Abstract
Study Objectives:
Prior research suggests that sleep deprivation is associated with declines in some aspects of emotional intelligence and increased severity on indices of psychological disturbance. Sleep deprivation is also associated with reduced prefrontal-amygdala functional connectivity, potentially reflecting impaired top-down modulation of emotion. It remains unknown whether this modified connectivity may be observed in relation to more typical levels of sleep curtailment. We examined whether self-reported sleep duration the night before an assessment would be associated with these effects.
Design:
Participants documented their hours of sleep from the previous night, completed the Bar-On Emotional Quotient Inventory (EQ-i), Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT), and Personality Assessment Inventory (PAI), and underwent resting-state functional magnetic resonance imaging (fMRI).
Setting:
Outpatient neuroimaging center at a private psychiatric hospital.
Participants:
Sixty-five healthy adults (33 men, 32 women), ranging in age from 18-45 y.
Interventions:
N/A.
Measurements and Results:
Greater self-reported sleep the preceding night was associated with higher scores on all scales of the EQ-i but not the MSCEIT, and with lower symptom severity scores on half of the psychopathology scales of the PAI. Longer sleep was also associated with stronger negative functional connectivity between the right ventromedial prefrontal cortex and amygdala. Moreover, greater negative connectivity between these regions was associated with higher EQ-i and lower symptom severity on the PAI.
Conclusions:
Self-reported sleep duration from the preceding night was negatively correlated with prefrontal-amygdala connectivity and the severity of subjective psychological distress, while positively correlated with higher perceived emotional intelligence. More sleep was associated with higher emotional and psychological strength.
Citation:
Killgore WDS. Self-reported sleep correlates with prefrontal-amygdala functional connectivity and emotional functioning. SLEEP 2013;36(11):1597-1608.
Keywords: Emotional intelligence, psychopathology, functional connectivity, prefrontal cortex, amygdala
INTRODUCTION
Sleep loss has a profound effect on elementary cognitive processes such as simple alertness, psychomotor vigilance, and response speed.1,2 However, there is now growing literature suggesting that sleep loss also has significant effects on mood and emotional functioning.3–6 Sleep deprivation increases physiological reactivity in response to emotional stressors,7 reduces the psychological threshold for coping with stress,8 and even leads to poorer frustration tolerance and an altered perception of the motives of others.9 Sleep deprivation impairs emotionally-based decision making10 and the ability to use emotions to effectively guide moral judgment.11–13 Moreover, extended sleep deprivation is associated with significant declines on standardized measures of coping skills and emotional intelligence (EI), a set of capabilities and traits that involve the ability to understand and regulate emotions.14 In one study, sleep deprivation was associated with significant declines in the ability to understand emotional responses in others and led to reduced self-reported interpersonal skills, including degraded empathy, stress management capacities, and impulse control.14 Even in healthy individuals, prolonged sleep deprivation leads to significant worsening on several standardized indices of psycho-pathology, including scales measuring somatic complaints, anxiety, depression, and paranoia.15 In short, when sleep is lacking, affective functioning becomes poorly regulated and emotionally salient stimuli may have a greater influence over cognitive processes.
Although the causal mechanisms for these mood and emotional changes are poorly understood, some evidence suggests that they may emerge from measurable alterations in brain functioning that occur following insufficient sleep or total sleep deprivation. Early brain imaging studies suggested that sleep deprivation is associated with significant declines in global cerebral energy metabolism.16 These declines are particularly notable within the prefrontal cortex, including the ventromedial regions,16 which are important to emotional regulation and behavioral control.17,18 More recent studies using functional magnetic resonance imaging (fMRI) suggest that the increased emotional responsiveness during sleep deprivation may be due in part to changes in the strength of functional connectivity between the emotional regulating regions of the medial prefrontal cortex and the amygdala, a structure involved in triggering emotional responses to salient stimuli.19 Other evidence suggests that this modified connectivity between the emotional regulation and emotional responsive regions of the brain may contribute to altered responsiveness to both positive and negatively valenced stimuli under conditions of sleep deprivation.20 Speculatively, such an alteration in the functional balance between these regions may affect higher-order regulation of emotional processes, which could conceivably be observed as declines in EI and even the emergence of symptoms of psychopathology.
Although the effects of total sleep deprivation on EI capacities and symptoms of psychopathology have been well documented within the confines of highly controlled laboratory environments,14,15 virtually no information exists regarding the relation between typical nightly sleep duration in the natural home environment and changes in emotional capacities. A recent fMRI study by our group demonstrated that self-reported sleep duration was reliably associated with next-day resting-state functional connectivity within the brain's default mode network, which includes regions of the medial prefrontal cortex and posterior cingulate cortex.21 It is, therefore, possible that subtle reductions in nocturnal sleep, even well within the normal range obtained by most healthy individuals on any given night, may be sufficient enough to be associated with variations in EI and psychopathology. Accordingly, we collected self-report information regarding typical and recent sleep patterns as well as standardized indices of EI and psychopathology in a sample of healthy participants. Based on our prior findings of changes in EI and psychopathology during total sleep deprivation, and other work showing altered prefrontal-amygdala connectivity under similar conditions, we hypothesized that (1) greater amounts of sleep reported for the night before an assessment would correlate with higher scores on measures of EI and lower scores on indices of psychopathology, (2) more sleep would be associated with increased negative functional connectivity between the ventromedial prefrontal cortex (vmPFC) and amygdala (i.e., suggesting a negative relationship between the intrinsic activation patterns of the amygdala and vmPFC in rested individuals), and (3) the strength of this negative functional connectivity would be directly related to higher EI and lower psychopathology scores in healthy adults.
METHOD
Participants
Sixty-five healthy adults (33 men, 32 women), ranging in age from 18-45 y (mean [M] = 30.2; standard deviation [SD] = 8.0) were recruited via internet advertisements and flyers from the vicinity of the Boston metropolitan area. Participants were screened via telephone interview using standard psychiatric diagnostic criteria22 and deemed to be free from any history of serious medical illnesses, including neurological, Axis I psychiatric, or substance use disorders (including alcohol and illicit drugs), or evidence of clinically significant sleep disorders. All 65 participants provided complete data for the questionnaires. A subsample (n = 58) of this group (29 men, 29 women), ranging in age from 18-45 y (M = 30.5; SD = 8.0) also provided usable resting state fMRI data that were correlated with the questionnaire data. All participants provided written informed consent and were compensated for their time. This research protocol was reviewed and approved by the Institutional Review Board of McLean Hospital.
Materials and Procedure
Sleep Questionnaires
Participants arrived at the laboratory between 09:00 and 11:00 in the morning. Upon arrival, each participant completed a brief questionnaire about their recent sleep schedule and typical habits. The primary question of interest simply asked participants: “How much sleep did you get last night?” This variable, identified as Sleep Last Night, was scored in hours. The questionnaire also included queries about typical bedtimes and wakeup times for weekdays and weekends. Based on this information an estimated Sleep Debt variable was also computed by subtracting a weighted average of typical sleep on weekdays and weekends from the Sleep Last Night variable. Additionally, participants also reported whether they had problems falling or staying asleep as a simple index of insomnia complaints. Participants also completed the Morningness-Eveningness Questionnaire (MEQ).23 Higher scores on the MEQ indicate a preference for earlier rise times and bedtimes and a tendency to function most effectively earlier in the day.
EI Scales
Participants completed two normed, well-validated, and commercially available measures of EI. As a mixed model, or Trait measure of EI, participants completed the Bar-On Emotional Quotient Inventory (EQ-i).24 A 125-item self-report measure, the EQ-i provides a global score of EI (Total EQ), as well as five composite subscales measuring various self-perceived facets of the construct, including the ability to relate well with others (Interpersonal), emotional self-awareness and self-confidence (Intrapersonal), emotional flexibility and problem solving (Adaptability), ability to cope with stress (Stress Management), and general optimism and contentedness (General Mood). The raw EQ-i scores were transformed into standard scores based on the general population norms provided by the test manual and scoring program.24 We also tested Ability EI using the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT),25 a performance-based test of the capacity to reason about and solve emotional problems. This test yields a Total EI score, as well as two Area scores. The first Area score measures the ability to perceive emotions and use that information to facilitate thought (Experiential EI), and is composed of two branch scores known as Perceiving and Facilitating emotions. The second Area score measures the ability to understand and control emotions (Strategic EI), and is composed of two branch scores known as Understanding and Managing emotions. Raw MSCEIT scores were transformed into standardized scores based on the general consensus scoring method (as opposed to expert consensus method) described in the test manual.25 Based on our prior published findings,14 we specifically hypothesized that greater sleep and increased functional connectivity variables would correlate with Total EQ, Intrapersonal, Interpersonal, and Stress Management variables from the EQ-i.
Psychopathology Scale
As a measure of psychopathologic symptom severity, participants completed the Personality Assessment Inventory (PAI), an objective measure that includes 344 statements that are self-rated on a four-point Likert scale.26 The PAI includes 18 primary scales: Somatic Complaints (SOM), which measures concerns about health and physical functioning; Anxiety (ANX), which measures general tension and negative affect; Anxiety Related Disorders (ARD), which assesses symptoms related to specific anxiety disorders; Depression (DEP), which measures common cognitive, affective, and physiological symptoms of depression; Mania (MAN), which measures common clinical features of hypomania and mania; Paranoia (PAR), which assesses paranoid features such as hypervigilance, resentment, and feelings of persecution; Schizophrenia (SCZ), which measures a variety of symptoms including unusual beliefs and perceptions, social deficits, and attentional problems; Borderline Features (BOR), which assesses broad problems with interpersonal functioning; Antisocial Features (ANT), which taps into constructs of adventuresomeness, egocentricity, lack of empathy, and antisocial attitudes; Alcohol Problems (ALC), which assesses behaviors and consequences associated with alcohol abuse and dependence; Drug Problems (DRG), which measures attitudes and behaviors related to drug abuse and dependence; Aggression (AGG), a scale assessing general attitudes conducive to aggressive behavior; Suicidal Ideation (SUI), which assesses thought content related to death and suicide; Stress (STR), which provides an index of current life stressors; Nonsupport (NON), which measures the perception of unavailability of social support; Treatment Rejection (RXR), which assesses a tendency to be satisfied with the current status quo and a disinterest in or unwillingness to participate in therapy; Dominance (DOM), an interpersonal scale that measures a bipolar dimension of dominance (versus submissiveness); and Warmth (WRM), an interpersonal scale that measures a bipolar dimension of empathy (versus rejecting). Raw scores on the PAI were transformed to standardized T-scores based on a normative sample of 1,000 community-dwelling adults as described in the test manual.26 According to prior published findings,15 we specifically hypothesized that greater sleep and increased functional connectivity variables would correlate with SOM, ANX, DEP, and PAR variables from the PAI.
Neuroimaging
Participants underwent a 6-min, eyes open, resting state fMRI scan between 13:00 and 15:00 in the afternoon. Due to the nature of the resting state scan (i.e., nontask engagement— mind wandering), we did not ask the participants to engage in any sort of vigilance control task during this data collection. Images were collected on a 3T Siemens Tim Trio scanner (Erlangen, Germany) and fitted with a 12-channel head coil. Standard structural images were acquired first for use in spatial normalization and for removal of tissue confounds. These images comprised a T1-weighted three-dimensional MPRAGE sequence (TR/TE/flip angle = 2.1s/2.25ms/12°), which yielded 128 sagittal slices (256 × 256 matrix) with a slice thickness of 1.33 mm and a voxel size of 1.33 × 1 × 1 mm. For the resting state scan, 180 images were collected (3.5-mm thickness, no skip; 22.4 cm field of view; 64 × 64 acquisition matrix) over 34 transverse interleaved slices using a T2*-weighted blood oxygen level dependent (BOLD) echoplanar imaging (EPI) sequence (TR/TE/flip angle = 2.0 sec/30 msec/90°).
Image Processing
Resting state data were preprocessed using standard algorithms in SPM8, including motion correction, slice-timing correction, anatomical co-registration, spatial normalization, and spatial smoothing (full width at maximum [FWHM] = 6 mm). Voxels were resliced to 2 × 2 × 2 mm. The time series of resting state data was analyzed using the Functional Connectivity (CONN) Toolbox27 version 13i (http://www.nitrc.org/projects/conn). As part of this process, the data were bandpass filtered (0.008, 0.10 Hz), and corrected for physiological noise using the aCompCor strategy.28 Major confounders were removed using principal components analysis to control for the effects of white matter and cerebrospinal fluid, and motion parameters were included as nuisance covariates; the resultant residual BOLD time series was used for subsequent functional connectivity analyses. To examine functional connectivity, four regions of interest (ROIs) were placed (right ventromedial prefrontal cortex [vmPFC] seed regions = left and right gyrus rectus; target regions = left [220 voxels] and right [248 voxels] amygdala) using the Automated Anatomical Labeling (AAL) Atlas.29 The gyrus rectus was selected based on recent work suggesting that gray matter of this region is associated with EI traits30 as well as sleep-related problems.31,32
Data Analysis
Questionnaires
Sleep variables from the questionnaires were evaluated for bivariate intercorrelations. Further, based on a tercile division of self-reported sleep (Sleep Last Night), participants were initially divided into three groups of low (≤ 6.5 h, n = 22), moderate (6.6-7.9 h, n = 21), or high (≥ 8 h, n = 22) sleep per night. Initial analyses were conducted using one-way analysis of variance to determine whether groups differed significantly on the primary EI and psychopathology variables. Secondary analyses were then undertaken using Pearson correlations to more closely examine the association between the sleep questionnaire item, Sleep Last Night, and scores on the primary and subscale scores of the EQ-i, MSCEIT, and PAI. Significance was evaluated at P < 0.05. Due to the large number of correlations, the P values were adjusted using a Bonferroni correction for all nonhypothesized associations within each analysis set.
Resting State Connectivity
Within the CONN Toolbox, the mean BOLD time series from the resting state scan was calculated for all voxels within the two seed regions for each participant. As a measure of functional connectivity, the zero-lagged bivariate correlation was calculated between the mean time course of each vmPFC seed region and every other voxel in the brain on a participant-by-participant basis. To improve normality before entry into the second level random effects general linear model, all bivariate correlation maps were Fisher transformed (i.e., z-score transformed) via an inverse hyperbolic tangent function.27 Each participant's self-reported sleep was then regressed against these individual beta maps to determine the correlation between functional connectivity and sleep. We corrected for all voxels in the bilateral amygdala ROI with a height threshold of P < 0.05 (family-wise error [FWE]-corrected), whereas spatial extent (i.e., minimum cluster size) was set at 10 voxels. The mean of the beta values from the resulting cluster was extracted for each individual and correlated with the indices of emotional intelligence and psychopathology.
RESULTS
Questionnaires
Sleep Last Night
As evident in Table 1, participants reported sleeping an average of 6.97 h (SD = 1.07) the night before the assessment session. Hours of reported sleep ranged from 4.0 to 9.0. From questions pertaining to estimated weekday and weekend sleep, participants reported generally sleeping 7.43 h (SD = 0.79) per night on average. These data were used to calculate an index of estimated Sleep Debt, which suggested that participants had reduced their sleep by approximately 26 min on the night preceding the scan compared with their typical sleep (Table 1). Sleep Last Night was not correlated with age, MEQ, or typical bed/wakeup times. As might be expected, however, MEQ was strongly correlated with typical bedtimes and wakeup times.
Table 1.
Means and intercorrelations among sleep variables
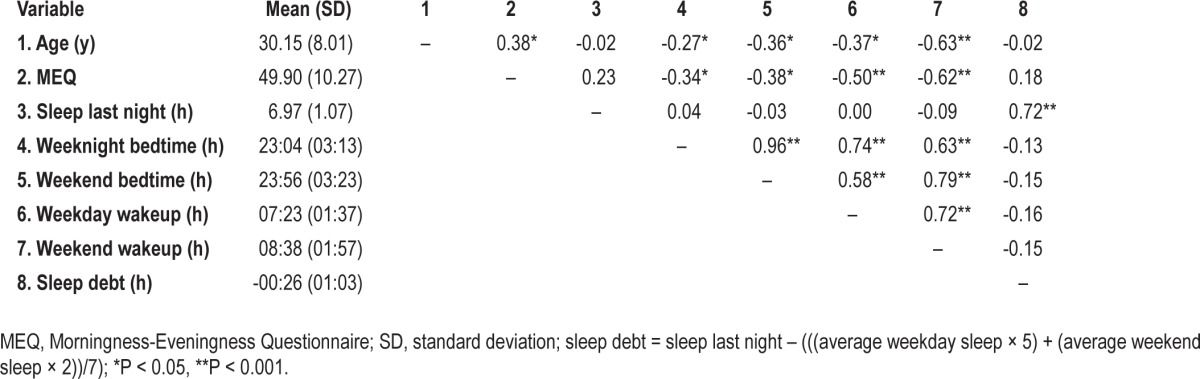
For preliminary analysis, Sleep Last Night was divided into three categories: low (≤ 6.5 h), moderate (6.6-7.9 h), or high (≥ 8 h). When divided in this manner, 22 participants were in the low sleep (M = 5.75 h, SD = 0.75), 21 were in the moderate sleep (M = 7.09 h, SD = 0.20), and 22 were in the high sleep (M = 8.08 h, SD = 0.23) groups. Because age is sometimes associated with sleep duration, this relationship was examined in the current dataset. However, age was not correlated with Sleep Last Night (r = -0.02, P = 0.88), so it was not included as a covariate in subsequent analyses. Finally, 43.8% of the sample (n = 28) reported that they occasionally had insomnia complaints (i.e., difficulty falling asleep or staying asleep). This variable was included as a nuisance covariate in subsequent partial correlation analyses involving emotional intelligence and psychopathology measures.
Emotional Intelligence
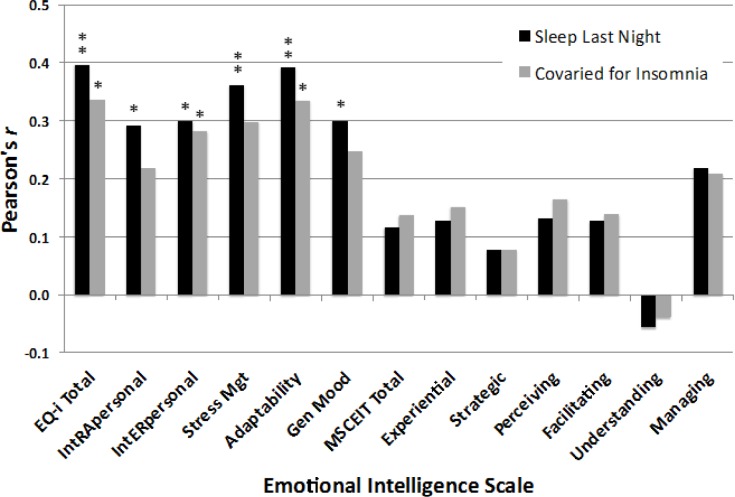
As evident in Figure 1A, there was a main effect of sleep category for Total EQ-i scores, F(2,62) = 3.64, P = 0.032, suggesting that a greater amount of sleep the preceding night was associated with higher Total EQ. Tukey post hoc tests showed that this effect was driven primarily by significantly higher EQ scores for the high versus low sleep group (P < 0.05). As shown in Figure 2, the number of h of Sleep Last Night was significantly correlated with higher scores for Total EQ (r = 0.396, P = 0.001), and all five composite EQ-i subscale scores, including Intrapersonal (r = 0.291, P = 0.019), Interpersonal (r = 0.299, P = 0.015), Stress Management (r = 0.362, P = 0.003), Adaptability (r = 0.392, P = 0.001; Bonferroni corrected P = 0.009), and General Mood (r = 0.300, P = 0.015; Bonferroni corrected P = 0.135), suggesting that more sleep was linearly associated with higher EQ-i scores. In contrast, MSCEIT scores did not differ significantly across sleep duration categories (Figure 1B), and none of the scales of the MSCEIT were significantly correlated with h of Sleep Last Night (Figure 2), (all r < 0.14, all P > 0.30). To address possible concern that this difference between the strength of correlations on the two scales might be accounted for by response biases affecting the self-report measures, the same analyses were conducted for the self-report scales using partial correlations to control for scores on the Negative Impression Index of the EQ-i (i.e., the tendency to “fake bad”). For the EQ-i, four of the six partial correlations remained significant after controlling for response bias, including Total EQ (r = 0.301, P = 0.015), Intrapersonal (r = 0.185, P = 0.142), Interpersonal (r = 0.272, P = 0.030), Stress Management (r = 0.306, P = 0.014), Adaptability (r = 0.297, P = 0.017; Bonferroni corrected P = 0.034), and General Mood (r = 0.239, P = 0.057; Bonferroni corrected P = 0.114). Finally, we explored the potential effect of insomnia complaints on these associations. Figure 2 shows that statistically controlling for insomnia complaints modestly reduced the strength of the correlations, although Total EQ and Adaptability remained significantly correlated with Sleep Last Night even after removing the influence of insomnia problems.
Figure 1.
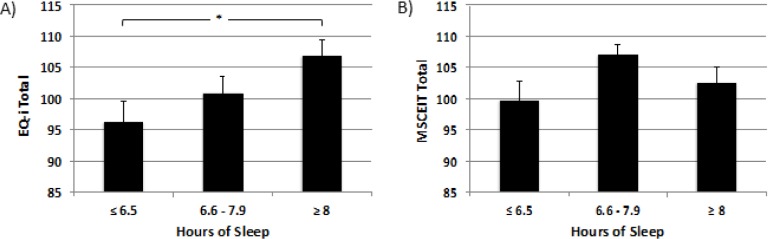
Mean emotional intelligence scores for the entire sample (n = 65) divided by terciles for Sleep Last Night, including Low Sleep (≤ 6.5 h, n = 22), Moderate Sleep (6.6-7.9 h, n = 21), and High Sleep (≥ 8 h, n = 22). (A) Analysis of variance indicated a significant main effect of sleep on scores on the Bar-On Emotional Intelligence Inventory (EQ-i) (P = 0.032), with a significant difference between the High and Low Sleep groups. (B) There was no main effect of sleep on the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT). *P < 0.05, corrected.
Figure 2.
Effect sizes of the correlations between h of self-reported sleep obtained the preceding night and scores on the Bar-On Emotional Intelligence Inventory (EQ-i) and the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT). Black bars: All scales of the EQ-i showed significant Pearson correlations with Sleep Last Night, whereas none of the MSCEIT scales showed significant correlations. Gray bars: Similar trends were observed after statistically controlling for insomnia complaints, but only Adaptability remained significant. *P < 0.05, **P < 0.005.
Psychopathology
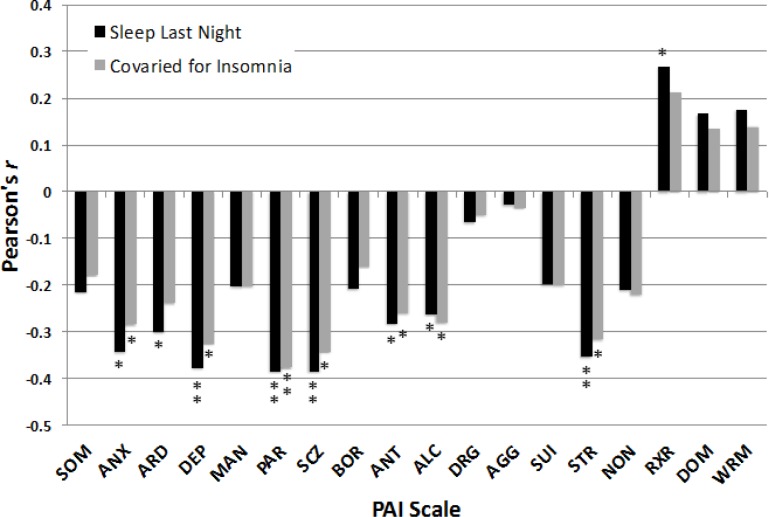
Linear associations were also obtained among several variables of the PAI with Sleep Last Night (Figure 3). Obtaining more h of Sleep Last Night correlated with significantly lower scores for ANX (r = -0.344, P = 0.007), ARD (r = -0.302, P = 0.018; Bonferroni corrected P = 0.252), DEP (r = -0.378, P = 0.003), PAR (r = -0.385, P = 0.002), SCZ (r = -0.385, P = 0.002; Bonferroni corrected P = 0.028), ANT (r = -0.284, P = 0.027; Bonferroni corrected P = 0.378), ALC (r = -0.263, P = 0.040; Bonferroni corrected P = 0.56), STR (r = -0.355, P = 0.005; Bonferroni corrected P = 0.07), and higher scores for RXR (r = 0.268, P = 0.037; Bonferroni corrected P = 0.518). In contrast, Sleep Last Night was not significantly correlated with scores for SOM, MAN, BOR, DRG, AGG, SUI, NON, DOM, and WRM (all r < |0.22|, all P > 0.09). Figure 3 also shows that statistically controlling for insomnia complaints had only minimal effects on the strength of most correlations between Sleep Last Night and PAI variables.
Figure 3.
Effect sizes of the correlations between h of self-reported sleep obtained the preceding night and scores on the Personality Assessment Inventory (PAI). Black bars: Greater sleep the preceding night was associated with lower scores on several indices of psychopathology based on bivariate correlations. Gray bars: Most of the correlations between Sleep Last Night and psychopathology remained significant after statistically controlling for insomnia complaints. See text for expansion of abbreviations. *P < 0.05, **P < 0.005.
Neuroimaging
VMPFC-Amygdala Functional Connectivity
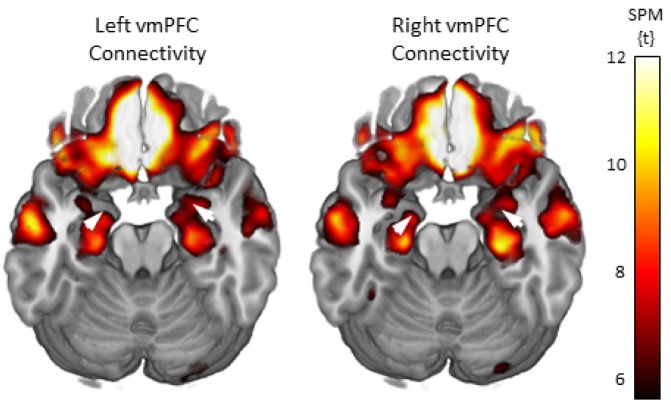
Whole brain connectivity maps through an axial slice showing the vmPFC and amygdala are displayed in Figure 4. These maps are corrected for multiple comparisons using FWE at P < 0.05 for the whole brain. From these maps, initial connectivity values (Fisher transformed correlation coefficients) were extracted for the left and right amygdala ROIs for all voxels exceeding the corrected threshold and are presented in Table 2.
Figure 4.
Functional connectivity maps for the left and right ventromedial prefrontal cortex (vmPFC) seed regions of interest (ROIs). The brain images show axial slices that include both the vmPFC and amygdala regions. The white arrows show the location of the amygdala target ROIs. The maps were set to a whole brain threshold of P < 0.05, family-wise error (FWE) corrected for multiple comparisons. SPM = statistical parametric mapping.
Table 2.
Connectivity values between the vmPFC and amygdala
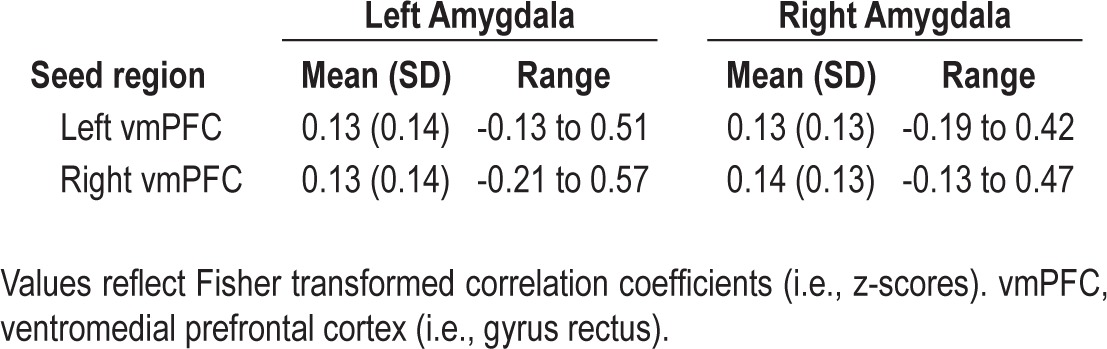
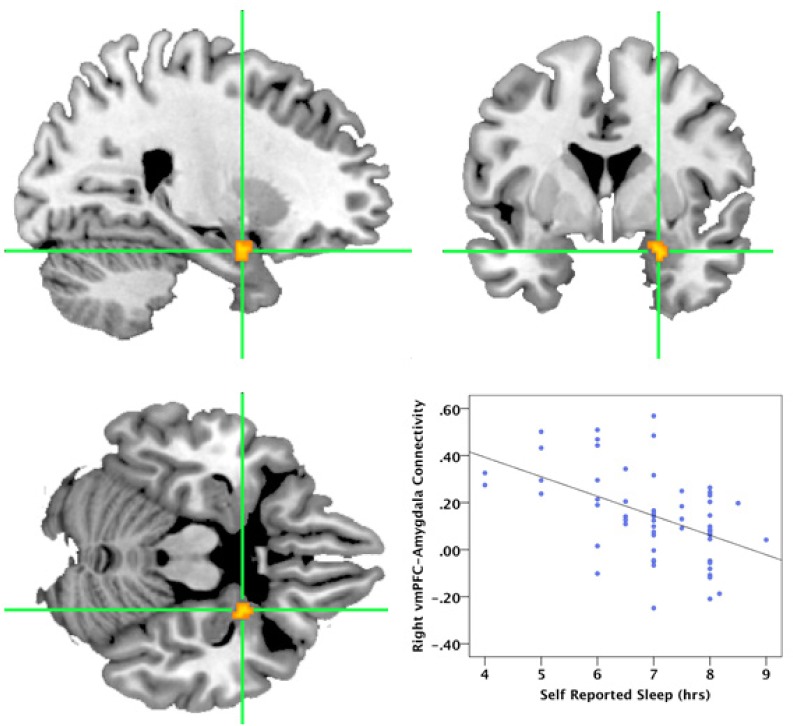
Functional connectivity between the left vmPFC seed region and voxels within either the right or left amygdala was not significantly correlated with Sleep Last Night. In contrast, greater Sleep Last Night was associated with greater negative functional connectivity between the right vmPFC seed region and voxels within the right amygdala (Figure 5). This analysis yielded a cluster of 10 voxels in the right amygdala ROI [Montreal Neurologic Institute (MNI) coordinates: x = 24, y = 2, z = -22]. Connectivity was negatively correlated with greater self-reported sleep time (T = 4.20, r = -0.49). Age was not associated with connectivity strength (r = -0.009, P = 0.94), so it was not included as a covariate in the analysis.
Figure 5.
Self-reported Sleep Last Night was significantly correlated with negative functional connectivity between the right ventromedial prefrontal cortex (vmPFC) and the right amygdala. The figure shows the cluster in the right amygdala [MNI coordinates: x = 24, y = 2, z = -22] that showed negative functional connectivity with the right vmPFC seed region as a function of greater reported sleep time. For visualization, the cluster is height thresholded at (P < 0.001, uncorrected, spatial extent P < 0.05 family-wise error [FWE] corrected). Figures are displayed in sagittal (top left), axial (bottom left), and coronal (top right) views. The scatterplot (bottom right) shows the linear relationship between hours of sleep and the connectivity values extracted from the displayed cluster.
Neuroimaging and Questionnaire Correlates
Connectivity Strength and EI
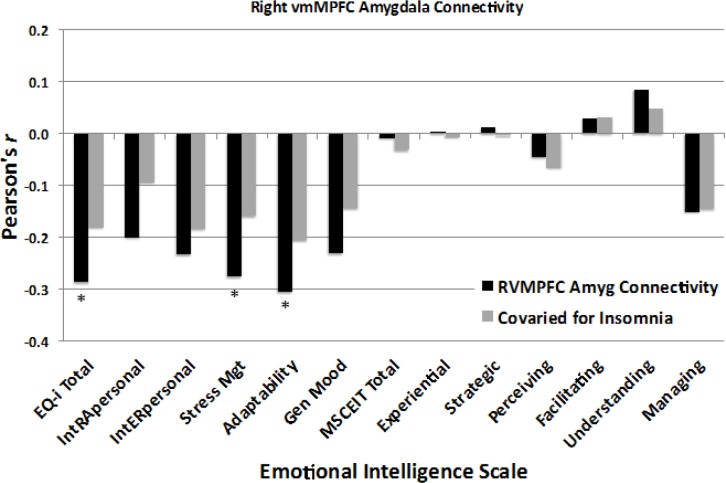
The beta values from the right amygdala cluster representing the strength of connectivity with the vmPFC that covaried with Sleep Last Night were extracted and entered into a correlation analysis with the various EI indices. As shown in Figure 6, the strength of the negative functional connectivity between the right vmPFC and right amygdala was linearly correlated with three EI measures, including Total EQ (r = -0.287, P = 0.029), Stress Management (r = -0.276, P = 0.036), and Adaptability (r = -0.305, P = 0.020; Bonferroni corrected P = 0.18). These findings suggest that greater negative connectivity between these regions was associated with higher EI scores on these scales, but not to the Intrapersonal, Interpersonal, or General Mood scales (all r < |0.34|, all P > 0.07). In contrast, the strength of connectivity between the right vmPFC and amygdala was unrelated to any of the scores on the MSCEIT, including Total EI, Experiential EI, Strategic EI, Perceiving EI, Facilitating EI, Understanding EI, Managing EI (all r < |0.16|, all P > 0.25). Figure 6 also shows that once insomnia complaints were statistically controlled, the strength of the correlation between EI scales and connectivity was reduced and none of the findings reached significance.
Figure 6.
Effect sizes show the correlations between the magnitude of ventromedial prefrontal cortex (vmPFC) – amygdala functional connectivity and scores on the Bar-On Emotional Intelligence Inventory (EQ-i) and the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT). Black bars: Total EQ-i, as well as composite scale scores for Stress Management and Adaptability showed significant negative bivariate correlations with functional connectivity, indicating that higher emotional intelligence on the EQ-i was associated with greater negative connectivity between these two regions. In contrast, MSCEIT scales were not significantly correlated with functional connectivity between these two regions. Gray bars: After controlling for insomnia complaints, the observed correlations with emotional intelligence were no longer significant. *P < 0.05.
Connectivity Strength and Psychopathology
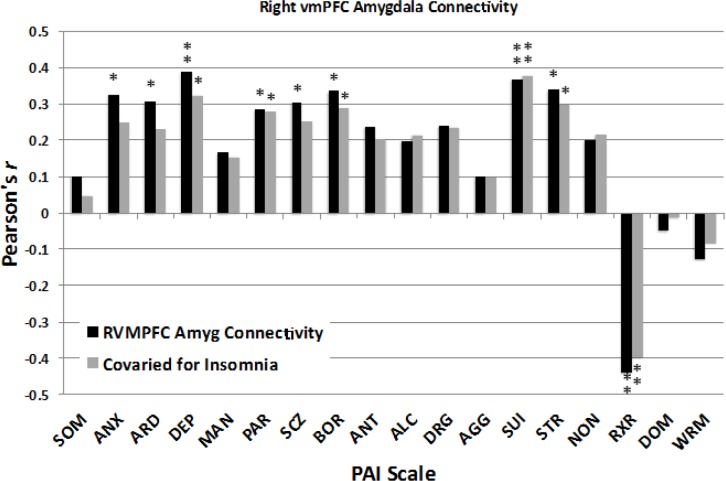
The extracted vmPFC-amygdala connectivity data were also correlated with the indices of psychopathology from the PAI. As evident in Figure 7, greater positive connectivity was associated with increased scores for ANX (r = 0.325, P = 0.013), ARD (r = 0.306, P = 0.021; Bonferroni corrected P = 0.294), DEP (r = 0.388, P = 0.003), PAR (r = 0.286, P = 0.031), SCZ (r = 0.305, P = 0.021; Bonferroni corrected P = 0.294), BOR (r = 0.335, P = 0.011; Bonferroni corrected P = 0.154), SUI (r = 0.368, P = 0.005; Bonferroni corrected P = 0.070), STR (r = 0.339, P = 0.010; Bonferroni corrected P = 0.140). In contrast, greater negative connectivity between vmPFC and amygdala was associated with healthier scores on RXR (r = -0.440, P = 0.001; Bonferroni corrected P = 0.014). Other scales were not significantly related to functional connectivity, including SOM, MAN, ANT, ALC, DRG, AGG, NON, DOM, and WRM (all r ≤ 0.24, all P > 0.07). Together, these findings suggest that when the vmPFC and amygdala covaried together positively, participants had higher psycho-pathology scores on several hypothesized scales, but as these regions showed greater negative connectivity, the severity of psychopathology scores was lower. Figure 7 shows that removing the effects of insomnia complaints had only minimal effects on the correlations between vmPFC-amygdala connectivity and PAI scores.
Figure 7.
Effect sizes show the correlations between the magnitude of ventromedial prefrontal cortex (vmPFC) – amygdala functional connectivity and scores on the Personality Assessment Inventory (PAI). Black bars: Scores on several PAI scales showed positive bivariate correlations with functional connectivity, indicating that symptoms of psychopathology tended to be higher as these two regions covaried positively together, whereas psychopathology was reduced as these two regions covaried negatively with one another. Gray bars: Partial correlations controlling for insomnia complaints remained significant for most PAI scales. See text for expansion of abbreviations. *P < 0.05, **P < 0.005.
DISCUSSION
Consistent with predictions from prior work,14,15 greater self-reported sleep the night before the assessment was significantly related to higher Trait EI scores and lower scores on several indices of psychopathology. Sleep duration the night before the scan was also negatively correlated with functional connectivity between the right vmPFC and amygdala. Moreover, the strength of this negative prefrontal-amygdala connectivity pattern was directly related to scores on Trait EI and psychopathology scales. Generally, the more strongly negative the functional connectivity, the higher the EI scores and less severe the psychopathology scores.
The current finding that greater self-reported nocturnal sleep duration was associated with higher Trait EI scores is in line with our previous work showing that total sleep deprivation was correlated with a decline on the same indices14 and other evidence suggesting a link between fatigue and lower EI.33 In our prior study, sleep deprivation led to degradation of several aspects of EI, including emotional self-awareness, perceived effectiveness in dealing with interpersonal relationship issues, and the ability to cope with stress. We currently show that EI is associated with the duration of sleep during a single night, as those who obtained fewer h of sleep the night before the assessment achieved lower Total EQ-i scores than those who obtained the most sleep. In fact, those participants obtaining 8 or more h of sleep typically had Total EQ-i scores about 10 points (i.e., about two thirds of an SD) higher than those obtaining 6.5 h of sleep or less the preceding night. Moreover, all five EQ-i composite subscales also showed significant positive correlations with sleep duration from the previous night. In contrast, we found no association between sleep and Ability EI, as assessed by the MSCEIT.
The current finding that Trait, but not Ability, EI is related to sleep duration is enlightening, as these two models conceptualize the EI construct in very different ways.34 Whereas Trait EI taps subjective emotional experience, global mood and optimism, self-confidence, self-awareness, and self-perceived interpersonal sensitivity,35 Ability EI involves the accuracy of emotional perception, the ability to use emotional information to facilitate performance, the quality of reasoning about emotional information, and the capacity to regulate or manage emotions.25 Our findings suggest that, within the range of sleep duration obtained by most people on a typical night, getting more sleep appears to be reliably associated with better self-perceived emotional functioning, interpersonal attunement, coping capacity, self-confidence, and self-awareness, but is not reliably related to performance based capacities, such as reasoning about emotional information and solving emotional problems. To the extent that these varied aspects of EI involve distinct neuroanatomical regions,30 it makes sense that sleep deprivation may have differential effects on such traits and capacities.
Greater sleep duration the night before the assessment was also associated with lower scores on several indices of psychopathology from the PAI. Specifically, increased sleep time was most strongly associated with reduced severity of complaints associated with anxiety, depression, paranoia, and schizophrenia. These findings are congruent with previous findings showing that total sleep deprivation was associated with increased symptoms of psychopathology.15 Together, these studies suggest that lack of sleep, or even modest curtailment of sleep, may be associated with a subtle nonclinical elevation of a number of emotional distress complaints, even among healthy normal individuals.
Although reduced sleep has long been perceived as a consequence or symptom of psychopathology, emerging evidence suggests that sleep disruption may also play a contributory role in the etiology of some psychiatric conditions. A recent large-scale study showed that behaviorally induced insufficient sleep among adolescents was associated with a significantly elevated risk of suicidal ideation.36 Currently, the precise neurobiological basis for the link between sleep loss and psychopathology remains uncertain, but some evidence suggests that lack of sleep may lead to altered neurochemistry within the prefrontal cortex,37 alterations in neurotransmitter receptor sensitivity,38–40 and increases cortical excitability, particularly in prefrontal regions.41 Considerable evidence points to dysfunction of the vmPFC during a number of psychopathological conditions including depression,42–45 anxiety disorders,46–49 and psychopathy.50 Metabolic activity in the vmPFC also appears to be particularly affected by sleep deprivation,16 and a number of studies have shown that tasks sensitive to vmPFC functioning are particularly impaired by lack of sleep.10,51–53 Notably, functional connectivity between the emotion regulating regions of the medial prefrontal cortex and the emotionally responsive regions of the limbic system, such as the amygdala and other cortical regions, appears to be altered by sleep deprivation.19,54
In the current study, the correlation between self-reported sleep duration and functional connectivity between the prefrontal cortex and amygdala was also investigated. With greater sleep duration the night before the scan, there was stronger negative functional connectivity between the vmPFC and amygdala in the right hemisphere. Emerging evidence suggests that the medial prefrontal regions are critical to normal top-down regulation of the amygdala and other limbic emotional engagement systems.55 Interestingly, this corticolimbic regulatory capacity can be depleted by overuse or fatigue.18 One interpretation of our findings, therefore, is that greater nocturnal sleep may facilitate the daily replenishment of this top-down regulatory capacity, leading to more effective modulation of affective responses by the prefrontal cortex. Of course, the correlational nature of the findings precludes the ability to draw directional conclusions. The current findings are consistent with those of Yoo and colleagues,19 who found that 35 h of sleep deprivation was associated with increased amygdala responsiveness to negative emotional stimuli and reduced functional connectivity between the medial prefrontal cortex and amygdala, but further extend these findings to more common levels of occasional sleep curtailment or the short sleep periods experienced periodically by most people. The right-lateralized nature of the finding was not hypothesized, but is interesting in light of other findings suggesting that sleep deprivation may have a greater impairing effect on cognitive processes mediated by the right compared with the left hemisphere.56–58
The final question we addressed was whether the sleep-related strength of functional connectivity between the vmPFC and amygdala might correlate directly with EI and psychopathology scores. The functional connectivity values between these two regions were extracted for each participant and used to predict scores on measures of EI and psychopathology. Higher Trait EI, Stress Management, and Adaptability scores were associated with a pattern of greater negative prefrontalamygdala functional connectivity. In contrast, no association was observed for Ability EI, suggesting that this aspect of the vmPFC-amygdala emotion regulation system appears to be more related to affective response traits rather than behaviorally measured emotional problem solving abilities. Similarly, we found that the strength of vmPFC-amygdala functional connectivity was modestly but significantly positively correlated with half of the psychopathology scales on the PAI, most notably depression, but also anxiety, paranoia, treatment rejection, and marginally to suicidal ideation. Although correlational in nature, these findings are consistent with a number of studies that have shown that affective regulation is associated with a negative relationship between the medial prefrontal cortex and the amygdala55,59 and that some forms of affective psychopathology may involve alteration or disruption of this neurocircuitry.46,60,61
Some limitations should be borne in mind when interpreting these findings. First, we used a self-report index of sleep, which is likely to suffer from some loss in precision and reliability, particularly when compared with objective methods such as ambulatory electroencephalographic or actigraphic monitoring. Future studies would benefit from the use of objective measurements of sleep. Second, although the current sample is relatively large for a neuroimaging study, our participants were all thoroughly screened to exclude clinical levels of psychopathology. Consequently, the current findings cannot be validly generalized to more severe forms of psychopathology. Third, because the findings from this study are correlational, it is not possible to determine the causal direction of the observed relationships or whether the findings may be due to an unmeasured third variable. Although insomnia complaints, response biases, and age did not seem to account for most of the findings, it is possible that other unexplored variables might have contributed. Furthermore, although we collected data regarding the amount of sleep obtained the preceding night and attempted to tightly control the time of scan administration, we did not specifically control for wakeup time on the day of the scan. Thus, it is possible that this could have added some uncontrolled variance to the data, potentially obscuring some important relationships. This is particularly important in light of the fact that we had no objective control for level of vigilance within the scanner, such as simultaneous electroencephalography. Thus, it is not possible to conclusively determine whether the observed differences in functional connectivity might have been due to fluctuations in vigilance occurring during the scan. Future studies would benefit from the use of simultaneous electroencephalography in this regard. Finally, we only examined functional connectivity between two regions, the vmPFC and amygdala. Emotional experience and regulation are extraordinarily complex processes and undoubtedly encompass a much larger neurocircuitry than the limited set of regions examined here. Future work will need to expand upon this neurocircuitry to include other candidate nodes such as the insular cortex, striatum, brainstem nuclei, and other higher-order associative regions.
Nonetheless, with appropriate consideration to the aforementioned limitations, we believe the current study advances our understanding of the association between recent sleep, prefrontal-amygdala connectivity, and emotional functioning. These data suggest that even small variations in sleep of only 1 or 2 h may be significantly associated with differences in some aspects of perceived emotional intelligence and the severity of psychological distress. Conversely, getting a full night of sleep appears to be connected with bolstered emotional strength and mental health.
DISCLOSURE STATEMENT
This was not an industry supported study. The author has indicated no financial conflicts of interest. This study was supported by a USAMRAA grant (W81XWH-09-1-0730).
ACKNOWLEDGMENTS
Zachary Schwab, BS, assisted in preprocessing of functional data and Melissa Weiner, BS, assisted in the collection of behavioral data.
REFERENCES
- 1.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 3.Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–29. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 4.Vandekerckhove M, Cluydts R. The emotional brain and sleep: an intimate relationship. Sleep Med Rev. 2010;14:219–26. doi: 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Penetar D, McCann U, Thorne D, et al. Caffeine reversal of sleep deprivation effects on alertness and mood. Psychopharmacology (Berl.) 1993;112:359–65. doi: 10.1007/BF02244933. [DOI] [PubMed] [Google Scholar]
- 6.Tempesta D, Couyoumdjian A, Curcio G, et al. Lack of sleep affects the evaluation of emotional stimuli. Brain Res Bull. 2010;82:104–8. doi: 10.1016/j.brainresbull.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Franzen PL, Gianaros PJ, Marsland AL, et al. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosom Med. 2011;73:679–82. doi: 10.1097/PSY.0b013e31822ff440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minkel JD, Banks S, Htaik O, et al. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12:1015–20. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn-Greene ET, Lipizzi EL, Conrad AK, Kamimori GH, Killgore WDS. Sleep deprivation adversely affects interpersonal responses to frustration. Pers Indiv Differ. 2006;41:1433–43. [Google Scholar]
- 10.Killgore WDS, Balkin TJ, Wesensten NJ. Impaired decision-making following 49 hours of sleep deprivation. J Sleep Res. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 11.Killgore WDS, Killgore DB, Day LM, Li C, Kamimori GH, Balkin TJ. The effects of 53 hours of sleep deprivation on moral judgment. Sleep. 2007;30:345–52. doi: 10.1093/sleep/30.3.345. [DOI] [PubMed] [Google Scholar]
- 12.Olsen OK, Pallesen S, Eid J. The impact of partial sleep deprivation on moral reasoning in military officers. Sleep. 2010;33:1086–90. doi: 10.1093/sleep/33.8.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tempesta D, Couyoumdjian A, Moroni F, Marzano C, De Gennaro L, Ferrara M. The impact of one night of sleep deprivation on moral judgments. Soc Neurosci. 2011 Sep 26; doi: 10.1080/17470919.2011.614002. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Killgore WDS, Kahn-Greene ET, Lipizzi EL, Newman RA, Kamimori GH, Balkin TJ. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 2007;9:517–26. doi: 10.1016/j.sleep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, Killgore WDS. The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 2007;8:215–21. doi: 10.1016/j.sleep.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 17.Sokol-Hessner P, Camerer CF, Phelps EA. Emotion regulation reduces loss aversion and decreases amygdala responses to losses. Soc Cogn Affect Neurosci. 2013;8:341–50. doi: 10.1093/scan/nss002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner DD, Heatherton TF. Self-regulatory depletion increases emotional reactivity in the amygdala. Soc Cogn Affect Neurosci. 2012 Aug 27; doi: 10.1093/scan/nss082. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep--a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–8. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011;31:4466–74. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killgore WD, Schwab ZJ, Weiner MR. Self-reported nocturnal sleep duration is associated with next-day resting state functional connectivity. Neuroreport. 2012;23:741–5. doi: 10.1097/WNR.0b013e3283565056. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. New York: Biometrics Research Department, New York State Psychiatric Institute; 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. (SCID-I/P) [Google Scholar]
- 23.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 24.Bar-On R. North Tonawanda, NY: Multi-Health Systems; 2002. BarOn emotional quotient inventory: a measure of emotional intelligence--technical manual. [Google Scholar]
- 25.Mayer JD, Salovey P, Caruso DR. North Tonawanda, NY: MHS; 2002. Mayer-Salovey-Caruso emotional intelligence test (MSCEIT) user's manual. [Google Scholar]
- 26.Morey LC. Lutz, FL: Psychological Assessment Resources, Inc; 1991. Personality assessment inventory. [Google Scholar]
- 27.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 28.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 30.Killgore WD, Weber M, Schwab ZJ, et al. Gray matter correlates of Trait and Ability models of emotional intelligence. Neuroreport. 2012;23:551–5. doi: 10.1097/WNR.0b013e32835446f7. [DOI] [PubMed] [Google Scholar]
- 31.Joo EY, Tae WS, Kim ST, Hong SB. Gray matter concentration abnormality in brains of narcolepsy patients. Korean J Radiol. 2009;10:552–8. doi: 10.3348/kjr.2009.10.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Killgore WDS, Schwab ZJ, Kipman M, DelDonno SR, Weber M. Voxel-based morphometric gray matter correlates of daytime sleepiness. Neurosci Lett. 2012;518:10–3. doi: 10.1016/j.neulet.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Brown RF, Schutte NS. Direct and indirect relationships between emotional intelligence and subjective fatigue in university students. J Psychosom Res. 2006;60:585–93. doi: 10.1016/j.jpsychores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Brackett MA, Mayer JD. Convergent, discriminant, and incremental validity of competing measures of emotional intelligence. Pers Soc Psychol Bull. 2003;29:1147–58. doi: 10.1177/0146167203254596. [DOI] [PubMed] [Google Scholar]
- 35.Bar-On R. The Bar-On model of emotional-social intelligence (ESI) Psicothema. 2006;(18 Suppl):13–25. [PubMed] [Google Scholar]
- 36.Lee YJ, Cho SJ, Cho IH, Kim SJ. Insufficient sleep and suicidality in adolescents. Sleep. 2012;35:455–60. doi: 10.5665/sleep.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernier D, Bartha R, Devarajan S, Macmaster FP, Schmidt MH, Rusak B. Effects of overnight sleep restriction on brain chemistry and mood in women with unipolar depression and healthy controls. J Psychiatry Neurosci. 2009;34:352–60. [PMC free article] [PubMed] [Google Scholar]
- 38.Novati A, Roman V, Cetin T, et al. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31:1579–85. doi: 10.1093/sleep/31.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roman V, Walstra I, Luiten PG, Meerlo P. Too little sleep gradually desensitizes the serotonin 1A receptor system. Sleep. 2005;28:1505–10. [PubMed] [Google Scholar]
- 40.Roman V, Hagewoud R, Luiten PG, Meerlo P. Differential effects of chronic partial sleep deprivation and stress on serotonin-1A and muscarinic acetylcholine receptor sensitivity. J. Sleep Res. 2006;15:386–94. doi: 10.1111/j.1365-2869.2006.00555.x. [DOI] [PubMed] [Google Scholar]
- 41.Huber R, Maki H, Rosanova M, et al. Human cortical excitability increases with time awake. Cereb Cortex. 2013;23:1–7. doi: 10.1093/cercor/bhs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 43.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 44.Drevets WC, Ongur D, Price JL. Reduced glucose metabolism in the subgenual prefrontal cortex in unipolar depression. Mol Psychiatry. 1998;3:190–1. doi: 10.1038/sj.mp.4000380. [DOI] [PubMed] [Google Scholar]
- 45.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad. Sci. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 46.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos Trans R Soc Lond B Biol Sci. 2008;363:2557–65. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chuah LY, Dolcos F, Chen AK, Zheng H, Parimal S, Chee MW. Sleep deprivation and interference by emotional distracters. Sleep. 2010;33:1305–13. doi: 10.1093/sleep/33.10.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Libedinsky C, Smith DV, Teng CS, et al. Sleep deprivation alters valuation signals in the ventromedial prefrontal cortex. Front Behav Neurosci. 2011;5:70. doi: 10.3389/fnbeh.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venkatraman V, Huettel SA, Chuah LY, Payne JW, Chee MW. Sleep deprivation biases the neural mechanisms underlying economic preferences. J Neurosci. 2011;31:3712–8. doi: 10.1523/JNEUROSCI.4407-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59:1745–51. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 55.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cote KA, Milner CE, Osip SL, Baker ML, Cuthbert BP. Physiological arousal and attention during a week of continuous sleep restriction. Physiol Behav. 2008;95:353–64. doi: 10.1016/j.physbeh.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Manly T, Dobler VB, Dodds CM, George MA. Rightward shift in spatial awareness with declining alertness. Neuropsychologia. 2005;43:1721–8. doi: 10.1016/j.neuropsychologia.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Pallesen S, Johnsen BH, Hansen A, et al. Sleep deprivation and hemispheric asymmetry for facial recognition reaction time and accuracy. Percept Mot Skills. 2004;98:1305–14. doi: 10.2466/pms.98.3c.1305-1314. [DOI] [PubMed] [Google Scholar]
- 59.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 60.Killgore WDS, Yurgelun-Todd DA. Ventromedial prefrontal activity correlates with depressed mood in adolescent children. Neuroreport. 2006;17:167–71. doi: 10.1097/01.wnr.0000198951.30939.73. [DOI] [PubMed] [Google Scholar]
- 61.Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–8. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]