Abstract
Gap junctions are assemblies of intercellular channels that regulate a variety of physiologic and developmental processes through the exchange of small ions and signaling molecules. These channels consist of connexin family proteins that allow for diversity of channel composition and conductance properties. The human connexin 43 gene, or GJA1, is located at human chromosome 6q22-q23 within the candidate region for the oculodentodigital dysplasia locus. This autosomal dominant syndrome presents with craniofacial (ocular, nasal, and dental) and limb dysmorphisms, spastic paraplegia, and neurodegeneration. Syndactyly type III and conductive deafness can occur in some cases, and cardiac abnormalities are observed in rare instances. We found mutations in the GJA1 gene in all 17 families with oculodentodigital dysplasia that we screened. Sixteen different missense mutations and one codon duplication were detected. These mutations may cause misassembly of channels or alter channel conduction properties. Expression patterns and phenotypic features of gja1 animal mutants, reported elsewhere, are compatible with the pleiotropic clinical presentation of oculodentodigital dysplasia.
Introduction
Oculodentodigital dysplasia (ODDD [MIM *164200]), also known as “oculodentoosseous dysplasia,” is an autosomal dominant disorder with high penetrance, intra- and interfamilial phenotypic variability, and advanced paternal age in sporadic cases. To date, >70 reports in the literature describe the clinical features of ODDD in >240 patients, the majority of whom were white (Gorlin et al. 2001; Loddenkemper et al. 2002). The typical craniofacial anomalies include a thin nose with hypoplastic alae nasi, small anteverted nares, prominent columnella, and microcephaly (fig. 1). Brittle nails and hair abnormalities of hypotrichosis and slow growth are present. Some cases have dysplastic ears and conductive hearing loss. Ophthalmic findings include microphthalmia, microcornea, fine porous spongy iris abnormalities, cataracts, glaucoma, and optic atrophy. Anomalies observed in the oral region are mandibular overgrowth and cleft palate. The majority of cases have abnormal primary and permanent dentition with microdontia, partial anodontia, enamel hypoplasia, multiple caries, and early tooth loss. Hand and foot abnormalities in ODDD include syndactyly involving the third, fourth, and fifth fingers and second to fourth toes, camptodactyly, and clinodactyly due to hypoplasia or aplasia of the middle phalanges. Other skeletal abnormalities are cranial hyperostosis, mandible with wide alveolar ridge, and broad tubular bones.
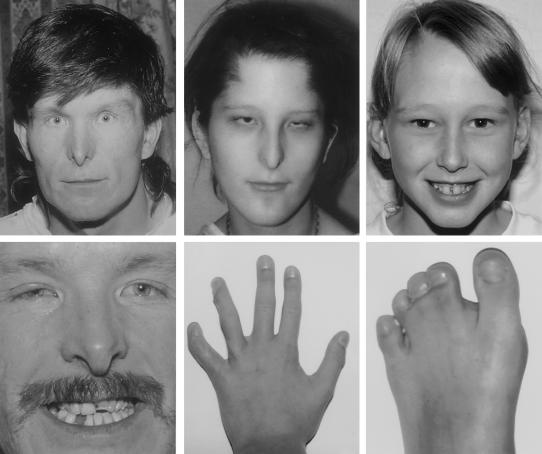
Figure 1.
ODDD phenotype. Across the top are an adult, a teenager, and a child with microcornea, narrow nose, and/or hypoplastic alae nasi. Across the bottom are additional features: partial ano- and microdontia and enamel hypoplasia, syndactyly, and clinodactyly. The child’s hand had surgical correction of the fourth and fifth finger syndactyly. Note that the child has a mild facial phenotype, but significant digital involvement, as shown in the bottom two right panels. The individuals in the top and bottom left panels are brothers with the Y17S mutation. The teenager has a G22E mutation, and the child has an Y98C mutation.
Neurologic symptoms are frequent in ODDD and include dysarthria, neurogenic bladder disturbances, spastic paraparesis, ataxia, anterior tibial muscle weakness, and seizures (Loddenkemper et al. 2002). Symptoms of spastic bladder or gait disturbances are the usual presenting neurologic manifestations evident by the 2nd decade of life. Mild mental retardation occurs infrequently. Brain magnetic resonance imaging studies of patients with ODDD have shown diffuse bilateral abnormalities in the subcortical cerebral white matter, which can define a slowly progressive leukodystrophy.
The ODDD locus was mapped to chromosome 6q22-q24 by linkage analysis (Gladwin et al. 1997), and the location of the disease gene was further refined between markers D6S266 and D6S1639 (Boyadjiev et al. 1999). A detailed physical map with a YAC contig of the region was created and fourteen candidate genes were identified and excluded by mutation analysis (Boyadjiev et al., in press; unpublished data). Another gene in the region is GJA1, which encodes gap junction protein alpha 1, a member of a large family of homologous connexin proteins.
GJA1 or connexin 43, like other connexin proteins, consists of an intracellular N-terminus, four transmembrane domains, two extracellular loops, one cytoplasmic loop, and an intracellular C-terminus (Kelsell et al. 2001). Six connexins can form a connexon, a specialized intracellular structure surrounding a pore. Two connexons in apposing cell membranes can align to form an intercellular gap junction. These channels provide a direct low-resistance intercellular pathway for the passage of ions and small molecules that confer distinct physiological properties. Gap junctions have been found in the majority of mammalian tissues. Most tissues express more than one type of connexin, and multiple types of connexins can assemble to form gap junctions between cells, with the diversity of combinations influencing the nature of the cell-to-cell communication. These properties are important for many physiological and developmental processes.
In mouse embryogenesis, Gja1 is expressed, beginning at the blastocyst stage, in discrete spatially restricted domains in the developing brain, neural tube, prevertebrae, and limb, and in various aspects of organogenesis (Ruangvoravat and Lo 1992). Its expression is associated with developmental processes mediated by inductive interactions, involving the branchial arches, eye, and otic vesicle, as well as migratory cells of the neural crest and sclerotomes. In postnatal tooth development, rat connexin 43 is demonstrated between the odontoblasts that actively secrete dentin matrix (Murakami et al. 2001), and its expression is seen in the epithelial-derived ameloblasts synthesizing and secreting enamel matrix proteins (Fried et al. 1996). A BLASTn search of the NCBI human est database, using the GJA1 mRNA sequence NM_000165, revealed GJA1 expression in libraries derived from placenta, brain, nerve, thyroid, lung epithelial cells, bone, bone marrow, testis, uterus, skin, and duodenal adenocarcinoma.
Despite the near-ubiquitous expression of the connexins, previous disorders associated with mutations in various connexins have a restricted range of phenotypic abnormalities, isolated deafness, skin abnormalities with or without deafness, or peripheral neuropathy. The human GJA1 gene is a candidate gene for the pleiotropic condition ODDD because of its map location and wide expression pattern. In this study, we screened the candidate gene GJA1 for mutations in families affected with ODDD.
Patients and Methods
We recruited seventeen unrelated white families affected with ODDD, with a total of 60 family members ranging in age from 2 wk to adulthood. All affected individuals had the typical ODDD craniofacial appearance and limb involvement. Members of nine of these families had neurologic manifestations. We obtained informed consent (protocol approved by the institutional review board of Johns Hopkins University) from all participants who underwent physical examination, photo documentation, and sample collection. DNA was isolated from patients’ blood samples or Epstein Barr virus–transformed lymphoblastoid cell lines. Centre d’Etude du Polymorphisme Humain (CEPH) DNAs were used as controls.
The organization of the GJA1 gene was determined from the human chromosome 6 sequence (map element NT_033944.2 in NCBI Sequence Viewer). The gene is composed of two exons with an intervening intron 11 kb in size. The mRNA sequence (NM_000165 or XM_027459) contains 191 bp of the first exon, which is untranslated. The second exon consists of 16 bp of additional 5′ untranslated sequence, 1,149 bp of coding sequence, and ∼1,732 bp of 3′ untranslated sequence. There are two potential poly (A) signal sites in the 3′ UTR at nucleotides 1759–1764 and at 3062–3067 of NM_000165 with the latter being utilized. There is a GJA1 pseudogene, GJA1P1, located within chromosome 5 at 5q21-5q22 (map element NT_006654.9).
PCR primers were designed with mismatches to the pseudogene sequence to specifically amplify GJA1 and avoid amplification of GJA1P1. A 925-bp product, which contains the coding sequence for the first 238 amino acids of the GJA1 protein, was amplified with forward primer 5′-GATCTTTTCTTCGTTGGC-3′ (within intron 1) and reverse primer 5′-CTCTTTCCCTTAACCCG-3′. A 1,079-bp product, which contains the coding region for the latter 178 amino acids of the GJA1 protein and extends into the 3′ UTR, was amplified with forward primer 5′-TTCCTCTCTCGCCCCAC-3′ and reverse primer 5′-GGCCTAGAAAGCTTACCTT-3′. Conditions of amplification were determined on a Bio-Rad Laboratories iCycler. All amplifications were performed using Platinum Taq High Fidelity DNA polymerase (Invitrogen). PCR products were sequenced in the forward and reverse directions by the dideoxy chain termination method on an ABI Prism 3700 automated fluorescent DNA analyzer (Applied Biosystems).
Six rare polymorphisms were observed within the 100 control alleles examined: IVS1-65G/A (1% incidence), IVS1-12T/A (1%), 717G/A (2%), 758C/T (1%), 11152_1153insA (2%), and 1322G/A (3%). Two of these were within the coding region, one resulting in a synonymous change, R239R (CGG→CGA), and the other resulting in A253V (GCG→GTG). The polymorphic amino acid change within the C-terminal domain, A253V, seen in one control allele, occurred in an amino acid that is not conserved among the GJA1 proteins of various species or among the human connexins. It should be noted that most of the variations listed in the GJA1 mRNA entry NM_000165 between nucleotides 160–1384 can be accounted for by nucleotide differences between GJA1 and its pseudogene and are not polymorphisms within GJA1.
Results and Discussion
GJA1 Mutations
We found mutations in 100% of the individuals studied who were affected with ODDD (table 1; fig. 2). A different missense mutation was found for each family, with the exception of a codon duplication in one family. These mutations segregated with the disease and were not found in any unaffected family members or in 100 control alleles from white subjects. The presence of different mutations in each family provides evidence that ODDD did not arise from a founder effect in the white population.
Table 1.
GJA1 Mutations in ODDD
| Family/Case | Inheritancea | NucleotideChangeb | Amino AcidChangeb | Reference(s)c |
| FOD1118-4881 | F* | 50A>C | Y17S | Rajic and deVeber 1966; Boyadjiev et al. 1999 |
| FOD1120-1902 | F* | 52T>C | S18P | Zellweger and Ionasescu 1974; Zach 1975; Judisch et al. 1979 |
| FODMS2630 | S* | 61G>A | G21R | This report |
| FODHN2441 | S | 65G>A | G22E | Traboulsi and Parks 1990 |
| FOD1083-4795 | S | 68A>C | K23T | Gorlin et al. 1963 |
| FODCL2603 | S* | 119C>T | A40V | This report |
| FODJC1858 | F* | 145C>A | Q49K | Boyadjiev et al. 1999 |
| FODLM1899 | F | 154_156dupTTT | F52dup | Gellis and Feingold 1974; Weintraub et al. 1975 |
| FOD1113-4855 | F* | 226C>A | R76S | Stanislaw et al. 1998; Boyadjiev et al. 1999 |
| FOD1140-4922 | F* | 268C>G | L90V | Mohr 1939; Opjordsmoen and Nyberg-Hansen 1980; Boyadjiev et al. 1999 |
| FOD1119-4896 | F* | 293A>G | Y98C | Wooldridge et al. 1977; Wooldridge 1993; Boyadjiev et al. 1999 |
| FODBC36590 | F* | 306G>C | K102N | This report |
| FODRB2514 | F | 389T>C | I130T | This report |
| FODOK2770 | S | 400A>G | K134E | This report |
| FOD975-4595 | F* | 412G>C | G138R | Shapiro et al. 1997; Boyadjiev et al. 1999 |
| FODHB00-153-0388 | F* | 605G>A | R202H | This report |
| FODJS2375 | F | 646G>T | V216L | Norton et al. 1995; Boyadjiev et al. 1999 |
F = familial; S = sporadic. An asterisk (*) denotes that more than one sample was available to document, in multiplex families, segregation of the mutation or, in sporadic cases, the absence of the mutation in both parents.
Mutation nomenclature is according to den Dunnen and Antonarakis (2000).
References are for families previously reported with clinical description or who have participated in linkage studies.
Figure 2.
Sequences of 17 GJA1 mutations found in families affected with ODDD. The top panels show the heterozygous mutations, with the exception of the sequence of the cloned duplication, 154_156dupTTT, and the bottom panels show the control sequences.
These amino acid changes are distributed throughout the protein, with the majority occurring in the amino terminal half (table 1; fig. 3). Mutations that produced pronounced changes—on the basis of differences in amino acid size and charge and lack of conservation among the connexins—are present in the cytoplasmic (Y17S, Y98C, I130T, and G138R), transmembrane (G21R and G22E), and extracellular (R76S) domains. Each mutation affects an amino acid highly conserved among the connexin 43 protein sequences for various species (fig. 4). Additionally, many of these same amino acids are conserved among the human connexin protein family, which are expressed from at least 20 different genes. Several of the amino acid alterations occur at the same residues that are mutated in other connexin (26, 31, and 32) disorders (table 2). The phenotypes do not correlate between those generated by connexin 43 mutations and by analogous amino acid mutations in other connexins. Multiple connexin 26 and 32 mutations in amino acids corresponding to connexin 43 R76 and R202 result in more than one phenotype.
Figure 3.
Diagram of the GJA1 protein. Locations of the amino acid changes are shown. The first and last amino acids of the protein and of each transmembrane domain are indicated as predicted by the program TMpred (see the TMpred Web site).
Figure 4.
Amino acid conservation in connexins. The amino acids of GJA1 that are affected in ODDD are aligned to the corresponding amino acids in gja1 in other species and in other human connexins. Accession numbers given (from NCBI's Entrez-Protein Web site) are for the versions used in the alignment. Connexin amino acid alignments were performed using the ClustalW program (see ClustalW Web site). Proteins are ordered according to homology. Gray shading indicates amino acid differences from the human GJA1 protein. Darker shading indicate amino acids observed only once in the connexins.
Table 2.
Connexin Mutations in Conserved Amino Acids[Note]
| Protein | Mutations | Phenotype | Reference | ||||||
| GJA1 (Cx43) | S18P | G22E | A40V | R76S | L90V | K134E | R202H | ODDD | This report |
| GJB2 (Cx26) | S17F | KIDS | Richard et al. 2002 | ||||||
| GJB2 (Cx26) | R75W | ADN-SHI and PPK | Richard et al. 1998a | ||||||
| GJB2 (Cx26) | R75Q | ADN-SHI | Connexin-Deafness Home Page | ||||||
| GJB2 (Cx26) | K122I | ARN-SSNHI | Connexin-Deafness Home Page | ||||||
| GJB2 (Cx26) | R184Q | ADN-SSNHI | Connexin-Deafness Home Page | ||||||
| GJB2 (Cx26) | R184P | ARN-SHI, compound heterozygote with delE138 | Connexin-Deafness Home Page | ||||||
| GJB2 (Cx26) | R184W | ARN-SHI, compound heterozygote with M34T | Connexin-Deafness Home Page | ||||||
| GJB3 (Cx31) | R180X | ADN-SHI | Connexin-Deafness Home Page | ||||||
| GJB1 (Cx32) | L89V | ADHI | Connexin-Deafness Home Page | ||||||
| GJB1 (Cx32) | G21D | A39P | R75P | L89P | R183C | CMT1X | CMT Mutations by Gene Web site | ||
| GJB1 (Cx32) | R75Q | R183H | CMT1X | CMT Mutations by Gene Web site | |||||
| GJB1 (Cx32) | R183S | CMT1X | CMT Mutations by Gene Web site | ||||||
| GJB1 (Cx32) | R75W | CMT1X and 48,XXYY | CMT Mutations by Gene Web site | ||||||
| GJB1 (Cx32) | A39V | CMT1X and CNS involvement | CMT Mutations by Gene Web site | ||||||
Note.— AD = autosomal dominant; AR = autosomal recessive; N-S = nonsyndromic; SN = sensorineural; HI = hearing impairment; PPK = palmoplantar keratoderma; CMTX1 = X-linked Charcot-Marie-Tooth syndrome.
Major Phenotypic Features (Syndactyly and Neurodegeneration) and GJA1 Genotype Analysis
Our mutation analysis supports clinical observations that ODDD is fully penetrant, since all carriers of mutations exhibited craniofacial and limb dysmorphisms. Intrafamilial variability of the major phenotypic characteristics was observed for all mutations segregating in multiplex families. For example, all of our probands have syndactyly involving at least the fourth and fifth fingers. Four individuals with sporadic disease have syndactyly type III (involvement of only the fourth and fifth fingers) and different missense mutations in the first transmembrane domain (G21R, G22E, K23T, and A40V). Two of our multiplex families have a proband with syndactyly type III but have other affected family members with additional digital involvement and mutations in domains beyond the first transmembrane (F52dup and R202H). These data suggest, as already stated in the literature, that ODDD and “isolated” syndactyly type III (MIM 186100) may represent a disease spectrum rather than separate genetic conditions (Schrander-Stumpel et al. 1993). The association of GJA1 mutations with abnormal limb development can be inferred by the strong expression of connexin 43 in the distal part of early limb buds and its restricted pattern in the developing digits and regions of precartilage condensation, as shown in Xenopus laevis, mouse, and chick (Ruangvoravat and Lo 1992; Meyers et al. 1997; van der Heyden et al. 2001).
It should also be noted from our data that specific phenotypic characteristics of ODDD did not appear to segregate with mutation(s) in specific protein domain(s). Thus, no obvious phenotype-genotype correlations were noted. As an example, nine of our families with neurologic symptoms, including five with documented white-matter degeneration, have mutations distributed in the N-terminal, membrane and intracellular domains (Y17S, G22E, K23T, L90V, K102N, I130T, K134E, G138R, and V216L). Epilepsy, which has been observed previously in ODDD, is present in two of our case subjects with the mutations R76S in the first extracellular loop and L90V in the second transmembrane domain. Two individuals with sporadic cases of ODDD and with G21R and A40V mutations do not manifest neurologic symptoms but are <2 years of age. It is of note that no neurologic symptoms were present among all affected adults in five of our affected families (S18P, Q49K, F52dup, Y98C, and R202H). Mutations in GJB1 (connexin 32), the major connexin of myelin-forming Schwann cells result in X-linked Charcot-Marie-Tooth disease (MIM 302800), a congenital demyelinating disorder of peripheral nerve (Bergoffen et al. 1993; Ressot and Bruzzone 2000). The type of degeneration in this disease differs from ODDD, since it involves myelin of peripheral nerves rather than myelin of the CNS.
In the CNS, connexin 43 is the major connexin of astrocytes and ependymocytes but is not expressed in oligodendrocytes or neurons. The role of connexin 43 in astrocyte gap junctions is not well understood, but they have been implicated in the propagation of cortical “calcium waves” and “spreading depression” (Martins-Ferrerira et al. 2000), and epilepsy is associated with cortical astrocytes with increased gap junction coupling (Lee et al. 1995). Astrocytes may also express limited quantities of other connexins, such as connexins 26, 30, and 45 (Dermietzel et al. 2000). Myelin-forming oligodendrocytes predominantly express connexin 32 but may also express connexin 45 (Dermietzel et al. 1997). Although astrocytes or oligodendrocytes form gap junctions with themselves, astrocytic-oligodendroglial gap junctions are also observed. The latter junctions may result from several possible heterotypic pairings, including connexin 43 with connexin 45 (Rash et al. 2001). Aberrant heterotypic pairings may be a molecular explanation of how mutations in connexin 43, which is normally expressed in astrocytes but not in oligodendroglial cells, can contribute to the dysfunction of CNS myelin observed in ODDD leukodystrophy.
Additional Phenotypic Features (Conductive Hearing Loss, Cataracts, Glaucoma, Keratoderma, and Cardiac Defects) and GJA1 Genotype Analysis
Eight of our families affected with ODDD have conductive hearing loss and mutations located in the N-terminal, transmembrane, and cytoplasmic loop regions (Y17S, S18P, G22E, K23T, L90V, Y98C, G138R, and V216L). GJA1 and other connexins are expressed in the cochlea (Rabionet et al. 2000), and mutations in other connexin proteins (connexin 32, 26, 31, and 30) have been associated with both autosomal dominant and autosomal recessive nonsyndromic hearing impairment, usually sensorineural. The type of hearing impairment in ODDD differs from the typical hearing loss associated with other connexin mutations, because it is conductive rather than sensorineural. Lui and his colleagues (2001) reported L11F and V24A mutations in connexin 43 in association with sensorineural recessive deafness. However, these mutations have subsequently been shown to involve the pseudogene of connexin 43 on chromosome 5 (W. E. Nance, personal communication).
One familial case had cataracts and the Y17S mutation. In the literature, there are mutations in GJA3 and GJA8 (connexins 46 and 50) that are found in patients with dominant zonular pulverulent cataracts (Shiels et al. 1998; Mackay et al. 1999). Glaucoma presented in this same individual with the Y17S mutation and in two other case individuals with G22E and L90V mutations. Connexin 43 plays an important role in eye development. It is expressed in the lens placode and during fetal stages in the anterior and lateral lens epithelium in the mouse, sheep, and chick (White 2002). Other connexins including connexin 46 and 50 are also expressed in the developing lens. In connexin 50 knockout mice, White et al. (1998) demonstrated that there was little if any prenatal effect, but postnatally two ocular abnormalities were present—microphthalmia and pulverulent cataracts. Similar features have been observed in ODDD patients. Connexin 43 is also localized in the outer pigmented cell layer of the ciliary body and pairs to the nonpigmented cell layer to form heterotypic junctions (Vaney et al. 2000). Glaucoma can result from an imbalance in intraocular fluid production by the ciliary body and outflow through the trabecular meshwork. Since connexin 43 deficient mice fail to thrive past birth, it can only be postulated that connexin 43 deletion or another type of mutation may unbalance connexin diversity and result postnatally in cataracts and glaucoma.
Some of our patients with ODDD exhibited phenotypic features rarely associated or not previously described for this condition. Two members of the family with the F52dup mutation had cleft palate. A familial case with the K102N mutation had plagiocephaly with no synostosis. One individual with sporadic ODDD had hyperkeratosis of the palms and soles and the K134E mutation. It is of interest that missense mutations in GJB2 (connexin 26) have been implicated in other skin conditions: palmar and plantar keratoderma with sensorineural deafness (Richard et al. 1998a), Vohwinkel syndrome (keratoderma, constriction of the digits, and sensorineural deafness) (Maestrini et al. 1999), and KIDS (keratitis, ichthyosis, and sensorineural deafness) (Richard et al. 2002). Mutations in GJB3 and GJB4 (connexin 31 and 30.3) can cause erythrokeratodermia variabilis (Richard et al. 1998b; Macari et al. 2000), and mutations in GJB6 (connexin 30) are present in patients with hidrotic ectodermal dysplasia (Lamartine et al. 2000).
Two of our families with ODDD had cardiac abnormalities. One individual with sporadic ODDD who had the G21R mutation had an atrioseptal defect. In addition, a familial case had recurrent ventricular tachycardia and an atrioventricular block with the I130T mutation. The latter proband’s paternal grandmother and father both died at 43 years of age, from cardiac arrhythmia and sudden cardiac death, respectively. Two paternal aunts have ventricular tachycardia and sick sinus syndrome. The proband and father have craniofacial features of ODDD. Although some of the family members with cardiac findings do not present with the craniofacial features of ODDD, they have not been screened for the mutation. The presence of congenital heart disease in ODDD has been noted previously. Schneider et al. (1977) reported a patient with an endocardial cushion defect, ostium primum atrial septal defect with a cleft mitral valve, which may result from a conotruncal heart defect. In addition, a heart murmur attributed to a ventricular septal defect was reported in an ODDD patient (Judisch et al. 1979).
The results of molecular studies are not clear as to the role that GJA1 mutations, rare or mosaic, may play in human cardiac malformations such as visceroatrial heterotaxia (Britz-Cunningham et al. 1995) and hypoplastic left heart (Dasgupta et al. 2001). However, it should be noted that a major site of expression of connexin 43 in mammals is cardiac muscle, where other connexins (37, 40, and 45) are also expressed (Kanter et al. 1993). Connexin 43 expression is not detected in the heart of day 8.5 mouse embryos, but it is quite prominent in the ventricle by day 10.5. Transgenic mice containing a cytomegalovirus (CMV) promoter driven connexin 43 construct caused Gja1 overexpression and conotruncal heart, right ventricle, pulmonary outflow tract, coronary vasculature, and neural tube defects (Ewart et al. 1997). The developmental abnormalities in the coronary vasculature are consistent with neural crest involvement, since deployment of the coronary vessels is dependent on neural crest–derived parasympathetic innervation in the heart. The Purkinje conduction fibers codistribute with the formation of coronary vasculature in avian embryos (Gourdie et al. 1995). Connexin 43 heterozygous knockout mice bred with connexin 40 null mice to yield haploinsufficiency of both genes show additive effects on ventricular (not atrial) conduction and cardiac morphogenesis of hypertrophy, in conjunction with atrioventricular junction or ventricular septal defects (Kirchhoff et al. 2000). The detection of the conduction system abnormalities in one of our families with ODDD may be consistent with altered expression of connexin 43. It is less clear whether the septal defect found in the other ODDD case mentioned above is directly related to connexin 43 dysfunction.
It was hypothesized that ODDD displayed the phenomenon of genetic anticipation (Shapiro et al. 1997). At least four ODDD families in the literature showed increasing severity of neurologic symptoms—such as white matter changes of the brain, spastic bladder, spastic paraplegia, and ataxia—in subsequent generations. Three families have shown more involvement of dysmorphic features, particularly syndactyly, in successive generations. However, mutation analysis of four of these families revealed changes (S18P, L90V, Y98C, and G138R) that cannot account for the genetic anticipation. Although unlikely, additional changes might be observed in the noncoding regions, which we did not screen, where many of the repeated trinucleotide sequences have been found for other disorders showing genetic anticipation. It is more likely that there are genetic and environmental modifiers that can account for the “observed” anticipation.
Relevance of Gja1 Null Mouse
Features observed in connexin 43 knockout mice are relevant but do not mimic the craniofacial, limb, or cardiac findings associated with ODDD. The lack of phenotypic correlation between the knockout mouse and ODDD suggests that loss of function of connexin 43 is not the mechanism by which missense mutations cause the human phenotype. For example, connexin 43 is the major gap junction protein present in osteoblasts (Civitelli et al. 1993). Lecanda et al. (2000) determined that, during embryonic development of connexin 43 null mutant mice, generalized intramembraneous and endochrondral bone development was delayed as a result of osteoblast dysfunction. At birth, these mice exhibited ossification defects of the skull, originating from hypoplastic, migratory neural crest cells, and mineralization defects in non–neural crest–derived bone. The mandible was small, with a rounded alveolar ridge and flattened arch that resulted in the development of small and pointed snouts and less prominent incisors. These features suggest that connexin 43 affects craniofacial development and normal osteogenesis, which is compatible with the ODDD phenotype. However, the craniofacial features are not consistent with those of ODDD, and limb abnormalities are lacking in the null mouse (Reaume et al. 1995; Lecanda et al. 2000). Furthermore, cardiac malformations involving blockage of the right ventricular outflow tract, present in the null mice (Reaume et al. 1995; Guerrero et al. 1997), are not obvious in the ODDD phenotype.
Potential Functional Consequences of Mutant GJA1
Mutations in the connexins can have variable functional consequences, which explains how mutations in different connexins or different mutations in the same connexin can cause an isolated or pleiotropic phenotype. In general, the deletion of one copy of a connexin gene does not appear to be of major consequence, as can be seen in animal-model knockouts as well as in human conditions that are recessive in nature. Deletion of both copies will be of consequence in cells and tissues where this connexin is vital and/or there is a lack of other connexins of compensatory function. In the case of missense mutations, cellular function of gap junctions may be affected in a number of ways. One possibility is that the protein alteration may cause mislocation to the cytoplasm and, for recessive mutations, failure to form connexons (either homotypic or of the appropriate heterotypic composition). Other protein alterations may allow mutant connexon formation, yet may disallow the formation of gap junctions with connexons of other cells. Lastly, some alterations may allow complete gap junctions to form across cells, but their properties of conductance, molecular permeability, phosphorylation, and voltage gating are significantly altered, and cellular function is affected either as a dominant negative or gain of function. In this regard, it is notable that five ODDD missense mutations (Y17S, S18P, G21R, G22E, and K23T) are clustered within a region homologous to a calmodulin-binding motif of connexin 32 (Peracchia et al. 2000). Gating of gap junctions by calmodulin is believed to occur in response to changes in intracellular calcium.
Pal et al. (1999) demonstrated that a connexin 50 mutation, P88S, failed to form functional gap junctions, as measured by conductance in Xenopus oocytes, when paired homotypically, and was able to inhibit the functional form of wild type connexin 50 in a dose-dependent fashion; Pal et al. concluded that only one mutant subunit per gap junction was necessary to abolish channel function. Richard et al. (1998a) had previously shown that the connexin 26 autosomal dominant mutation R75W (analogous to connexin 43 residue R76, which is mutated to a serine in ODDD) (table 2) also acted in a dominant-negative fashion against wild-type connexin 26; however, the autosomal recessive mutant W77R, though failing to form functional gap junctions itself, did not greatly interfere with wild-type connexin as measured by conductance in Xenopus oocytes. Rouan et al. (2001) further demonstrated trans-dominant inhibition of connexin 43 by mutant connexin 26 in Xenopus oocytes. Richard et al. (2002) showed lack of coupling and dye transfer in HeLa cells expressing mutant connexin 26 S17F (analogous to connexin 43 residue S18, which is mutated to a proline in ODDD) (table 2) and proposed a trans-dominant inhibition of connexin 30.
One can, then, speculate that the GJA1 missense mutations found in ODDD have a dominant negative effect. A single hit that alters the function of the mutant allele causes the pleiotropic phenotype of ODDD because of the wide expression pattern of GJA1, and this allele may then cause aberrant connexons with the wild-type allele and other connexins. However, we cannot exclude the possibility that these missense mutations result in gain of function. Additionally, there must be modifiers, perhaps in different racial groups that enhance the degree of pleiotropy and variability of expression observed in ODDD. The complex combinatorial interactions among the connexins suggest that additional mutational analysis of connexins other than connexin 43 in ODDD patients may give us insights into which specific connexins may modify the functional consequences of the GJA1 missense mutations. Also, future studies using GJA1 site-directed mutagenesis in combination with other normal or mutant connexins in cellular and animal model systems will shed light on how the observed missense mutations contribute to the pathogenesis of ODDD.
Acknowledgments
We thank the families with ODDD who participated in this research effort and James Casey, Norma Chow, Chong Ae Kim, Alan P. Mersch, Robert Saul, Torberg Torbergsen, and Robert Gorlin, for their patient referrals and interest in our research. We thank George X. Zhang, David L. Ash, and Cristi Radford for technical assistance. The work was supported by National Institutes of Health grants R01 DE13849, R01 HD24061, and M01 RR0052.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- ClustalW, http://www.ebi.ac.uk/clustalw/ (for ClustalW program)
- CMT Mutations by Gene, http://molgen-www.uia.ac.be/CMTMutations/DataSource/MutByGene.cfm
- Connexin-Deafness Home Page, http://www.crg.es/deafness
- Entrez-Protein, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=protein (for accession numbers in )
- NCBI Sequence Viewer, http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?val=22056350 (for human chromosome 6 sequence)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for ODDD [MIM *164200], “isolated” syndactyly type III [MIM 186100], and X-linked Charcot-Marie-Tooth disease [MIM 302800])
- TMpred Server, http://www.ch.embnet.org/software/TMPRED_form.html (for TMpred program)
References
- Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH (1993) Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262:2039–2042 [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA, Jabs EW, LaBuda M, Jamal JE, Torbergsen T, Ptacek LJ 2nd, Rogers RC, Nyberg-Hansen R, Opjordsmoen S, Zeller CB, Stine OC, Stalker HJ, Zori RT, Shapiro RE (1999) Linkage analysis narrows the critical region for oculodentodigital dysplasia to chromosome 6q22-q23. Genomics 58:34–40 [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA, Chowdry AB, Shapiro RE, Paznekas WA, Wandstrat AE, Choi JW, Kasch L, Zhang G, Wollnik B, Burgess CE, Schalling M, Lovett M, Jabs EW. Physical map of the chromosome 6q22 region containing the oculodentodigital dysplasia locus: analysis of thirteen candidate genes and identification of novel ESTs and DNA polymorphisms. Cytogenet Genome Res (in press) [DOI] [PubMed] [Google Scholar]
- Britz-Cunningham SH, Shah MM, Zuppan CW, Fletcher WH (1995) Mutations of the connexin43 gap-junction gene in patients with heart malformations and defects of laterality. N Engl J Med 332:1323–1329 [DOI] [PubMed] [Google Scholar]
- Civitelli R, Beyer EC, Warlow PM, Robertson AJ, Geist ST, Steinberg TH (1993) Connexin 43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest 91:1888–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta C, Martinez A-M, Zuppan CW, Shah MM, Bailey LL, Fletcher WH (2001) Identification of connexin43 (α1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE). Mut Res 479:173–186 [DOI] [PubMed] [Google Scholar]
- den Dunnen JT, Antonararkis SE (2000) Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 15:7–12 [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Farooq M, Kessler JA, Althaus H, Hertzberg EL, Spray DC (1997) Oligodendrocytes express gap junction proteins connexin32 and connexin45. Glia 20:101–114 [PubMed] [Google Scholar]
- Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MV, Spray DC (2000) Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Rev 32:45–56 [DOI] [PubMed] [Google Scholar]
- Ewart JL, Cohen MF, Meyer RA, Huang GY, Wessels A, Gourdie RG, Chin AJ, Park SM, Lazatin BO, Villabon S, Lo CW (1997) Heart and neural tube defects in transgenic mice overexpressing the Cx43 gap junction gene. Development 124:1281–1289 [DOI] [PubMed] [Google Scholar]
- Fried K, Mitsiadis TA, Guerrier A, Haegerstrand A, Meister B (1996) Combinatorial expression patterns of the connexins 26, 32, and 43 during development, homeostasis, and regeneration of rat teeth. Int J Dev Biol 40:985–995 [PubMed] [Google Scholar]
- Gellis SS, Feingold M (1974) Oculodentodigital dysplasia. Am J Dis Child 128:81–82 [DOI] [PubMed] [Google Scholar]
- Gladwin A, Donnai D, Metcalfe K, Schrander-Stumpel C, Brueton L, Verloes A, Aylsworth A, Toriello H, Winter R, Dixon M (1997) Localization of a gene for oculodentodigital syndrome to human chromosome 6q22-q24. Hum Mol Genet 6:123–127 [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, Meskin LH, Geme JW (1963) Oculodentodigital dysplasia. J Ped 63:69–75 [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, Cohen MM Jr, Hennekam RCM (2001) Oculodentoosseous dysplasia (oculodentodigital syndrome). In: Syndromes of the head and neck, 4th ed. Oxford University Press, New York, pp 290–292 [Google Scholar]
- Gourdie RG, Mima T, Thompson RP, Mikawa T (1995) Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development 121:1423–1431 [DOI] [PubMed] [Google Scholar]
- Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA (1997) Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest 99:1991–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judisch GF, Martin-Casals A, Hanson JW, Olin WH (1979) Oculodentodigital dysplasia; four new reports and a literature review. Arch Ophthal 97:878–884 [DOI] [PubMed] [Google Scholar]
- Kanter HL, Laing JG, Beyer EC, Green KG, Saffitz JE (1993) Multiple connexins colocalize in canine ventricular myocyte gap junctions. Circ Res 73:344–350 [DOI] [PubMed] [Google Scholar]
- Kelsell DP, Dunlop J, Hodgins MB (2001) Human diseases: clues to cracking the connexin code. Trends Cell Biol 11:2–6 [DOI] [PubMed] [Google Scholar]
- Kirchhoff S, Kim J-S, Hagendorff A, Thonnissen E, Kruger O, Lamers WH, Willecke K (2000) Abnormal cardiac conduction and morphogenesis in Connexin40 and Connexin43 double-deficient mice. Circ Res 87:399–405 [DOI] [PubMed] [Google Scholar]
- Lamartine J, Essenfelder GM, Kibar Z, Lanneluc I, Callouet E, Laoudj D, Lemaitre G, Hand C, Haylick SJ, Zonana J, Antonarakis S, Radhakrishna U, Kelsell DP, Christianson AL, Pitaval A, Der Kaloustian V, Fraser C, Blanchet-Bardon C, Rouleau GA, Waksman G (2000) Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat Genet 26:142–144 [DOI] [PubMed] [Google Scholar]
- Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R (2000) Connexin 43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol 151:931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Magge S, Spencer DD, Sontheimer H, Cornell-Bell AH (1995) Human epileptic astrocytes exhibit increased gap junction coupling. Glia 15:195–202 [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xia XJ, Adams J, Chen ZY, Welch KO, Tekin M, Ouyang XM, Kristiansen A, Pandya A, Balkany T, Arnos KS, Nance WE (2001) Mutations in GJA1 (connexin 43) are associated with non-syndromic autosomal recessive deafness. Hum Mol Genet 10:2945–2951 [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Grote K, Evers S, Oelerich M, Stogbauer F (2002) Neurological manifestations of the oculodentodigital dysplasia syndrome. J Neurol 249:584–595 [DOI] [PubMed] [Google Scholar]
- Macari F, Landau M, Cousin P, Mevorah B, Brenner S, Panizzon R, Schorderet DF, Hohl D, Huber M (2000) Mutation in the gene for connexin 30.3 in a family with erythrokeratodermia variabilis. Am J Hum Genet 67:1296–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattacharya S (1999) Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet 64:1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestrini E, Korge BP, Ocana-Sierra J, Calzolari E, Cambiaghi S, Scudder PM, Hovnanian A, Monaco AP; Munro CS (1999) A missense mutation in connexin 26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel's syndrome) in three unrelated families. Hum Molec Genet 8:1237–1243 [DOI] [PubMed] [Google Scholar]
- Martins-Ferreira H, Nedergaard M, Nicholson C (2000) Perspectives on spreading depression. Brain Res Rev 32:215–234 [DOI] [PubMed] [Google Scholar]
- Meyers RA, Cohen MF, Recalde S, Zakany J, Bell SM, Scot WJ Jr, Lo CW (1997) Developmental regulation and asymmetric expression of the gene encoding Cx43 gap junctions in the mouse limb bud. Dev Genet 21:290–300 [DOI] [PubMed] [Google Scholar]
- Mohr O (1939) Dominant acrocephalosyndactyly. Hereditas 25:193–203 [Google Scholar]
- Murakami S, Muramatsu T, Shimono M (2001) Expression and localization of connexin 43 in rat incisor odontoblasts. Anat Embryol 203:367–374 [DOI] [PubMed] [Google Scholar]
- Norton KK, Cary JC, Gutmann DH (1995) Oculodentodigital dysplasia with cerebral white matter abnormalities in a two-generation family. Am J Med Genet 57:458–461 [DOI] [PubMed] [Google Scholar]
- Opjordsmoen S, Nyberg-Hansen R (1980) Hereditary spastic paraplegia with neurogenic bladder disturbances and syndactylia. Acta Neurol Scandinav 61:35–41 [DOI] [PubMed] [Google Scholar]
- Pal JD, Berthoud VM, Beyer EC, Mackay D, Shiels A, Ebihara L (1999) Molecular mechanism underlying a Cx50-linked congenital cataract. Am J Physiol 276:C1443–C1446 [DOI] [PubMed] [Google Scholar]
- Peracchia C, Sotkis A, Wang XG, Peracchia LL, Persechini A (2000) Calmodulin directly gates gap junction channels. J Biol Chem 275:26220–26224 [DOI] [PubMed] [Google Scholar]
- Rabionet R, Gasparini P, Estivill X (2000) Molecular genetics of hearing impairment due to mutations in gap junction genes encoding beta connexins. Hum Mutat 16:190–202 [DOI] [PubMed] [Google Scholar]
- Rajic DS, deVeber LL (1966) Hereditary oculodentoosseous dysplasia. Ann Radiol 9:224–231 [Google Scholar]
- Rash JE, Yasumura T, Davidson KG, Furman CS, Dudek FE, Nagy JI (2001) Identification of cells expression Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Adhes Commun 8:315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J (1995) Cardiac malformation in neonatal mice lacking connexin 43. Science 267:1831–1834 [DOI] [PubMed] [Google Scholar]
- Ressot C, Bruzzone R (2000) Connexin channels in Schwann cells and the development of the X-linked form of Charcot-Marie-Tooth disease. Brain Res Rev 32:192–202 [DOI] [PubMed] [Google Scholar]
- Richard G, White TW, Smith LE, Bailey RA, Compton JG, Paul DL, Bale SJ (1998a) Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Hum Genet 103:393–399 [DOI] [PubMed] [Google Scholar]
- Richard G, Smith LE, Bailey RA, Itin P, Hohl D, Epstein EH Jr, DiGiovanna JJ, Compton JG, Bale SJ (1998b) Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nat Genet 20:366–369 [DOI] [PubMed] [Google Scholar]
- Richard G, Rouan F, Willoughby CE, Brown N, Chung P, Ryynanen M, Jabs EW, Bale SJ, DiGiovanna JJ, Uitto J, Russell L (2002) Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet 70:1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangvoravat CP, Lo CW (1992) Connexin 43 expression in the mouse embryo: localization of transcripts within developmentally significant domains. Dev Dyn 194:261–281 [DOI] [PubMed] [Google Scholar]
- Rouan F, White TW, Brown N, Taylor AM, Lucke TW, Paul DL, Munro CS, Uitto J, Hodgins MB, Richard G (2001) trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. J Cell Sci 114:2105–2113 [DOI] [PubMed] [Google Scholar]
- Schneider JA, Shaw GG, Van Reken DE (1977) Congenital heart disease in oculodentodigital dysplasia. Va Med 104:262–263 [PubMed] [Google Scholar]
- Schrander-Stumpel CT, De Groot-Wijnands JB, De Die-Smulders C, Fryns JP (1993) Type III syndactyly and oculodentodigital dysplasia: a clinical spectrum. Genet Couns 4:271–276 [PubMed] [Google Scholar]
- Shapiro RE, Griffin JW, Stine OC (1997) Evidence for genetic anticipation in the oculodentodigital syndrome. Am J Med Genet 71:36–41 [PubMed] [Google Scholar]
- Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S (1998) A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet 62:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw CL, Narvaez C, Rogers RG, Woodard CS (1998) Oculodentodigital dysplasia with cerebral white matter abnormalities: an additional case. Proc Greenwood Genet Center 17:20–24 [Google Scholar]
- Traboulsi EI, Parks MM (1990) Glaucoma in oculo-dento-osseous dysplasia. Am J Ophthalmol 109:310–313 [DOI] [PubMed] [Google Scholar]
- van der Heyden MAG, Roeleveld L, Peterson J, Destree OHJ (2001) Connexin43 expression during Xenopus development. Mech Dev 108:217–220 [DOI] [PubMed] [Google Scholar]
- Vaney DI, Weiler R (2000) Gap junctions in the eye: evidence for heteromeric, heterotypic and mixed-homotypic interactions. Brain Res Rev 32:115–120 [DOI] [PubMed] [Google Scholar]
- Weintraub DM, Baum JL, Pashayan HM (1975) A family with oculodentodigital dysplasia. Cleft Palate J 12:323–329 [PubMed] [Google Scholar]
- White TW (2002) Unique and redundant connexin contributions to lens development. Science 295:319–320 [DOI] [PubMed] [Google Scholar]
- White TW, Goodenough DA, Paul DL (1998) Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol 143:815–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge WE, Anthony DD, Olson ER, Bates GP, Sammon TJ (1977) Oculodentodigital dysplasia. Mol Med 74:379–383 [PubMed] [Google Scholar]
- Wooldridge WE (1993) Oculodentodigital dysplasia. In: Demis DJ (ed) Clinical dermatology. Harper and Row, New York pp 1–4 [Google Scholar]
- Zach GA (1975) Oculodento-osseus dysplasia syndrome. Oral Surg 40:122–125 [DOI] [PubMed] [Google Scholar]
- Zellweger H, Ionasescu V (1974) Familial oculo-dento-digital dysplasia. Am J Hum Genet 26:97A [Google Scholar]