Abstract
Infection with bovine papillomavirus type 1 (BPV1) or BPV2 induces fibropapillomas in cows and skin sarcoids in horses. Prophylactic vaccination targeting BPV1 and BPV2 may reduce the incidence of these economically important diseases. The L1 major capsid proteins of BPV1 and BPV2 were expressed in Sf-9 insect cells and both self-assembled into virus-like particles (VLPs). Using conformation-dependent monoclonal antibodies (mAb) both type-specific and shared epitopes were detected. Antisera were raised against BPV1 or BPV2 VLP using alum adjuvant, and their (cross)neutralization capacity was tested by C127 neutralization assays using native BPV1 and BPV2 virions, or by BPV1 pseudovirion assay. Antisera induced by either VLP vaccine were able to robustly (cross-)neutralize heterologous as well as homologous types, indicating that BPV1 and BPV2 are closely related serotypes. These results suggest that a monovalent BPV1 (or BPV2) VLP vaccine may potentially protect against both BPV1 and BPV2 infections and associated diseases.
Keywords: Bovine papillomavirus (BPV), Vaccine, Sarcoids, Fibropapilloma, Serotypes
Introduction
Papillomaviruses (PV) comprise a large family of small DNA tumor viruses that infect the epithelia of skin and mucosa of many animal species and humans causing benign hyperproliferations, papillomas or warts. Respective ten bovine papillomavirus types (BPV1-10) have been characterized, 9 of which cause papillomas of skin and teats (BPV1-3 and BPV5-10), whereas BPV4 causes tumors of the upper gastrointestinal tract in animals feeding bracken fern (Nasir and Campo, 2008). The most important types BPV1 and BPV2 (genus delta) are unusual in their ability to infect both the epithelial cells and the fibroblasts of the underlying dermis, causing fibropapillomas of the skin, teats and udders, as well as urinary bladder cancer in the cow (Nasir and Campo, 2008). Similar to other papillomaviruses BPV1/2 replication and virion production is confined to the epithelial portion of the lesion, while fibroblastic infection is non-productive. Lesions normally regress as a result of a cellular immune response, which appears to protect against re-infection with that type. However, in some animals papillomas persist or even widely spread due to the inability to reject the infection, thus necessitating slaughtering of the animal. In addition, BPV1/2 may persist as latent infection and become reactivated by immunosuppression and/or physical trauma (Campo et al., 1994). Similar to human papillomavirus (HPV) infections (Frazer, 2009) genetic host factors that control innate immunity and effector T-cell responses may determine the increased risk of persisting infection.
The only known natural infection outside the primary host is infection of horses and other ungulates with BPV1 or BPV2 causing the development of sarcoids, a non-productive chronic infection of the skin. The ability of BPV1 and BPV2 to non-productively transform non-epithelial cells is not species-specific, although the increased host range is limited to fibroblasts as further shown by the induction of experimental fibroblastic tumors in hamsters, and focal transformation of cultured rodent cells (Howley and Lowy, 2007). Sarcoids are locally invasive fibroblastic skin tumors with reported prevalence from 13–67%, representing the most common equine neoplasm (Nasir and Campo, 2008). Genomic DNA of BPV1 or BPV2 can be detected episomally (non-integrated into the host genome) in the vast majority of these tumors. Currently, there is no effective therapy for sarcoids. Thus worldwide sarcoids result in significant economic losses both in the agrarian economy of developing countries (by affecting donkeys at an estimated 0.6 cases/animal/year) and the horse industry (Nasir and Campo, 2008).
Similar to results in later HPV vaccine studies, prophylactic vaccination with recombinantly expressed L1 virus-like particles (VLPs) of BPV4, which is not closely related to BPV1 or BPV2, protected cows against experimental BPV4 challenge with high efficacy, but had no therapeutic effect on established tumors (Kirnbauer et al., 1996). Efficacy of VLP vaccination is correlated with the induction of neutralizing antibodies, which are mainly restricted to the types present in the vaccine, but may partially extend to closely related types (Brown et al., 2009; Wheeler et al., 2009).
BPV1 and BPV2 are distinct genotypes, as defined by their 84% nucleotide homology of the L1 gene. Their L1 amino acid sequence identity reaches 92%, which is comparable to the similarity of HPV6/11 (92%) or HPV18/45 (87%). Limited studies have been performed on the serological relationship between BPV1 and BPV2 (Dvoretzky et al., 1980). However, the ability of a BPV1 or BPV2 VLP vaccine to induce cross-neutralization of the respective heterologous type has not been examined.
A prophylactic VLP-based vaccine to prevent equine sarcoids should prevent both BPV1 and BPV2 infections. Here we evaluated the serotypes of BPV1 and BPV2 by probing the capsids with neutralizing monoclonal and polyclonal antibodies. In addition, neutralization assays were performed with homologous and heterologous anti-VLP antisera using infectious virions in focus-forming neutralization assays, or using a recently available BPV1 pseudovirion assay. The results demonstrate that BPV1 and BPV2 are closely related serotypes, indicating that a monovalent BPV1 (or BPV2) VLP vaccine may suffice to protect against both BPV1 and BPV2 infections and associated diseases.
Results and discussion
BPV1 and BPV2 VLPs partly share neutralization epitopes
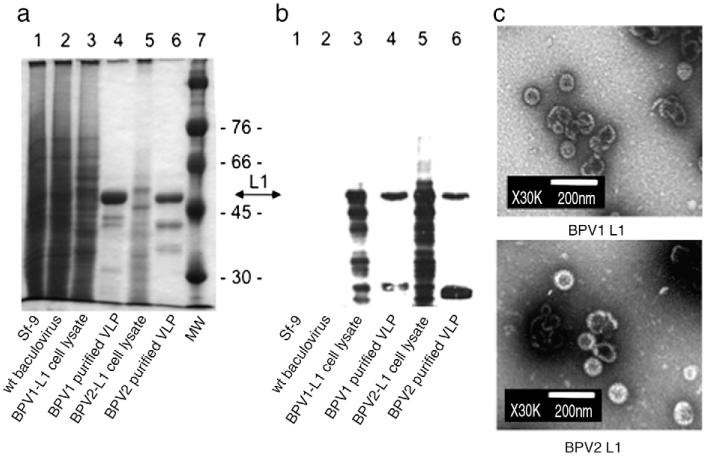
BPV1 VLPs were generated by expression of L1 in insect cells and purified on density gradients as described previously (Kirnbauer et al., 1992). To generate BPV2 VLP, the L1 open reading frame (ORF) was amplified from genomic BPV2 DNA in an analogous manner, cloned into the baculovirus expression vector pEVmod, and verified by sequencing. Following the generation of recombinant baculoviruses, BPV2 L1 was expressed in Sf-9 insect cells and verified by SDS-PAGE and Coomassie staining, and Western blotting using the non-neutralizing monoclonal antibody (mAb) AU-1 (raised against BPV1). AU-1 recognizes the linear BPV1-L1 epitope DTYRYI, which is predicted to also exist in BPV2-L1. As expected by their close homology, purified BPV1 and BPV2-L1 proteins migrated similarly as 55 kD proteins on a Coomassie stained gel (Fig. 1a, lanes 4 and 6). Western blotting demonstrated a major immunoreactive band of 55 kD in cell lysates and purified VLP preparation for both BPV types (Fig. 1b, lanes 3–6), as predicted, but not in control lysates of uninfected or wild type baculovirus-infected Sf-9 cells (Fig. 1b, lanes 1 and 2). Smaller bands likely represent degraded proteins, as observed for over-expressed L1 proteins of various papillomavirus types. Purified BPV1 and BPV2 preparations were further examined by negative staining and transmission electron microscopy (TEM), demonstrating self-assembly into VLP of about 60 nm in diameter and a morphology similar to other papillomavirus types (Fig. 1c).
Fig. 1. Expression of major capsid protein L1 of BPV1 and BPV2 in Sf-9 insect cells.
L1 of BPV1 and BPV2 were expressed by recombinant baculoviruses and VLPs were purified by density gradient centrifugation. Crude cell lysates and purified VLPs were separated by SDS-PAGE and visualized by Coomassie staining (a), or Western blotting (b) using mAb AU-1 raised against BPV1 L1 (at a dilution of 1/10,000). Both L1 proteins migrated as 55 kD proteins. Faster migrating immunoreactive bands likely represent degradation products. Molecular weight (MW) markers are indicated. Non-infected (Sf-9) or wt baculovirus-infected cells served as controls. For transmission electron microscopy (TEM) (c) purified VLPs were negatively stained with 1% uranylacetate and visualized at a magnification × 30,000. Scale bar represents 200 nm.
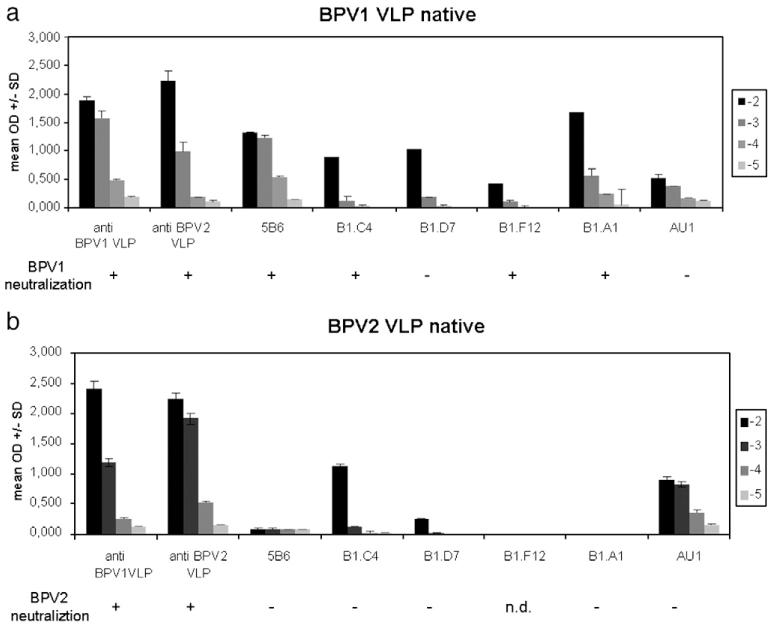
The major neutralization epitopes of papillomaviruses are conformation-dependent and located on the surface loops of the assembled L1 capsid or pentamers. To examine if BPV1 and BPV2 VLPs share cross-reactive epitopes, ELISA were performed under non-denaturing (native) conditions (Fig. 2). Sera of rabbits vaccinated with L1 VLP of either BPV1 or BPV2 were analyzed for their ability to react to the homologous and heterologous types. In addition, VLPs were probed with mAb to conformation-dependent epitopes of BPV1. Particles were dispersed into the wells and incubated in triplicates with 10-fold serial antibody dilutions ranging from 10−2 to 10−5 (Figs. 2a, b). Both antisera recognized BPV1 and BPV2 VLPs by ELISA with a titer of 10,000, indicating, as expected, that antibodies can be generated in rabbits that cross-react to the respective heterologous type. However, it is likely that at least part of the observed cross-reactivity may be attributable to linear non-neutralizing epitopes that are exposed on partially disassembled VLP or denatured protein. This is in agreement with the reactivity for both BPV types to AU-1, which recognizes a linear L1 epitope (Figs. 1, 2) (Ghim, Young, and Jenson, 1996). MAb B1.C4, B1.D7, and B1.F12 have been raised to BPV1 VLP, and mAb B1. A1 and 5B6 have been raised to BPV1 virions. As shown in Figs. 2a, b, two types of antibody binding pattern were observed. MAb B1.C4 and B1.D7 recognize both BPV1 and BPV2-L1 VLPs, with B1.D7 being less reactive to BPV2 as compared to BPV1. In contrast, 5B6, B1.F12, and B1.A1 reactivity was restricted to BPV1 VLP. These results indicated that BPV1 and BPV2 VLPs share conformation-dependent epitopes that are detectable by some mAb on both VLPs. In addition, other epitopes exist that are type-specific as shown by mAb reactivity restricted to BPV1.
Fig. 2. Serologic analysis of BPV1 and BPV2 by VLP ELISA and virion neutralization assays.
BPV1 VLP (a) or BPV2 VLP (b) were analyzed under non-denaturing (native) conditions using antisera raised to BPV1-L1 and BPV2-L1 VLPs, and mAb directed to conformational BPV1 epitopes. VLPs were incubated with 10-fold serial dilutions (10−2 to 10−5) of antibodies. Data are expressed as mean OD±SD of triplicate wells. Neutralization capacity of antibodies at final dilution of 1:100 was determined by C127 focus-forming neutralization assays using BPV1 (a) or BPV2 (b) native virions. +, Neutralizing, −, non-neutralizing; n.d., not determined.
BPV1/BPV2 virion and BPV1 pseudovirion neutralization assays
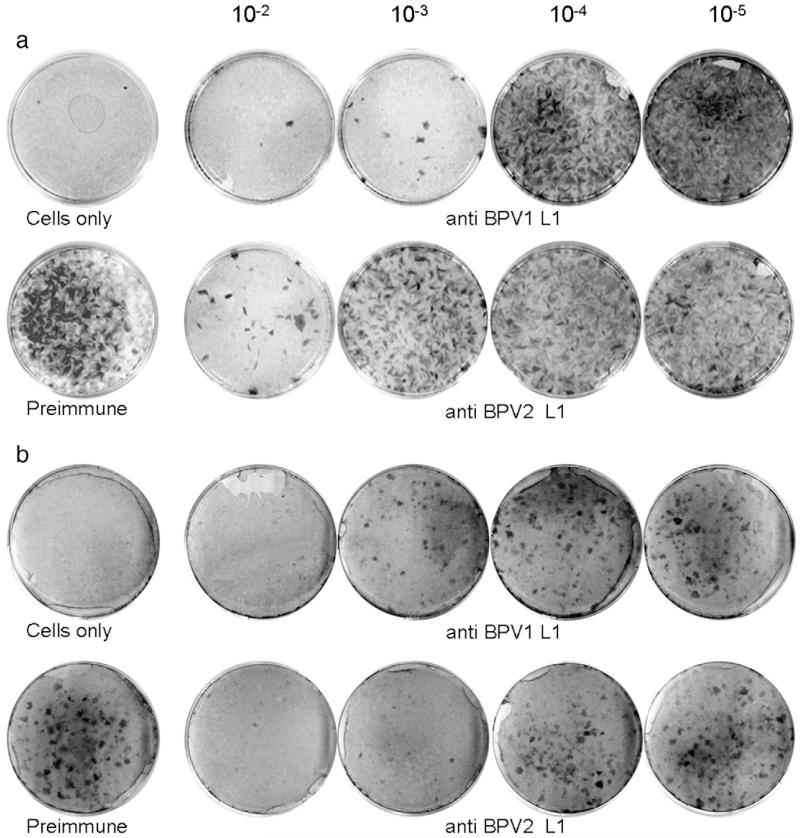
Efficacy of papillomavirus vaccines correlates with their capacity to induce neutralizing serum antibodies, which are sufficient to protect against experimental infection (Breitburd et al., 1995; Suzich et al., 1995). Thus we next determined whether antisera induced by VLP vaccination were neutralizing against the respective homologous and heterologous types. Infectious BPV1 or BPV2 virions were incubated with antisera raised against homologous or heterologous VLP type for 1 h before infection of C127 mouse fibroblasts. Following 3 weeks of maintenance, cultures were stained to detect focally transformed cells indicating successful infectious events (Dvoretzky et al., 1980). As shown in Fig. 3a, innumerable foci developed in cultures infected with BPV1 virions incubated with preimmune serum. In contrast anti-BPV1 serum neutralized with a titer of 1000, whereas anti BPV2 antisera neutralized with a titer of 100 (≥ 50% reduction of the number of foci obtained with preimmune serum). Likewise, when analyzed for BPV2 neutralization (Fig. 3b) both anti-BPV1 and anti-BPV2 sera neutralized with a titer of 1000 (one representative of at least 3 independent experiments for both types is shown). As shown previously, antisera raised against denatured VLP or heterologous HPV16 VLP do not neutralize BPV1 virions (Kirnbauer et al., 1992; Roden et al., 1996). We also employed BPV1 pseudovirions (Buck et al., 2005) for testing rabbit antisera to BPV2- and BPV1-L1 VLPs for neutralization capacity. Antisera to BPV1 and BPV2 VLPs (cross)neutralized BPV1 pseudovirions with a titer of 1000 and 100, respectively, whereas an anti-HPV16 VLP antiserum used as control was non-neutralizing (not shown).
Fig. 3. Comparison of antisera to BPV1 and BPV2 VLPs by focus-forming neutralization assays.
Infectious BPV1 (a) or BPV2 virions (b) purified from cow warts were pre-incubated with immune sera, plated onto C127 fibroblasts, maintained for 3 weeks with regular feeding, and stained. Control plates received either no virus (cells only) or virus incubated with preimmune serum at 10−2 dilution. Antisera raised against BPV1- or BPV2-L1 VLP were serially diluted 10-fold from 10−2 to 10−5 as indicated. Neutralization titers are scored visually as the reciprocal of the highest serum dilution that results in ≥50% reduction in the number of foci obtained with preimmune serum.
These results show that both BPV1 and BPV2 VLPs can induce neutralizing antisera to their homologous type, as well as cross-neutralization to the heterologous type. Given that the neutralizing activity for both BPV1 VLP and BPV2 VLP antisera is no more than one order of magnitude less against the heterologous virus compared with that against the homologous virus, this clearly indicated that BPV1 and BPV2 are closely related serotypes. An implication of these results is that a monovalent vaccine, composed of VLPs from BPV1 or BPV2, is likely to confer a similar degree of protection against the heterologous virus as against the homologous virus.
We next determined the relative capacity of the mAb to BPV1 to neutralize infectious BPV1 and BPV2. Native virions were extracted from two different cow warts and viral DNAs genotyped by L1 sequencing. Results indicated the presence of BPV1 or BPV2, respectively, with similar degree of nucleotide relatedness as those in GenBank (not shown). As indicated by focus-forming neutralization assays, mAb 5B6, B1.C4, B1.F12, and B1.A1 (dilution 1:100) neutralized BPV1 virions, whereas B1.D7 was non-neutralizing (Fig. 2a). In contrast, none of the mAb was able to neutralize BPV2 (not tested for B1.F12), whether or not they were reactive to BPV2 VLP by ELISA (Fig. 2b). The type-specificity of the monoclonal as compared to the polyclonal antibodies reflects the differences in epitope diversity recognized by these two reagents. Monoclonal antibodies recognize a single epitope, and a single amino acid change within this epitope can eliminate this binding (as we have shown in the case of mAb H16.E70 binding to HPV16 L1 of one variant but not another (Roden et al., 1997)). It is noteworthy that the principal immunodominant neutralizing epitopes of L1 contain the great majority of amino acid changes between variants and types. By contrast, polyclonal antibodies recognize a plurality of epitopes, such that a single change in one epitope is unlikely to impact its reactivity significantly (consistent with the cross-neutralization of all HPV16 variants tested by antisera to a single HPV16 variant L1 VLP (Cheng et al., 1995)). We also envision other less likely explanations, including immunization bias in mice towards generating a type-specific rather than cross-neutralizing immune response, or selection bias for hybridomas by using BPV1 capsids. Surprisingly, B1.C4 that bound both VLP and virion types by ELISA (Fig. 2) or FACS (not shown), proved only neutralizing to BPV1, but not BPV2 virions, indicating a subtle difference in the epitopes’ sequence, structure or function between the types.
By analogy to the results of VLP vaccines in humans, and experimental vaccination in cows, it is likely that a VLP vaccine will protect against both epidermal and dermal infections in cows (Campo, 1997; Kirnbauer et al., 1996; Paavonen et al., 2007; Villa et al., 2005). However, it is unknown whether it would protect against dermal infection in horses and thus be as effective in protecting against sarcoids, as it seems possible that details of the pathogenesis of the dermal infection in cows and horses may be different in ways that might have implications for vaccine efficacy. In cows most dermal infection may be derived from progeny virus that has replicated in the overlying epidermis. VLP vaccines may be highly effective in part because they interfere with the ability of progeny virus to spread to secondary sites of infection. In horses, by contrast, there is no progeny virus that is known to develop, in the epidermis or the dermis. Therefore, it is likely that the dermal infection in horses arises de novo from external virions. Although these incoming virions would be neutralized by the systemic antibodies, this supposition remains to be proven. We are currently conducting BPV1 VLP vaccination trials to evaluate efficacy against experimental challenge in horses.
Human VLP vaccine studies have shown stabilized vaccine titers and high protective efficacy at least 6 years after vaccination. By analogy, this is well within the time range of lactation in cows requiring effective protection. The potential for long-term vaccine efficacy in horses, mules and donkeys, which may stay up to several decades in use remains to be determined.
The N-terminus of L2 contains broadly cross-neutralization epitopes that are shared by cutaneous and mucosal types. Vaccinations with the L2 minor capsid protein induced low-titer antibodies and protected cattle and rabbits against challenge with the homologous virus type at both cutaneous and mucosal sites (Campo, 1997; Embers et al., 2002; Pastrana et al., 2005; Roden et al., 1994). Although BPV1 and BPV2 appear as the most important types causing diseases in ungulates, a vaccine including L2 may offer even broader protection against additional pathogenic BPV types (Nasir and Campo, 2008).
Materials and methods
Recombinant baculovirus expression vectors
To generate recombinant baculovirus for expression of the major capsid protein L1 of BPV2, the L1 open reading frame was amplified from genomic clone pAT153-BPV2 (kindly provided by Saveria Campo, Glasgow) between nucleotides (nt) 5601–7139, by polymerase chain reaction (PCR) using a combination of high-fidelity (proofreading) Pwo Isis and Taq DNA polymerases (Qbiogene, MP Biomedicals, Irvine, California) at a ratio of 5:1. Primers incorporate 5′ an EcoRI restriction enzyme (RE) site (underlined) CCGCTGAATTCAATATGGCGTTGTGGCAACAAG, and 3′ a KpnI site GCGGTGGTACCGTTGACTTACCTTATGGTTCAC. The amplimer was cloned into the baculovirus transfer vector pEV mod (Kirnbauer et al., 1992). Final clones were verified by RE digest and DNA sequencing.
Following co-transfection of Sf-9 insect cells with transfer vector and linearized baculovirus DNA (BaculoGold, BD Biosciences Pharmingen, San Diego, CA), recombinant baculoviruses were obtained by plaque purification, and high-titer viral stocks generated. Sf9 insect cells were infected at high multiplicity of infection (MOI) for three days, lysed by sonication, and high molecular mass structures separated by sucrose and cesium chloride gradient ultra-centrifugation as described (Shafti-Keramat et al., 2003). The virus-like particles (VLP)-containing band was collected and dialyzed against phosphate buffered saline (PBS)/0.5 M NaCl/1 mM CaCl2/0.01% Brij 58. Generation of BPV1 L1-expressing baculovirus has been reported previously (Kirnbauer et al., 1992). To analyze integrity of purified VLP, particle preparations were negatively stained with 1% uranyl acetate and analyzed by transmission electron microscopy (TEM) using a JEOL 1010 electron microscope at 80 kV and × 30,000 magnification.
SDS-PAGE and Western blot
Aliquots of purified VLP or crude cell lysates were analyzed by sodium dodecyl-sulfate (SDS)-polyacrylamide gel electrophoreses (PAGE) and Western blot. Briefly, samples were denatured in SDS sample buffer containing 2% beta-mercaptoethanol and electrophoresed on 10% SDS-PAGE. Following separation proteins were either stained with Coomassie brilliant blue, or immunoblotted and probed overnight at 4 °C with monoclonal antibody (mAb) AU-1 (1/10,000). Finally blots were incubated with second-step peroxidase-labeled goat-anti-mouse (1/40,000) (BIO-RAD Laboratories, Hercules, CA) and developed using the ECL-system (Pierce, Biotechnologies, Rockford, IL).
Polyclonal and monoclonal antibodies
Fifty μg of purified VLP was administered to NZW rabbits (Charles River, Kisslegg, Germany) four times, the first boost four weeks after first injection, then in two week intervals, using aluminum hydroxide as adjuvant. Monoclonal antibodies B1.C4, B1.D7, and B1.F12 have been raised to BPV1 VLP, and mAb B1.A1 and 5B6 have been raised to BPV1 virions (Christensen and Kreider, 1993; Roden et al., 1994), all of which recognize conformational epitopes. Non-neutralizing mAb AU-1 recognizes the linear BPV1-L1 epitope DTYRYI (BabCo, Berkeley Antibody, Richmond, CA). MAb H16.V5 and 5B6 are neutralizing for HPV16 and BPV 1, respectively.
Isolation of BPV1 and BPV2 virions from cow warts
Cow warts containing BPV1 or BPV2 infectious virions were kindly provided by Richard Roden, Johns Hopkins University, and Wayne Lancaster, Wayne State University. Warts were minced with a scalpel, re-suspended in PBS and frozen in liquid nitrogen. Subsequently the tissue was mechanically disrupted for 3 min using a Swing mill MM200 (Retsch, Haan, Germany), centrifuged at 10,000 g, 4 °C, for 5 min, and the supernatant containing virions was stored in aliquots at −70 °C. To verify the BPV type, viral DNA was isolated by proteinase K digest, the L1 ORF was amplified by PCR using primers GCATCCCTCCTTGTTGAGG (nt 5543–5561) and GTTGACTTACCTTATGGTTCAC (nt 7118–7139) and the DNA sequence was obtained.
VLP enzyme-linked immunosorbent assay (ELISA)
Native L1 VLPs were used as antigen in enzyme-linked immunosorbent assay (ELISA) as described (Kirnbauer et al., 1994). For denaturing L1 ELISA, proteins were dried onto the ELISA plate in denaturing buffer (0.2 M NaHCO3/0.01 M DTT/pH 10.6) at 37 °C overnight.
BPV 1 pseudovirion neutralization assay
Pseudovirions were generated by co-transfection of 293 TT cells with plasmids pSheLL and pSEAP, and neutralization assays were performed as described (Buck et al., 2005). BPV 1 pseudovirions were incubated for 1 h at 37 °C/5% CO2 with serial dilutions of mAb or rabbit immune sera and placed on 293 TT cells. Incubation with AU-1 (BabCo, Richmond, CA), H16.V5, 5B6, antisera to BPV1-L1 VLP and preimmune sera of the same animals served as appropriate controls. Secreted alkaline phosphatase (SEAP) content in the supernatants was determined using p-nitrophenyl phosphate (Sigma, St. Louis, MO) dissolved in diethanolamine (Acros Organics, NJ), and OD values measured at 405 nm. Immune IgG dilutions showing at least 50% reduction in SEAP activity as compared to preimmune IgG were considered neutralizing. Data shown are the mean ± SD of triplicate wells of a representative experiment.
BPV1 and BPV2 virion neutralization assays (C127 focus formation)
BPV1 or BPV2 virions were incubated with ten-fold serial dilutions of immune sera in 1 ml serum-free RPMI medium (Gibco) and plated onto sub-confluent mouse C127 fibroblasts in 60 mm dishes for 1 h at 37 °C (Dvoretzky et al., 1980). RPMI medium supplemented with 5% FCS was added, plates were maintained for about 3 weeks with periodic feeding, and stained with methanol/5% methylene blue/2.5% carbolfuchsin. Endpoint neutralization titers were scored as the reciprocal of the highest serum dilution that resulted in ≥ 50% reduction in the number of foci obtained with preimmune sera.
Flow cytometric analysis (FACS)
HaCaT cells were trypsinized and incubated in RPMI medium/5% FCS for 1 h at 37 °C. VLP (BPV1-L1 or BPV2-L1) or virions (BPV1 or BPV2) were bound to 1×105 HaCaT cells for 1 h at 4 °C in FC buffer (PBS/2% fetal bovine serum/0.01% sodium azide) as described (Day et al., 2007). Cells were washed to remove unbound particles and mAb 5B6, B1.C4, anti BPV1-L1, or mAb H16.V5 (HPV16-neutralizing) as a control, respectively, were added at a final concentration of 1 μg/ml and incubated for 1 h at 4 °C in FC buffer. Cells were washed again with FC buffer, incubated with fluorescein isothiocyanate-labeled goat-anti-mouse IgG (H+L) (Alexa Fluor 488; Molecular Probes) for 1 h at 4 °C, and analyzed on a Becton Dickinson FACScan.
Acknowledgments
This research was supported by a grant to RK from the FWF-Austrian Science Foundation (P18990-B13) and a grant to SB and RK by the Viennese Center of Innovation and Technology (ZIT). We thank Doug Lowy and John Schiller for critical reading of the manuscript.
References
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 1995;69(6):3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Steben M, Bosch FX, Dillner J, Joura EA, Kurman RJ, Majewski S, Munoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Lupinacci LC, Giacoletti KE, Sings HL, James M, Hesley TM, Barr E. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J. Infect. Dis. 2009;199(7):926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 2005;119:445–462. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- Campo MS. Vaccination against papillomavirus in cattle. Clin. Dermatol. 1997;15(2):275–283. doi: 10.1016/s0738-081x(96)00165-4. [DOI] [PubMed] [Google Scholar]
- Campo MS, Jarrett WF, O’Neil W, Barron RJ. Latent papillomavirus infection in cattle. Res. Vet. Sci. 1994;56(2):151–157. doi: 10.1016/0034-5288(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Cheng G, Icenogle JP, Kirnbauer R, Hubbert NL, St Louis ME, Han C, Svare EI, Kruger Kjaer S, Lowy DR, Schiller JT. Divergent human papillomavirus type 16 variants are serologically cross-reactive. J. Inf. Dis. 1995;172:1584–1587. doi: 10.1093/infdis/172.6.1584. [DOI] [PubMed] [Google Scholar]
- Christensen ND, Kreider JW. Monoclonal antibody neutralization of BPV-1. Virus Res. 1993;28(2):195–202. doi: 10.1016/0168-1702(93)90136-b. [DOI] [PubMed] [Google Scholar]
- Day PM, Thompson CD, Buck CB, Pang YY, Lowy DR, Schiller JT. Neutralization of human papillomavirus with monoclonal antibodies reveals different mechanisms of inhibition. J. Virol. 2007;81(16):8784–8792. doi: 10.1128/JVI.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I, Shober R, Chattopadhyay SK, Lowy DR. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Embers ME, Budgeon LR, Pickel M, Christensen ND. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of L2, the minor capsid protein. J. Virol. 2002;76(19):9798–9805. doi: 10.1128/JVI.76.19.9798-9805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384(2):410–414. doi: 10.1016/j.virol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Ghim SJ, Young R, Jenson AB. Antigenicity of bovine papillomavirus type 1 (BPV-1) L1 virus-like particles compared with that of intact BPV-1 virions. J. Gen. Virol. 1996;77(Pt 2):183–188. doi: 10.1099/0022-1317-77-2-183. [DOI] [PubMed] [Google Scholar]
- Howley PM, Lowy DR. Papillomavirus. In: Knipe DM, Howley PM, editors. Fields Virology. 5 ed. Vol. 2. Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 2299–2354. [Google Scholar]
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. U. S. A. 1992;89(24):12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Hubbert NL, Wheeler CM, Becker TM, Lowy DR, Schiller JT. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J. Natl. Cancer Inst. 1994;86(7):494–499. doi: 10.1093/jnci/86.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Chandrachud L, O’Neil B, Wagner E, Grindlay G, Armstrong A, McGarvie G, Schiller J, Lowy D, Campo M. Virus-like particles of bovine papillomavirus type-4 in prophylactic and therapeutic immunization. Virology. 1996;219(1):37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- Nasir L, Campo MS. Bovine papillomaviruses: their role in the aetiology of cutaneous tumours of bovids and equids. Vet. Dermatol. 2008;19(5):243–254. doi: 10.1111/j.1365-3164.2008.00683.x. [DOI] [PubMed] [Google Scholar]
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, de Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, Christensen ND, Lowy DR, Schiller JT, Roden RB. Cross-neutralization of cutaneous and mucosal papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337(2):365–372. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Roden RB, Weissinger EM, Henderson DW, Booy F, Kirnbauer R, Mushinski JF, Lowy DR, Schiller JT. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J. Virol. 1994;68(11):7570–7574. doi: 10.1128/jvi.68.11.7570-7574.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden R, Greenstone H, Kirnbauer R, Booy F, Joel J, Lowy D, Schiller J. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J. Virol. 1996;70(9):5875–5883. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden RB, Armstrong A, Haderer P, Christensen ND, Hubbert NL, Lowy DR, Schiller JT, Kirnbauer R. Characterization of a human papillomavirus type 16 variant-dependent neutralizing epitope. J. Virol. 1997;71(8):6247–6252. doi: 10.1128/jvi.71.8.6247-6252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J. Virol. 2003;77(24):13125–13135. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzich JA, Ghim S, Palmer-Hill FJ, White WI, Tamura JK, Bell J, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, Skjeldestad FE, Olsson SE, Steinwall M, Brown DR, Kurman RJ, Ronnett BM, Stoler MH, Ferenczy A, Harper DM, Tamms GM, Yu J, Lupinacci L, Railkar R, Taddeo FJ, Jansen KU, Esser MT, Sings HL, Saah AJ, Barr E. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, Garcia P, Ault KA, Garland SM, Leodolter S, Olsson SE, Tang GW, Ferris DG, Paavonen J, Steben M, Bosch FX, Dillner J, Joura EA, Kurman RJ, Majewski S, Munoz N, Myers ER, Villa LL, Taddeo FJ, Roberts C, Tadesse A, Bryan J, Lupinacci LC, Giacoletti KE, James M, Vuocolo S, Hesley TM, Barr E. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J. Infect Dis. 2009;199(7):936–944. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]