Abstract
Study Objective:
To investigate the longitudinal relationships between actigraph-derived sleep duration, fragmentation, and lipid levels.
Design and Setting:
Longitudinal data from the Coronary Artery Risk Development in Young Adults Sleep Study (2003-05), an observational cohort at the Chicago site.
Participants:
There were 503 black and white adults, ages 32-51 years, with no prior history of cardiovascular disease.
Interventions:
N/A.
Measurement and Results:
Sleep duration and fragmentation were measured using 6 days of wrist actigraphy. Sleep quality was measured with the Pittsburgh Sleep Quality Index. The outcome variables, measured at 3 examinations over 10 years (Baseline [2000-01], 5-year [2005-06], and 10-year follow-up [2010-11]), were total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), and TC/HDL ratio. The associations between each sleep parameter and 10-year change in lipids were analyzed with generalized estimating equation models adjusting for relevant confounders. After adjustment, each hour increase in sleep duration was significantly associated with higher TC (5.2 mg/dL, 95%CI: 1.7, 8.6) and LDL (3.4 mg/dL, 95%CI: 0.2, 6.6) in the total sample, a 1.1 mg/dL increase in TG (95%CI: 1.0, 1.1) among men, and a borderline significant greater odds for a TC/HDL ratio ≥ 5 among men (OR: 1.37, 95%CI: 0.99, 1.90). Overall, sleep fragmentation and sleep quality scores were not associated with change in lipids.
Conclusions:
Beyond relevant covariates, over a 10-year follow-up, longer objective sleep duration was longitudinally and significantly associated with a poorer lipid profile. Greater objective sleep fragmentation and self-reported poor sleep quality were not related to a poorer lipid profile.
Citation:
Petrov MER; Kim Y; Lauderdale D; Lewis CE; Reis JP; Carnethon MR; Knutson K; Glasser SJ. Longitudinal associations between objective sleep and lipids: The CARDIA Study. SLEEP 2013;36(11):1587-1595.
Keywords: Sleep duration; sleep fragmentation, lipids, sleep quality, ethnicity, sex
INTRODUCTION
Over the last two decades, studies documented an association between insufficient and disrupted sleep with cardiovascular disease (CVD) and cardiovascular-related mortality.1–14 Because the mechanisms underlying these relationships are unclear, recent studies have investigated links between sleep and risk factors for CVD, particularly lipoproteins15–27 and triglycerides.15–21,25,26 These studies yielded inconsistent results and were limited by self-reported, retrospective sleep measures, little assessment beyond sleep duration, cross-sectional designs, and samples lacking ethnic diversity. Two cross-sectional studies examined the relationship between sleep and lipids using objective sleep measures,16,23 but little is known about the longitudinal relationships.
Our study group, using data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, prospectively demonstrated significant associations between shorter objective sleep duration or greater sleep fragmentation and incident coronary artery calcification28 and five-year change in blood pressure.29 In the present study we aimed to determine whether objectively measured sleep parameters are longitudinally associated with lipid profiles among persons without a prior history of CVD. We also tested whether these associations vary by sex and race because disrupted sleep and lipid levels vary by these groups.30,31
METHODS
Study Sample and Design
The CARDIA study was initiated across 4 US sites in 1985-86. The study recruited 18- to 30-year-olds balanced by age (18-24, 25-30 years), race (black, white), sex, and education level (< high school, ≥ high school) at each study site. The CARDIA study procedures have been described previously.32 The present analysis includes participants from the ancillary CARDIA Sleep Study at the Chicago site who were not pregnant during the year 15 (2000-01) clinical examination. Written consent was provided by 670 participants (82%). Three days of wrist actigraphy and self-reported sleep were collected in 2 waves one year apart between 2003 and 2005. Participants reporting a history of one or more of the following conditions at year 15 were excluded from the analyses: high blood pressure; heart problems; peripheral vascular disease; stroke or transient ischemic attack; blood clot in leg, veins, or lungs requiring blood thinning medicine; high systolic (≥ 140 mm Hg) and diastolic blood pressure (≥ 90 mm Hg); hypertension medication use; and lipid-lowering medication use. After exclusions, 503 participants qualified for the present study (75.1%). The term “baseline” in this study refers to data collected at the year 15 clinical examination. Longitudinal analyses investigated change in lipid levels across clinical examination years 15 (baseline), 20 (5-year follow-up), and 25 (10-year follow-up). For each examination, records were omitted from the analyses if participants reported being pregnant or using lipid-lowering medications. This approach maximized the number of participants with eligible records instead of excluding anyone who met exclusion criteria at the 3 time points. The ancillary CARDIA Sleep Study was approved by the institutional review boards at Northwestern University and the University of Chicago.
Outcome Measures
At baseline, 5-year, and 10-year follow-up, participants had blood drawn by certified technicians in the early to mid-morning after 12 h of fasting. Levels of total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides (TG) were measured at the Northwest Lipids Research Laboratory in Seattle, WA. Cholesterol and TG were analyzed with the Abbott Spectrum diagnostic system using the Trinder-type method and the ultraviolet method, respectively.33 LDL was computed with the Friedewald equation34 for only those participants with TG levels below 400 mg/dL. CARDIA uses an extensive quality control program to ensure comparability of lipid values across examination years.35 The TC/HDL ratio was also evaluated as a dichotomous variable (i.e., < 5.0 vs. ≥ 5.0) because the clinical recommendation by the American Heart Association is to maintain a ratio below 5 to 1.36 The TC/HDL ratio is a useful value for gauging a patient's CVD risk.
Sleep Measures
The 3 sleep measures were wrist actigraphy-derived average sleep duration and sleep fragmentation, and the global score from the Pittsburgh Sleep Quality Index (PSQI).37 The wrist actigraphs (Actiwatch-16, Mini-Mitter Inc, Bend, OR) were worn for up to 6 days from 2003 to 2005. The consistency in sleep duration and fragmentation values was high; therefore, the averages for all available days were computed.38 Wrist activity was measured in 30-sec epochs using highly sensitive omnidirectional accelerometers. Validated manufacturer software was used to calculate average sleep duration and fragmentation. Sleep duration was the time from sleep onset to final awakening minus the amount of time spent awake during the night. Sleep fragmentation was a measure of the restlessness of the sleep period, from sleep onset to sleep termination, by summing the percentage of time the participant was moving and the percentage of time spent immobile for ≤ 1 min to create a sleep fragmentation score. More detailed information on the actigraphy methods used were previously described.30 The PSQI is a well-validated measure of sleep quality and disturbances over the past month.37 Scores > 5 indicate poor sleep quality, and higher scores indicate greater severity (Range: 0-21).
Covariates
Sociodemographic covariates were age, sex, race (self-reported; black or white), income (self-reported; 4-level ordinal variable: < $16K; $16-34,999K; $35-74,999K; ≥ $75K), and education (self-reported; 3-level categorical variable: < high school; high school degree; some college or greater). Poor lipid risk factors were smoking status; physical activity; body mass index (BMI); alcohol use; symptoms of depression; C-reactive protein (CRP); history of diabetes, thyroid problems, or kidney problems; and sleep apnea risk.
Smoking status was classified as never smoked, former, or current smoker. Self-reported physical activity was assessed using the CARDIA Physical Activity History Questionnaire, an instrument that has demonstrated good psychometric properties.39 Intensity scores assigned to 13 measured physical activities were summated into a continuous measure of “exercise units.” Alcohol use was a continuous measure of the average mL/day. Symptoms of depression were measured with the Center for Epidemiologic Studies Depression scale (CES-D).40 CRP levels were determined using an ultrasensitive ELISA assay41 derived from purified protein and polyclonal anti-C-reactive protein antibodies (Calbiochem). History of diabetes, thyroid, or kidney problems was determined by self-report to the question “has a doctor or nurse ever said that you have…” any of these conditions. Apnea risk was determined by the Berlin Questionnaire.42 The Berlin Questionnaire categorizes individuals by risk for having sleep apnea if they endorse experiencing ≥ 2 of 3 of the following: frequent daytime sleepiness, loud or frequent snoring, and having either high blood pressure or BMI > 30 kg/m2. Since persons with high blood pressure were excluded from the study and BMI was already controlled for, only daytime sleepiness and snoring were controlled in the models.
Statistical Analyses
Sample characteristics for the whole sample and their relation to each continuous sleep variable were analyzed with one-way ANOVA for categorical variables and Pearson correlation coefficients for continuous variables. Linear regression models were used to investigate the associations between all continuous sleep parameters and lipid levels—including the TC/HDL ratio—at baseline after adjusting for covariates in a sequence of nested models. Model 1 consisted of the unadjusted relationship between each sleep parameter with lipid levels. Model 2 added sociodemographic factors to Model 1. Model 3 added poor lipid profile risk factors to Model 2. Given the time difference between baseline covariate assessment (2000-01) and the sleep assessment (2003-05), we also analyzed the associations using the 5-year follow-up examination as baseline data to determine any differences between the 2 analysis approaches. Sleep duration and fragmentation were not included in the same models because of a significant inverse relationship between them (r = -0.44, P < 0.001). TG, CRP, and physical activity values were log-normalized due to non-normal distributions.
Generalized estimating equation regression models were used to estimate the relation between each sleep parameter and 10-year change in lipids and the TC/HDL ratio, while controlling for the effect of time on lipids and the correlations between the 3 lipid measurements to correct the standard errors of the time-dependent covariates. The correction for the within-subjects correlations used an unstructured covariance structure. The same sequence of nested models described earlier was used in these analyses. To verify the presence of linear relationships, quadratic associations between sleep duration and lipids were also tested. We also assessed if the associations varied by race and race-sex groups by adding interaction terms into separate fully adjusted models. If significant interactions were discovered, then stratified analyses by race or race-sex groups were conducted while adjusting for all covariates including sex in sleep-race stratified models. Given known differences in lipid levels between men and women and change in lipids over time,31 sex-stratified models were conducted even in the absence of significant interactions. In the sex-stratified models, additional time-varying covariates were accounted for in the models on women. These covariates were hormone medication use (dichotomous “yes” or “no” variable), menopause status (i.e., postmenopausal or not), and oral contraceptive medication use (dichotomous “yes” or “no” variable). All regression coefficients were tested for significance at the 0.05 α level using 2-sided tests. Statistical analyses were performed using SAS 9.2 (SAS Institute, Inc., Gary, NC).
RESULTS
Sample Characteristics
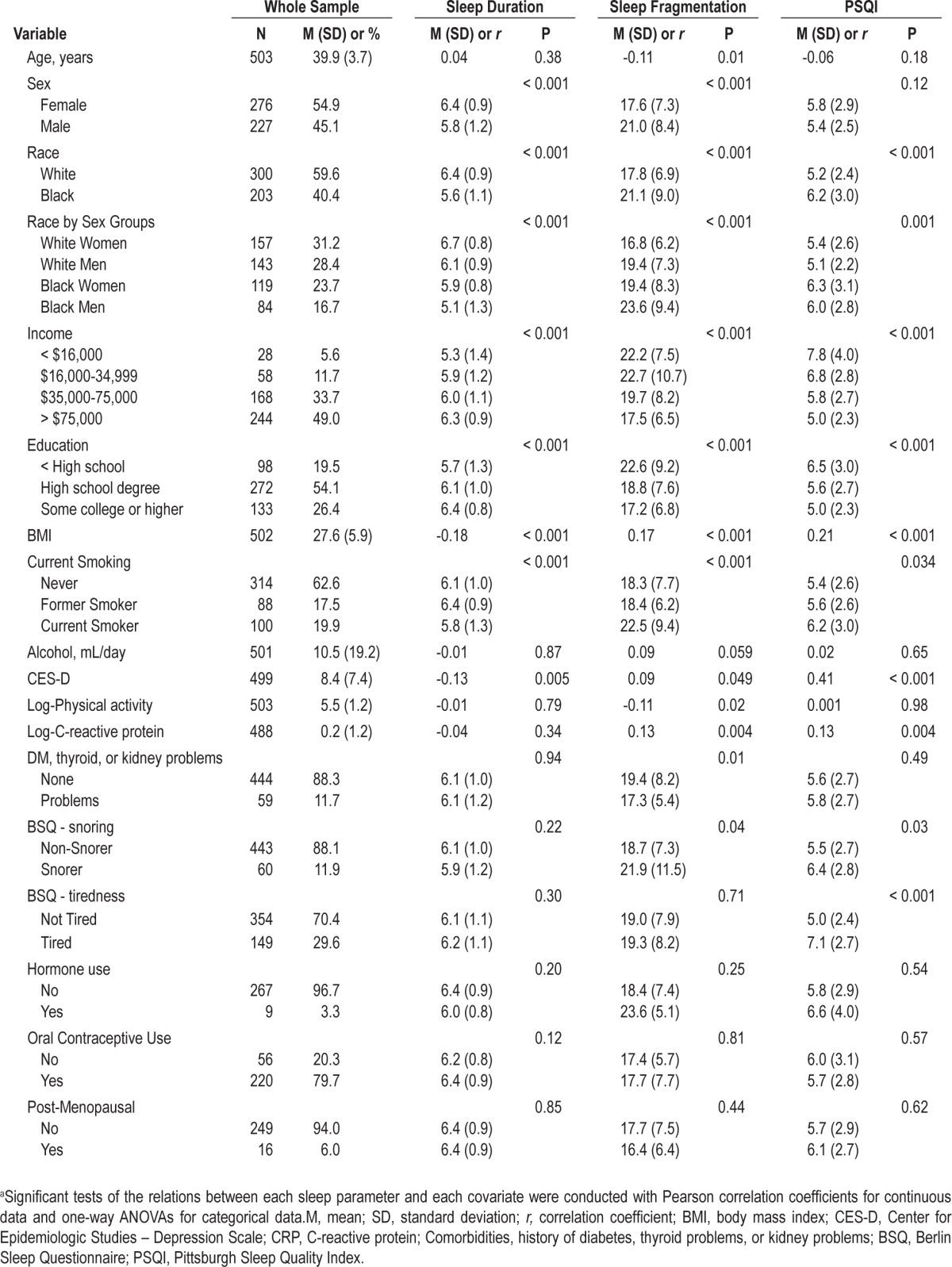
In Table 1, sample characteristics for all predictors and their relation to each sleep variable are displayed. Baseline mean age for the sample was 39.9 years (SD 3.7; range: 32-51). Women represented 54.9% of the sample (n = 276), and 40.4% were black (n = 203; black women n = 119; black men n = 84). Most participants reported moderate-to-high incomes and a high-school level of education or higher. At baseline, many of the covariates were significantly related to at least one of the sleep variables, with the exceptions of alcohol consumption in the total sample and reproductive-related factors among women.
Table 1.
Sample characteristics and their relation with each sleep parameter at baselinea
Sleep at Baseline and Lipids Changes over 10-year Follow-up
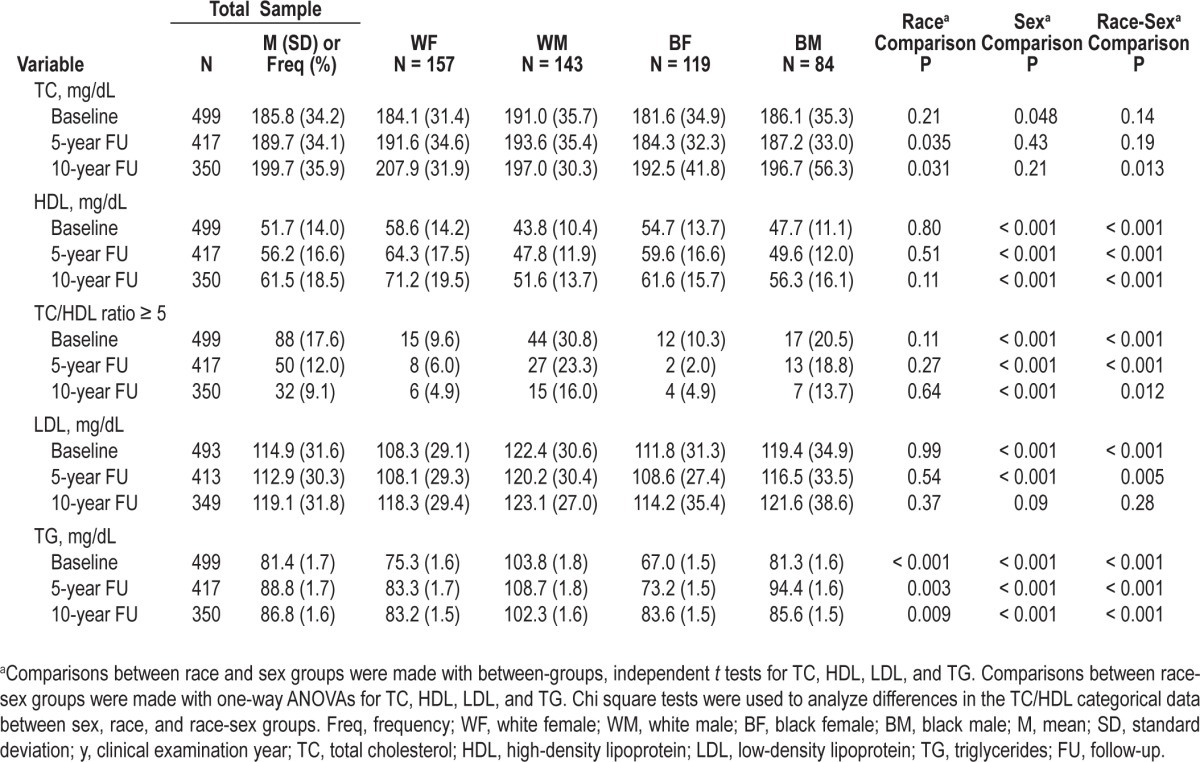
In the sample, 90.5% of the participants (n = 456) provided 6 nights of recorded sleep, and 99.0% (n = 499) provided ≥ 3 nights. The sample slept on average 6.1 h (SD 1.1, range: 1.8-8.8) per night with an average sleep fragmentation score of 19.1% (SD 8.0, range: 4.4-63.9). The sample perceived their sleep quality to be borderline poor (M = 5.6, SD 2.7, range: 0-16). Half of the sample reported poor sleep quality (> 5; n = 249). Table 2 displays changes in lipid levels for each examination year in the total sample and by race-sex groups. In general, all lipid levels increased over the 10-year interval, although the proportion of participants with a TC/HDL ratio ≥ 5.0 decreased. TC, LDL, and HDL increased the most in white women, and TG increased the most in black women. The proportion of participants with a TC/HDL ratio ≥ 5 reduced for all groups, but white males consistently had the highest proportion over time. Men had significantly greater TC and LDL than women at baseline, but this difference became nonsignificant by 10-year follow-up. However, race differences in TC emerged over time, with whites having greater TC than blacks. HDL over 10-year follow-up was consistently greater among women than men, with white females having the greatest levels. White males consistently had the greatest TG levels over time compared to all other race-sex groups.
Table 2.
Sleep and lipid level characteristics by examination year in the total sample and by race-sex groups
Linear Associations between Sleep Variables and Lipid Values at Baseline
Results from the analyses between the sleep variables and baseline and 5-year follow-up examination lipid values were similar. Therefore, we present the results of the linear models, using lipid data at baseline (2000-01) with the sleep data. Adjusting for all covariates, there was a significant positive association between sleep duration and baseline TC (3.6 mg/dL per hour, t456 = 2.1, P = 0.039). In addition there was a sex-sleep duration interaction on HDL (P = 0.018), and race-sleep fragmentation interactions on TC (P = 0.011) and LDL (P = 0.006). Stratified analyses, however, did not reveal any significant associations by subgroups (data not presented). There were no other significant associations or interactions noted.
10-year Longitudinal Associations
Sleep Duration
The associations between sleep duration and 10-year change in lipids in the total sample and by sex are described in Table 3. In the overall sample, each hour increase in sleep duration was significantly associated with a 5.2 mg/dL increase in TC (P = 0.003) and a 3.3 mg/dL per hour increase in LDL (P = 0.039) over 10 years in fully adjusted models. Longer sleep duration was also associated with a 27% greater odds of incident TC/HDL ratio ≥ 5.0 (95%CI: 0.99, 1.63) and a 1.0 mg/dL increase in TG in fully-adjusted models, but both results were borderline significant. There were no significant associations with HDL after adjustment. However, there was a significant interaction by sex for HDL (P = 0.031).
Table 3.
Sequentially adjusted association between one hour increments of sleep duration and 10-year change in lipids in the total sample and by sexa
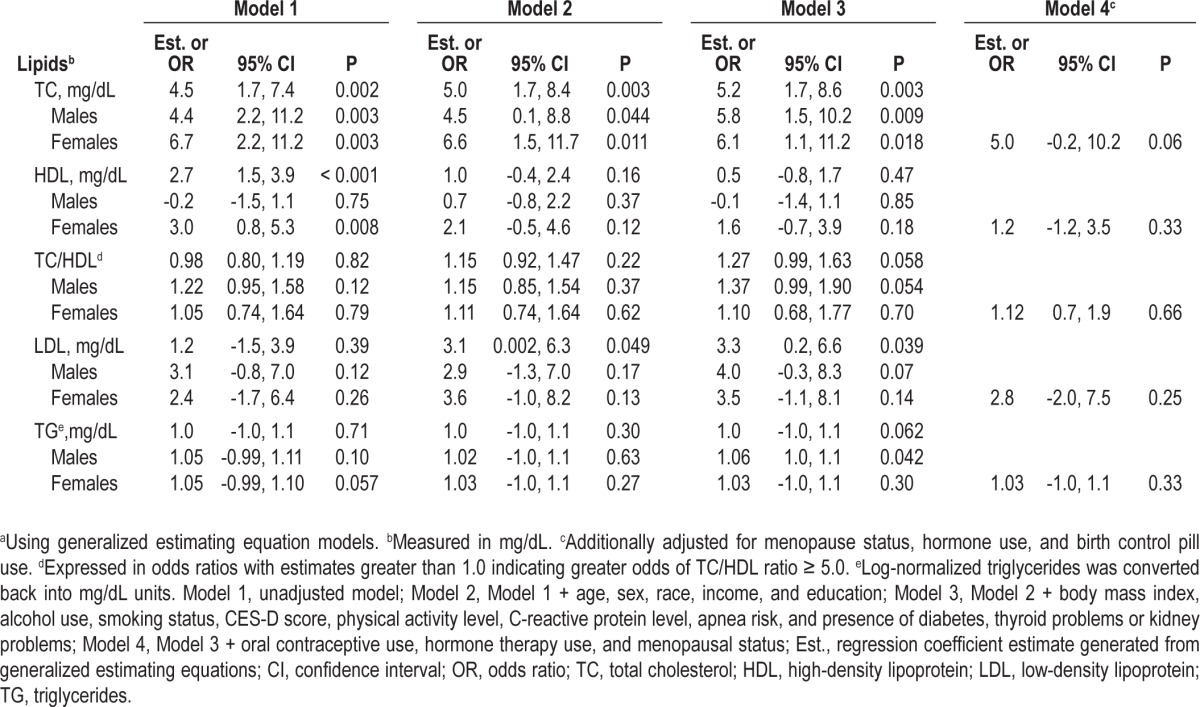
In sex-stratified analyses with additional adjustment for reproductive-related factors among women, an hour increase in sleep duration among women was associated with a 1.2 mg/ dL increase in HDL and a 0.1 mg/dL decrease among men. Neither of these associations reached statistical significance. In sex-stratified analyses for other outcomes, the positive association between sleep duration and TC remained significant for males but not significant for females once the model adjusted for reproductive-related factors (5.0 mg/dL, 95%CI: -0.2, 10.2, P = 0.06). Adjustment for reproductive-related factors among women also attenuated the association between sleep duration and LDL (2.8 mg/dL, 95%CI: -2.0, 7.5, P = 0.25). Among males, there was a borderline significant positive association between sleep duration and odds of TC/HDL ratio ≥ 5.0 (P = 0.054), and a significant positive association between sleep duration and TG (1.1 mg/dL, 95%CI: 1.0, 1.1, P = 0.042). There were no significant interactions by race or race-sex groups, nor were there any significant quadratic associations.
Sleep Fragmentation
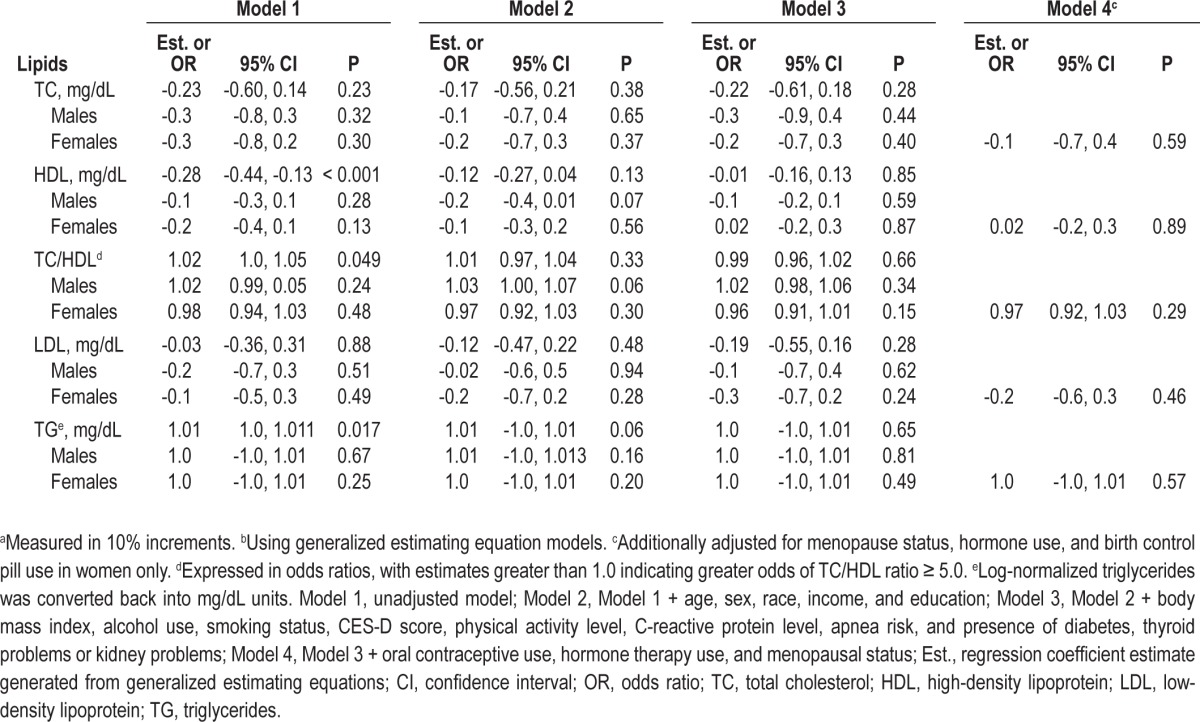
Table 4 displays the associations between sleep fragmentation and 10-year change in lipid levels in the total sample and by sex. Sleep fragmentation was not associated with change in lipid levels after full adjustment in the overall sample or by sex group. There were significant interactions by race for TC (P = 0.009) and LDL (P = 0.003). However, in race-stratified analyses, the separate associations between sleep fragmentation, TC, and LDL for whites and blacks were not significant (data not presented).
Table 4.
Sequentially adjusted association between sleep fragmentationa and 10-year change in lipids in the total sample and by sexb
PSQI
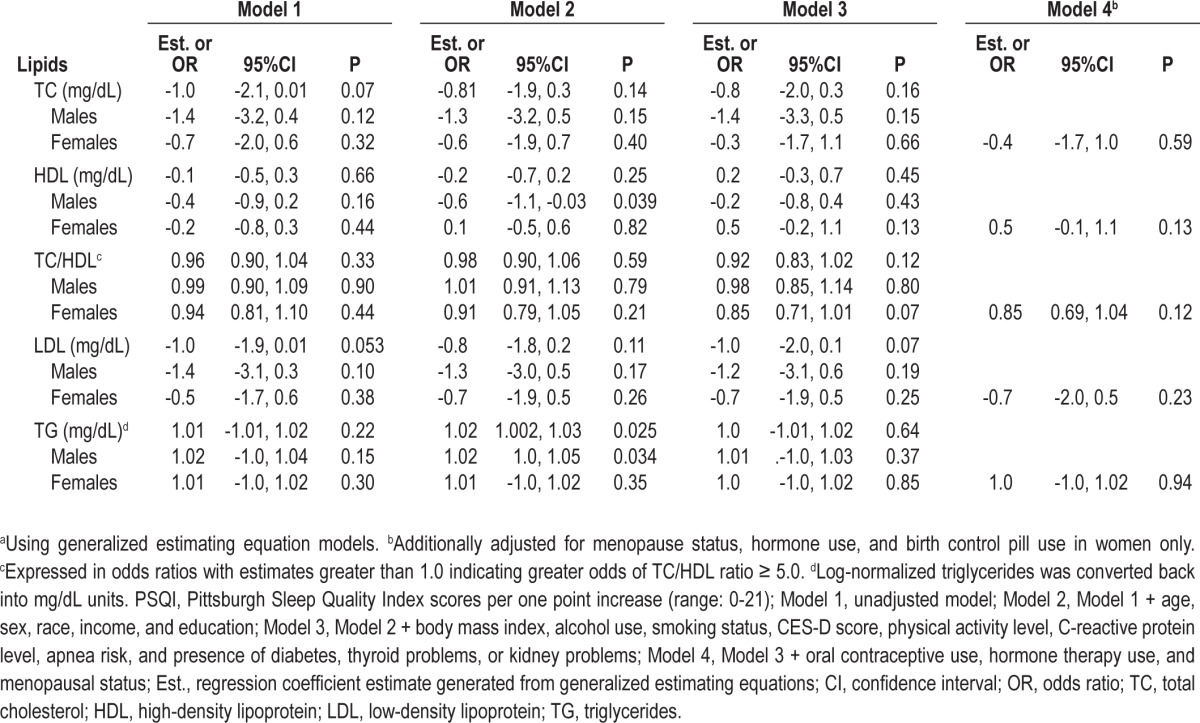
Table 5 displays the associations between PSQI scores and 10-year change in lipid levels in the total sample and by sex. PSQI scores were not significantly associated with change in lipid levels in the total sample. There were no interactions by sex, race, or race-sex groups.
Table 5.
Sequentially adjusted association between PSQI scores and 10-year change in lipids in the total sample and by sexa
DISCUSSION
In a sample of early middle-aged black and white adults free of CVD and lipid-lowering medications, we demonstrated that over 10 years, longer sleep duration was associated with significant increases in TC and LDL in the overall sample, significant increases in TG among men, and positive but not significant associations with TC/HDL ratio ≥ 5.0 among men. With further adjustment for reproductive-related factors, the positive associations between sleep duration and TC and LDL among women were attenuated. Sleep fragmentation was not related to 10-year increases in lipid levels in the overall sample. Poor self-reported sleep quality, as measured by PSQI scores, was not associated with 10-year changes in lipid levels.
Our study is one of the first to determine longitudinal associations between objectively derived sleep measures and lipid levels. While several significant and borderline significant associations were noted between the objective sleep measures and changes in lipids, none were found for self-reported sleep quality. This result is in concert with one other study that found nonsignificant associations between PSQI scores and HDL and TG in a cross-sectional, community-based study.22 Previous studies that examined the cross-sectional associations between self-reported sleep variables and lipids produced inconsistent results.15–27 These inconsistencies may have been due to the biased, retrospective nature of self-report measures. For example, self-reported sleep duration has been found to represent an overestimate of actual measured sleep by approximately one hour.30 All of these limitations may account for the inconsistent results in the literature, and the lack of significant associations between PSQI scores and changes in lipids compared to the objective measures in the present study. Furthermore, objective sleep may have a mechanistic role in changes in lipids over time, whereas poor sleep quality may reflect poor health in general rather than serve as a predictive risk factor.
Only two previous studies have measured objective measures of sleep in relation to lipids.16,23 Using polysomnography, Ekstedt and colleagues found among 24 adults aged 24-43 years that greater sleep fragmentation was cross-sectionally related to higher TC and LDL and lower HDL; while total sleep time was positively associated with LDL-to-HDL ratio, a clinical marker of CVD risk.16 The second study used actigraphic measures in 768 community-dwelling adults and determined greater sleep duration and time in bed were cross-sectionally related to higher TC and TC-to-HDL ratio in adults aged 57-65 years.23 Our findings were similar to the results for sleep duration found by both of these studies. All three studies used different age groups, suggesting that longer sleep duration is independently associated with higher cholesterol and LDL in young to middle-aged adults. It is important to note that the average sleep durations in these studies, including the present sample, were fairly low, and the maximums reported were not excessive. Thus, even modestly long sleep durations are related to alterations in lipid levels. In contrast, one longitudinal study found short sleep duration increased the odds of incident hypercholesteremia, but this study was among adolescents and hypercholesterolemia was self-reported.24
Although there is much evidence on the mechanisms that may explain a relationship between sleep loss and poor lipid profiles,43 the literature is scant on the biological mechanisms that elucidate the relationship between longer sleep duration and greater cholesterol levels. Sleep disordered breathing was hypothesized to be a confounder in the relationship,11 but our study controlled for subjective high risk of sleep disordered breathing. Causal pathways cannot be ascertained with the present study; however, we propose the following potential pathways: (a) a mediated relationship by physiological alterations in mechanisms of lipid metabolism; (b) mediated relationship by an unhealthy lifestyle, e.g., excessive time in bed, low physical activity, or high stress that induce longer sleep durations and detrimental lipid levels; or (c) high cholesterol induces sickness that affects sleep duration. In the context of our study, excessive time in bed is an unlikely mediator, given the short length of the mean sleep duration in the sample. Pathway (c) is also improbable because participants were relatively young, and free of CVD and lipid-lowering medications.
In this study, longer sleep duration was associated with significant 10-year increases in TC and LDL, but these associations were attenuated among women once reproductive-related factors were adjusted for in the model. Premenopausal women tend to have lower TC, LDL, and HDL concentrations than men, which have been related to differences in sex hormones and their effects on lipid metabolism; but as we age, these differences diminish.44,45 In the study sample, 10-year changes in lipid levels between men and women did tend to converge, with women approaching male levels. Longer sleep duration was related to increases in TC and LDL in women, suggesting sleep duration may have a role in this convergence beyond the effects of age and other cardiac risk factors. However, the literature suggests this convergence may be more related to the menopausal transition.46 Given the attenuation in the associations between sleep duration, TC and LDL when reproductive-related factors were accounted for in the models for women, it can be deduced that the menopausal transition may explain the increases in lipids more so than sleep duration. However, it may be possible that sleep duration modifies sex hormone concentrations during the menopausal transition, which in turn affects lipid metabolism. To date, there have been no experimental studies on the effects of sleep duration on sex hormones and metabolic control among women.
A major strength of our study is it addresses many voids present in the current literature. It also recruited an ethnically diverse sample with no baseline CVD and controlled for the symptoms of sleep disordered breathing; although this was assessed by self-report. The study also examined lipid profiles over a long follow-up time frame and used objective sleep metrics as predictors.
However, there are pertinent limitations to the study. The sample was relatively small and located at one regional site, which may attenuate the generalization of these results. The use of actigraph-assessed sleep was a definite improvement upon previous studies given its strong congruence with gold standard polysomnography.47 However, polysomnography would have allowed for a more accurate classification of sleep disordered breathing, and the examination of sleep stages. While self-reported symptoms of sleep disordered breathing with the Berlin Questionnaire improved our model, it is not a true substitute for validated diagnosis. Our study also suggests that objective sleep parameters may be predictive of lipid changes whereas self-reported sleep parameters are not. This result further implies that an objective measure of sleep disordered breathing may be necessary for accurate estimation in future studies. Despite the robust associations found between sleep and lipid levels over time, causality also cannot be substantiated. Sleep was also measured after the original study's defined “baseline.” To mitigate this limitation we evaluated cross-sectional associations between sleep and 5-year follow-up lipid values and found the results to be equivalent to the results using baseline lipid values, suggesting similar longitudinal results would have likely been procured using this data (data not presented). Unmeasured and residual confounding factors, such as self-reported stress, may have also imposed changes in the present results.
In conclusion, our study found over a ten-year period that longer sleep duration is related to significant increases in LDL and TC across race-sex groups, and significant increases in TG among men. Future projects should examine the biological mechanisms that may explain the association between objective sleep and lipid profiles across ethnically diverse high risk groups for CVD.
DISCLOSURE STATEMENT
This was not an industry supported study. Poster presented at the Academy Health 2012 Annual Research Meeting, Orlando, FL, 6/25/2012. Dr. Petrov receives training support from AHRQ (5 T32 HS013852-09) and NCMHD (3 P60 MD000502-08S1). Dr. Lewis receives funding from Novo Nordisk. Dr. Glasser receives salary support from AMGON. The other authors have indicated no financial conflicts of interest. CARDIA is supported by US Public Health Service contracts NO1-HC-48047, NO1-HC-48048, NO1-HC-48049, NO1-HC-48050, and NO1-HC-95095 from the National Heart, Lung, and Blood Institute, and grant AG 11412 from the National Institute on Aging. Yongin Kim had full access to the data and is responsible for the data analyses presented.
REFERENCES
- 1.Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: The MORGEN study. Sleep. 2011;34:1487–92. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eguchi K, Pickering TG, Schwartz JE, et al. Short sleep duration as an independent predictor of cardiovascular events in Japanese patients with hypertension. Arch Intern Med. 2008;168:2225–31. doi: 10.1001/archinte.168.20.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 6.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: Analyses of the First National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 7.Ferrie JE, Shipley MJ, Franscesco P, et al. A prospective study of change in sleep duration: Associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–66. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 9.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 10.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 11.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 12.Lanfranchi PA, Pennestri MH, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep. 2009;32:760–6. doi: 10.1093/sleep/32.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3:489–94. [PMC free article] [PubMed] [Google Scholar]
- 14.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haseli-Mashhadi N, Dadd T, Pan A, Yu Z, Lin X, Franco OH. Sleep quality in middle-aged and elderly Chinese: distribution, associated factors and associations with cardio-metabolic risk factors. BMC Pub Health. 2009;9:130. doi: 10.1186/1471-2458-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekstedt M, Åkerstedt T, Söderström S. Microarousals during sleep are associated with increased levels of lipids, cortisol, and blood pressure. Psychosom Med. 2004;66:925–31. doi: 10.1097/01.psy.0000145821.25453.f7. [DOI] [PubMed] [Google Scholar]
- 17.Bjorvatn B, Sagen EM, Øyane N, et al. The association between sleep duration, body mass index and metabolic measures in the Hordaland Health Study. J Sleep Res. 2007;16:66–76. doi: 10.1111/j.1365-2869.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams CJ, Patel SR, Hu FB, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with Type 2 diabetes. Diabetes Care. 2007;30:1233–40. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 19.Kaneita Y, Uchiyama M, Yoshiike N, Ohida T. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 2008;31:645–52. doi: 10.1093/sleep/31.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerkhofs M, Boudjeltia KZ, Stenuit P, Brohée D, Cauchie P, Vanhaeverbeek M. Sleep restriction increases blood neutrophils, total cholesterol and low density lipoprotein cholesterol in postmenopausal women: a preliminary study. Maturitas. 2007;56:212–15. doi: 10.1016/j.maturitas.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jennings JR, Muldoon MF, Hall M, Buysse DJ, Manuck SB. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30:219–23. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- 23.Van Den Berg JF, Miedema HM, Tulen JH, et al. Long sleep duration is associated with serum cholesterol in the elderly: the Rotterdam Study. Psychosom Med. 2008;70:1005–11. doi: 10.1097/PSY.0b013e318186e656. [DOI] [PubMed] [Google Scholar]
- 24.Gangwisch JE, Malaspina D, Babiss LA, et al. Short sleep duration as a risk factor for hypercholesterolemia: analyses of the National Longitudinal Study of Adolescent Health. Sleep. 2010;33:956–61. doi: 10.1093/sleep/33.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi KM, Lee JS, Park HS, Baik SH, Choi DS, Kim SM. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes. 2008;32:1091–7. doi: 10.1038/ijo.2008.62. [DOI] [PubMed] [Google Scholar]
- 26.Chaput JP, Després JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Québec Family Study. Obesity. 2007;15:253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 27.Sabanayagam C, Shankar A. Sleep duration and hypercholesterolaemia: results from the National Health Interview Survey 2008. Sleep Med. 2012;13:145–50. doi: 10.1016/j.sleep.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–66. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: The CARDIA Sleep study. Arch Intern Med. 2009;169:1055–61. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 31.Johnson JL, Slentz CA, Duscha BD, et al. Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis. 2004;176:371–7. doi: 10.1016/j.atherosclerosis.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 33.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. In: Albers JJ, Segrest JP, editors. Methods in enzymology. Plasma lipoproteins, Part B: characterization, cell biology, and metabolism (Volume 129, pp. 101-123) New York, NY: Academic Press, Inc.; 1986. [DOI] [PubMed] [Google Scholar]
- 34.Friedewald WT, Levy RI, Frederickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–501. [PubMed] [Google Scholar]
- 35.Bild DE, Jacobs DR, Liu K, et al. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: the CARDIA Study. Ann Epidemiol. 1996;6:235–45. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 36.American Heart Association [Internet] Dallas, TX: The Association; c2013 [updated: 2011 Oct 11; cited: 2013 Feb 6]. [about 5 screens]. Available from: http://www.heart.org/HEARTORG/Conditions/Cholesterol/AboutCholesterol/What-Your-Cholesterol-Levels-Mean_UCM_305562_Article.jsp. [Google Scholar]
- 37.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 38.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA Study. Sleep. 2007;30:793–6. doi: 10.1093/sleep/30.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs DR, Jr, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehab. 1989:448–59. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radloff LS. The CES-D scale: a self-reported depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 41.Macy E, Hayes T, Tracy R. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference interval and epidemiological applications. Clin Chem. 1997;43:52–8. [PubMed] [Google Scholar]
- 42.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 43.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazzard WR. Atherogenesis: why women live longer than men. Geriatrics. 1985;40:42–51. [PubMed] [Google Scholar]
- 45.Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Artherosclerosis. 1993;98:83–90. doi: 10.1016/0021-9150(93)90225-j. [DOI] [PubMed] [Google Scholar]
- 46.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–73. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]


