Abstract
Objectives:
Antidepressants, among the most commonly prescribed medications, trigger symptoms of REM sleep behavior disorder (RBD) in up to 6% of users. Idiopathic RBD is a very strong prodromal marker of Parkinson disease and other synuclein-mediated neurodegenerative syndromes. It is therefore critically important to understand whether antidepressant-associated RBD is an independent pharmacologic syndrome or a sign of possible prodromal neurodegeneration.
Design:
Prospective cohort study.
Setting:
Tertiary sleep disorders center.
Participants:
100 patients with idiopathic RBD, all with diagnosis confirmed on polysomnography, stratified to baseline antidepressant use, with 45 matched controls.
Measurements/Results:
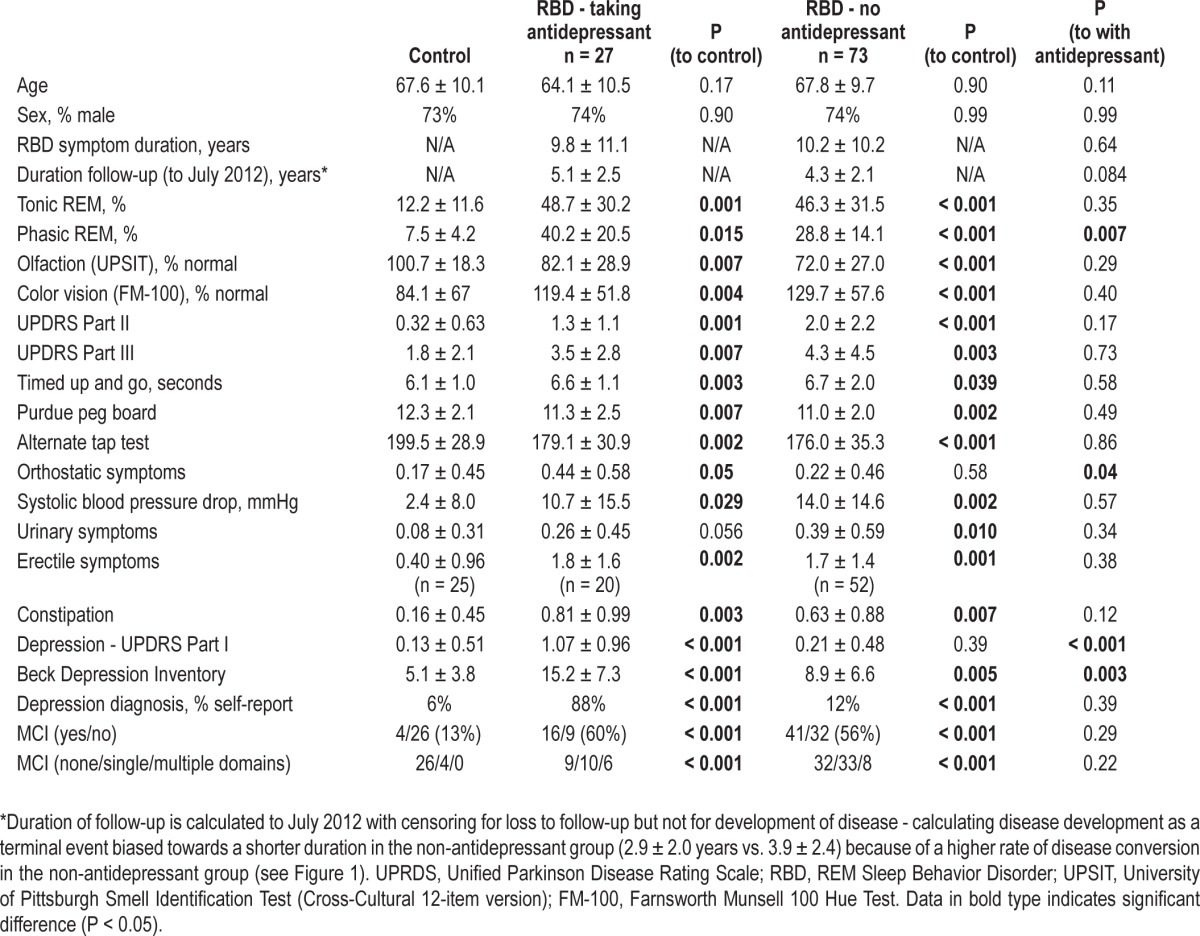
Of 100 patients, 27 were taking antidepressants. Compared to matched controls, RBD patients taking antidepressants demonstrated significant abnormalities of 12/14 neurodegenerative markers tested, including olfaction (P = 0.007), color vision (P = 0.004), Unified Parkinson Disease Rating Scale II and III (P < 0.001 and 0.007), timed up-and-go (P = 0.003), alternate tap test (P = 0.002), Purdue Pegboard (P = 0.007), systolic blood pressure drop (P = 0.029), erectile dysfunction (P = 0.002), constipation (P = 0.003), depression indices (P < 0.001), and prevalence of mild cognitive impairment (13% vs. 60%, P < 0.001). All these abnormalities were indistinguishable in severity from RBD patients not taking antidepressants. However, on prospective follow-up, RBD patients taking antidepressants had a lower risk of developing neurodegenerative disease than those without antidepressant use (5-year risk = 22% vs. 59%, RR = 0.22, 95%CI = 0.06, 0.74).
Conclusions:
Although patients with antidepressant-associated RBD have a lower risk of neurodegeneration than patients with “purely-idiopathic” RBD, markers of prodromal neurodegeneration are still clearly present. Development of RBD with antidepressants can be an early signal of an underlying neurodegenerative disease.
Citation:
Postuma RB; Gagnon JF; Tuineaig M; Bertrand JA; Latreille V; Desjardins C; Montplaisir JY. Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal? SLEEP 2013;36(11):1579-1585.
Keywords: REM sleep behavior disorder, antidepressants, Parkinson disease
INTRODUCTION
REM sleep behavior disorder (RBD) is a parasomnia characterized by loss of the normal atonia of REM sleep, such that patients appear to act out their dreams.1 Behaviors include yelling, laughing or crying, complex voluntary movements, falling out of bed, and even violent behaviors with injury. RBD is not rare—the prevalence of severe and violent RBD is approximately 1/2002; milder forms may increase this estimate considerably. Most patients with RBD do not seek medical attention, and lack of awareness among physicians can exacerbate this under-recognition.1 RBD has received considerable recent attention because it is strongly associated with synuclein-mediated neurodegenerative diseases such as Parkinson disease (PD), dementia with Lewy Bodies (DLB), and multiple system atrophy. Of special interest, RBD can predict these diseases; patients with idiopathic RBD have > 50% risk of developing neurodegenerative disease over 10 years.3–6 Therefore, the majority of idiopathic RBD patients in sleep clinics are actually in prodromal stages of neurodegenerative disease. The very high conversion rate to defined disease, combined with the long latency to disease make RBD patients unique as windows into early stages of neurodegenerative disease, and as potential candidates for development and eventual use of neuroprotective therapy.7
Whereas RBD is often idiopathic, numerous studies have found that antidepressants can produce dream-enactment behavior and loss of normal REM sleep atonia. This is common; symptoms of dream enactment occur in up to 6% of patients prescribed antidepressants (prevalence is higher in older patients).8–17 The reason for this connection is unclear, particularly in relation to neurodegenerative disease. There are several potential mechanisms, each of which would have different effects upon neurodegeneration risk and the presence of neurodegenerative markers. These include:
Antidepressants independently cause an idiopathic-like RBD disorder that is completely independent of associated synucleinopathy. If this were the case, the risk of neurodegenerative disease would be low, and there would be few signs of neurodegenerative synucleinopathy in RBD patients taking antidepressants.
Antidepressants do not cause RBD per se, but augment/ trigger an RBD that is subclinical, resulting in an earlier clinical presentation than would otherwise have occurred. If this were the case, risk of neurodegenerative disease would be lower than in pure idiopathic RBD, but markers of neurodegeneration would be present (although abnormalities may be less advanced than in other idiopathic RBD patients).
Within RBD, antidepressants are simply a proxy marker of another prodromal sign of neurodegeneration (i.e., depression18–20). If this were the case, risk of disease may be higher than in idiopathic RBD, and markers would be present to at least the same degree (or more) than the rest of the idiopathic RBD cohort.
Given that antidepressants are commonly prescribed in the general population, and given the profound impact of synuclein-mediated neurodegenerative diseases, understanding the connection between antidepressants and RBD is of considerable clinical and scientific importance. Since 2004, we have been prospectively following a large cohort of patients with idiopathic RBD21–23 to assess risk of disease and test predictive markers of neurodegeneration. We therefore compared neurodegenerative markers in RBD patients taking antidepressants, and then prospectively assessed neurodegenerative disease risk over an 8-year follow-up.
METHODS
Patients with idiopathic RBD were recruited from the sleep disorders laboratory at the Hôpital du Sacré Coeur, Montreal, Quebec. Ethics approval was obtained from the research ethics board, and all patients provided written informed consent. RBD was defined according to standard ICSD-II criteria24 as excessive chin EMG activity during REM sleep,25 and either history of elaborate motor activity during sleep associated with dream content or documentation of behavioral manifestations occurring during REM sleep on polysomnographic recording. All patients were asked to hold all medications and substances used for sleep for 2 weeks before the recording, including sedating antidepressants used for insomnia (patients could continue other antidepressants). Controls were selected from the general population, and were frequency-matched for age and sex. All controls had a PSG documenting the absence of RBD.
Baseline characteristics, ancillary testing, and neurologic follow-up of the cohort have been described extensively elsewhere.26,27 All evaluations were performed by a movement disorders neurologist (RP) and a neuropsychologist (JAB, CD, or JFG), specifically testing markers of early neurodegenerative disease. All participants underwent a systematic medical history and a complete neurological examination that included all components of the Unified Parkinson Disease Rating Scale (UPDRS).28 Three additional quantitative motor indices were used23: the alternate tap test,29 the Purdue Peg Board,30 and the “timed up and go” test.31 Olfaction was assessed with the brief 12-item Cross-Cultural Smell Identification Test (UPSIT-12).22,32 Color vision testing was performed using the Farnsworth-Munsell 100 (FM-100) Hue test.22,33,34 Symptoms of autonomic dysfunction were assessed with a structured clinical interview based upon the multiple system atrophy rating scale; orthostatic symptoms, urinary dysfunction, constipation, and erectile dysfunction were graded from 0-4.35 Blood pressure was measured in the supine position and after standing for one minute, and the orthostatic systolic blood pressure drop was calculated. Cognition was assessed with complete neuropsycho-logical examination and diagnosis of mild cognitive impairment was made according to single or multiple domains, as described elsewhere.27,36 Depression was assessed with the UPDRS Part I (annually) the Beck Depression Inventory (baseline only), and by self-report of physician-diagnosed depression (annually).
Patients were then followed on an annual basis, with the same comprehensive protocol to assess development of a defined neurodegenerative disease (recruitment into the cohort is rolling, so maximum potential follow-up duration ranged from 1-8 years). On annual examinations, parkinsonism was diagnosed according to the UK brain bank criteria bradykinesia in association with rest tremor, rigidity, or postural instability.9,37 Diagnosis of dementia was determined by consensus between the neuropsychologist (JFG) and the neurologist (RP), according to Movement Disorders Criteria for PD dementia and consensus criteria for DLB.38,39
All patients who took antidepressants at baseline examination or were taking antidepressants at the time of RBD symptom onset were included in the antidepressant group. In the primary analysis, all patients with antidepressant use at baseline (i.e., “antidepressant-associated” RBD) were included, regardless of temporal relationship to RBD symptoms (because the time of RBD symptoms can be unreliable for many patients, and also to allow evaluation of a potential role of antidepressants as proxy disease marker). However, those with reported RBD symptom onset > 1 year before disease were excluded from a subgroup analysis assessing possible “antidepressant-triggered” RBD. For comparison of neurodegenerative markers, statistical analysis was performed using logistic regression, adjusting for age and sex (all analyses 2-tailed). For estimating risk of neurodegenerative disease, analysis was with Cox proportional hazards (antidepressant use as independent variable), also adjusting for age and sex.
RESULTS
One hundred patients were evaluated and met full criteria for RBD (Table 1). Of these, 27 reported antidepressant use at or before baseline examination and 73 did not. Seven took paroxetine, 4 sertraline, 5 citalopram, 1 escitalopram, 5 venlafaxine, 1 mirtazapine, 1 phenelzine, 3 buspirone (2 of whom took other antidepressants), 1 clomipramine, 1 amitriptyline, 1 trazodone, and 1 desipramine. There was no difference in age or sex between those taking or not taking antidepressants; mean age of those taking antidepressants was 64.5 ± 10.5 years. Of the 27 with reported antidepressant use, 8 reported probable onset of RBD symptoms > 1 year before initial prescription of antidepressant—these were removed in a separate subgroup analysis. All 100 were evaluated in a full baseline neurological evaluation; in addition, 84 completed additional prospective follow-up ≥ 1 year (mean = 4.5 years to September 2012, range = 1-8 years). For comparison of markers of neurodegeneration, 44 age- and sex-matched controls were also evaluated.
Table 1.
Neurodegenerative markers in RBD patients according to antidepressant use
Are antidepressants associated with neurodegenerative markers in RBD?
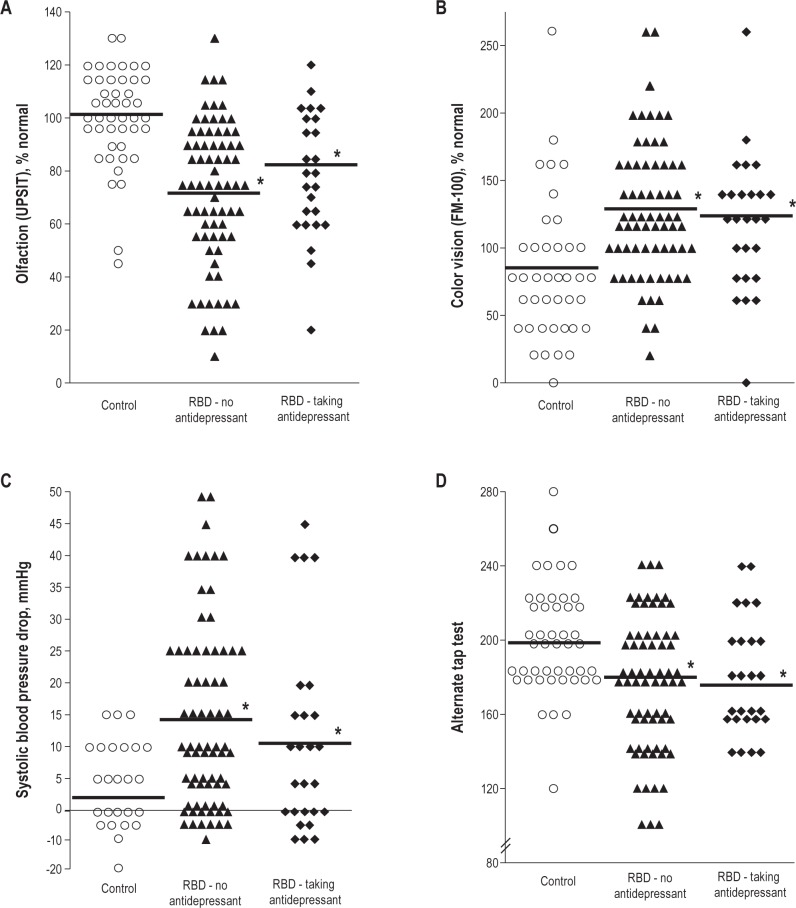
If antidepressants cause an RBD syndrome unrelated to underlying synucleinopathy, then other markers of neurodegenerative synucleinopathy should be absent (i.e., similar to age-matched controls). However, patients with antidepressant-associated RBD demonstrated significant abnormalities of neurodegenerative markers compared to controls (see Figure 1). Patients demonstrated mild motor slowing on all quantitative tests of motor function (UPDRS Part III = 3.5 ± 2.8 vs. 1.8 ± 2.1 [P = 0.007], alternate tap test 179.1 ± 30.9 vs. 199.5 ± 28.9 taps [P = 0.002], Purdue Pegboard 11.3 ± 2.5 vs. 12.3 ± 2.1 pegs [P = 0.007], and timed up-and-go 6.6 ± 1.1 vs. 6.1 ± 1.0 seconds [P = 0.003]). Olfactory function, possibly the most well-established non-motor predictor of Parkinson disease22,40–43 was decreased compared to controls (UPSIT-12 = 8.1 ± 2.5 vs. 9.7 ± -2.3, P = 0.007). Consistent with RBD as a predictor of DLB, patients had a higher prevalence of mild cognitive impairment on neuropsychological examination (60% vs. 13%, P < 0.001). Color vision, a predictor of neurodegenerative disease in RBD (especially DLB),22,44 was also abnormal compared to controls (FM-100 error score = 144.4 ± 54.8 vs. 98.5 ± 74.2, P = 0.004). Rating scales of constipation, a known prodromal marker of PD45–47 were increased (symptom score = 0.81 ± 0.99 vs. 0.16 ± 0.45, P = 0.003), as was orthostatic blood pressure drop (0.029), and erectile dysfunction symptom scores (1.8 ± 1.6 vs. 0.40 ± 0.96, P = 0.002). RBD patients taking antidepressants had increased depressive symptoms (Beck Depression Inventory = 15.2 ± 7.3 vs. 5.1 ± 3.8, P < 0.001). Notably, there were no significant differences in any variable between RBD patients taking vs. not taking antidepressants, with the exception of orthostatic symptoms (more severe in patients taking antidepressants [P = 0.042]) and depression symptoms (more severe in patients taking antidepressants). Removing patients with an unlikely temporal relation for antidepressant-triggered RBD did not alter results (Table S1). Patients taking antidepressants had higher percentage of phasic (but not tonic) REM sleep EMG activity (P = 0.007), consistent with a direct effect of antidepressants upon phasic REM.
Figure 1.
Selected prodromal markers of synuclein-mediated neurodegeneration in controls and in idiopathic RBD patients according to antidepressant use. Olfaction (A), color vision (B), orthostatic blood pressure drop (C), motor speed (alternate tap test) (D). For figure legibility, individual results have been rounded to the nearest integer. *Significant difference compared to controls (there were no significant differences between RBD patients taking vs. not taking antidepressants).
Therefore, there was clear evidence of abnormalities of numerous markers of synuclein-mediated neurodegeneration in our patients with antidepressant-RBD. These were indistinguishable from RBD patients without associated antidepressant use.
Do RBD patients taking antidepressants have the same risk of neurodegeneration?
Although RBD patients with antidepressant use might have evidence of prodromal neurodegenerative synucleinopathy, this does not necessarily imply that disease risk would be the same. It is possible that antidepressants triggered a clinical presentation of RBD that occurred earlier than it would have if antidepressants were not used. If so, risk of disease would remain, but might be lower than in those without this symptomatic trigger.
Over 8 years follow-up, 36 patients developed a defined neurodegenerative syndrome. Eighteen developed primary parkinsonism (PD = 15, possible multiple system atrophy = 3), and 18 developed dementia (all 18 met possible DLB criteria,39,48 13 met probable DLB criteria). Over the total prospective period, 32/61 (52%) patients not taking antidepressants developed neurodegenerative disease, compared to 4/23 (17%) of those taking antidepressants (P = 0.006, Fischer exact test). On Kaplan-Meier survival analysis, the estimated 5-year risk of developing neurodegeneration in patients not taking antidepressants was 59%, compared to 22% if taking antidepressants (RR = 0.22, 95% CI = 0.06, 0.74 - see Figure 2). The 7-year risk was 84% compared to 22%; however, numbers at these longer intervals were low (7 and 5 in each group). Removal of those who reported RBD symptom onset > 1 year before first prescription of antidepressants did not appreciably change results (59% vs. 32%). Self-report of diagnosed depression did not significantly predict risk of developing defined neurodegeneration (5-year risk with depression = 41%, without depression = 53%, P = 0.23). Cox proportional hazards analysis, adjusting for age and sex, found a significant association between antidepressants and a lower risk of developing defined neurodegeneration (adjusted RR = 0.30, 95% CI = 0.10, 0.86, P = 0.026).
Figure 2.
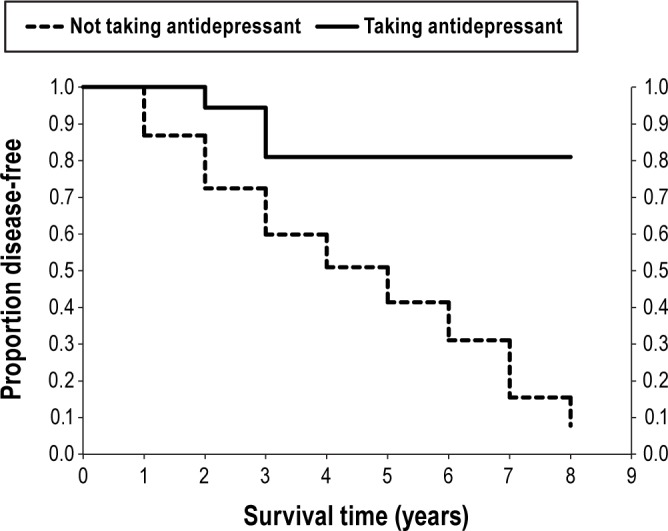
Kaplan-Meier life table analysis of disease-free survival in patients with idiopathic RBD according to history of antidepressant use. Cox proportional hazards P = 0.016 for antidepressant vs. no antidepressant.
Therefore, although some RBD patients taking antidepressants eventually developed a defined neurodegenerative synucleinopathy, risk was lower than for other idiopathic RBD patients.
DISCUSSION
An association between antidepressants and RBD has been documented by numerous groups.8–15 Given the vast number of antidepressants prescribed worldwide, it is of critical clinical importance to understand whether RBD occurring with prescription of antidepressants is a relatively benign side effect, or is a marker of prodromal neurodegenerative disease that requires further evaluation and follow-up. The existence of a large RBD cohort extensively evaluated for neurodegenerative markers, combined with a prospective longitudinal follow-up, allowed us to test possible explanations for the association between RBD and antidepressants. If antidepressants were associated with a separate “purely pharmacologic” syndrome, we would have anticipated a lower risk of neurodegeneration (observed), but also few ancillary signs of neurodegeneration (not observed). If antidepressants simply marked depression or anxiety as a prodromal marker of neurodegeneration, we would have anticipated ancillary signs of neurodegeneration (observed), but also a higher (or at least equal) risk of developing neurodegenerative disease (not observed). The finding of a lower risk of neurodegenerative disease, yet combined with clear evidence of markers of neurodegeneration suggests that antidepressants primarily trigger early clinical presentation of an RBD that is nonetheless still due to underlying neurodegeneration.
Our finding is consistent with previous observations that although clinical RBD can be triggered by antidepressants, withdrawal of antidepressants may not reverse the loss of REM sleep atonia.13,14 Therefore, these studies suggest that antidepressants unmask an already-present subclinical loss of REM sleep atonia (which persists after antidepressant withdrawal). It is also consistent with the fact that only a minority of patients taking antidepressants present with clinical RBD, and that this is more common in older individuals (who would be more likely to have an underlying neurodegenerative disease).11 Studies systematically assessing emergence of dream enactment with new antidepressant prescription would be of considerable interest, to assess the true risk of RBD and risk factors for its development.
These results have important clinical implications. Anti-depressants are among the most commonly-prescribed medications in clinical medicine, commonly used for both depression and anxiety. Symptoms of RBD will usually not be mentioned by patients, and even when reported may be dismissed by health professionals as a benign or “normal” phenomenon. Our findings suggest that the dream enactment behavior occurring with antidepressant use may be an important signal of an impending neurodegenerative disease, especially in older individuals (significance in young individuals is unclear, as they were not represented in this cohort). Therefore, surveillance for this side effect is warranted, and if detected, requires diagnostic evaluation by referral to a qualified sleep center. If RBD is confirmed, referral to a neurologist for prospective clinical follow-up may be recommended to detect and treat early manifestations of disease (although no established neuroprotective therapy to prevent PD is available, early symptomatic treatment can considerably improve quality of life49). Among sleep centers already treating patients with RBD, patients taking antidepressants might be advised that their risk of neurodegenerative disease is lower than in other RBD patients (possibly simply because their antidepressants triggered earlier clinical presentation of their prodromal disease). Nevertheless, clinical follow-up is still warranted, given that a neurodegenerative etiology for their symptoms may still be present. As a final clinical point, since antidepressants also substantially reduce REM sleep time, they can also paradoxically reduce RBD or withdrawal of antidepressants can augment symptoms (note that all patients in our study had full clinical symptoms of RBD).
Some limitations should be noted. We used a broad panel of markers of neurodegenerative disease to assess underlying synucleinopathy; although olfactory, color vision, constipation, and motor markers are established markers of prodromal synucleinopathy,22,23 some autonomic and cognitive markers have not yet been proven to mark disease. Therefore the significance of these latter abnormalities is less certain. Our study analyzed overall outcomes and neurodegenerative markers in the aggregate—we aimed to assess the predominant pattern of antidepressant-associated RBD, without assuming that all patients have the same underlying pathophysiology. The interaction between depression, antidepressants and RBD is complex,14,16,50,51 and it is certainly plausible that our cohort contained a smaller subset of individuals with a “pure antidepressant-caused” RBD that was not associated with synucleinopathy, or a small subset of patients for whom antidepressants marked depression as a prodromal symptom. Note that patients taking antidepressant still had higher Beck scores despite treatment—this may not be surprising, considering that medications may not be completely efficacious. It does raise the possibility that depression may have confounded the cognitive examination (which, however, would have resulted in a higher likelihood of dementia). Of note, our cohort was older and male-predominant (as are most cohorts with idiopathic RBD4,6,52), and did not include the young, predominantly female antidepressant users reported by other groups8,10 (we had 5 patients under 50, only one of whom was female); such a subgroup certainly may have a separate pathophysiology. Again, it would be of considerable interest in the future to prospectively evaluate patients in psychiatry clinics to systematically assess the prevalence and risk factors for emergence of RBD symptoms with new antidepressant prescription. Mainly for compliance and ethical issues, we did not systematically evaluate results of polysomnography with and without antidepressants. Finally, the sample size was too small to provide sufficient power to assess subgroups such as those with young-onset RBD, women, or patients taking different types of antidepressants.
Although patients with antidepressant-associated RBD have a lower risk of neurodegeneration than those without antidepressants, markers of prodromal neurodegeneration are still clearly present. This suggests that antidepressants predominantly trigger clinical presentation of a previously subclinical loss of REM sleep atonia due to an underlying synucleinopathy. Dream-enactment behavior occurring with antidepressants may be an important early signal of neurodegenerative disease.
DISCLOSURE STATEMENT
This study was supported by the Fonds de la Recheche en Sante Quebec and the Canadian Institute of Health Research; the authors have no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years. Dr. Postuma has received travel and speaker fees from Novartis Canada and Teva Neurosciences, and has received research support from the Fonds de la Recherche en Santé du Québec, the Parkinson Society of Canada, the Webster Foundation, and from the Canadian Institutes of Health Research. Dr. Gagnon has received research support from the Fonds de la Recherche en Santé du Québec, and from the Canadian Institutes of Health Research. Dr. Montplaisir has received compensation as a consultant to Impax Pharma, Servier, Jazz, Merk, and Valeant; speaker fees from Valeant; research support from Merck, GlaxoSmithKline, the Fonds de la Recherche en Santé du Québec, and the Canadian Institutes of Health Research. The other authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
Neurodegenerative markers in patients with antidepressants according to temporal relation to RBD symptoms
REFERENCES
- 1.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in sleep. Sleep. 2002;25:120–38. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, Caulet M, Priest RG. Violent behavior during sleep. J Clin Psychiatry. 1997;58:369–76. [PubMed] [Google Scholar]
- 3.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–93. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 4.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 5.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenck CH, Boeve B, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older males initially diagnosed with idiopathic REM sleep behavior disorder (RBD): 16 year update on a previously reported series. Sleep Med. 2013;14:744–8. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Postuma RB, Gagnon JF, Montplaisir J. Clinical prediction of Parkinson's disease - planning for the age of neuroprotection. J Neurol Neurosurg Psychiatry. 2010;81:1008–13. doi: 10.1136/jnnp.2009.174748. [DOI] [PubMed] [Google Scholar]
- 8.Teman PT, Tippmann-Peikert M, Silber MH, Slocumb NL, Auger RR. Idiopathic rapid-eye-movement sleep disorder: associations with antidepressants, psychiatric diagnoses, and other factors, in relation to age of onset. Sleep Med. 2009;10:60–5. doi: 10.1016/j.sleep.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Lam SP, Fong SY, Ho CK, Yu MW, Wing YK. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69:1374–82. doi: 10.4088/jcp.v69n0904. [DOI] [PubMed] [Google Scholar]
- 10.Ju YE, Larson-Prior L, Duntley S. Changing demographics in REM sleep behavior disorder: possible effect of autoimmunity and antidepressants. Sleep Med. 2011;12:278–83. doi: 10.1016/j.sleep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Winkelman JW, James L. Serotonergic antidepressants are associated with REM sleep without atonia. Sleep. 2004;27:317–21. doi: 10.1093/sleep/27.2.317. [DOI] [PubMed] [Google Scholar]
- 12.Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67:742–7. doi: 10.1212/01.wnl.0000233926.47469.73. [DOI] [PubMed] [Google Scholar]
- 13.Schenck CH, Mahowald MW, Kim SW, O'Connor KA, Hurwitz TD. Prominent eye movements during NREM sleep and REM sleep behavior disorder associated with fluoxetine treatment of depression and obsessive-compulsive disorder. Sleep. 1992;15:226–35. doi: 10.1093/sleep/15.3.226. [DOI] [PubMed] [Google Scholar]
- 14.Lam SP, Zhang J, Tsoh J, et al. REM sleep behavior disorder in psychiatric populations. J Clin Psychiatry. 2010;71:1101–3. doi: 10.4088/JCP.l05877gry. [DOI] [PubMed] [Google Scholar]
- 15.Frauscher B, Gschliesser V, Brandauer E, et al. REM sleep behavior disorder in 703 sleep-disorder patients: the importance of eliciting a comprehensive sleep history. Sleep Med. 2010;11:167–71. doi: 10.1016/j.sleep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Lam SP, Li SX, Chan JW, et al. Does REM sleep behavior disorder exist in psychiatric populations? A clinical and polysomnographic case-control study. Sleep Med. 2013;14:788–94. doi: 10.1016/j.sleep.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Schenck CH, Hurwitz TD, Mahowald MW. REM sleep behavior disorder. Am J Psychiatry. 1988;145:652. doi: 10.1176/ajp.145.5.652a. [DOI] [PubMed] [Google Scholar]
- 18.Alonso A, Rodriguez LA, Logroscino G, Hernan MA. Use of antidepressants and the risk of Parkinson's disease: a prospective study. J Neurol Neurosurg Psychiatry. 2009;80:671–4. doi: 10.1136/jnnp.2008.152983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisskopf MG, Chen H, Schwarzschild MA, Kawachi I, Ascherio A. Prospective study of phobic anxiety and risk of Parkinson's disease. Mov Disord. 2003;18:646–51. doi: 10.1002/mds.10425. [DOI] [PubMed] [Google Scholar]
- 20.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–31. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845–51. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]
- 22.Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir J. Olfaction and color vision identify impending neurodegeneration in REM behavior disorder. Ann Neurol. 2011;69:811–8. doi: 10.1002/ana.22282. [DOI] [PubMed] [Google Scholar]
- 23.Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain. 2012;135:1860–70. doi: 10.1093/brain/aws093. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The international classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- 25.Montplaisir J, Gagnon JF, Fantini ML, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010;25:2044–51. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 26.Postuma RB, Gagnon JF, Vendette M, Montplaisir J. Markers of neurodegeneration in idiopathic REM sleep behavior disorder and Parkinson disease. Brain. 2009;132:2298–307. doi: 10.1093/brain/awp244. [DOI] [PubMed] [Google Scholar]
- 27.Gagnon JF, Vendette M, Postuma RB, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol. 2009;66:39–47. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- 28.Fahn S, Elton R, members of the UDC . The Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent developments in Parkinson's disease. Florham Park, NJ: MacMillan HealthCare Information; 1987. pp. 153–63. [Google Scholar]
- 29.Nutt JG, Lea ES, Van HL, Schuff RA, Sexton GJ. Determinants of tapping speed in normal control subjects and subjects with Parkinson's disease: differing effects of brief and continued practice. Mov Disord. 2000;15:843–9. doi: 10.1002/1531-8257(200009)15:5<843::aid-mds1013>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Desrosiers J, Hebert R, Bravo G, Dutil E. The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil. 1995;17:217–24. doi: 10.3109/09638289509166638. [DOI] [PubMed] [Google Scholar]
- 31.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 32.Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT) Laryngoscope. 1996;106:353–6. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Farnsworth D. The Farnsworth 100-hue test and dichotomous tests for color vision. J Optom Soc Am. 1943;33:568–78. [Google Scholar]
- 34.Kinnear PR, Sahraie A. New Farnsworth-Munsell 100 hue test norms of normal observers for each year of age 5-22 and for age decades 30-70. Br J Ophthalmol. 2002;86:1408–11. doi: 10.1136/bjo.86.12.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tison F, Seppi K, Sampaio C, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS) Mov Disord. 2004;19:1391–402. doi: 10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]
- 36.Massicotte-Marquez J, Decary A, Postuma RB, et al. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology. 2008;70:1250–7. doi: 10.1212/01.wnl.0000286943.79593.a6. [DOI] [PubMed] [Google Scholar]
- 37.Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain. 2012;135:1860–70. doi: 10.1093/brain/aws093. [DOI] [PubMed] [Google Scholar]
- 38.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–24. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 39.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 40.Postuma RB, Aarsland D, Barone P, et al. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov Disord. 2012;27:617–26. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- 41.Ross W, Petrovitch H, abbott RD, et al. Association of olfactory dysfunction with risk of future Parkinson's disease. Mov Disord. 2005;20(Supplement 10):S129–S30. [Google Scholar]
- 42.Berendse HW, Booij J, Francot CM, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson's disease patients' relatives with a decreased sense of smell. Ann Neurol. 2001;50:34–41. doi: 10.1002/ana.1049. [DOI] [PubMed] [Google Scholar]
- 43.Siderowf A, Jennings D, Connolly J, Doty RL, Marek K, Stern MB. Risk factors for Parkinson's disease and impaired olfaction in relatives of patients with Parkinson's disease. Mov Disord. 2007;22:2249–55. doi: 10.1002/mds.21707. [DOI] [PubMed] [Google Scholar]
- 44.Bertrand JA, Bedetti C, Postuma RB, et al. Color discrimination deficits in Parkinson's disease are related to cognitive impairment and white-matter alterations. Mov Disord. 2012;27:1781–8. doi: 10.1002/mds.25272. [DOI] [PubMed] [Google Scholar]
- 45.Savica R, Carlin JM, Grossardt BR, et al. Medical records documentation of constipation preceding Parkinson disease: A case-control study. Neurology. 2009;73:1752–8. doi: 10.1212/WNL.0b013e3181c34af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbott RD, Ross GW, Petrovitch H, et al. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord. 2007;22:1581–6. doi: 10.1002/mds.21560. [DOI] [PubMed] [Google Scholar]
- 47.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology. 2001;57:456–62. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 48.Postuma RB, Gagnon JF, Vendette M, Montplaisir JY. Idiopathic REM sleep behavior disorder in the transition to degenerative disease. Mov Disord. 2009;24:2225–32. doi: 10.1002/mds.22757. [DOI] [PubMed] [Google Scholar]
- 49.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 50.Lam SP, Li SX, Mok V, Wing YK. Young-onset REM sleep behavior disorder: Beyond the antidepressant effect. Sleep Med. 2012;13:211. doi: 10.1016/j.sleep.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 51.Wing YK, Li SX, Mok V, et al. Prospective outcome of rapid eye movement sleep behaviour disorder: psychiatric disorders as a potential early marker of Parkinson's disease. J Neurol Neurosurg Psychiatry. 2012;83:470–2. doi: 10.1136/jnnp-2011-301232. [DOI] [PubMed] [Google Scholar]
- 52.Boot BP, Boeve BF, Roberts RO, et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol. 2012;71:49–56. doi: 10.1002/ana.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neurodegenerative markers in patients with antidepressants according to temporal relation to RBD symptoms