Abstract
Study Objectives:
Photic and non-photic stimuli have been shown to shift the phase of the human circadian clock. We examined how photic and non-photic time cues may be combined by the human circadian system by assessing the phase advancing effects of one evening dose of exogenous melatonin, alone and in combination with one session of morning bright light exposure.
Design:
Randomized placebo-controlled double-blind circadian protocol. The effects of four conditions, dim light (∼1.9 lux, ∼0.6 Watts/m2)-placebo, dim light-melatonin (5 mg), bright light (∼3000 lux, ∼7 Watts/m2)-placebo, and bright light-melatonin on circadian phase was assessed by the change in the salivary dim light melatonin onset (DLMO) prior to and following treatment under constant routine conditions. Melatonin or placebo was administered 5.75 h prior to habitual bedtime and 3 h of bright light exposure started 1 h prior to habitual wake time.
Setting:
Sleep and chronobiology laboratory environment free of time cues.
Participants:
Thirty-six healthy participants (18 females) aged 22 ± 4 y (mean ± SD).
Results:
Morning bright light combined with early evening exogenous melatonin induced a greater phase advance of the DLMO than either treatment alone. Bright light alone and melatonin alone induced similar phase advances.
Conclusion:
Information from light and melatonin appear to be combined by the human circadian clock. The ability to combine circadian time cues has important implications for understanding fundamental physiological principles of the human circadian timing system. Knowledge of such principles is important for designing effective countermeasures for phase-shifting the human circadian clock to adapt to jet lag, shift work, and for designing effective treatments for circadian sleep-wakefulness disorders.
Citation:
Burke TM; Markwald RR; Chinoy ED; Snider JA; Bessman SC; Jung CM; Wright Jr KP. Combination of light and melatonin time cues for phase advancing the human circadian clock. SLEEP 2013;36(11):1617-1624.
Keywords: Light response, zeitgeber, phase shift
INTRODUCTION
The mammalian master circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus.1,2 The SCN provides environmental and biological timing information to the rest of the body so that physiology and behavior are coordinated for optimal functioning relative to the time of day.3,4 The SCN receives input about environmental time through photic pathways via rod, cone, and melanopsin photoreceptors in the retina.5,6 The SCN also receives input about behavioral and physiological states through non-photic pathways (e.g., serotonergic input from the raphe nucleus).7–10 Misalignment between environmental time and internal biological timing (e.g., shift work, jet lag, circadian sleep-wakefulness disorders) can result in adverse psychological, neurobehavioral, and physiological consequences.11–16 Photic and non-photic stimuli have both been used to phase shift the human circadian clock; however, there is limited information about how combinations of phase-shifting stimuli influence the timing of the circadian clock in humans. Findings from research in non-humans suggest the combination of photic and non-photic stimuli interact to increase or attenuate the magnitude of circadian phase shifts17–21 and contribute to circadian entrainment.22 In humans, Wirz-Justice et al.23 examined the combination of exogenous melatonin, timed to advance the circadian clock, and bright light exposure, timed to delay the circadian clock, and found an additive interaction such that the combination resulted in no phase shift relative to the control condition. These findings suggest that information from photic and non-photic stimuli may be combined by the human circadian system. We hypothesized that combinations of properly timed photic and non-photic stimuli will induce a greater phase shift response than individual stimuli. Such potential combined effects of photic and non-photic stimuli by the human circadian clock has important implications for entrainment of the human circadian clock to 24-hour and near-24-hour day lengths such as required by some orbital space flight missions or by a mission to the planet Mars,24–27 for the treatment of circadian sleep-wakefulness disorders,28 and for circadian adaptation to jet lag and shift work schedules.16,29
Although light is a strong synchronizer of the SCN to the external environment,30,31 non-photic stimuli, such as activity, exercise, restricted food availability, and exogenous melatonin have also been shown to shift the timing of the mammalian circadian system.32–37 Both light and melatonin have been found to phase shift the circadian system of humans; in the majority of studies conducted to date, subjects were exposed to multiple days of light exposure or melatonin administration.38–48 Fewer investigations have examined the influence of one session of light exposure or one dose of melatonin.23,49–55 Nonetheless, findings in general are consistent in showing that bright light exposure in the evening produces the largest phase delays and bright light exposure in the early morning produces the largest phase advances. Melatonin administration on the other hand produces the largest phase advances in the late afternoon/early evening, and melatonin administration in the early morning following habitual wake time produces the largest phase delays.38,44,47 Few studies have directly compared the phase resetting response to one day of light exposure and one day of melatonin administration.
Using a circadian protocol to evaluate the phase resetting response of the human circadian clock to photic and non-photic stimuli, we sought to examine the influence of one session of bright light exposure and one dose of exogenous melatonin, alone and in combination. This experiment was based upon previous research in humans showing that light and melatonin alone can phase shift circadian rhythms23,34,49,56–63 and findings from animal and human studies showing that melatonin can attenuate light induced phase shifts.23,33,50,64 This study tested the hypothesis that the circadian phase advance induced by the combination of morning bright light exposure and late afternoon exogenous melatonin administration would induce a greater phase shift than either stimulus alone. Four experimental conditions were compared: dim light-placebo (DLP), dim light-melatonin (DLM), bright light-placebo (BLP), and bright light-melatonin (BLM). Timing of photic and nonphotic stimuli was based on existing light and melatonin phase response curves (PRCs) and was selected to induce large phase shifts. Specifically, to achieve a large phase advance, a single 3-h light exposure with an intensity of ∼3000 lux was applied in the early morning when longer light exposures show the largest phase advances,51,53 and a single 5 mg dose of melatonin was administered in the late afternoon/early evening when multiple consecutive days of exogenous melatonin administration shows the largest phase advances.38,44,47
METHODS
Participants
Thirty-six young healthy subjects (18 females, 18 males) aged 22.0 ± 3.8 y, body mass index (BMI) 22.3 ± 2.1 (mean ± SD) participated. Prior to the study, subjects underwent detailed health screening. Health of subjects was determined by medical evaluation at the Clinical and Translational Research Center (CTRC) and Sleep and Chronobiology Laboratory at the University of Colorado Boulder based on physical exam, blood chemistries, clinical electrocardiography, and medical, psychiatric, and sleep histories. Exclusion criteria included: known medical, psychiatric or sleep disorders, abnormal blood chemistries, illicit drug or nicotine use, habitual sleep duration < 7 h or > 9 h, medication use (exception for oral contraceptives), BMI outside the range of 18.5 to 27, shift work within one year prior, or travel across more than one time zone in the three weeks prior to in-laboratory procedures. Study procedures were approved by the Institutional Review Board at the University of Colorado Boulder and the Scientific Advisory and Review Committee of the Colorado Clinical and Translational Sciences Institute. Subjects gave written informed consent and were compensated for their participation.
Experimental Design
Pre-Study Control: Ambulatory Wakefulness-Sleep-Activity Recordings
Subjects maintained a regular ∼8-h sleep-wakefulness schedule based on their habitual sleep and wake times for one week prior to the in-laboratory study. Regular sleep-wakefulness schedules were verified via sleep logs, time-stamped voice-recorder of bed and wake times, and wrist actigraphy recordings (Actiwatch-L, Philips Mini Mitter, Bend, OR). Subjects were instructed to refrain from over-the-counter medications, supplements, and caffeine for two weeks prior, naps one week prior, exercise three days prior, and alcohol two days prior to the in-laboratory protocol. Subjects self-reported compliance with the above requests. Additionally, upon admission to the in-laboratory protocol, urine toxicology and an alcohol breath test (Lifeloc Technologies Model FC10, Wheat Ridge, CO) were performed and female subjects were given a pregnancy test to verify absence of pregnancy. Five of the female subjects were taking oral contraceptives; two were randomized to the DLM, and one each to each of the other conditions.
In-Laboratory Protocol
Subjects were studied individually in specially designed sleep and circadian research suites that provided an environment free of external time cues for five calendar days. Following pre-study assessments (Days 1-7), subjects were scheduled to arrive at the Sleep and Chronobiology Laboratory ∼4 h prior to habitual sleep time (Figure 1). All protocol events such as meal times, pill administration, light exposure, and sleep opportunities were scheduled relative to the subject's habitual wake time. Ambient temperature was maintained in thermoneutral range (∼22.2°C). Except for the bright light exposure conditions, lighting in the angle of gaze was maintained at dim levels equivalent to candle light (∼1.9 lux; ∼0.6 Watts/m2) during scheduled wakefulness and darkness during scheduled sleep opportunities. The first day of the in-laboratory protocol, Day 8, consisted of a habituation episode followed by an 8-h nighttime sleep opportunity. Days 9-10 consisted of a 28-h modified constant routine (CR1).31 The constant routine protocol is used to estimate circadian phase while controlling for the effects of environmental and behavioral influences. Subjects maintained wakefulness while being exposed to dim light under bedrest conditions with the head of the bed raised to ∼35°. Brief bathroom breaks, using a commode ∼1 m from the bed, were scheduled so that they did not occur within the 15 min before a saliva sample; bedpans and urinals were provided at unscheduled times, otherwise constant posture was maintained. Isocaloric, hourly snacks prepared by the CTRC nutritionist, were used to equally distribute food and fluid intake over the constant routine. Melatonin or placebo was administered on Day 10. Exposure to bright light occurred on Days 10-11. This was followed by a second 19-h constant routine (CR2) on Day 11 to reassess circadian melatonin phase.
Figure 1.
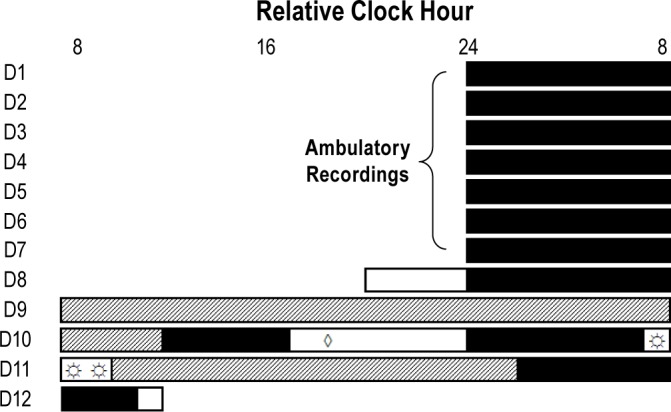
12 daylong phase-shifting protocol. The first 7 days (D1-7) of study were ambulatory recording of subjects maintaining consistent sleep-wakefulness schedules. This was followed by 5 days living in the laboratory (D8-12). Example times for a subject with a habitual bedtime at midnight. Black bars represent scheduled sleep opportunities, open bars represent scheduled wakefulness not part of constant routines, and the hatched bars represent constant routines. The protocol consisted of 2 constant routines which were used to estimate circadian phase. Subjects received melatonin or rice powder-filled placebo (◊) 5.75 h prior to habitual bedtime. Subjects were awakened from sleep early so that exposure to bright or dim light (☼) could begin 1 h prior to habitual wake time.
Experimental Conditions
Subjects were randomly assigned to experimental condition: DLP, DLM, BLP, BLM. Pill administration of either placebo or 5 mg melatonin was double-blind and scheduled in the late afternoon 5.75 h prior to habitual bedtime. Implementation of pill allocation was performed by the CTRC pharmacist who provided pills identical in appearance. Pills consisted of 5 opaque capsules of either 1 mg immediate release melatonin (5 mg total dose; Life Extension Foundation, Inc.), or identical looking capsules of rice flour placebo. The Clinical Trials. gov ID of this study was NCT00387179 and we administered melatonin under FDA IND 76168. The allocation sequence was concealed until interventions were assigned and data were prepared for statistical analysis.
Subjects were awakened 1 h earlier than habitual wake time on Day 10-11 and exposed to either 3 h of continuous bright light or dim light. Commercially available ceiling mounted fluorescent lamps (Sylvania Octron 32W T8 bulb; Danvers, MA, USA) provided broad spectrum white light exposure, similar to natural, midday, daylight (6500-K color temperature). During the light exposure, subjects were under the direct supervision of research assistants who remained in the suite to ensure the intended intensity of illumination was achieved. Subjects wore clear Uvex glasses (Uvex Winter Optical, Smithfield, RI, USA) to further block any UV light and maintained constant posture while alternating between fixing their gaze on a target for 6 min or free gaze for 6 min.51,53 Average light intensities during the fixed gaze were 2984 ± 367 lux (∼7 Watts/m2) for the bright light conditions and 1.9 ± 0.4 lux (∼0.6 Watts/m2) for the dim light conditions. Illuminance in the angle of gaze at eye level was measured with a research photometer (International Light, Newburyport, MA, USA) and irradiance was measured with a HOBO Micro Station Data Logger with HOBO Silicon Pyranometer Smart Sensor (Onset, Pocasset, MA, USA).
Circadian Phase Assessment and Analysis
Salivary melatonin was collected every 30 min at night and every 60 min during the day (hours 3-10 of habitual wakefulness) of each CR. Collected saliva was centrifuged and then frozen at -80°C until assayed. Salivary melatonin concentration was determined by ELISA assay according to manufacturer instructions (IBL International, Hamburg, Germany).
We first tested whether the phase angle of entrainment and thus the timing of the photic and non-photic stimuli was similar among conditions. We calculated the salivary dim-light melatonin onset (DLMO) to bedtime phase angle of entrainment as the timing of the DLMO minus bedtime, and thus negative numbers indicate a DLMO prior to bedtime.25,27,65 Circadian phase shifts were determined by the change in the timing of the salivary DLMO between CR1 and CR2. The salivary DLMO was defined as the linearly interpolated time point when melatonin levels exceeded and remained 2 standard deviations above the stable baseline mean.66–69 Circadian phase shifts were analyzed with a mixed model ANOVA assigning drug and light conditions as fixed factors. Planned one-tailed independent t-tests were used to determine significance for our directional hypotheses. Specifically we hypothesized: (1) that melatonin and bright light experimental conditions would induce significant phase advance shifts compared to the dim light-placebo control condition, which was expected to show a delay drift due to the on average longer than 24-h circadian period;25,70–72 (2) that bright light-placebo would induce a significant phase advance shift compared to dim-light melatonin; and (3) that bright light-melatonin would induce a significant phase advance shift compared to dim light-melatonin and bright light-placebo. In addition to the above statistical tests, effect sizes (Cohen d) were calculated to determine the size of phase resetting effects. Standard interpretations of effect size were used:73 small, d = 0.2; moderate, d = 0.5; large, d = 0.8.
RESULTS
Average DLMO Threshold and Phase Angle of Entrainment
Using the current DLMO definition, an average DLMO threshold level of 10.4 pg/mL was observed. This level is consistent with prior findings of an average 10.2 pg/mL salivary melatonin threshold using the same DLMO definition.66
The phase angle of entrainment between DLMO and bedtime was not statistically different among conditions prior to treatment (P = 0.19): DLP -2.36 ± 0.75 h (mean ± SD; 95%CI: -2.94 to -1.79 h); DLM -2.68 ± 0.41 h (95%CI: -3.00 to -2.37 h); BLP -2.79 ± 1.04 h (95%CI: -3.59 to -1.99 h); BLM -2.02 ± 0.89 h (95%CI: -2.70 to -1.34 h). The average phase angle was -2.46 h (± 0.83 h SD) with a maximum phase angle of -4.96 h and a minimum phase angle of -0.65 h. Post hoc analyses did not find association between initial phase angle and the resulting phase shift reported below when testing all three treatments combined in one analysis or when testing each treatment individually (all P > 0.25).
Phase Resetting Response
Figure 2 shows circadian phase shifts for individual subjects and the condition means for the melatonin and light conditions. Significant main effects for bright light (F1,32 = 14.04, P < 0.001) and for melatonin (F1,32 = 10.58, P < 0.01) were observed for circadian phase shifts. The interaction effect between light exposure and pill condition was not significant (F1,32 = 0.29, P = 0.60). We found a mean phase delay in the DLP condition of ∼26 min. Significant circadian phase advances were found for DLM, BLP, and BLM conditions compared to DLP (P < 0.05) with large effect sizes, d = 1.40, 1.53, and 2.81, respectively. There were no statistical differences found between DLM and BLP (P = 0.37) conditions with a less than small effect size, d = 0.16. The largest phase advance shift was found for the BLM condition, which induced a phase shift significantly larger than DLM and BLP (P < 0.05) with large effect sizes, d = 1.12 and 0.93, respectively.
Figure 2.
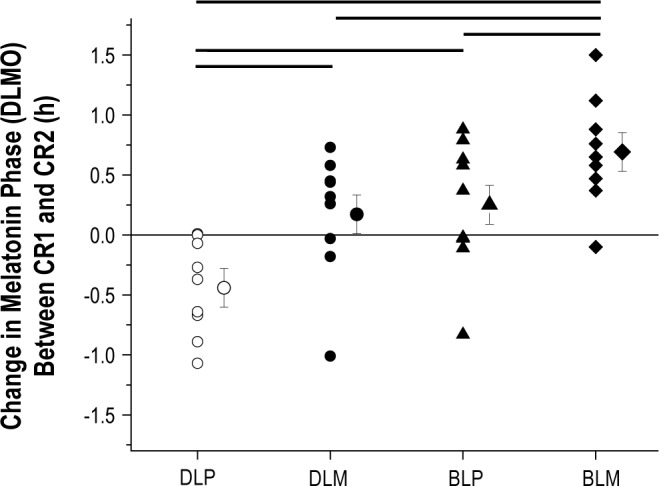
Phase resetting response to bright light and melatonin. On the y-axis, phase advances are plotted as positive values and phase delays are plotted as negative values with a value of zero representing no phase shift. Plotted are phase shifts for individual subjects within each condition as well as the condition mean ± SEM. DLP, dim light-placebo; DLM, dim light-melatonin; BLP, bright light-placebo; BLM, bright light-melatonin. Thicker lines above plot represent significant effects between conditions at either end of the line (P < 0.05). On average, DLM, BLP, and BLM induced ∼37 min, ∼42 min, and ∼68 min phase advances relative to DLP, respectively.
DISCUSSION
We found that photic and non-photic time cues are combined by the internal circadian clock in humans, furthering our knowledge about basic circadian principles as applied to humans and providing evidence for the effectiveness of combined treatments to induce desired phase shifts of circadian timing. Uniquely, and consistent with our hypotheses, the current findings indicate that a single exposure to 3 h of ∼3000 lux bright light in the morning combined with a single 5 mg dose of exogenous melatonin in the late afternoon/early evening can be combined to induce a greater circadian phase advance of the human circadian clock than either treatment alone. Our findings also reveal that under controlled dim light conditions, a single administration of 5 mg melatonin in the early evening is able to induce a comparable phase advance to that induced by a single 3-h exposure to ∼3000 lux bright light in the morning, inconsistent with our hypothesis that light exposure would induce a greater shift than melatonin. These findings have important implications for understanding fundamental physiological principles of the human circadian timing system, for adapting the human circadian clock to 24-hour and near-24-hour day lengths,24–27 and for the treatment of circadian sleep-wakefulness disorders (e.g., adapting to eastward travel and treatment of delayed sleep phase disorder).28,43 The combination of properly timed light exposure and melatonin administration may provide an effective means of advancing the human circadian clock such that the combination can produce a phase shift that is greater than that of either given alone.
Large effect sizes of all conditions relative to dim light-placebo suggest the strong ability of light and melatonin stimuli for shifting the human circadian clock. Relative to dim light-placebo, the magnitude of the response for exogenous melatonin (5 mg) and bright light exposure (∼3000 lux) were similar. This result suggests that under controlled environmental conditions at the specific times of the circadian and sleep-wakefulness cycle, and at the dose of melatonin and intensity and duration of light studied, melatonin may be as effective as bright light exposure to shift the human circadian clock. The finding that photic and non-photic stimuli are combined by the human circadian time keeping system also highlights the need to consider light exposure when using melatonin to shift the circadian clock. For example, uncontrolled evening light exposure in the work/home environment would likely reduce the effectiveness of evening melatonin administration when using melatonin to phase advance the clock.23 Knowledge of light and melatonin PRCs and the respective crossover times for phase advances and delays is important for optimal timing of treatments, since at most circadian phases light and melatonin induce phase shifts in the opposite direction; although there are narrow circadian phase windows where both appear to induce phase shifts in the same direction.29,38
Circadian phase angles of entrainment were on average more than two hours prior to habitual bedtime. The average and range of phase angles observed in the current study are generally consistent with prior findings when circadian phase is assessed using other DLMO thresholds and tested upon entry into the laboratory following at least one week of maintaining habitual ∼8-h sleep schedules.65 The finding that the dim light-placebo condition showed an average phase delay is consistent with previous findings of an average delay drift in phase of the human circadian clock in an environment absent of time cues,31,52,54 which is likely due to the longer than average circadian period in humans.25,70–72 Individual differences in the observed circadian phase resetting responses to melatonin and bright light stimuli are also likely driven by individual differences in circadian period.52 Assessment of circadian period and factoring it out statistically, or exposure of all individuals to each experimental condition would be necessary to control for effects of individual differences in circadian period on the phase resetting response.
Findings from studies of non-humans indicate that nonphotic time cues can facilitate entrainment to shifted light-dark cycles74 such as those induced by jet lag and shift work. Findings from the current study indicate that combined treatments may also facilitate circadian adaptation in humans to such schedules. Findings from two previous studies that examined the combination of photic and non-photic time cues indicate: (1) that during simulated night shift work, five days of exposure to intermittent bright light (5000 lux) in a gradually delaying light-dark cycle induced large and significant phase delay shifts and that the addition of 1.8 mg sustained release melatonin prior to a daytime sleep opportunity did not facilitate the phase resetting response40; and (2) that three days of exposure to intermittent bright light (5000 lux) in a gradually advancing light-dark cycle combined with either 0.5 mg or 3.0 mg melatonin 5 h or 7 h prior to bedtime, respectively, induced relatively large and significant phase advance shifts as compared to a dim light-placebo condition.75 The latter study by Revell et al., however, did not include dim light-melatonin alone conditions, thus it is unknown whether the combination of the treatments tested would be more effective than either alone. Given the number of treatment days, exposure to light alone may be sufficient to induce large phase shifts reducing the ability to detect differences among conditions due to ceiling effects. In the combined melatonin and light condition of the current study, it is likely that the evening melatonin dose, administered 12.75 hours prior to the beginning of the morning light exposure session, induced a phase advance shift of the circadian clock prior to light exposure and contributes to the current findings. Findings from a study by Paul et al.76 show that afternoon administration of sustained release 3 mg melatonin and morning exposure to moderate levels of green light (500 nm), combined with an advanced 13.5-h sleep opportunity, induced a significant phase advance shift relative to green light alone and to a dim light-placebo control. Green light alone did not induce a significant phase shift relative to dim light-placebo, whereas melatonin alone induced a significant phase advance relative to dim light-placebo, but not green light alone. Green light exposure was timed 1 h earlier in the combined condition than in the green light alone condition and could have contributed to their findings. Nonetheless, these findings are generally consistent with our findings of the effectiveness of combined treatments of photic and non-photic time cues for phase shifting the human circadian clock. Findings from Crowley et al.40 also show that multiple days of exposure to bright light are more effective than multiple days of administration of melatonin. Thus, the addition of melatonin when using multiple days of light exposure may not provide additional benefit, assuming that light exposure is properly timed.
Consistent with the present findings of combined light and melatonin stimuli, further evidence for combination of information by the human circadian clock comes from findings that the circadian system is capable of temporal integration of multiple pulses of light in the same session.54,55
Phase Resetting with Exogenous Melatonin
In the present study, melatonin administration was timed to induce a maximal phase advance shift based on multiple pulse phase response curves for melatonin.38,44 More recently, a three-pulse phase response curve has been published comparing a 3 mg dosage of melatonin to 0.5 mg of melatonin,47 suggesting that a lower dosage of melatonin is as effective at inducing phase shifts of the human circadian clock as the higher dose if timed appropriately. Specifically, Burgess et al. reported 0.5 mg of melatonin had a similar phase shift magnitude as compared to that induced by 3 mg of melatonin; however, the maximal phase shift occurred ∼2 h later in the smaller dose relative to DLMO.47 The latter finding suggests that the 5 mg melatonin dose may have induced a larger phase advance if it were administered earlier relative to DLMO in the current study. Revell et al.75 found that when the various doses were timed differently relative to DLMO, with the smaller dose closer to DLMO, no dose-relationship was found.
There have been several studies aimed at exploring the ability of a single administration of exogenous melatonin to induce a circadian phase advance. For example, findings from a study by Deacon and Arendt63 indicate that the phase shift response to a single administration of immediate release melatonin is dose dependent with mean phase advances of plasma melatonin onset of 22, 42, and 86 min, relative to placebo for 0.05, 0.5, and 5 mg doses, respectively. In recent studies by Paul et al.,69,76 a single administration of either 3 mg immediate release, 3 mg sustained release, or a combined 1 mg immediate release with 2 mg sustained release melatonin preparations induced phase advances between ∼29-50 min as compared to placebo. Single administration of 5 mg immediate release melatonin has previously been found to produce an average phase advance of ∼44 min relative to a pooled placebo.34 Our finding of an average ∼37 min phase advance relative to placebo following the single administration of 5 mg immediate release melatonin in the later afternoon/early evening is consistent with previous findings.
We did not assess the influence of exogenous melatonin on sleepiness and performance in the current study. Given that properly timed administration of lower doses of melatonin induce phase advances of a similar phase shift magnitude induced by higher doses in sighted47 and blind humans,77 and that melatonin may induce dose-dependent impairments in performance and increases in sleepiness,78 it is important to use the lowest effective dose possible to reduce the risk of accident. If melatonin is taken during the daytime, patients should be warned about potential safety risks (e.g., drowsy driving, occupational accidents).
Phase Resetting with Bright Light Exposure
Light exposure for the current study was timed according to the single pulse 6.7-h light PRC to induce a maximal phase advance shift.53 Light intensity was selected to be on the asymptotic portion of the luminance intensity curve based on a single 6.5-h light pulse.51 Thus, had we selected a dimmer light intensity or shorter duration,79 it is possible that differences in the magnitude of the circadian phase resetting response would have been found when comparing the light and melatonin stimuli. Our light exposure duration was less than half that used in the studies noted, and we did not invert the sleep-wakefulness cycle to have light exposure be in the middle of each day. Instead, subjects maintained their habitual sleep-wakefulness schedule and were awakened 1 h earlier than habitual wake time for light exposure. We avoided starting light exposure earlier so that we reduced the risk of exposing subjects to light on the crossover point between phase delays and advances.53 Findings from a recent study examining phase delays of the human circadian system varying the intensity (2000, 4000, and 8000 lux) and duration (1, 2, and 3 h) of light exposure showed an effect of duration with larger phase shifts observed with longer exposures, but no significant influence of intensity of light exposure.80 The latter is consistent with findings that ∼1230 lux and ∼5700 lux light intensities produced similar phase delays.81 These studies are consistent with prior findings that light exposures of the intensities tested, including the light intensity used in the current study, are above the saturating response for inducing phase shifts.51
There have also been prior studies in which the ability of a single exposure to light to induce a circadian phase advance has been explored.49,51,53,61,76 Our finding of an average ∼42 min phase advance following a single 3-h morning exposure to ∼3000 lux broad spectrum white light relative to placebo is smaller than that reported for 4-6.7-h morning exposures to 3000-12000 lux broad spectrum white light49,51,53,61,76 and larger than that reported for a 1-h exposure to 350 lux green light.76 As the circadian phase resetting response to light in humans is also dependent on the spectral characteristics of the light exposure,82 further research is needed to examine combinations of various lighting regimens and non-photic time cues.
Implications for Use of Photic and Non-Photic Stimuli to Reset Circadian Phase
The phase shifts induced by the photic and non-photic stimuli tested in the current study are physiologically and clinically meaningful. For example, the average sighted human requires a daily phase advance shift of ∼9 minutes every day to entrain or synchronize to the 24-h day.25,31,70–72 Estimates of circadian period that were derived from forced desynchrony protocols25–27,70–72 and validated by demonstration of the near-24-h entrainment limits of the human circadian clock in dim light,25 indicate that upper range of circadian periods observed in healthy, sighted humans is near 24.6 h.72 Thus, the current light and melatonin treatments tested would be sufficient to entrain most sighted, and many blind, humans who show a longer than 24-h circadian period, to the 24-h day.28,83,84
As outlined in reviews by the American Academy of Sleep Medicine,28,43 and in recommended practice parameters,85 evidence exists showing that light and melatonin treatments are effective and are considered as standard treatment, guideline, or optional treatment for a variety of circadian sleep-wake disorders including jet lag disorder, shift work disorder, delayed and advanced sleep phase disorders, and blind and sighted patients with non-24-hour sleep-wake rhythm disorder. Much of the evidence for light and melatonin treatments are based on basic circadian science, and thus these treatments are also likely to be useful in helping to adapt individuals who do not meet the criteria for a clinical diagnosis to jet lag and shift work. It is important to note, however, that large trials are needed to assess the effectiveness of combined light and melatonin treatments, as much as possible, during real world conditions outside of the controlled laboratory. Such evidence exists for timed daily melatonin administration, which is indicated for the therapy of non-24-hour sleep-wake disorder in blind individuals.28,85–87 In addition, light and melatonin treatments have been used to treat winter depression, which is thought to have a circadian component to its pathophysiology and treatment.88 When advancing the human circadian clock is desired, such as when adapting to early morning shift work and eastward jet travel, or when treating delayed sleep phase disorder, our findings indicate that the combination of bright light exposure in the early morning and exogenous melatonin in the evening would provide the greatest phase shift treatment response.
DISCLSOURE STATEMENT
This study was supported by NIH RO1 HL081761 and Colorado CTSI Grant Number UL1 TR000154 from NIH/ NCATS. Contents are the authors' sole responsibility and do not necessarily represent official NIH views. Mr. Snider reports he was a consultant for Zeo, Inc. Dr. Wright Jr serves as a consultant for Takeda Pharmaceuticals and Chair of the External Advisory Board of the Northwestern American Waterways Project. He served as Chair of the Scientific Advisory Board and as a consultant for Zeo, Inc. and has received honorarium from Potomac Center for Medical Education, the Associated Professional Sleep Societies, and the National Institutes of Health. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Experiments were performed in the Sleep and Chronobiology Laboratory at the University of Colorado Boulder. All authors contributed to the following parts: (1) Conception and/ or design of the experiment; (2) Collection, analysis and/or interpretation of data; (3) Drafting the article or revising it critically for important intellectual content. All authors approved the final submitted version.
ABBREVIATIONS
- CR
constant routine
- CTRC
Clinical Translational Research Center
- BLM
bright light-melatonin
- BLP
bright light-placebo
- DLM
dim light-melatonin
- DLMO
dim light melatonin onset
- DLP
dim light-placebo
- PRC
phase response curve
- SCN
suprachiasmatic nucleus
REFERENCES
- 1.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–6. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 2.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 3.Pittendrigh CS. Temporal organization: reflections of a Darwinian clockwatcher. Annu Rev Physiol. 1993;55:17–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 4.DeCoursey PJ, Walker JK, Smith SA. A circadian pacemaker in free-living chipmunks: essential for survival? J Comp Physiol. 2000;186:169–80. doi: 10.1007/s003590050017. [DOI] [PubMed] [Google Scholar]
- 5.Guler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–5. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–5. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hastings MH, Mead SM, Vindlacheruvu RR, Ebling FJ, Maywood ES, Grosse J. Non-photic phase shifting of the circadian activity rhythm of Syrian hamsters: the relative potency of arousal and melatonin. Brain Res. 1992;591:20–6. doi: 10.1016/0006-8993(92)90973-d. [DOI] [PubMed] [Google Scholar]
- 8.Maywood ES, Mrosovsky N. A molecular explanation of interactions between photic and non-photic circadian clock-resetting stimuli. Gene Expr Patterns. 2001;1:27–31. doi: 10.1016/s1567-133x(01)00005-9. [DOI] [PubMed] [Google Scholar]
- 9.Challet E, Pevet P. Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals. Front Biosci. 2003;8:S246–57. doi: 10.2741/1039. [DOI] [PubMed] [Google Scholar]
- 10.Novak CM, Ehlen JC, Albers HE. Photic and nonphotic inputs to the diurnal circadian clock. Biol Rhyth Res. 2008;39:291–304. [Google Scholar]
- 11.Knutsson A, Akerstedt T, Jonsson BG, Orthgomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;2:89–91. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 12.Wright KP, Jr, Hull JT, Hughes RJ, Ronda JM, Czeisler CA. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci. 2006;18:508–21. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- 13.Barger LK, Lockley SW, Rajaratnam SM, Landrigan CP. Neurobehavioral, health, and safety consequences associated with shift work in safety-sensitive professions. Curr Neurol Neurosci Rep. 2009;9:155–64. doi: 10.1007/s11910-009-0024-7. [DOI] [PubMed] [Google Scholar]
- 14.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava UR. Shift work related to stress, health and mood states: a study of dairy workers. J Health Manag. 2011;12:173–200. [Google Scholar]
- 16.Wright KP, Jr, Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep Med Rev. 2013;17:41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Benloucif S, Masana MI, Yun K, Dubocovich ML. Interactions between light and melatonin on the circadian clock of mice. J Biol Rhythms. 1999;14:281–9. doi: 10.1177/074873099129000696. [DOI] [PubMed] [Google Scholar]
- 18.Smart CM, Biello SM. WAY-100635, a specific 5-HT1A antagonist, can increase the responsiveness of the mammalian circadian pacemaker to photic stimuli. Neurosci Lett. 2001;305:33–6. doi: 10.1016/s0304-3940(01)01797-9. [DOI] [PubMed] [Google Scholar]
- 19.Lall GS, Biello SM. Attenuation of phase shifts to light by activity or neuropeptide Y: a time course study. Brain Res. 2002;957:109–16. doi: 10.1016/s0006-8993(02)03610-7. [DOI] [PubMed] [Google Scholar]
- 20.Lall GS, Biello SM. Attenuation of circadian light induced phase advances and delays by neuropeptide Y and a neuropeptide Y Y1/Y5 receptor agonist. Neurosci. 2003;119:611–8. doi: 10.1016/s0306-4522(02)00811-4. [DOI] [PubMed] [Google Scholar]
- 21.Yannielli P, Harrington ME. Let there be “more” light: enhancement of light actions on the circadian system through non-photic pathways. Prog Neurobiol. 2004;74:59–76. doi: 10.1016/j.pneurobio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Boothroyd CE, Wijnen H, Naef F, Saez L, Young MW. Integration of light and temperature in the regulation of circadian gene expression in Drosophila. PLoS Genet. 2007;3:e54. doi: 10.1371/journal.pgen.0030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wirz-Justice A, Krauchi K, Cajochen C, Danilenko KV, Renz C, Weber JM. Evening melatonin and bright light administration induce additive phase shifts in dim light melatonin onset. J Pineal Res. 2004;36:192–4. doi: 10.1111/j.1600-079x.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 24.Dijk DJ, Neri DF, Wyatt JK, et al. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle missions. Am J Physiol. 2001;281:R1647–64. doi: 10.1152/ajpregu.2001.281.5.R1647. [DOI] [PubMed] [Google Scholar]
- 25.Wright KP, Jr, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci U S A. 2001;98:14027–32. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gronfier C, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci U S A. 2007;104:9081–6. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheer FA, Wright KP, Jr, Kronauer RE, Czeisler CA. Plasticity of the intrinsic period of the human circadian timing system. PLoS ONE. 2007;2:e721. doi: 10.1371/journal.pone.0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30:1480–97. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drake CL, Wright KP., Jr . Shift work, shift work disorder, and jet lag. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. St Louis: Saunders; 2011. pp. 784–98. [Google Scholar]
- 30.Czeisler CA, Wright KP., Jr . Influence of light on circadian rhythmicity in humans. In: Turek FW, Zee PC, editors. Regulation of sleep and circadian rhythms. New York: Marcel Dekker Inc; 1999. pp. 149–80. [Google Scholar]
- 31.Duffy JF, Wright KP., Jr Entrainment of the human circadian system by light. J Biol Rhythms. 2005;28:326–38. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 32.Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neur Biol. 1979;25:545–54. doi: 10.1016/s0163-1047(79)90332-7. [DOI] [PubMed] [Google Scholar]
- 33.Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc. 1996;71:343–72. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 34.Krauchi K, Cajochen C, Mori D, Graw P, Wirz-Justice A. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. Am J Physiol Regul Integr Comp. 1997;41:R1178–88. doi: 10.1152/ajpregu.1997.272.4.R1178. [DOI] [PubMed] [Google Scholar]
- 35.Youngstedt SD, Kripke DF, Elliott JA. Circadian phase-delaying effects of bright light alone and combined with exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2002;282:R259–66. doi: 10.1152/ajpregu.00473.2001. [DOI] [PubMed] [Google Scholar]
- 36.Barger LK, Wright KP, Jr, Hughes RJ, Czeisler CA. Daily exercise facilitates phase delays of circadian melatonin rhythm in very dim light. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1077–84. doi: 10.1152/ajpregu.00397.2003. [DOI] [PubMed] [Google Scholar]
- 37.Muscat L, Morin LP. Intergeniculate leaflet: contributions to photic and non-photic responsiveness of the hamster circadian system. Neurosci. 2006;140:305–20. doi: 10.1016/j.neuroscience.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 38.Lewy AJ, Bauer VK, Ahmed S, et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 39.Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–28. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18:513–23. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- 41.Rajaratnam SM, Dijk DJ, Middleton B, Stone BM, Arendt J. Melatonin phase-shifts human circadian rhythms with no evidence of changes in the duration of endogenous melatonin secretion or the 24-hour production of reproductive hormones. J Clin Endocrinol Metab. 2003;88:4303–9. doi: 10.1210/jc.2003-030460. [DOI] [PubMed] [Google Scholar]
- 42.Zeitzer JM, Khalsa SB, Boivin DB, et al. Temporal dynamics of late-night photic stimulation of the human circadian timing system. Am J Physiol Regul Integr Comp Physiol. 2005;289:R839–44. doi: 10.1152/ajpregu.00232.2005. [DOI] [PubMed] [Google Scholar]
- 43.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30:1456–79. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586:639–47. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffy JF, Czeisler CA. Effect of light on human circadian physiology. Sleep Med Clin. 2009;4:165–77. doi: 10.1016/j.jsmc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajaratnam SM, Cohen DA, Rogers NL. Melatonin and melatonin analogues. Sleep Med Clin. 2009;4:179–93. [Google Scholar]
- 47.Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab. 2010;95:3325–31. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crowley SJ, Eastman CI. Melatonin in the afternoons of a gradually advancing sleep schedule enhances the circadian rhythm phase advance. Psychopharmacol. 2013;225:825–37. doi: 10.1007/s00213-012-2869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buresová M, Dvoráková M, Zvolsky P, Illnerová H. Early morning bright light phase advances the human circadian pacemaker within one day. Neurosci Lett. 1991;121:47–50. doi: 10.1016/0304-3940(91)90646-b. [DOI] [PubMed] [Google Scholar]
- 50.Deacon S, Arendt J. Adapting to phase shifts, II. Effects of melatonin and conflicting light treatment. Physiol Behav. 1996;59:675–82. doi: 10.1016/0031-9384(95)02148-5. [DOI] [PubMed] [Google Scholar]
- 51.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright KP, Jr, Czeisler CA. Absence of circadian phase resetting in response to bright light behind the knees. Science. 2002;297:571. doi: 10.1126/science.1071697. [DOI] [PubMed] [Google Scholar]
- 53.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gronfier C, Wright KP, Jr, Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004;287:E174–81. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. Response of the human circadian system to millisecond flashes of light. PLoS One. 2011;6:e22078. doi: 10.1371/journal.pone.0022078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennaway DJ, Earl CR, Shaw PF, Royles P, Carbone F, Webb H. Phase delay of the rhythm of 6-sulphatoxy melatonin excretion by artificial light. J Pineal Res. 1987;4:315–20. doi: 10.1111/j.1600-079x.1987.tb00869.x. [DOI] [PubMed] [Google Scholar]
- 57.Sack RL, Lewy AJ, Hoban TM. Free-running melatonin rhythms in blind people: phase shifts with melatonin and triazolam administration. In: Rensing L, an der Heiden U, Mackey MC, editors. Temporal disorder in human oscillatory systems. Heidelberg: Springer-Verlag; 1987. pp. 219–24. [Google Scholar]
- 58.Honma K, Honma S. A human phase response curve for bright light pulses. Jpn J Psychiatry Neurol. 1988;42:167–8. [Google Scholar]
- 59.Mallo C, Zaidan R, Faure A, Brun J, Chazot G, Claustrat B. Effects of a four-day nocturnal melatonin treatment on the 24 h plasma melatonin, cortisol and prolactin profiles in humans. Acta Endocrinol. 1988;119:474–80. doi: 10.1530/acta.0.1190474. [DOI] [PubMed] [Google Scholar]
- 60.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 61.Dawson D, Lack L, Morris M. Phase resetting of the human circadian pacemaker with use of a single pulse of bright light. Chronobiol Int. 1993;10:94–102. doi: 10.3109/07420529309059697. [DOI] [PubMed] [Google Scholar]
- 62.Van Cauter E, Sturis J, Byrne MM, et al. Preliminary studies on the immediate phase-shifting effects of light and exercise on the human circadian clock. J Biol Rhythms. 1993;8:S99–108. [PubMed] [Google Scholar]
- 63.Deacon S, Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 1995;688:77–85. doi: 10.1016/0006-8993(95)96872-i. [DOI] [PubMed] [Google Scholar]
- 64.Cagnacci A, Soldani R, Yen SS. Contemporaneous melatonin administration modifies the circadian response to nocturnal bright light stimuli. Am J Physiol Regul Integr Comp Physiol. 1997;272:R482–6. doi: 10.1152/ajpregu.1997.272.2.R482. [DOI] [PubMed] [Google Scholar]
- 65.Wright KP, Jr, Gronfier C, Duffy JE, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–77. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–66. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 67.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227–36. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 68.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–88. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 69.Paul MA, Miller JC, Gray GW, Love RJ, Lieberman HR, Arendt J. Melatonin treatment for eastward and westward travel preparation. Psychopharmacol. 2010;208:377–86. doi: 10.1007/s00213-009-1737-7. [DOI] [PubMed] [Google Scholar]
- 70.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 71.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 72.Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108:15602–8. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New Jersey: Lawrence Erlbaum; 1988. [Google Scholar]
- 74.Mrosovsky N, Salmon PA. A behavioural method for accelerating re-entrainment of rhythms to new light-dark cycles. Nature. 1987;330:372–3. doi: 10.1038/330372a0. [DOI] [PubMed] [Google Scholar]
- 75.Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006;91:54–9. doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paul MA, Gray GW, Lieberman HR, et al. Phase advance with separate and combined melatonin and light treatment. Psychopharmacol. 2011;214:515–23. doi: 10.1007/s00213-010-2059-5. [DOI] [PubMed] [Google Scholar]
- 77.Lewy AJ, Emens JS, Lefler BJ, Yuhas K, Jackman AR. Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol Int. 2005;22:1093–106. doi: 10.1080/07420520500398064. [DOI] [PubMed] [Google Scholar]
- 78.Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci U S A. 1994;91:1824–8. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang AM, Santhi N, St Hilaire M, et al. Human responses to bright light of different durations. J Physiol. 2012;590:3103–12. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dewan K, Benloucif S, Reid K, Wolfe LF, Zee PC. Light-induced changes of the circadian clock of humans: increasing duration is more effective than increasing light intensity. Sleep. 2011;34:593–9. doi: 10.1093/sleep/34.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martin SK, Eastman CI. Medium-intensity light produces circadian rhythm adaptation to simulated night-shift work. Sleep. 1998;21:154–65. [PubMed] [Google Scholar]
- 82.Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emens J, Lewy AJ, Laurie AL, Songer JB. Rest-activity cycle and melatonin rhythm in blind free-runners have similar periods. J Biol Rhythms. 2010;25:381–4. doi: 10.1177/0748730410379080. [DOI] [PubMed] [Google Scholar]
- 84.Eastman CI, Molina TA, Dziepak ME, Smith MR. Blacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans) Chronobiol Int. 2012;29:1072–7. doi: 10.3109/07420528.2012.700670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30:1445–59. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skene DJ, Lockley SW, Arendt J. Use of melatonin in the treatment of phase shift and sleep disorders. Adv Exp Med Biol. 1999;467:79–84. doi: 10.1007/978-1-4615-4709-9_10. [DOI] [PubMed] [Google Scholar]
- 87.Lewy AJ, Emens J, Jackman A, Yuhas K. Circadian uses of melatonin in humans. Chronobiol Int. 2006;23:403–12. doi: 10.1080/07420520500545862. [DOI] [PubMed] [Google Scholar]
- 88.Lewy AJ, Emens JS, Songer JB, et al. Winter depression: integrating mood, circadian rhythms, and the sleep/wake and light/dark cycles into a bio-psycho-social-environmental model. Sleep Med Clin. 2009;4:285–99. doi: 10.1016/j.jsmc.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


