Abstract
A growing body of evidence suggests that podocyte apoptosis is a major cause of decreased podocyte number, which leads to albuminuria and glomerular injury. The aim of this study was to clarify the molecular mechanisms of angiotensin II (Ang II)-induced apoptosis in cultured mouse podocytes. We examined the effects of Ang II (100 nmol/L) on apoptosis, superoxide anions, and cytosol pH in podocytes. For intracellular pH measurements, image analysis was conducted using confocal laser microscopy after incubation with carboxy-seminaphthorhodafluor-1. Superoxide anions and intracellular pH were elevated with Ang II treatment. Apoptotic cell numbers, as measured by TUNEL staining and caspase 3 activity, were also augmented in the Ang II–treated group. Pre-treatment with olmesartan (100 nmol/L, an Ang II type 1–receptor blocker), apocynin (50 µmol/L, NADPH oxidase inhibitor), or 5-N, N hexamethylene amiloride [30 µmol/L, Na+/H+ exchanger type 1 (NHE-1) inhibitor] abolished Ang II-induced podocyte apoptosis, whereas NHE-1 mRNA and protein expression was not affected by Ang II treatment. Moreover, Ang II increased NHE-1 phosphorylation. These results suggest that superoxide production, NHE-1 activation, and intracellular alkalization were early features prior to apoptosis in Ang II– treated mouse podocytes, and may offer new insights into the mechanisms responsible for Ang II–induced podocyte injury.
Keywords: angiotensin II, Na+/H+ exchanger type 1 (NHE-1), intracellular pH, apoptosis, podocyte
Introduction
Podocytes are highly specialized, terminally differentiated, epithelial cells that play a key role in both maintenance of the glomerular filtration barrier and structural integrity (1). There is an increasing body of experimental and clinical evidence indicating the contribution of decreased podocyte number to the progression of chronic kidney disease (2, 3). The consequences of reduced podocyte number include albuminuria and glomerulosclerosis. Conversely, angiotensin II (Ang II) is an important risk factor in the progression of chronic kidney disease (4, 5). We recently reported that Ang II blockade prevents the onset of albuminuria via its protective effects on podocytes (6). Additionally, in vitro experiments revealed that Ang II induces a large number of non-hemodynamic effects, including induction of oxygen radicals and stimulation of cell apoptosis (7, 8). Ang II induces podocyte apoptosis both in vivo and in vitro (9, 10); however, the molecular mechanisms responsible for Ang II–induced podocyte apoptosis are not entirely clear.
Reactive oxygen species (ROS), especially the superoxide anion, also play an important role in cell survival and apoptosis (11, 12). We and others reported that Ang II mediates NADPH-derived superoxide production via Ang II type 1 (AT1) receptor in various types of cells and subsequently induces renal injury (13– 15). Recent reports revealed that apoptosis contributed to the downregulation of antioxidant protein in podocytes, indicating the involvement of ROS production in podocyte apoptosis (16).
Na+/H+ exchanger type 1 (NHE-1) is a member of the solute carrier family 9A, and it is a ubiquitously distributed protein with central roles in the control of cell volume and intracellular pH (pHi). The activation of NHE-1 and subsequent alkalinization of the cytosol generally precedes the activation of many cellular functions, including intracellular signaling pathways associated with apoptosis (17). It has been also reported that Ang II activates NHE-1 in various tissues (18, 19). NHE-1 is predominately expressed in differentiated podocytes (20), although its functions, including in the involvement of podocyte apoptosis, are still unclear.
In the present study, we hypothesized that Ang II induces podocyte apoptosis via NHE-1-induced pHi changes and NADPH oxidase–derived ROS production. To test this hypothesis, we examined the effects of the NHE-1 inhibitor and an antioxidant on Ang II–induced apoptotic changes in cultured mouse podocytes.
Materials and Methods
Cell culture
Cell culture of conditionally immortalized mouse podocytes was performed, as previously reported (21). To propagate podocytes, cells were grown on type I collagen–coated dishes at 33°C in the presence of 10 U/mL of mouse recombinant interferon-γ (Sigma-Aldrich, St. Louis, MO, USA), which enhances the expression of a thermosensitive T-antigen, in RPMI 1640 (Sigma-Aldrich). To induce differentiation, cells were maintained at 37°C without interferon-γ for 10 – 14 days. All experiments were performed with differentiated podocytes.
Superoxide anion measurement
Superoxide anion production was determined with dihydroethidium (DHE) staining, as described previously (22). Briefly, cells were plated onto a glass-bottom dish (Asahi Glass, Tokyo). At the appropriate time after stimulation, DHE (10 µmol/L) was added into the medium, and the incubation was continued for 30 .min. Then, cells were washed with PBS, and images were obtained with a laser scanning confocal microscope system (LSM 700; Carl Zeiss, Oberkochen, Germany). The averages of fluorescence intensity values were calculated using NIH ImageJ software.
pHi measurement
For pHi imaging, podocytes were grown on 2-well glass chamber slides (BD Bioscience, Franklin Lakes, NJ, USA). Cells were incubated with the acetoxymethyl ester of the pH-reporter dye carboxy-seminaphthorhodafluor (SNARF)-1 (Life Technologies, Carlsbad, CA, USA) at 10 µmol/L for 30 min as previously reported (23). Excess extracellular dye was washed away with medium. The pHi dye was excited using a 488 nm argon laser, and fluorescence was detected with confocal microscopy at 580 and 640 nm. The 580/640 nm fluorescence ratio was then converted into pHi using a calibration curve. The standard curve was obtained by measuring the ratio signals of carboxy-SNARF-1–loading cells in high-potassium buffers (25 mmol/L HEPES, 145 mmol/L KCl, 0.8 mmol/L CaCl2, 5.5 mmol/L glucose) at different pHs (pH 6.7, 7.0, 7.3, 7.6, or 7.9) in the presence of 10 µg/mL of nigericin, an electroneutral H+/K+ ionophore.
Real-time RT-PCR
For real-time RT-PCR analysis, total RNAs were extracted using ISOGEN (Nippon Gene, Tokyo). cDNA (from 1 µg RNA) was synthesized, as described previously (24). The expression of mRNA was analyzed with a Light Cycler Fast Start DNA Master SYBR Green I kit (Life Technologies). RT-PCR was performed using pre-designed primers for mouse NHE-1 (TaqMan Gene expression Assays: Life Technologies) and mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (F: 5’-TGAACGGGAAGCTCACTGG-3’ and R: 5’-TCCACCACCCTGTTGCTGTA-3’) (25). All data were normalized to the expression of GAPDH.
Western blot analysis
Cells at 80% – 90% confluence were made quiescent by incubation with medium containing 0.1% FBS for 24 h. Cells were lysed as described previously (26); solubilized proteins were isolated by centrifugation (12,500 rpm, 10 min) and quantified by the Bradford assay. Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. After blocking, the membranes were incubated with primary antibodies against NHE-1 (Cell Signaling Technology, Beverly, MA, USA). The membranes were then embedded with Infrared Dye were visualized with the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). To confirm equal protein loading, each membrane was re-probed with an anti-β-actin antibody (Sigma-Aldrich). Band intensities were quantified by immunoblot densitometry using NIH ImageJ software.
Immunoprecipitation
We determined NHE-1 phosphorylation by co-immunoprecipitation and western blotting, as reported previously (26, 27). Protein samples (130 µg) from podocytes were immunoprecipitated by overnight incubation with anti-phospho-Ser 14- 3-3 antibody (Cell Signaling Technology), followed by western blot analysis with the antibody against NHE-1.
TUNEL method
TUNEL staining was performed using a MEBSTAIN Apoptosis Kit Direct (MBL, Nagoya), as previously reported (28). This method detects nucleosome-sized DNA fragments by tailing their 3′-OH ends with digoxi-genin nucleotides using terminal deoxynucleotidyl transferase (TdT). After treatment, the cells were fixed with 4% paraformaldehyde and incubated with TdT buffer. Cells were double-labeled with 1 µg/mL of DAPI for 15 min at 37°C. The numbers of TUNEL-positive cells were counted using fluorescence microscopy.
Caspase 3 activity
An APOPCYTO caspase 3 colorimetric assay kit (MBL) was used for the measurement of caspase activities, as previously reported (28). Briefly, podocytes were plated in 100-mm dishes and cultured in medium. After treatment, the cells were lysed with 150 µL of ice-cold lysis buffer. After centrifugation at 10,000 g at 4°C for 5 min, protein concentrations in the supernatants were quantified using the Bradford assay. A 4-amino acid sequence was labeled with p-nitroanilide (pNA) at the C-terminal side. Free pNA was released from the labeled synthetic substrate following cleavage with active caspase. The lysates were incubated with the reaction buffer and then incubated with DEVD-pNA substrate for 2 h at 37°C. Absorbance was monitored at a wavelength of 405 nm.
Statistical analyses
Values are presented as means ± S.E.M. Multiple-group comparisons were made using one-way analyses of variance (ANOVA), followed by a Bonferroni’s test. Student’s t-tests were performed to compare the means when the experimental design comprised of two individual groups. P < 0.05 was considered statistically significant.
Results
Effects of Ang II on superoxide production in podocytes
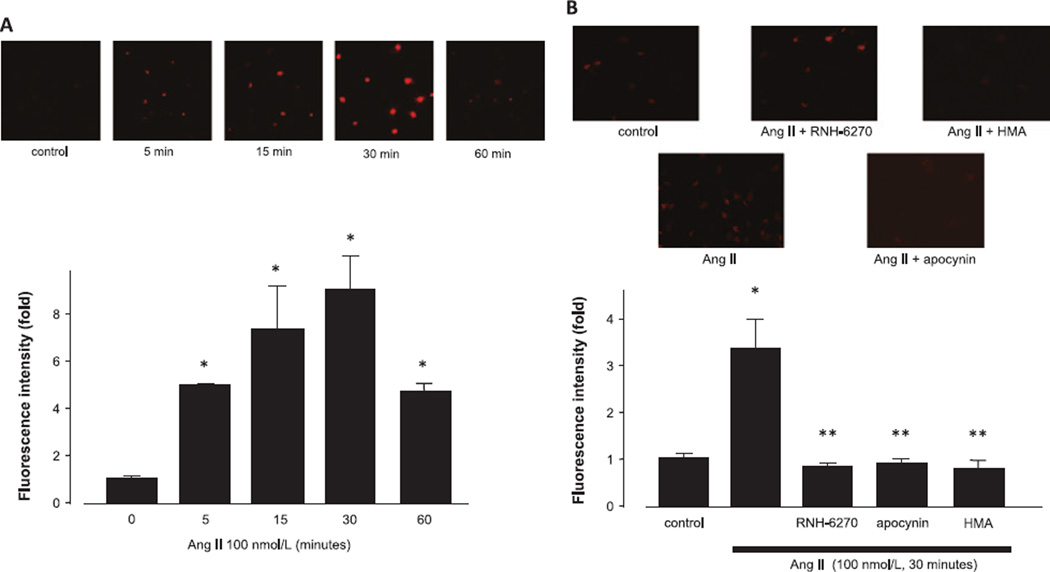
An incubation of podocytes with 100 nmol/L of Ang II resulted in a significant increase in DHE-positive areas compared with the control group (Fig. 1A). Pre-incubation with the appropriate inhibitors [i.e., olmesartan (RNH-6270, an active form of olmesartan medoxomil, 100 nmol/L), an AT1-receptor blocker; apocynin (50 µmol/L), an NADPH oxidase inhibitor; or 5-N , N-hexamethylene amiloride (HMA, 30 µmol/L), an NHE-1 inhibitor], inhibited superoxide production induced by Ang II, indicating the involvement of AT1 receptors, NADPH oxidase, and NHE-1 in the Ang II–mediated increases in ROS production.
Fig. 1.
Effects of Ang II on superoxide production. A: Podocytes were incubated with 100 nmol/L Ang II for the indicated times. B: Podocytes were pre-treated with olmesartan (100 nmol/L), apocynin (50 µmol/L), or HMA (30 µmol/L) for 1 h, and then stimulated with 100 nmol/L of Ang II for 30 min prior to DHE staining. DHE fluorescence was used to evaluate superoxide production in podocytes. Representative photographs of DHE-stained podocytes are shown. Data are reported as the mean ± S.E.M. (n = 6), expressed as fold change compared with unstimulated cells. *P < 0.05 vs. control podocytes, **P < 0.05 vs. Ang II alone.
Effects of Ang II on pHi in podocytes
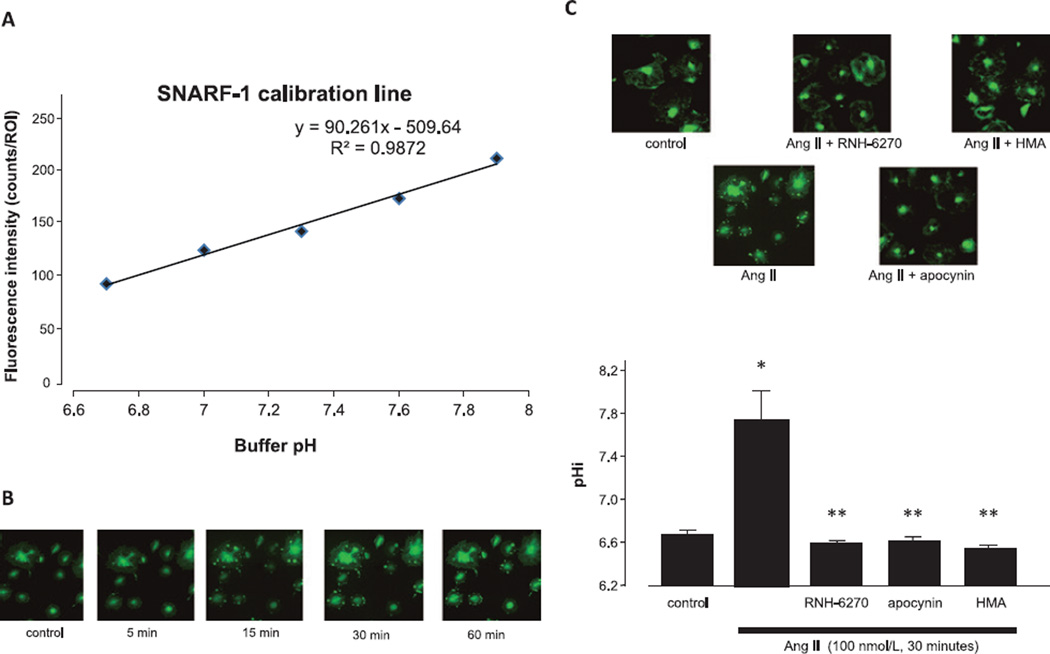
We used SNARF-1 staining to obtain a calibration line to measure pHi (Fig. 2A). Treatment of podocytes with 100 nmol/L Ang II caused an increase in pHi (Fig. 2B). Ang II–induced intracellular alkalinization in podocytes was inhibited by HMA (Fig. 2C). Olmesartan and apocynin also prevented Ang II–induced intracellular alkalinization, indicating that Ang II activates NHE-1 via the AT1 receptor and NADPH oxidase.
Fig. 2.
Effects of Ang II on intracellular pH. A: The calibration line was obtained by measuring the signals of carboxy-SNARF-1-loading cells at different pHs in the presence of 10 µg/mL of nigericin. B: Podocytes were incubated with 100 nmol/L Ang II for the indicated times. C: Podocytes were pre-treated with olmesartan (100 nmol/L), apocynin (50 µmol/L), or HMA (30 µmol/L) for 1 h and then stimulated with 100 nmol/L Ang II for 30 min before SNARF-1 staining. Representative photographs of SNARF-1-stained podocytes are shown. Data are reported as the mean ± S.E.M. (n = 6). *P < 0.05 vs. control podocytes, **P < 0.05 vs. Ang II alone.
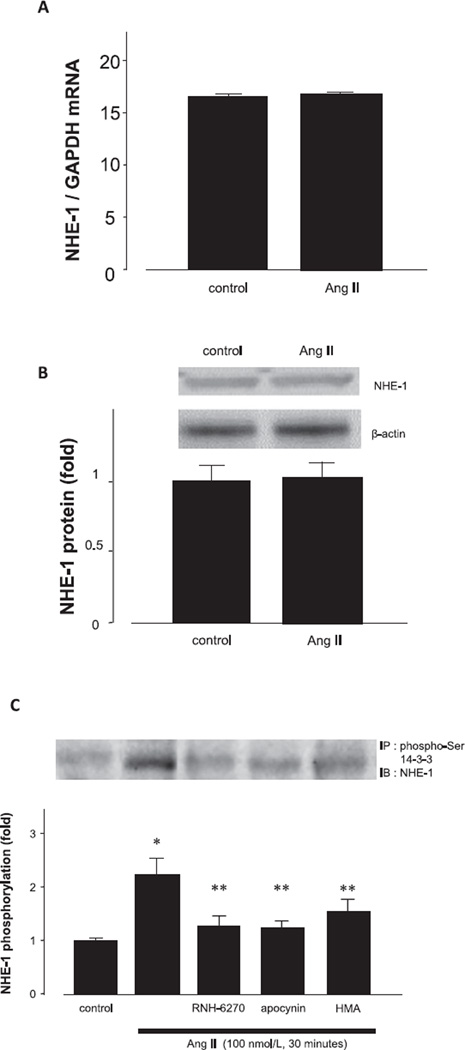
Furthermore, to clarify the mechanisms behind the pHi changes induced via NHE-1, we measured NHE-1 mRNA expression by RT-PCR. We found that NHE-1 was expressed in cultured podocytes under basal condition. However, Ang II failed to increase NHE-1 mRNA expression (Fig. 3A) or affect NHE-1 protein expression (Fig. 3B). By contrast, Ang II augmented NHE-1 phosphorylation, which was abolished by pretreatment with inhibitors (Fig. 3C). These results suggest that intracellular alkalinization was primarily the result of NHE-1 activation and not due to an increase in NHE-1 expression.
Fig. 3.
Effects of Ang II on NHE-1 mRNA, protein, and phosphorylation. Podocytes were treated with 100 nmol/L Ang II for 24 h (A and B) or 30 min (C). A: NHE-1 mRNA expression was measured by RT-PCR. B: NHE-1 protein expression was measured by western blot analysis. C: Protein samples of podocytes were immunoprecipitated with anti-phospho-Ser 14-3˓ antibody, followed by western blot analysis with the antibody against NHE-1. Data are reported as the mean ± S.E.M. (n = 6). *P < 0.05 vs. control podocytes, **P < 0.05 vs. Ang II alone.
Effects of Ang II on apoptosis in podocytes
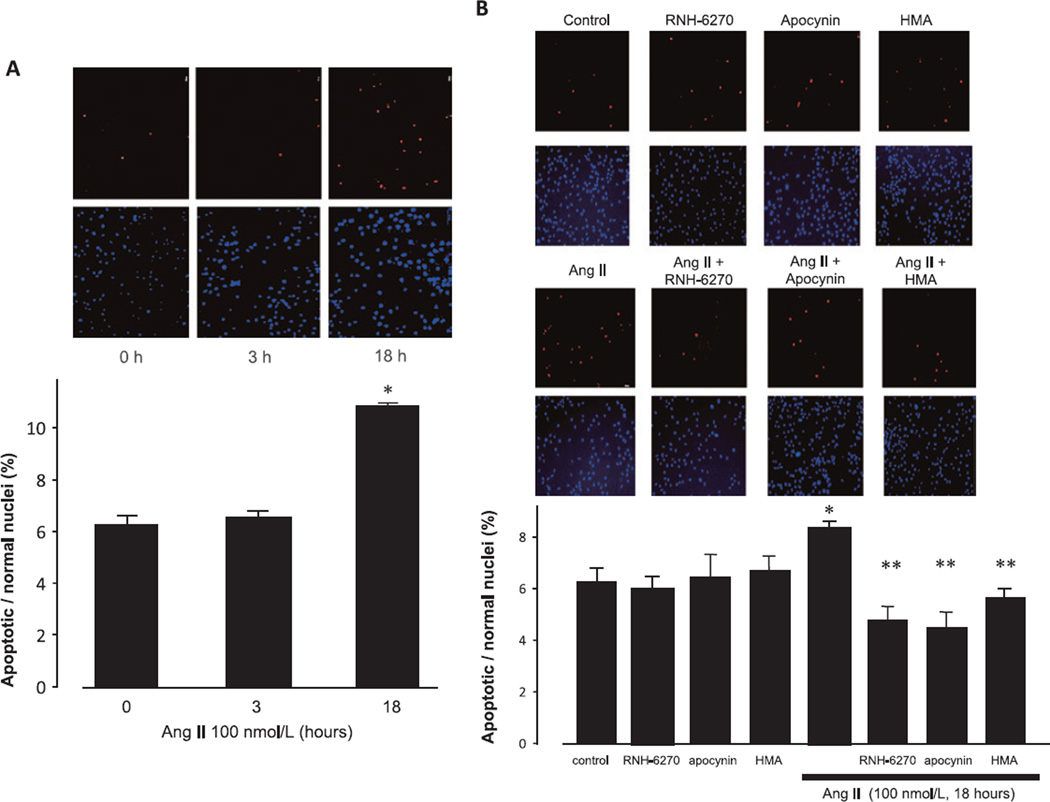
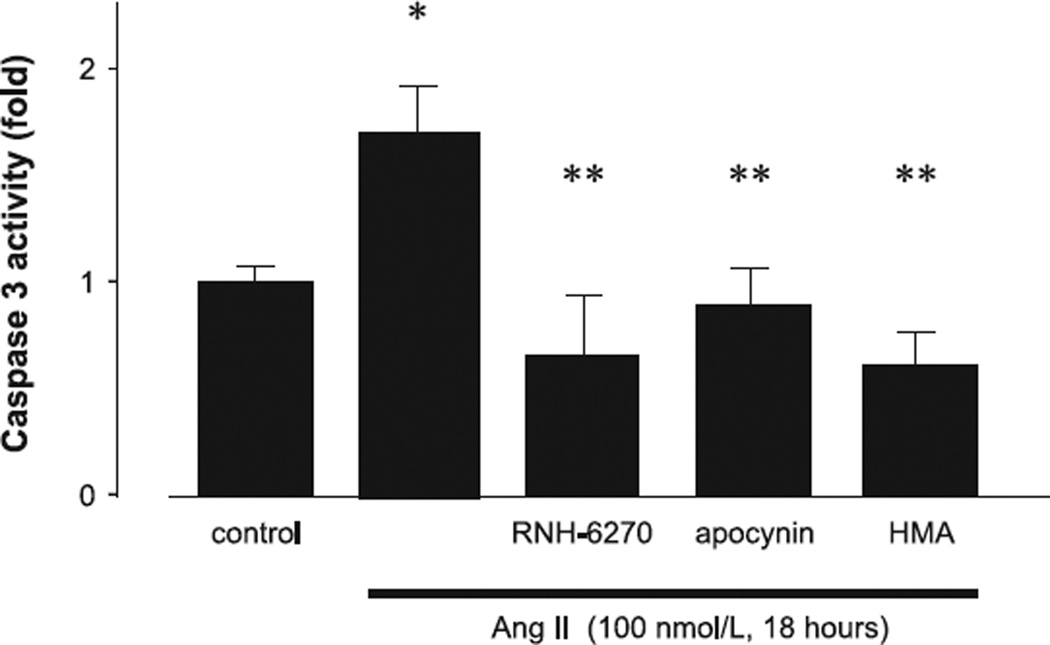
The incubation of podocytes with 100 nmol/L Ang II for 18 h resulted in a significant increase in apoptosis compared with the control, as determined via TUNEL staining (Fig. 4A). Pre-treatment with olmesartan, apocynin, or HMA inhibited Ang II–induced podocyte apoptosis (Fig. 4B). The findings with respect to caspase 3 activity were similar to those obtained with TUNEL staining, where Ang II was found to augment caspase 3 activity, and this was completely inhibited by olmesartan, apocynin, and HMA (Fig. 5). These results suggest that both NHE-1 and ROS promote apoptosis in mouse podocytes.
Fig. 4.
Effects of Ang II on TUNEL staining. A: Podocytes were incubated with 100 nmol/L Ang II for the indicated times. B: Podocytes were treated with 100 nmol/L Ang II for 18 h prior to TUNEL staining. Blue: DAPI stained normal nuclei and Red: TUNEL-positive nuclei. Representative photographs of TUNEL staining are shown. Data are reported as the mean ± S.E.M. (n = 6), expressed as apoptotic/normal nuclei. *P < 0.05 vs. control podocytes. **P < 0.05 vs. Ang II alone.
Fig. 5.
Effects of Ang II on caspase 3 activity. Podocytes were treated with 100 nmol/L of Ang II for 18 h prior to caspase 3 assay. Data are reported as the mean ± S.E.M. (n = 6), expressed as fold change compared with unstimulated cells. *P < 0.05 vs. control podocytes, **P < 0.05 vs. Ang II alone.
Discussion
Ang II is directly involved in podocyte injury (6, 29). Studies have indicated that the major cause of Ang II– induced podocyte injury is due to apoptosis in podocytes (9, 10, 16). A reduced podocyte number due to apoptosis induces proteinuria and subsequent glomerulosclerosis (2, 30). Although Ang II induces podocyte apoptosis, its molecular mechanisms are not fully elucidated. In the present study, we revealed that Ang II–induced podocyte apoptosis is associated with NHE-1 activation, intracellular alkalization, and an accumulation of intracellular ROS. These findings reveal the possible mechanisms of Ang II–induced podocyte injury via pHi-mediated apoptosis.
NHE-1, which participates in the regulation of pHi by mediating the exchange of Na+ for H+ across the plasma membrane (31), is activated by a wide range of stimuli, including many growth factors and hormones, cell adhesion, and stress stimuli (32, 33). Ang II also activates NHE-1 in various tissues. We demonstrated that 100 nmol/L Ang II induces intracellular alkalinization via NHE-1 activation in cultured mouse podocytes. Furthermore, Ang II–induced intracellular alkalinization was reduced with a selective NHE-1 inhibitor, indicating that NHE-1 stimulation was primarily involved. We also demonstrated that there was a remarkable increase of pHi in Ang II–treated cells in conjunction with ROS production and apoptosis. Several reports suggested that NHE-1 promotes apoptosis via actively inducing intracellular alkalinization, activation of Bax (34), the inhibition of mitochondrial ADP transport (35), and endonucleases (36). However, the mechanisms behind alkalinizationrelated apoptosis still need to be evaluated. Conversely, although our findings clearly reveal the involvement of pHi in podocyte apoptosis, it is difficult to evaluate pH changes of podocytes in in vivo experiments. Several studies have reported on organ injury via pH changes in animal model (37, 38); however, measurements of pHi in podocytes have not yet been evaluated. Therefore, podocyte-specific NHE-1 inhibition may be useful in revealing the effects of NHE-1–mediated pHi in glomerular injury, instead of in vivo pH measurements.
Ang II is known to induce oxidative stress in a variety of renal cells, including podocytes (39). Ang II augments ROS production via the activation of NADPH oxidase through the AT1 receptor (13). Moreover, increased ROS production has been implicated in the pathogenesis of Ang II–induced apoptosis in cardiovascular and renal tissues (40, 41). In the present study, Ang II augmented ROS generation in podocytes. We also found that the AT1-receptor antagonist and NADPH-oxidase inhibitor blocked Ang II–induced apoptosis. These results indicate that Ang II–induced podocyte apoptosis is mediated via NADPH-dependent ROS production through the AT1 receptor. Our results also demonstrated that the inhibition of NADPH oxidase in the presence of Ang II caused an increase in pHi, indicating that ROS production may be upstream from NHE-1 activation, as previously reported in cardiomyocytes (42).
In conclusion, our results highlight the role of NHE-1 stimulation, intracellular alkalization, and accumulation of intracellular ROS in the Ang II–induced, caspase-3– dependent apoptosis of podocytes. The inhibition of NHE-1 and NADPH oxidase may have protective effects against podocyte apoptosis, thereby improving renal injury. Although further studies are necessary to fully elucidate the mechanisms of Ang II–induced podocyte injury, the inhibition of these pathways may potentially serve as clinical therapeutic targets.
Acknowledgments
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20590253 and 22790792) and a Sanju Alumni Research Grant. We are grateful to Daiichi-Sankyo Co. for supplying olmesartan.
References
- 1.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 2.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 3.Steffes MW, Schmidt D, McCrery R, Basgen JM. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59:2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 4.Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim HN, Rosenberg ME, Hostetter TH. Role of the renin-angiotensin-aldosterone system in the progression of renal disease: a critical review. Semin Nephrol. 1997;17:431–440. [PubMed] [Google Scholar]
- 6.Sofue T, Kiyomoto H, Kobori H, Urushihara M, Nishijima Y, Kaifu K, et al. Early treatment with olmesartan prevents juxtamedullary glomerular podocyte injury and the onset of microalbuminuria in type 2 diabetic rats. Am J Hypertens. 2012;25:604–611. doi: 10.1038/ajh.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf G, Butzmann U, Wenzel UO. The renin-angiotensin system and progression of renal disease: from hemodynamics to cell biology. Nephron Physiol. 2003;93:3–P13. doi: 10.1159/000066656. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 9.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, et al. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol. 2002;283:F173–F180. doi: 10.1152/ajprenal.00240.2001. [DOI] [PubMed] [Google Scholar]
- 10.Jia J, Ding G, Zhu J, Chen C, Liang W, Franki N, et al. Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol. 2008;28:500–507. doi: 10.1159/000113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 12.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 13.Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin II and oxidative stress. Curr Opin Cardiol. 2007;22:311–315. doi: 10.1097/HCO.0b013e3281532b53. [DOI] [PubMed] [Google Scholar]
- 14.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan YY, Kohno M, Nakano D, Ohsaki H, Kobori H, Suwarni D, et al. Cilnidipine suppresses podocyte injury and proteinuria in metabolic syndrome rats: possible involvement of N-type calcium channel in podocyte. J Hypertens. 2010;28:1034–1043. doi: 10.1097/hjh.0b013e328336ade3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu HH, Hoffmann S, Di Marco GS, Endlich N, Peter-Katalinic J, Weide T, et al. Downregulation of the antioxidant protein peroxiredoxin 2 contributes to angiotensin II-mediated podocyte apoptosis. Kidney Int. 2011;80:959–969. doi: 10.1038/ki.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, Obejero-Paz CA, et al. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem. 2004;279:26280–26286. doi: 10.1074/jbc.M400814200. [DOI] [PubMed] [Google Scholar]
- 18.Paletas K, Sailer X, Rizeq L, Dimitriadi A, Koliakos G, Kaloyianni M. Angiotensin-II-dependent NHE1 activation in human monocytes. J Am Soc Hypertens. 2008;2:173–181. doi: 10.1016/j.jash.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 20.Coaxum SD, Garnovskaya MN, Gooz M, Baldys A, Raymond JR. Epidermal growth factor activates Na(+/)H(+) exchanger in podocytes through a mechanism that involves Janus kinase and calmodulin. Biochim Biophys Acta. 2009;1793:1174–1181. doi: 10.1016/j.bbamcr.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, et al. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol. 2005;16:2906–2912. doi: 10.1681/ASN.2005040390. [DOI] [PubMed] [Google Scholar]
- 23.Balut C, vandeVen M, Despa S, Lambrichts I, Ameloot M, Steels P, et al. Measurement of cytosolic and mitochondrial pH in living cells during reversible metabolic inhibition. Kidney Int. 2008;73:226–232. doi: 10.1038/sj.ki.5002632. [DOI] [PubMed] [Google Scholar]
- 24.Moriwaki K, Kiyomoto H, Hitomi H, Ihara G, Kaifu K, Matsubara K, et al. Interferon-gamma enhances superoxide production in human mesangial cells via the JAK-STAT pathway. Kidney Int. 2006;70:788–793. doi: 10.1038/sj.ki.5001639. [DOI] [PubMed] [Google Scholar]
- 25.Zhang GX, Ohmori K, Nagai Y, Fujisawa Y, Nishiyama A, Abe Y, et al. Role of AT1 receptor in isoproterenol-induced cardiac hypertrophy and oxidative stress in mice. J Mol Cell Cardiol. 2007;42:804–811. doi: 10.1016/j.yjmcc.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1142–1147. doi: 10.1161/01.ATV.0000164313.17167.df. [DOI] [PubMed] [Google Scholar]
- 27.Snabaitis AK, D’Mello R, Dashnyam S, Avkiran M. A novel role for protein phosphatase 2A in receptor-mediated regulation of the cardiac sarcolemmal Na+/H+ exchanger NHE1. J Biol Chem. 2006;281:20252–20262. doi: 10.1074/jbc.M600268200. [DOI] [PubMed] [Google Scholar]
- 28.Kaifu K, Kiyomoto H, Hitomi H, Matsubara K, Hara T, Moriwaki K, et al. Insulin attenuates apoptosis induced by high glucose via the PI3-kinase/Akt pathway in rat peritoneal mesothelial cells. Nephrol Dial Transplant. 2009;24:809–815. doi: 10.1093/ndt/gfn598. [DOI] [PubMed] [Google Scholar]
- 29.Durvasula RV, Shankland SJ. The renin-angiotensin system in glomerular podocytes: mediator of glomerulosclerosis and link to hypertensive nephropathy. Curr Hypertens Rep. 2006;8:132–138. doi: 10.1007/s11906-006-0009-8. [DOI] [PubMed] [Google Scholar]
- 30.Zhou LL, Hou FF, Wang GB, Yang F, Xie D, Wang YP, et al. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int. 2009;76:1148–1160. doi: 10.1038/ki.2009.322. [DOI] [PubMed] [Google Scholar]
- 31.Schelling JR, Abu Jawdeh BG. Regulation of cell survival by Na+/H+ exchanger-1. Am J Physiol Renal Physiol. 2008;295:F625–F632. doi: 10.1152/ajprenal.90212.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eguti DM, Thieme K, Leung GP, Mello-Aires M, Oliveira-Souza M. Regulation of Na+/H+ exchanger isoform 1 (NHE1) by calmodulin-binding sites: role of angiotensin II. Cell Physiol Biochem. 2010;26:541–552. doi: 10.1159/000322322. [DOI] [PubMed] [Google Scholar]
- 33.Bianchini L, Pouyssegus J. Regulation of the Na+/H+ exchanger isoform NHE1: role of phosphorylation. Kidney Int. 1996;49:1038–1041. doi: 10.1038/ki.1996.151. [DOI] [PubMed] [Google Scholar]
- 34.Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci U S A. 1999;96:14476–14481. doi: 10.1073/pnas.96.25.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khaled AR, Reynolds DA, Young HA, Thompson CB, Muegge K, Durum SK. Interleukin-3 withdrawal induces an early increase in mitochondrial membrane potential unrelated to the Bcl-2 family. Roles of intracellular pH, ADP transport, and F(0) F(1)-ATPase. J Biol Chem. 2001;276:6453–6462. doi: 10.1074/jbc.M006391200. [DOI] [PubMed] [Google Scholar]
- 36.Wyllie AH, Arends MJ, Morris RG, Walker SW, Evan G. The apoptosis endonuclease and its regulation. Semin Immunol. 1992;4:389–397. [PubMed] [Google Scholar]
- 37.Onken H, Parks SK, Goss GG, Moffett DF. Serotonin-induced high intracellular pH aids in alkali secretion in the anterior midgut of larval yellow fever mosquito Aedes aegypti L. J Exp Biol. 2009;212:2571–2578. doi: 10.1242/jeb.030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrovic S, Barone S, Wang Z, McDonough AA, Amlal H, Soleimani M. Slc26a6 (PAT1) deletion downregulates the apical Na+/H+ exchanger in the straight segment of the proximal tubule. Am J Nephrol. 2008;28:330–338. doi: 10.1159/000111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhaskaran M, Reddy K, Radhakrishanan N, Franki N, Ding G, Singhal PC. Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol Renal Physiol. 2003;284:F955–F965. doi: 10.1152/ajprenal.00246.2002. [DOI] [PubMed] [Google Scholar]
- 40.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439–2446. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 41.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(Suppl 2):S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 42.Cingolani OH, Perez NG, Ennis IL, Alvarez MC, Mosca SM, Schinella GR, et al. In vivo key role of reactive oxygen species and NHE-1 activation in determining excessive cardiac hypertrophy. Pflugers Arch. 2011;462:733–743. doi: 10.1007/s00424-011-1020-8. [DOI] [PubMed] [Google Scholar]