Abstract
Objective:
To examine whether a social cognitive therapy (SCT) intervention increases continuous positive airway pressure (CPAP) use compared to equivalent social interaction (SI) time.
Participants:
Individuals with obstructive sleep apnea (OSA) referred for CPAP therapy.
Intervention:
Participants received a 30-min group education session regarding OSA and CPAP. Groups of three to four participants were then randomly assigned to an SCT session or social interaction.
Measurements:
CPAP usage was assessed at 7 nights, then 1, 3, and 6 months. The two primary outcomes were adherence, usage ≥ 4 h per night at 6 months, and uptake of CPAP. Questionnaires were given pretreatment and posttreatment.
Results:
Two hundred six individuals were randomized to SI (n = 97) or SCT (n = 109). CPAP uptake was not different between groups (82% in SI, 88% in SCT groups, P = 0.35). There were no differences between groups in adherence: 63-66% at 1 week, and at 6 months 55-47% (P = 0.36). Higher pretreatment apnea-hypopnea index, higher baseline self-efficacy, and use of CPAP (≥ 4 h) at 1 week were independent predictors of CPAP adherence at 6 months. CPAP adherence increased by a factor of 1.8 (odds ratio = 1.8, 95% confidence interval 1.1-3.0) for every one-unit increase in self-efficacy. There was no difference between groups postintervention in self-efficacy scores, sleepiness, mood, or sleep quality.
Conclusions:
In this randomized trial, a single SCT application did not increase adherence when compared with SI time. Although self-efficacy scores prior to CPAP predicted adherence, self-efficacy was not increased by the interventions. Increasing intensity and understanding of SCT interventions may be needed to improve CPAP adherence.
Clinical Trials Registration:
Australian New Zealand Clinical Trials Registry, ACTRN12607000424404.
Citation:
Bartlett D; Wong K; Richards D; Moy E; Espie CA; Cistulli PA; Grunstein R. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: a randomized trial. SLEEP 2013;36(11):1647-1654.
Keywords: Continuous positive airway pressure, obstructive sleep apnea, patient adherence, self-efficacy
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep disorder affecting 2-7% of the population and when untreated has significant morbidity and mortality.1–3 Continuous positive airway pressure (CPAP)4 when used on a nightly basis is currently considered the most effective treatment for OSA, improving neurocognitive function, quality of life, cardiovascular disease risk, and mood outcomes.5
Due to technological improvements over the past 20 years, adherence to CPAP can be accurately and objectively measured. Adherence to CPAP has been arbitrarily defined as usage of at least 4 h per night, 5 nights per week with most individuals either remaining intermittent users, discontinuing treatment, or not accepting the treatment following CPAP titration.6–8 Dropout rates range from 5% to 50% in 1 week to 6 mo.9,10 Studies of CPAP use have shown variable limitations, including use of selected cohorts or very little information available on those who do not start CPAP or discontinue its use very early. Few studies report adherence at > 6 h every night despite that amount of uninterrupted sleep being seen as an important cutoff level in studies linking short sleep duration to impaired health.11 CPAP adherence is important because greater degrees of CPAP use are associated with better neurocognitive and cardiovascular outcomes12 and quality of life measures, including daytime sleepiness.9,13,14
Factors influencing CPAP acceptance and adherence have been recently reviewed extensively,15 and include older age and female sex. greater pretreatment OSA severity, greater pretreatment daytime sleepiness, subjective resolution of daytime symptoms when using CPAP, initial experience of using CPAP and resolution of problems associated with CPAP usage (mask fitting, leaks, nasal congestion, claustrophobia), health beliefs and attitudes, partner support/bed sharing, socioeconomic status, and educational interventions.
Another suggested predictor of CPAP adherence is patient self-efficacy. This metric includes the individual's belief in his or her ability to successfully engage in a novel behavior such as CPAP in the future by being prepared to use it every night (self-regulatory skills), looking after the machine, mask, and tubing (setting achievable goals, making specific plans), and observing successful but realistic models of successful CPAP users.16,17 However, the value of self-efficacy as a predictor of CPAP adherence is uncertain, possibly in part because of differences in timing of measurement of this metric prior to or after behavioral and other interventions.9,15,18–20
A range of nontechnological interventions have been designed to improve adherence to CPAP. Purely educational strategies have limited effect on adherence.15,21 Behavioral interventions, based on health psychology approaches to nonadherence to CPAP, appear more promising.21 However, the optimum content, delivery, timing, and intensity of such interventions are unknown. Our group has previously found two separate social cognitive theory (SCT) sessions significantly increased CPAP uptake and nightly usage by 2.9 h at 1 mo compared to treatment as usual (TAU), which included mask fitting and information about CPAP only.18 The intervention was based on SCT.16,17 A potential limitation of this study was the unequal intervention time spent with these two groups. The SCT group received an additional 2 h of contact compared with the TAU group. This increased attention may have also influenced uptake and adherence outcomes.
The aim of the current study was to compare a pragmatic application of a single SCT group session to an equivalent time of social interaction (SI) to investigate CPAP usage in a larger patient sample over a longer time period (6 mo). We hypothesized that a single SCT session prior to commencing CPAP would improve CPAP uptake and compliance and therefore provide a simple and potentially cost-effective strategy to improve CPAP adherence. Both groups received an enhanced behavioral and education session (BES), now routine at our centers, that incorporated some elements of the active intervention in our previous study.18
METHODS
Participants
Participants were recruited following diagnosis with moderate to severe OSA and referral for a CPAP-titration study from three geographically distinct clinics in Sydney, New South Wales (Royal Prince Alfred Hospital, Royal North Shore Hospital and the Woolcock Institute of Medical Research). Recruitment took place between November 2007 and August 2009. Follow-up was completed by May 2010. Individuals were asked to participate in a study that would be valuable in helping them to use CPAP. They were informed they would be randomized to one of two slightly different educational treatment interventions, SCT or SI, after the routine BES. Participants attended the intervention predominantly in groups of four, and occasionally fewer due to nonattendance. Participants were informed they would be asked to complete a number of questionnaires and a neurobehavioral test battery at 1 mo and 6 mo. Results from the neurobehavioral battery are currently being prepared in a separate publication. Participants arrived at the sleep laboratory at 12:30, gave consent to participate, completed baseline questionnaires (including the self-efficacy scale) and the neurobehavioral test battery, and undertook CPAP education, followed by mask fitting, trial of CPAP at 4 cm H2O (acclimatization) and randomization. Although the afternoon schedule was intense it was designed to minimize study visits compared with our previous study.18 The SCT group was shown an additional slide presentation and video whereas the SI group had afternoon tea and viewed a video of an individual undergoing a diagnostic and CPAP titration night at one of the participating clinics. Exclusion criteria included an inability to understand fluent English and any previous use of CPAP. This study was approved by the Ethics Review Committee of Sydney South West Area Health Service Sydney and is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12607000424404).
Randomization, Allocation Concealment, and Blinding
Prior to recruitment, a randomization sequence by group using random permuted blocks with a 1:1 allocation ratio to SI and SCT interventions was generated by an independent investigator. Although participants were undergoing mask fitting with the sleep technologist, the psychologist responsible for the interventions opened a sequentially numbered, opaque, sealed envelope containing the randomization allocation. Participants were then presented with the SCT or SI protocol. Staff administering the SCT or SI were not blinded to the intervention, but did not have ongoing clinical contact with the patients. Other staff members interacting with the patients in subsequent visits were blinded to group allocation.
Questionnaires
All questionnaires were given at baseline, 1 mo, and 6 mo. The SCT questionnaire assesses the likelihood of an individual taking up and using CPAP.22 Only three subscales (self-efficacy, social support, and outcome expectancy) were used in this trial. Self-efficacy measures the individual's belief that he/she will be able to successfully use CPAP at some time in the future. Social support measures the level of support the person would expect from a bed partner and/or members of the individual's family or social network encouraging the use of CPAP every night. Outcome expectancy measures the expectation that using CPAP successfully will result in improvement in OSA daytime deficits and general health.
Other questionnaires administered included the Epworth Sleepiness Scale (ESS),23 Depression Anxiety Stress Scale (DASS),24 Fatigue Severity Scale (FSS),25 Pittsburgh Sleep Quality Index (PSQI),26 and Functional Outcomes Sleep Questionnaire (FOSQ).27 Baseline questionnaires were given prior to receiving any CPAP education or randomization. The patients were specifically asked to ensure they completed the SCT questionnaire prior to the BES or randomization and these were collected prior to provision of any content.
Interventions
Behavioral and Educational Session
This intervention was delivered to both arms. Based on previous research findings,18 the standard CPAP education now included information about CPAP machines and masks, a standardized slide presentation containing information about normal sleep, daytime and nighttime health consequences of untreated OSA (cardiovascular and driving/occupational accident risks), and the effectiveness of CPAP treatment. As part of the CPAP-titration process, participants were fitted with a comfortable, well-sealed mask and familiarized with all the equipment (CPAP machine and humidifier) by one of our sleep technologists. Side effects encountered when using CPAP were highlighted and discussed. All participants had a trial of CPAP at a pressure of 4 cm H20 in a supine position and were encouraged to handle the equipment and to ask questions. Throughout this session participants were strongly encouraged by staff members to seek help and support for any mechanical difficulties throughout the study period.
Social Cognitive Therapy
The intervention was predominantly based on social cognitive theory factors (increased perceived self-efficacy, outcome expectations, and social support) and was delivered by one of two experienced psychologists. At the beginning of the intervention participants listed a goal for using CPAP, reinforcing the message that in using CPAP they would have more positive health options in the future. Throughout the slide presentation it was stressed how unhelpful thoughts can reduce CPAP usage; for example, focusing on side effects of CPAP such as a congested nose might override any long-term perceived positive results from using CPAP. The 20-min slide-based SCT intervention promoted future health and safety benefits of using CPAP every night such as being more involved with partners/family/friends, feeling less sleepy, and more likely to feel like exercising. A 15-min video (made especially for the study) showed two female and two male CPAP users (one younger and one older role model for each sex) who described their personal experiences of learning to manage CPAP, and to persevere as the long-term health benefits (reduced sleepiness, more energy, better memory and mood) were worth the effort. Simple relaxation strategies were also given, as this was used in the previously published study.18 Handouts of the slides and an additional booklet, containing information about sleep, OSA/CPAP, and general health (exercise, diet, effects of alcohol) was provided to the SCT group only. Images of the successful role models were used as symbols of success and unhelpful thoughts associated with CPAP treatment were reframed into more helpful or realistic thoughts/goals.
Social Interaction
SI was a social intervention used to ensure equal time was spent with all participants. The SI group was provided with afternoon tea (20 min) including biscuits (cookies) and decaffeinated tea and coffee. The SI group was also shown a 15-min video provided by a local television station that followed a patient from his baseline diagnostic sleep study to being diagnosed with OSA and undergoing a CPAP titration study.
Both interventions were of equal length and scripted by the content of the slides and or the different video presentations.
CPAP Titration and Apparatus
Patients underwent an in-laboratory manual titration of their CPAP pressure with polysomnography (PSG). CPAP-therapy pressure was titrated to treat apnea, hypopnea, and flow limitation, with a therapy target of apnea-hypopnea index (AHI) < 5 per hour of sleep. A prescription for an appropriate CPAP pressure and well sealing mask were provided for participants. Participants were required to purchase their own mask and tubing, but a CPAP flow generator was provided for the duration of the study at no cost.
All participants were provided with either a REMstar Pro CPAP (Respironics Inc., Murrysville PA) device with a data-storage Smart-Card, or a Sandman Goodnight 420 Series Auto HH (Covidien, Mansfield, MA) which allowed usage data to be directly downloaded from the machine or via a memory key. The data card/key recorded run time while the mask was on the patient's face. The returned data was downloaded by the research assistant and automatically analyzed using software provided by the respective device manufacturers.
Procedures and Ongoing Interactions
The Behavioral and Educational Session and either the SI or SCT interventions, together with the CPAP titration study, were conducted during the baseline visit. After participants had used their CPAP for 7 nights, a sleep unit staff member (blinded to intervention) telephoned participants to request the data card from the device be returned by mail in the envelope provided. The staff member also asked participants to report on how their CPAP therapy was going and if there were any difficulties, which was followed by appropriate troubleshooting. This telephone follow-up protocol was also carried out at 3 mo. The participants returned to the clinic for a final visit at 6 mo to complete questionnaires, repeat the neurobehavioral test battery, have their CPAP usage measured, and to return the CPAP flow generator and undergo repeat PSG. Participants also attended medical follow-up during this period as per routine practice.
Statistical Analysis
Usage data were downloaded from the CPAP flow generators at 1 week, 1 mo, 3 mo, and 6 mo. The data collected at 1 week included the first 7 days of therapy, whereas subsequent data collection periods included all days starting from the day after the previous data collection (for example, the data collection at 1 mo included the period from days 8 to 30). The mask-on time for each period was divided by the number of nights to obtain a mean daily usage. The two primary outcome measures were the proportion accepting and implementing CPAP, which was defined by the purchase of a CPAP mask and subsequently obtaining a CPAP flow generator from the clinic, and the proportion of adherent participants, defined as using CPAP for an average ≥ 4 h and ≥ 6 h a night, at 6 mo. Secondary outcomes were mean hours of CPAP usage (measured by mask-on time) at 1 week, 1 mo, 3 mo, and 6 mo.
Adherence to CPAP and other binary variables were presented as proportions (percentages), whereas average nightly CPAP use and other continuous variables were described by mean and standard deviation. Differences in proportions between the two intervention groups were analyzed by the chi-square test. Differences in continuous variables were analyzed by unpaired t-test. The analysis was by intention to treat. Participants who withdrew from the study were asked to attend the 6-mo follow-up visit to collect outcome data. In cases where CPAP usage data from device download was missing, usage was imputed to be zero hours/night if it was confirmed with the participant that had they had refused or abandoned CPAP therapy, or else missing values were imputed by carrying forward the last observation. Because this strategy might lead to an overestimate of CPAP usage, sensitivity analyses were conducted, specifically by imputing zero usage to all missing data, or confining analysis to cases with complete data. Predictors of CPAP adherence at 6 mo were explored by logistic regression, and the effect of the SCT intervention was also evaluated, adjusting for significant predictors of adherence or variables found to be different between the intervention groups at baseline. P values < 0.05 were considered significant. Statistical analyses were conducted using SAS (SAS Institute, Cary, NC, USA), and R (R Foundation for Statistical Computing, Vienna, Austria).
Sample Size
Assuming the proportion using CPAP therapy in the SI group to be between 20% and 70%, in order to detect a difference in proportions of 20%, 62 to 97 patients would be required per group, based on a two-group comparison with 80% power and two-tailed significance of 0.05. We aimed to recruit a total of 220 patients to account for loss to follow-up.
RESULTS
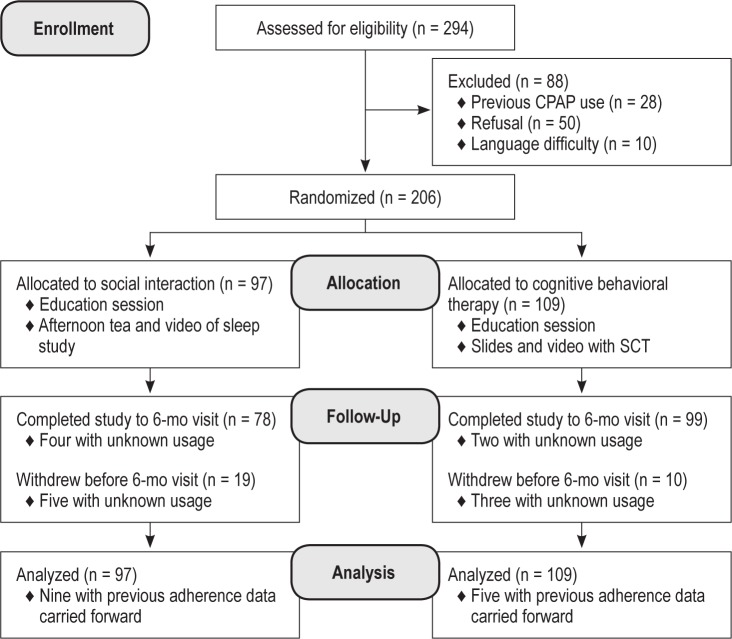
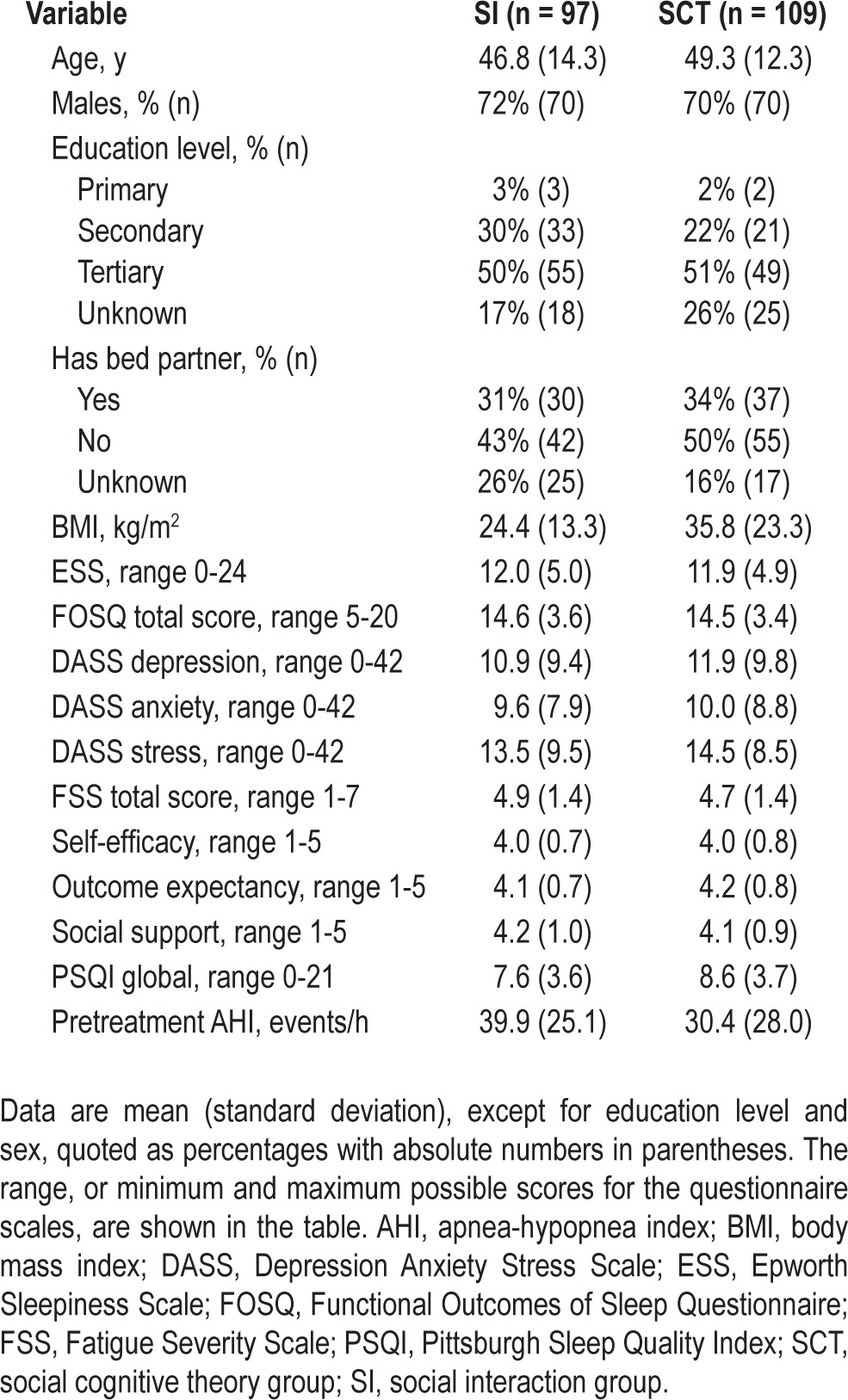
After obtaining consent, 206 participants were randomized to either the SI (n = 97) or the SCT group (n = 109). Figure 1 describes the flow of participants through the study. Baseline characteristics of the 206 individuals are shown in Table 1. Sleep apnea severity prior to treatment, measured by AHI (P = 0.02) and arousal index (P = 0.04) were slightly higher in the SI group compared with the SCT group. No differences were found at baseline in sex, education level, presence of a bed partner, body mass index, daytime sleepiness, or any psychological factors as measured by questionnaire data.
Figure 1.
Participant flow diagram. CPAP, continuous positive airway pressure; SCT, social cognitive theory group.
Table 1.
Baseline participant characteristics
Twenty-four patients (10 in the SCT group, 14 in the SI group) were confirmed on direct questioning to have ceased CPAP therapy during the follow-up period, and in the absence of usage data download from their CPAP device, had their nightly usage assigned as zero hours. Withdrawal rate was 11% in the SCT group (12 participants), as compared with 20% (19 participants) in the SI group (P = 0.13). Of these patients withdrawing prior to 6 mo of follow-up, CPAP usage data were unknown in three patients in the SCT group and five in the SI group. In those completing follow-up, an additional six patients (two in the SCT, four in the SI group) had data cards that were missing or corrupted.
CPAP Uptake
The proportion of patients in the SCT group who collected their loaner CPAP machine after having purchased their own mask (88%, 96 patients) did not differ from the SI group (82%, 80 patients, P = 0.35).
Adherence
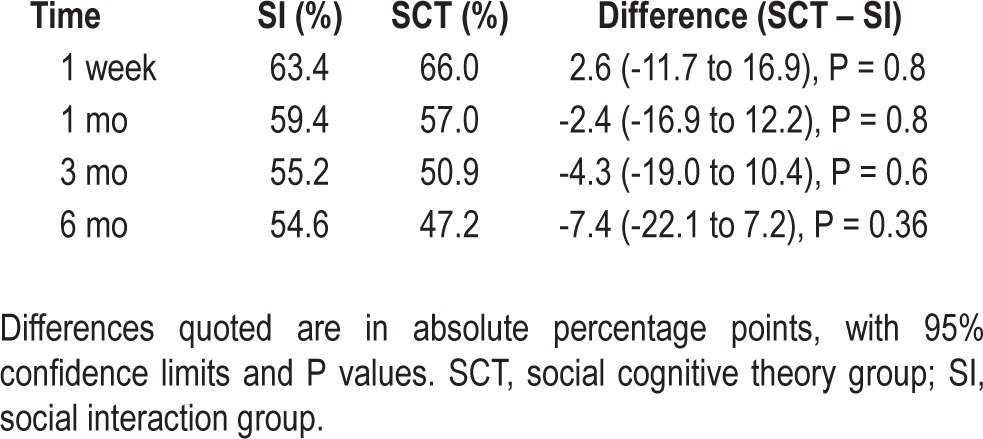
Table 2 shows the proportions of patients using CPAP for an average of 4 h or greater per night, at the 1 week, 1 mo, 3 mo, and 6 mo follow-up time points. There were no differences in CPAP adherence between groups at each of the follow-up points. At 6 mo, 54.6% of patients were adherent to CPAP in the social interaction group, as compared with 47.2% in the SCT group (difference, 7.4 percentage points, 95% confidence interval [CI], 22.1-7.2, P = 0.4). A higher rate of missing adherence data in the SI group might serve to attenuate any positive effect of the SCT intervention, particularly if missing data were related to poor adherence. Nevertheless, the finding of no differences in adherence between intervention groups was robust to sensitivity analyses assigning a value of zero to missing usage data, or examining complete data only.
Table 2.
Adherence to continuous positive airway pressure, as defined by average nightly usage ≥ 4 h
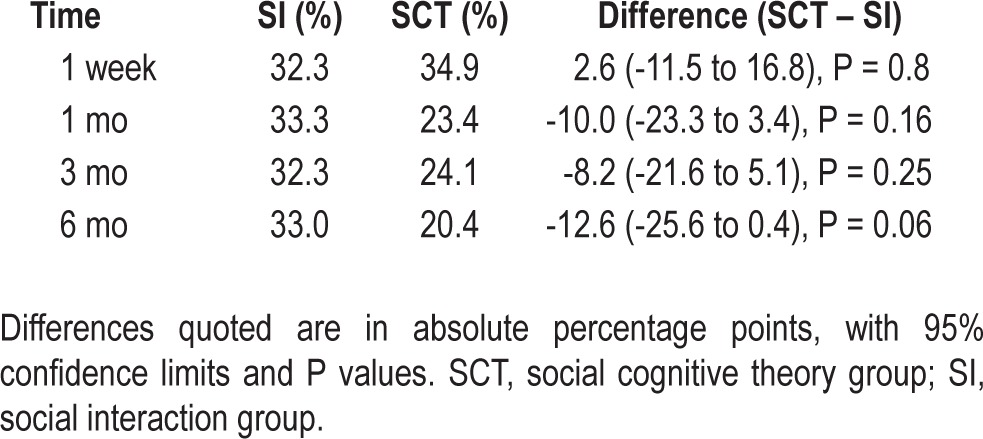
At 6 mo (Table 3), 33% of patients in the social interaction group were using CPAP for ≥ 6 h a night, as opposed to 20.4% in the SCT group (P = 0.06). Mean nightly usage of CPAP was 4.1 h in the SI group and 3.5 h in the SCT group, with the difference between groups being 0.64 h (95% CI, 0.10-1.37, P = 0.09).
Table 3.
Adherence to continuous positive airway pressure, as defined by average nightly usage ≥ 6 h
Logistic regression modeling was used to explore other predictors of adherence (Table 4). In univariate analyses, pretreatment AHI and self-efficacy were significant predictors of adherence. In multivariate models, they remained significant predictors with greater OSA severity (odds ratio [OR] 1.1 per 10 event/h increase in AHI, 95% CI 1.01-1.29, P = 0.04) and higher self-efficacy (OR 2.7 per unit increase score, 95% CI 1.7-4.3, P < 0.0001) both associated with treatment adherence at 6 mo. When adjusted for AHI and self-efficacy, there was no effect of SCT, compared with SI on adherence (OR 0.90, 95% CI 0.5-2.1, P = 0.7). The other variables considered for the regression model included sex, age, education level (primary, secondary or tertiary), and the outcomes of the symptom questionnaires.
Table 4.
Univariate and multivariate logistic regression models examining predictors of continuous positive airway pressure adherence at 6 mo
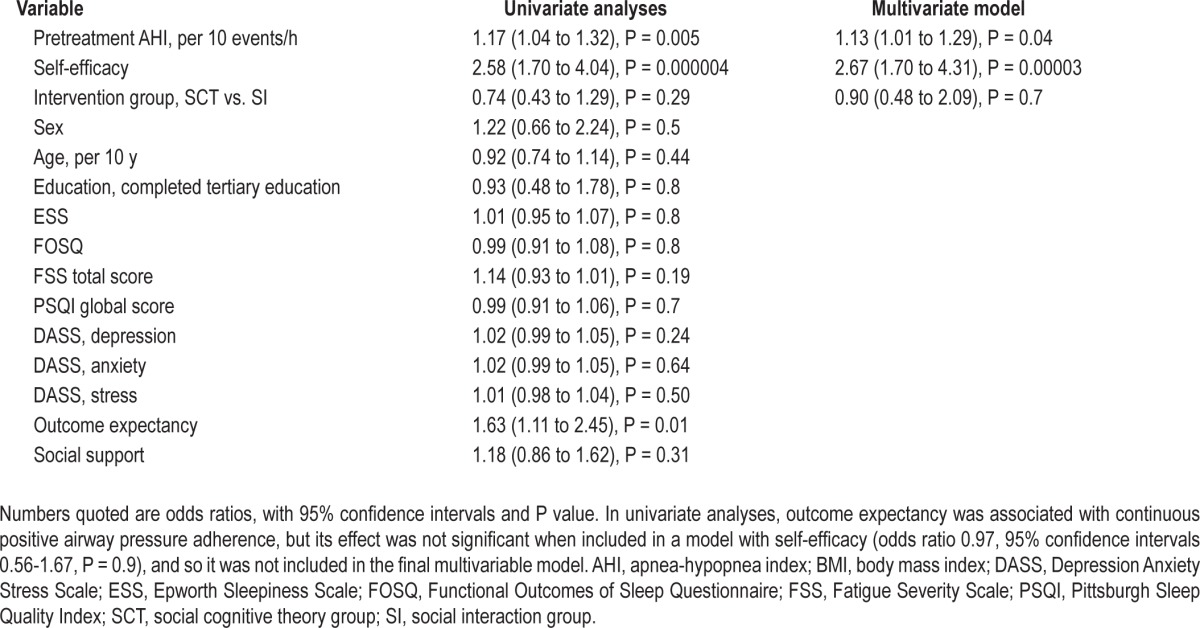
Although baseline self-efficacy was associated with increased adherence to CPAP, neither intervention appeared to change self-efficacy, when the baseline measurement was compared to 1-mo follow-up. In addition, the effect of the interventions on CPAP adherence did not appear to be influenced by the degree of self-efficacy at baseline (interaction effect of group by self-efficacy, P = 0.9), this does not support the notion that individuals with lower self-efficacy might obtain a greater benefit from the SCT intervention.
Symptoms and Questionnaire Data
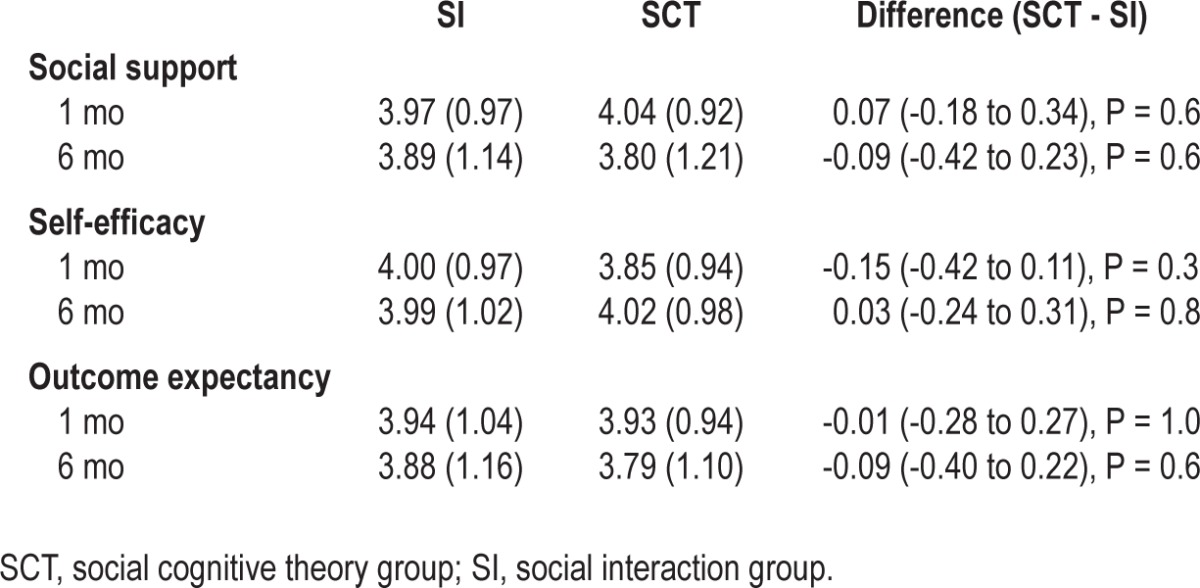
At follow-up, there was no difference between the groups in ESS FOSQ, DASS subscales, FSS PSQI, Social support, Self-efficacy, and Outcome expectancy (Tables 5 and 6).
Table 5.
Questionnaire scales at 6 mo
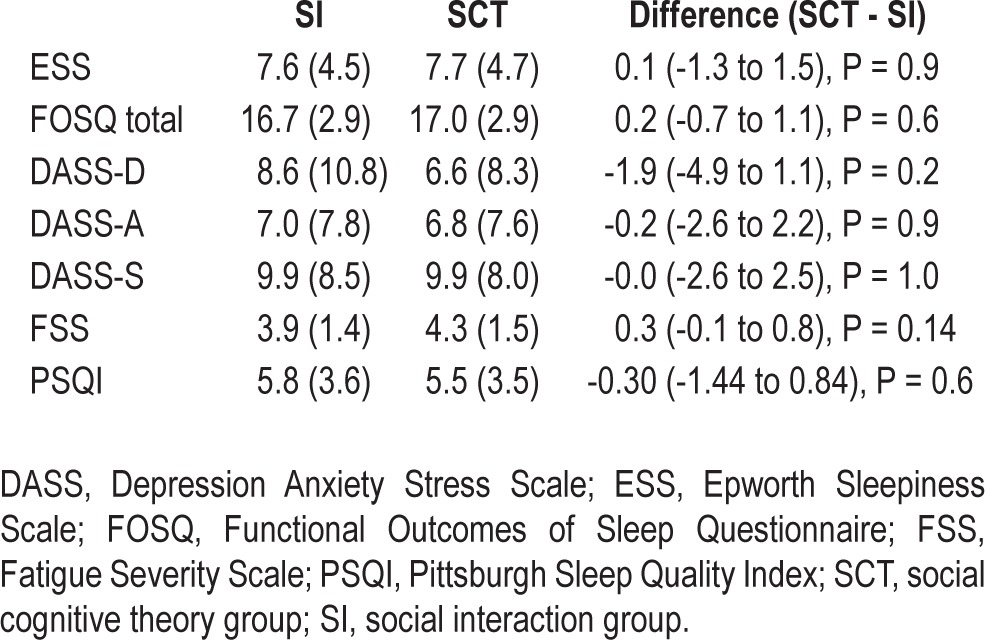
Table 6.
Social support, self-efficacy and outcome expectancies at 1 and 6 mo
DISCUSSION
Previous positive findings from limited randomized controlled studies suggested a cognitive behavioral therapy (CBT) intervention with a social cognitive component enhanced CPAP adherence.15,18 However, contrary to our hypothesis, a single CPAP-specific group SCT intervention did not improve CPAP uptake or adherence compared with an equivalent time period of social interaction, which was in addition to our behavioral and education session. Moreover, there was no difference in effect of either arm of the study on a range of subjective sleep and psychosocial outcomes. Although higher self-efficacy scores at baseline, prior to titration, strongly predicted CPAP adherence, neither intervention increased the self-efficacy score at 6 mo. As expected, temporal patterns of nonadherence showed that most of patients who were nonadherent to CPAP treatment at 6 mo were already nonadherent at 1 week.
The “active” intervention was a single SCT group session with a cognitive component28 delivered in a group setting to increase the uptake and usage of CPAP in patients with OSA. The key component of the SCT session was a video of four individuals who had overcome considerable difficulties and through perseverance were now using CPAP successfully. This was a pragmatic choice given such an intervention could be delivered as a low-cost, low-intensity adjunct to CPAP treatment and was feasible in many clinical settings. Previous work from our group had suggested such an intervention delivered prior to the first night of CPAP was effective at 1 mo and improved CPAP adherence.18 Despite these previous positive findings, we observed no difference between the SCT and SI arms.
There are a number of possible explanations for the difference between our previous findings and the current study. This study had a time-matched control arm whereas previously the intervention involved extra sessions compared to a TAU arm. It is possible that the simple act of having a group session with patients interacting and discussing their common health problem may not be an inert control. We observed a trend toward the patients in the SI arm having better adherence at 6 mo, if we used a more rigorous adherence criteria of > 6 h CPAP usage/night (33% versus 20.4%, P = 0.06). Previously, the active intervention group attended two sessions on separate occasions prior to CPAP titration and partners were encouraged to participate. The current study consisted of an intensive afternoon consisting of questionnaire completion, a neurobehavioral testing battery (reported in another study), CPAP training, and group sessions (usual education and either SCT or SI) followed by CPAP titration. The relative intensity and timing (12:30-06:00) of the program in patients with untreated OSA and possible neurobehavioral impairment may have attenuated the efficacy of any active behavioral intervention such as group SCT would achieve in comparison with our previous approach (two separate CBT sessions at night or Sunday lunchtime). The SI group had equal time but a less intense intervention, possibly allowing them more time to integrate information. The behavioral educational content common to both arms was more detailed compared with the previous study and it may have influenced CPAP uptake and adherence. Early afternoon educational interventions in untreated individuals with OSA may not be optimal. In addition, the educational background of patients in this study was broader; however, even in the current study 78% completed high school and 51% had university degrees. Finally, partners were not able to be directly involved in the education sessions in the current study due to the study protocol timetable. In the previous study18 partners were encouraged to be present at the then-two CBT/SCT sessions, and social support was significantly higher in this group compared with the TAU control group. Previous research29 has found that having a partner was more likely to result in higher CPAP usage, whereas other research found increased disease severity and a more collaborative spousal relationship positively influenced adherence.30 In the previous study18 the follow-up calls were carried out by a CPAP therapist and staff member who was known to the participants, although they were blinded to participant group. Developing a rapport with the participants, where the same staff member interacts on a regular basis, is an important factor in CPAP uptake and adherence.31
Recent Cochrane and other reviews15,21 identified two other studies from the same institution that have tested SCT/CBT-like interventions to increase CPAP adherence. In the first study, 12 patients were randomized to either two 45-min individual sessions of symptom discussion/goal setting or an attention control.32 There was a marked improvement in CPAP adherence with the active intervention. The second study randomized 142 patients to either motivational enhancement,33 enhanced education, or a TAU arm. By the end of the 13-week data collection period, 51% of the individuals in the control group had discontinued CPAP compared with 30% of those in the enhanced education group and 26% in the motivational enhancement group.
However, differences were not seen between the two interventions and control group in adherence at 13 weeks. These two studies used different interventions and shorter follow-up periods. In both of these studies, interventions were delivered after CPAP titration unlike in the current study where interventions were delivered the afternoon prior to titration. These data, taken together with the current finding, question the effectiveness of currently available behavioral interventions in improving CPAP adherence. Some specific (e.g., SCT) interventions may work only in certain settings or need to be administered repeatedly over a longer period of time to improve CPAP usage in patients with OSA.
Importantly, self-efficacy measured prior to CPAP titration strongly predicted adherence at 6 mo. For every unit increase on the baseline self-efficacy scale, hours of CPAP use nearly doubled (OR = 1.8) irrespective of the group intervention. The self-efficacy questionnaire was a subset of one previously used in CPAP research28 and was specifically given prior to any education or randomization. This approach was taken to ensure consistency with our previous protocol where self-efficacy was also predictive of CPAP adherence.18 In other protocols examining CPAP adherence, self-efficacy is typically measured after the behavioral intervention or after a short period of time on CPAP.9,15,19,20 In these studies self-efficacy has been variably related to CPAP adherence.
One potential application of using patient-reported self-efficacy prior to commencing CPAP treatment is to differentiate which patients may require more or less intensive support with CPAP treatment. This approach would support the use of a pragmatic and economically sensible stepped care model as suggested for insomnia treatment.34
Overall CPAP adherence at 6 mo in both arms of this study (47.2-54.6% using 4 h/night cutoff) was consistent with a number of recent larger trials examining interventions to increase CPAP usage. Using an automated telemedicine intervention for 6-12 mo, 44.7% of patients adhered to CPAP compared with 34.5% in an attention control group.35 Another study comparing motivational enhancement, enhanced education, or control showed adherence rates of 61-67% but this was measured at 12 weeks.33 In observational follow-up of 330 US veterans, CPAP adherence ≥ 4 h occurred in 48.9% after the first week of therapy,36 whereas in another study of 91 sleep clinic patients followed for the same duration, 39.6% used CPAP for > 4 h/night. Given the evidence indicating that greater hours of CPAP use result in better psychosocial and other outcomes, some have questioned the value of the > 4 h adherence metric.15 Moreover, there is reasonable consensus that 6 h or more of normal quality sleep is required for normal neurobehavioral functioning and lesser degrees of sleep contribute to a range of poor health outcomes.13 We observed that, depending on the arm of study, only 20.4-33% of patients used CPAP > 6 h/night. Others have found both higher (32-45%)33 or lower (23.1%)20 levels of this adherence cutoff. These data clearly indicate that a large proportion of patients prescribed CPAP titration by their doctors do not use CPAP or use it suboptimally, providing strong impetus to develop better interventions to improve adherence or to validate therapies that are as equally effective as CPAP.
The data presented in this study are not questioning the efficacy of all behavioral interventions in patients commencing with CPAP treatment. We only used a single-group SCT session at baseline, and more frequent use of such interventions early in treatment to consolidate an understanding of the treatment process is likely to increase CPAP adherence. We used a multi-component approach that included some aspects of SCT but also relaxation. This was not personalized to each individual and thus may have diminished the potential effectiveness of this intervention. Such interventions using a “one size fits all” approach for all patients are expensive (including time factors). It is likely the best use of behavioral treatments will require a more intensive approach in a subgroup of patients predicted to be nonadherent to CPAP by questionnaires or other unidentified markers. Moreover, the high rate of nonadherence identified by this and other studies in relatively unselected populations emphasises the challenges in treating OSA with CPAP.
A single CPAP-specific group SCT intervention did not improve CPAP uptake or adherence compared with an equivalent time period of social interaction. Such approaches may well be cognitively challenging for patients with OSA, and future research should focus on determining optimal timing and repetition of both educational information and other behavioral interventions. Other factors requiring investigation include time of day when the intervention sessions take place, partner support, use of known health care providers/staff and/or “health coaches.” Randomized controlled trials testing the value of self-efficacy as a predictor of CPAP adherence in a potential stepped care model also needs to be considered.
DISCLOSURE STATEMENT
Professor Colin is Clinical and Scientific Director of Sleepio Ltd and a shareholder in the company, but has not received any income from this source. He has acted as a consultant and speaker for Boots UK and Novartis. The other authors have indicated no financial conflicts of interest. Continuous positive airway pressure machines were provided without charge by Phillips Respironics and Covidien.
ACKNOWLEDGMENTS
Drs. Bartlett and Wong are equal first authors. Work for this study was carried out at the Woolcock Institute of Medical Research and University of Sydney and Royal Prince Alfred Hospital in Sydney, Australia. This work was supported by National Health and Medical Research Council (NHMRC) Project Grant 457355, NHMRC CCRE for Interdisciplinary Sleep Health and an NHMRC Practitioner Fellowship to Dr. Grunstein.
REFERENCES
- 1.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med. 1995;151:1459–65. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 2.Marshall N, Wong K, Liu P, Cullen S, Knuiman M, Grunstein R. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 3.Punjabi N, Newman A, Young T, Resnick H, Sanders M. Sleep-disordered breathing and cardiovascular disease: An outcome-based definition of hypopneas. Am J Resp Crit Care Med. 2008;177:1150–5. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan C, Issa F, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 5.McDaid C, Durée K, Griffin S, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009;13:427–36. doi: 10.1016/j.smrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Barone-Kribbs N, Pack A, Kline L, et al. Effects of one night without CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;47:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 7.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with CPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J. 2000;16:921–7. doi: 10.1183/09031936.00.16592100. [DOI] [PubMed] [Google Scholar]
- 8.Weaver T, Kribbs N, Pack A, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 9.Weaver T, Grunstein R. Adherence to continuous positive airway pressure therapy: The challenges to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engleman H, Wild M. Improving CPAP use by patients with the sleep apnea/hypopnea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 11.Grandner M, Patel N, Gehrman P, Perlis M, Pack A. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010;14:239–47. doi: 10.1016/j.smrv.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Spanish Sleep and Breathing Network. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 13.Weaver T, Chasens E. Continuous positive airway pressure for sleep apnea in older adults. Sleep Med Rev. 2007;11:99–111. doi: 10.1016/j.smrv.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antic N, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–9. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawyer A, Gooneratne N, Marcus C, Ofer D, Richards K, Weaver T. A systematic review of CPAP adherence across age groups: Clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–56. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandura A. Social learning theory. Englewood Cliffs, NJ: Prentice Hall; 1977. [Google Scholar]
- 17.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44:1175–84. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 18.Richards D, Bartlett D, Wong K, Malouff J, Grunstein R. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomised trial. Sleep. 2007;30:635–40. doi: 10.1093/sleep/30.5.635. [DOI] [PubMed] [Google Scholar]
- 19.Weaver T. Predicting adherence to continuous positive airway pressure-the role of patient perception. J Clin Sleep Med. 2005;1:354–6. [PubMed] [Google Scholar]
- 20.Ye L, Pien G, Ratcliffe S, Weaver T. Gender differences in obstructive sleep apnea and treatment response to continuous positive airway pressure. J Clin Sleep Med. 2009;5:512–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Smith I, Lasserson T. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2009 Oct 7;:CD003531. doi: 10.1002/14651858.CD003531.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Stepnowsky C, Marler M, Ancoli-Israel S. Determinants of nasal CPAP compliance. Sleep Med. 2002;3:239–47. doi: 10.1016/s1389-9457(01)00162-9. [DOI] [PubMed] [Google Scholar]
- 23.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20:844–9. doi: 10.1093/sleep/20.10.844. [DOI] [PubMed] [Google Scholar]
- 24.Lovibond S, Lovibond P. Manual for the depression anxiety stress scales. 2 nd ed. Sydney: The Psychology Foundation of Australia Inc. University of New South Wales; 1995. [Google Scholar]
- 25.Schwartz J, Jandorf L, Krupp L. The measurement of fatigue: a new instrument. J Psychosomatic Res. 1993;37:753–62. doi: 10.1016/0022-3999(93)90104-n. [DOI] [PubMed] [Google Scholar]
- 26.Buysse D, Reynolds C, Monk T, Berman S, Kupfer D. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Weaver T, Laizner A, Evans L, et al. An Instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 28.Stepnowsky C, Bardwell W, Moore P, Ancoli-Israel S, Dimsdale J. Psychologic correlates of compliance with continuous positive airway pressure. Sleep. 2002;25:758–62. doi: 10.1093/sleep/25.7.758. [DOI] [PubMed] [Google Scholar]
- 29.Lewis K, Bartle I, Watkins A, Seale L, Ebden P. Simple interventions improve re-attendance when treating the sleep apnoea syndrome. Sleep Med. 2006;7:241–47. doi: 10.1016/j.sleep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Baron K, Berg C, Czajkowski L, Smith T, Gunn H, Jones C. Self-efficacy contributes to individual differences in subjective improvements using CPAP. Sleep Breath. 2011;15:599–606. doi: 10.1007/s11325-010-0409-5. [DOI] [PubMed] [Google Scholar]
- 31.Sin D, Mayers I, Man G, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea. Chest. 2002;121:430–5. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 32.Aloia M, Di Dio L, Ilniczky N, Perlis M, Greenblatt D, Giles D. Improving compliance with nasal CPAP and vigilance in older adults with OSAHS. Sleep Breath. 2001;5:13–21. doi: 10.1007/s11325-001-0013-9. [DOI] [PubMed] [Google Scholar]
- 33.Aloia M, Arendt J, Millman R, et al. Brief Behavioral Therapies reducs early positive airway pressure discontinuation rates in sleep apnea syndrome: preliminary findings. Behav Sleep Med. 2007;5:89–104. doi: 10.1080/15402000701190549. [DOI] [PubMed] [Google Scholar]
- 34.Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549–58. doi: 10.1093/sleep/32.12.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sparrow D, Aloia M, DeMolles D, Gottlieb D. A telemedicine intervention to improve adherence to continuous positive airway pressure: a randomised controlled trial. Thorax. 2010;65:1061–6. doi: 10.1136/thx.2009.133215. [DOI] [PubMed] [Google Scholar]
- 36.Platt A, Field S, Asch D, et al. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32:799–806. doi: 10.1093/sleep/32.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]