 neutrophil per high power field) in histopathology sections of periprosthetic tissues.
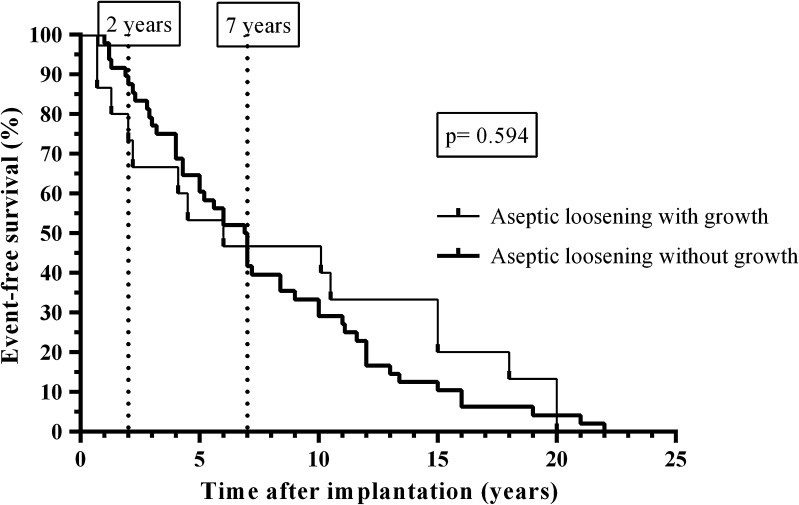
No difference in survival time of the prostheses was found (p = 0.594) between patients with aseptic loosening without growth and patients with aseptic loosening with growth in one periprosthetic tissue and or in sonication fluid culture less than 50 CFU/mL (Fig. 2). None of the patients with aseptic failure or PJI required a prosthetic joint revision after a minimum followup of 1.3 years (median, 2.1years; range, 1.3–2.9 years).The causative microorganisms grew at less than 50 CFU/mL in two PJI (Table 2); one was hematogenous PJI caused by coagulase-negative staphylococci (related to catheter-associated infection) and another acute PJI was caused by Pasteurella multocida after a cat bite. No patient with mechanical failure or aseptic loosening had two or more periprosthetic tissues positive or 50 CFU/mL or greater, except one patient with positive sonication fluid culture with Corynebacterium species, which was considered contamination during the sonication procedure. All aseptic failures were negative by multiplex PCR. P acnes was isolated in 12 of 63 cases (19%) of aseptic loosening, no P acnes was isolated in 17 cases of mechanical failure, and only two in 63 cases of PJI (p < 0.05).
Fig. 2.
The Kaplan-Meier survival analysis is shown for the 63 patients (63 prostheses) with aseptic loosening stratified according to microbiological findings (48 without growth and 15 with growth of periprosthetic tissue and/or sonication fluid culture). The vertical spikes denote censored cases. The vertical line indicates the survival 2 years after prosthesis implantation.
Table 2.
Positivity of the periprosthetic tissue culture and sonication fluid culture
| Type of sample | Prosthetic joint infection (n = 32) | Mechanical failure (n = 17) | Aseptic loosening (n = 63) |
|---|---|---|---|
| Periprosthetic tissue culture | |||
| 1 sample positive | 2 (6%)* | 2 (12%)† | 8 (13%)‡ |
| ≥ 2 samples positive | 24 (75%) | 0 | 0 |
| Sonication culture | |||
| < 50 CFU/mL | 2 (3%)§ | 2 (12%)∥ | 7 (11%)# |
| ≥ 50 CFU/mL | 22 (69%) | 0 | 1 (2%)& |
* E. coli (n = 1); coagulase-negative staphylococcus (n = 1); †coagulase-negative staphylococci (n = 2); ‡coagulase-negative staphylococci (n = 2), viridans group streptococcus (n = 1) and P. acnes (n = 5); § P. multocida (n = 1), coagulase-negative staphylococcus (n = 1), both patients received antibiotics before surgery; ∥coagulase-negative staphylococci (n = 2); # P. acnes (n = 7), these cases did not correspond with any positive periprosthetic tissue culture; & Corynebacterium sp. (n = 1), growth was considered a contamination during sonication procedure.
Discussion
An accurate diagnosis of the reason for prosthesis failure is crucial, particularly the differentiation between aseptic loosening and PJI because the treatment differs profoundly between these two clinical entities. A missed diagnosis of PJI can lead to a relapse of infection and early prosthesis failure [4, 9, 10, 30]. We found that the need for revision surgery within 2 years after implantation is associated with high probability of infection. This underlines the importance of a systematic search for infection in all early failures (within the first 2 years of implantation), despite the absence of typical clinical or laboratory findings suggestive of infection. Moreover, we observed that growth of low-virulence organisms below the established microbiological criteria for positivity is not associated with shorter event-free survival of the prosthesis.
A limitation of our study was the low number of patients in the groups with and without microbiological growth (15 and 48 cases, respectively), which may represent a bias. Second, we were not able to determine whether these organisms represent contaminants or asymptomatic colonization of the prosthesis (“silent” biofilms) as was previously reported for electrophysiologic cardiac devices [24] and breast implants [23]. A longer followup (> 2 years) of these patients may clarify the importance of positive cultures without clinical symptoms.
In our study, aseptic loosening was the most common reason for revision surgery. However, if the arthroplasty revision was performed within the first 2 years of implantation, the etiology of failure was more likely to be PJI than aseptic loosening. In contrast, all PJI occurring after the second year of implantation were acute infections. Therefore, the differential diagnosis between PJI and aseptic loosening after the second year was not a challenge. Along the same line, the time to failure was almost four times longer in patients with aseptic loosening than in those whose failures were caused by PJI. This is in accordance with another study [26] and underlines the importance of a systematic search for infection in all early failures (within the first 2 years of implantation) despite the absence of typical clinical or laboratory findings suggestive of infection. Therefore, the AAOS clinical practice guidelines [5] may need to be revisited to avoid misclassification of PJI as aseptic loosening. Some authors reported that low-grade PJI often are misclassified as aseptic loosening when obvious clinical signs of infection are missing [2, 17]. An accurate diagnosis of the reason of prosthesis failure is important, particularly the differentiation between aseptic loosening and PJI, because the treatment differs profoundly between these two clinical entities. A missed diagnosis of PJI often leads to a relapse of infection and early failure [4].
However, the presence of low-virulence microorganisms was reported in 21% to 57% of aseptic loosening cases [9, 27], but their relevance is questionable. In our study, an optimized diagnostic procedure was used, including sonication of the removed implant, prolonged incubation of multiple cultures, and multiplex PCR. We found that growth of low-virulence organisms below the established microbiological criteria for positivity is not associated with a shorter, event-free survival of the prosthesis. To improve detection of low-grade infections, multiplex PCR of the sonication fluid was used and the PCR was negative in all cases of aseptic failure. However, this PCR kit does not include specific primers for detection of P acnes and Corynebacterium species. A modified primer kit including primers for low-virulence organisms frequently involved in PJI is needed [3]. The causative microorganisms grew at less than 50 CFU/mL in two cases of PJI. One of these cases was a hematogenous PJI and the other was an acute infection caused by P multocida. These findings are in agreement with our theory that in acute PJI, the involved biofilms have few layers of immature biofilm, so sonication does not show superior sensitivity [22]. Interestingly, microorganisms that grew below the cut-off differed among the three patient groups. Whereas P acnes was isolated in 19% of patients with aseptic loosening, no P acnes was isolated in patients with mechanical failure.
Revision surgery attributable to PJI occurred predominantly within the first 2 years after implantation, whereas aseptic loosening occurred after a median of more than 7 years after implantation. The role of P acnes in shoulder prostheses is well known [12, 20]. However, the relevance of isolated positive cultures of P acnes in presumably cases of aseptic loosening and its potential clinical relevance in the pathogenesis of PJI need to be determined.
Acknowledgments
We thank Andrej Trampuz MD for comments to the manuscript.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Surgical and antimicrobial treatments were performed at Hospital del Mar, Barcelona, Spain. Microbiological analyses were performed at the Reference Laboratory of Cataluña, Barcelona, Spain.
References
- 1.Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol. 2010;48:1208–1214. doi: 10.1128/JCM.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bori G, Soriano A, Garcia S, Gallart X, Casanova L, Mallofre C, Almela M, Martinez JA, Riba J, Mensa J. Low sensitivity of histology to predict the presence of microorganisms in suspected aseptic loosening of a joint prosthesis. Mod Pathol. 2006;19:874–877. doi: 10.1038/modpathol.3800606. [DOI] [PubMed] [Google Scholar]
- 3.Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs. 2012;35:923–934. doi: 10.5301/ijao.5000168. [DOI] [PubMed] [Google Scholar]
- 4.Del Pozo JL, Patel R. Clinical practice: infection associated with prosthetic joints. N Engl J Med. 2009;361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Della Valle C, Parvizi J, Bauer TW, DiCesare PE, Evans RP, Segreti J, Spangehl M, Watters WC 3rd, Keith M, Turkelson CM, Wies JL, Sluka P, Hitchcock K; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am. 2011;93:1355–1357. [DOI] [PubMed]
- 6.Dodson CC, Craig EV, Cordasco FA, Dines DM, Dines JS, Dicarlo E, Brause BD, Warren RF. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg. 2010;19:303–307. doi: 10.1016/j.jse.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 7.Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;311:46–53. [PubMed] [Google Scholar]
- 8.Havelin LI, Engesaeter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties. Acta Orthop Scand. 2000;71:337–353. doi: 10.1080/000164700317393321. [DOI] [PubMed] [Google Scholar]
- 9.Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res. 2011;29:617–622. doi: 10.1002/jor.21286. [DOI] [PubMed] [Google Scholar]
- 10.Ince A, Rupp J, Frommelt L, Katzer A, Gille J, Lohr JF. Is “aseptic” loosening of the prosthetic cup after total hip replacement due to nonculturable bacterial pathogens in patients with low-grade infection? Clin Infect Dis. 2004;39:1599–1603. doi: 10.1086/425303. [DOI] [PubMed] [Google Scholar]
- 11.Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty: a register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91:38–47. doi: 10.2106/JBJS.G.01686. [DOI] [PubMed] [Google Scholar]
- 12.Kelly JD, 2nd, Hobgood ER. Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res. 2009;467:2343–2348. doi: 10.1007/s11999-009-0875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144–151. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- 15.Malchau H, Herberts P, Eisler T, Garellick G, Soderman P. The Swedish Total Hip Replacement Register. J Bone Joint Surg Am. 2002;84(suppl 2):2–20. doi: 10.2106/00004623-200200002-00002. [DOI] [PubMed] [Google Scholar]
- 16.Moran E, Byren I, Atkins BL. The diagnosis and management of prosthetic joint infections. J Antimicrob Chemother. 2010;65(suppl 3):iii45–54. [DOI] [PubMed]
- 17.Nelson CL, McLaren AC, McLaren SG, Johnson JW, Smeltzer MS. Is aseptic loosening truly aseptic? Clin Orthop Relat Res. 2005;437:25–30. doi: 10.1097/01.blo.0000175715.68624.3d. [DOI] [PubMed] [Google Scholar]
- 18.Paxton EW, Furnes O, Namba RS, Inacio MC, Fenstad AM, Havelin LI. Comparison of the Morwegian knee arthroplasty register and a United States arthroplasty registry. J Bone Joint Surg Am. 2011;93(suppl 3):20–30. doi: 10.2106/JBJS.K.01045. [DOI] [PubMed] [Google Scholar]
- 19.Piper KE, Fernandez-Sampedro M, Steckelberg KE, Mandrekar JN, Karau MJ, Steckelberg JM, Berbari EF, Osmon DR, Hanssen AD, Lewallen DG, Cofield RH, Sperling JW, Sanchez-Sotelo J, Huddleston PM, Dekutoski MB, Yaszemski M, Currier B, Patel R. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One. 2010;5:e9358. doi: 10.1371/journal.pone.0009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol. 2009;47:1878–1884. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portillo ME, Salvado M, Sorli L, Alier A, Martinez S, Trampuz A, Gomez J, Puig L, Horcajada JP. Multiplex PCR of sonication fluid accurately differentiates between prosthetic joint infection and aseptic failure. J Infect. 2012;65:541–548. doi: 10.1016/j.jinf.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Portillo ME, Salvado M, Trampuz A, Plasencia V, Rodriguez-Villasante M, Sorli L, Puig L, Horcajada JP. Sonication versus vortexing of implants for diagnosis of prosthetic joint infection. J Clin Microbiol. 2013;51:591–594. doi: 10.1128/JCM.02482-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieger UM, Pierer G, Luscher NJ, Trampuz A. Sonication of removed breast implants for improved detection of subclinical infection. Aesthet Plast Surg. 2009;33:404–408. doi: 10.1007/s00266-009-9333-0. [DOI] [PubMed] [Google Scholar]
- 24.Rohacek M, Weisser M, Kobza R, Schoenenberger AW, Pfyffer GE, Frei R, Erne P, Trampuz A. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation. 2010;121:1691–1697. doi: 10.1161/CIRCULATIONAHA.109.906461. [DOI] [PubMed] [Google Scholar]
- 25.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869–1875. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 26.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper: Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Sierra JM, Garcia S, Martinez-Pastor JC, Tomas X, Gallart X, Vila J, Bori G, Macule F, Mensa J, Riba J, Soriano A. Relationship between the degree of osteolysis and cultures obtained by sonication of the prostheses in patients with aseptic loosening of a hip or knee arthroplasty. Arch Orthop Trauma Surg. 2011;131:1357–1361. doi: 10.1007/s00402-011-1307-4. [DOI] [PubMed] [Google Scholar]
- 28.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 30.Trampuz A, Zimmerli W. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly. 2005;135:243–251. doi: 10.4414/smw.2005.10934. [DOI] [PubMed] [Google Scholar]
- 31.Trebse R. The diagnostic protocol for evaluation of periprosthetic joint infection. Hip Int. 2012;22(suppl 8):25–35. doi: 10.5301/HIP.2012.9567. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]