Abstract
Study Objectives:
To examine the role of genetic and environmental factors on sleep behavior in 12-year-old twins matched for family environment.
Design:
Population-based twin cohort.
Setting:
Participants were assessed in their home environment.
Patients or Participants:
One hundred thirty-two adolescent twins comprising 25 monozygotic (MZ) and 41 dizygotic (DZ) twin pairs; aged 12.2 ± 0.1 y (mean ± standard deviation).
Interventions:
N/A.
Measurements and Results:
For 2 weeks in their home environment, participants wore a wrist activity monitor and completed a daily sleep diary. Sleep diaries included reports of bedtime, wake time, and estimated sleep onset time. Mean timing, duration, and quality of sleep during the 2 weeks were calculated for each individual and compared within twin pairs. MZ twin correlations were higher than the DZ correlations for total sleep time (MZr = 0.64; DZr = 0.38) and sleep onset latency (MZr = 0.83; DZr = 0.53) and significantly higher for wake after sleep onset (MZr = 0.66; DZr = 0.04) and sleep efficiency (MZr = 0.82; DZr = 0.10). Univariate modeling showed additive genetic factors accounted for 65% of the variance in total sleep time, 83% in sleep onset latency, and 52% and 57% of the variance in wake after sleep onset and sleep efficiency, respectively. A predominant influence of shared environment was found on the timing of sleep (67% for sleep start time, 86% for sleep end time).
Conclusions:
There is a strong genetic influence on the sleep-wake patterns of 12-year-old adolescents. Genes have a greater influence on sleep initiation and sleep maintenance and a smaller role in sleep timing, likely to be influenced by family environment.
Citation:
Sletten TL; Rajaratnam SMW; Wright MJ; Zhu G; Naismith S; Martin NG; Hickie I. Genetic and environmental contributions to sleep-wake behavior in 12-year-old twins. SLEEP 2013;36(11):1715-1722.
Keywords: Adolescence, environmental influences, genetics, heritability, sleep, twins
INTRODUCTION
Sleep architecture and patterns of sleep behavior including the timing, duration, and quality of sleep demonstrate considerable differences between individuals.1,2 Variability in sleep need has also been reported, with some individuals apparently requiring considerably less sleep (“short sleepers”) than others.3–5 Individual preference in the timing of sleep, or chronotype, has also been reported.6,7 Interindividual variability in sleep is not only due to environment and the demands of daily life but may result from heritable factors. Numerous specific genes have been indicated to have a strong influence in sleep disorders including a deficiency of the hypocretin signal in narcolepsy,8 a mutation in the beta3 subunit of the gamma-aminobutyric acid (GABA)A receptor in chronic insomnia,9 a PER2 gene mutation in advanced sleep phase disorder,10 and a polymorphism in PER3 in delayed sleep phase disorder.11 The role of genetics in individual variability in normal sleep, however, is not well understood.
Studies of twins who are reared together and thereby well matched for family and social environments provide an opportunity to examine the contribution of genetic and environmental factors in normal sleep.12,13 Separation of heritable and environmental factors is possible as monozygotic (MZ) twins are genetically identical, whereas dizygotic (DZ) twins share an average of 50% of their genes. In analyses of within-pair differences, a trait influenced by genotype should thus demonstrate more similarity in MZ than in DZ twins. In contrast, if the extent of such differences is the same for MZ and DZ twins, it would be concluded that the phenotype is predominantly influenced by the common environment.
Studies of twins using polysomnography have indicated heritability in sleep architecture. Polysomnographic recordings of sleep in the laboratory environment revealed body movements and densities of stage 2, slow wave, and rapid eye movement (REM) sleep are genetically determined.14–18 One night of polysomnographic recordings in 14 MZ and DZ adult twin pairs demonstrated stronger similarities in MZ twins than DZ twins for sleep latency, awakenings, duration of sleep cycles, and amount of REM.18
Although objective polysomnographic studies indicate a role of genetics in the organization of sleep stages, they are usually limited to 1 night of recording and are often conducted in the laboratory setting. Given that sleep can demonstrate high night-to-night variability,19 assessment of a single night may not be representative. Monitoring of patterns in sleep-wake behavior across time would be preferable. Numerous studies based on subjective measures collected over multiple nights have examined the heritability of sleep behavior. Self-reported habitual bedtime and wake time, sleep duration, and subjective sleep quality in adults hold considerable heritability.20–22 Likewise, reports on sleep in 2,238 MZ and 4,545 DZ adult twins revealed significant heritability estimates for sleep length (h2 = 0.44) and sleep quality (h2 = 0.44).22 Genetic differences account for similar proportions of variance in sleep quality and sleep disturbance (30-57%), daytime sleepiness (29-38%), chronotype (44-54%) and in patterns of sleep (40%) in adult twins.21,23–26
The aforementioned studies of twins provide evidence that normal sleep patterns are strongly influenced by heritability. Conversely, the reported effect of genetics on the variation in sleep duration is low (9-17%)21,23 and environmental factors have been indicated as more important than genetic factors for parent and child reports of sleep problems in adolescents.27 Most investigations of the role of genetics on sleep behavior, however, have predominantly relied on subjective reporting of typical sleep patterns or subjective mood after night sleep and have not objectively examined sleep over a period beyond a single night.21,22,28,29 Assessment of sleep in twins has also primarily been conducted in individuals older than 16 y. Objective assessments of the role of genetics in sleep-wake patterns in adolescents are limited. The aim of the current study was to examine the relative contributions of genetic and environmental factors on phenotypic sleep-wake patterns in 12-year-old MZ and DZ twins matched for family and social environment.
METHODS
Participants
Participants were a subsample of adolescent twins from the Brisbane Adolescent Twin Study,30 recruited through primary and secondary schools in South East Queensland, personal referrals, or via the Queensland Twin Registry (QTwin). Zygosity in same-sex twins was initially determined by a clinical nurse following observation of physical similarity and conversation with twins and parents. Subsequently, in 12 of the 25 MZ pairs we determined zygosity from DNA using a commercial profiler kit (AmpFISTR Profiler Plus Amplification Kit, Applied Biosystems, Foster City, CA, USA). Confirmation of zygosity assignment will be made in all remaining same-sex pairs once further genotyping becomes available. Even so, our previous studies show that zygosity assignment from a clinical nurse has a low probability of error (2.4%).31
The current protocol was approved by the Queensland Institute of Medical Research Human Research Ethics Committee. Both the twins and a parent provided written informed consent. Participants were compensated $50 for participation.
Procedures
Twelve-year-old twins participating in the Twin Mole Study30 at the Queensland Institute of Medical Research between May 2010 and January 2012 were invited to participate in the current study. One hundred thirty-nine twin pairs from a total of 150 pairs (92.7%) agreed to participate. Data from seven pairs were excluded prior to analysis due to problems with acti-graphic data collection, insufficient data or health issues likely to influence sleep behavior.
Twins wore a wrist actigraph or activity monitor (Acti-watch-64, Respironics Inc, Bend, OR, USA), and completed a sleep diary for 2 weeks in their home environment. The activity monitor was placed on the nondominant wrist and was worn at all times except when likely to be damaged by contact with water (i.e., when showering, bathing, or swimming) or by sport involving high physical contact. Each co-twin's activity monitor was identified by name and a different colored band to prevent exchange of monitors. Twins were asked to complete the sleep diary each morning after they woke and got out of bed, reporting the time of going to bed, the time taken to fall asleep and the time of waking in the morning. If they woke during the night, participants reported the reason for waking. They also reported caffeine consumption in the 3 h prior to bedtime and reported if they had a nap during the previous day.
Measures
Actigraphic and sleep diary data were deidentified to prevent matching of twin pairs during data cleaning and extraction procedures. Bedtime, rise time, time in bed, and subjective sleep onset latency were determined from sleep diary reports. Bedtimes and rise times reported in diaries were used to identify sleep episodes for actigraphic analysis. Discrepancies of at least 30 min between sleep diaries and actigraphy required correction to match actigraphy in 10.2% of total sleep periods. Actigraphy data were extracted and examined using Actiware 5 software (Respironics Inc, Bend, OR, USA). Software sensitivity was set to medium (40 activity counts) to establish each 1-min epoch as sleep or wake. Objective sleep parameters calculated for main sleep periods included sleep onset time and wake time, sleep onset latency (min taken to fall asleep), total sleep time (sleep onset to wake time minus awakenings), sleep efficiency (proportion of time between sleep onset and wake that was sleep), wake after sleep onset (min of wakefulness after sleep onset), and sleep fragmentation (sum of percent mobile and the ratio of percent immobile to percent mobile bouts). Sleep onset was scored as the first epoch in a series of ≥ 10 min in which ≤ 1 epoch contained measured activity. Wake was determined as the last immobile epoch in a series of ≥ 10 minutes, in which ≤ 1 epoch contained measured activity. Napping by participants was infrequent during the 2-week period, reported on 3.0% of all study days. On 23.6% of days adolescents reported consumption of caffeine, including chocolate within 3 h of bedtime. Napping and caffeine consumption by participants were not included in the analysis.
Statistical Analysis
For each participant, the mean and standard deviation (SD) of each sleep measure was calculated for all nights of the 2-week participation interval, and separately for school and non-school nights. Nonschool nights were those that occurred prior to weekends, school holidays, and public holidays. All measures were normally distributed with no outliers (i.e. more than +3 SD from the mean). Genetic analyses were carried out using structural equation modeling in the statistical software package Mx.32 First, a model that estimated all parameters freely (saturated model) was fitted to the data. We then tested whether the means and variances of the MZ and DZ twins could be equated, as well as any fixed effects for sex, body mass index (BMI), or shared room status. Using analysis of variance we also tested whether the absolute intrapair difference for any of the objective sleep measures was dependent on whether co-twins shared a bedroom or had their own separate room. Higher MZ compared to DZ correlations indicate an importance of genetic factors. Similar MZ and DZ correlations indicate that shared environmental factors account for the variability in sleep behavior. A MZ correlation of less than 1 implicated an influence of nonshared environment (plus measurement error).
For all nights of participation, variation in each of the seven objective sleep measures (sleep start time, sleep end time, total sleep time, wake after sleep onset, sleep onset latency, sleep efficiency, sleep fragmentation) was decomposed into sources due to additive genetic (A), common environmental (C) and unique/nonshared environmental (E) factors (and measurement error). An initial unrestricted Cholesky ACE model was fit to the each variable so that variances, covariances and means could be estimated freely by minimizing the -2 times log likelihood of data (-2InL). Reduced models (AE, CE) were examined to determine whether they provided a better fit than the Cholesky using the likelihood ratio test, and AE and CE models were compared using Akaike's information criteria (AIC). Lower AIC values indicated a better fit. In addition, heritability was estimated using Falconer's formula [h2 = 2(rMZ – rDZ)].
RESULTS
One hundred thirty-two (50 boys, 82 girls) young adolescent twins including 25 MZ and 41 DZ pairs, aged 12.2 ± 0.1 y (mean ± SD) with BMI between 12.8 and 30.3 kg/m2 participated in the study. MZ twins comprised of 13 male and 12 female pairs. DZ twins included 6 male, 23 female, and 12 opposite sex pairs. Table 1 presents the mean ± SD for measures of sleep timing, duration, and quality during the 2-week assessment period for the 66 twin pairs. DZ twins went to bed an average of 16 min later than MZ twins (P = 0.03). Other sleep measures were not significantly different between the two zygosity types. Participants slept for an average of 7.8 h per night. Table 1 also reports sleep measures separately for school nights and nonschool nights. For all twin pairs, bedtime and rise time occurred significantly later [by 50 min (P < 0.0001) and 44 min (P < 0.0001), respectively] on nonschool nights compared to school nights. The total sleep time of sleep episodes on nonschool nights was 12 min less than on school nights (P = 0.02).
Table 1.
Mean ± standard deviation for sleep parameters averaged for school nights, nonschool nights, and across 2 weeks for individuals in monozygotic (MZ) and dizygotic (DZ) twin pairs and for the full sample (n = 132; 50 boys, 82 girls)
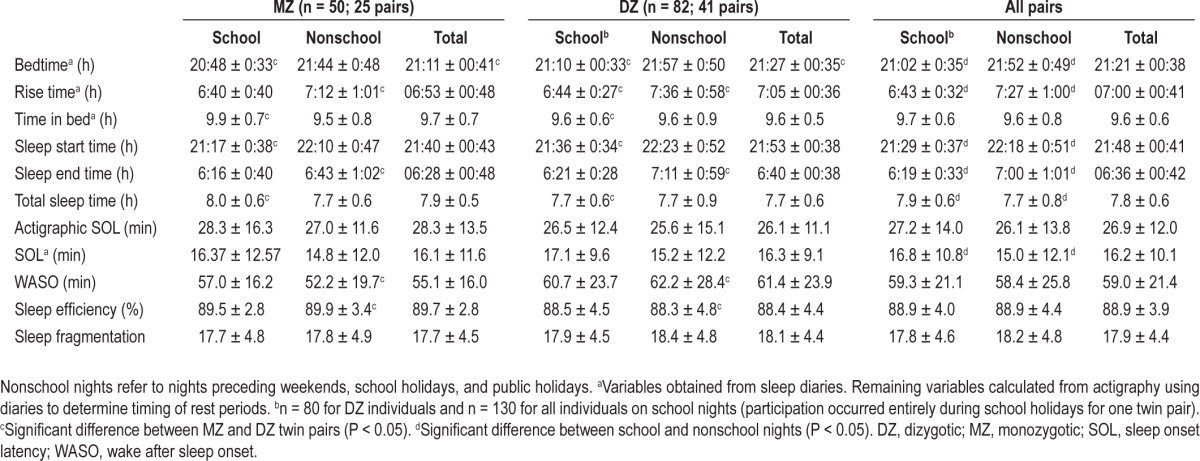
Shared room status did not affect any sleep measure, as assessed using Mann-Whitney U tests on data from all nights of participation. In addition, absolute intrapair differences in all sleep measures were not significantly different for the twin pairs that shared a bedroom (0.37 to 0.83) compared with those twins who did not share a bedroom (0.25 to 0.72). Spearman rho correlations revealed BMI was significantly correlated with sleep start time (r = 0.231, P < 0.01) and total sleep time (r = 0.272, P < 0.01). BMI was included as a covariate in each univariate model. MZ and DZ twins did not differ in age, height, weight, or BMI. There was also no difference in measures of sleep timing, duration or quality for MZ and DZ twins. Further, of the 14 nights that data were collected, on average 2.96 (± 4.22) were prior to school or public holidays. No difference in the mean number of holidays was found between MZ and DZ twin pairs, and for all sleep measures the number of holidays had no significant effect when included as a fixed effect.
Maximum likelihood twin correlations for all nights are presented in Table 2. Correlation coefficients for sleep start time (MZr = 0.94; DZr = 0.81) and sleep end time (MZr = 0.90; DZr = 0.83) were high and not significantly different (confidence intervals overlap) between MZ and DZ pairs, indicating shared environmental factors strongly influence these measures across all nights of participation. For total sleep time the correlation was almost twice as large in the MZ (r = 0.64) compared with the DZ pairs (r = 0.38) but the difference between correlations was not significant. For indicators of sleep quality including wake after sleep onset, sleep efficiency, and sleep fragmentation, MZ correlations were several times larger than correlations for DZ twins and the DZ correlations for the sleep quality phenotypes were not significant (Table 2) because the confidence intervals included zero. For sleep onset latency, although the MZ correlation was larger than the DZ correlation, the DZ correlation was significant (r = 0.53).
Table 2.
Maximum likelihood monozygotic and dizygotic twin correlations (with 95% confidence intervals) for total nights of participation
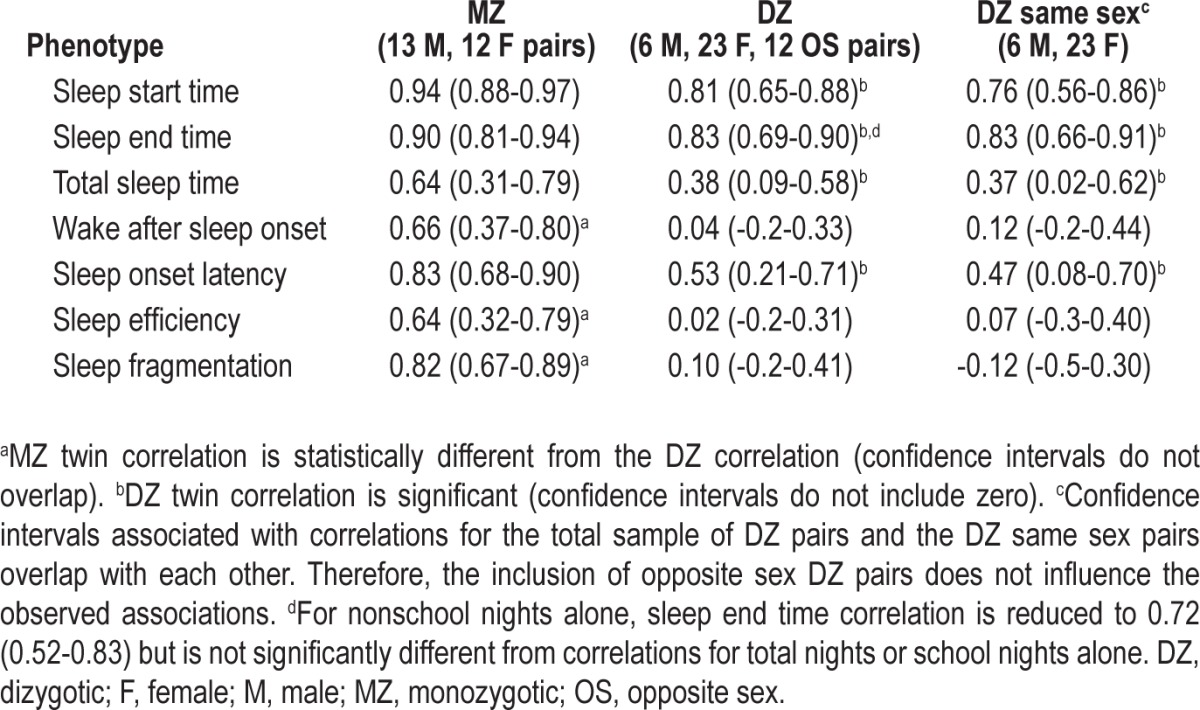
Table 3 summarizes the best-fitting univariate models and parameter estimates for each sleep related phenotype. For comparison, Falconer's heritability estimates based on the zygosity differences in the twin correlations are also presented. For sleep start time the best fitting model included A, C, and E where the heritability estimate was 0.28 but with more variability (67%) due to common environment than genetics (28%). There was a much smaller influence of genetic factors for sleep end time with only 15% of the variance being due to genes (estimated from the ACE model) with most of the variance being due to shared (76-86%) environmental factors.
Table 3.
Model fit and proportion of variance in sleep phenotypes accounted for by additive genetic, common environment, and nonshared environment (and measurement error) for total nights of participation
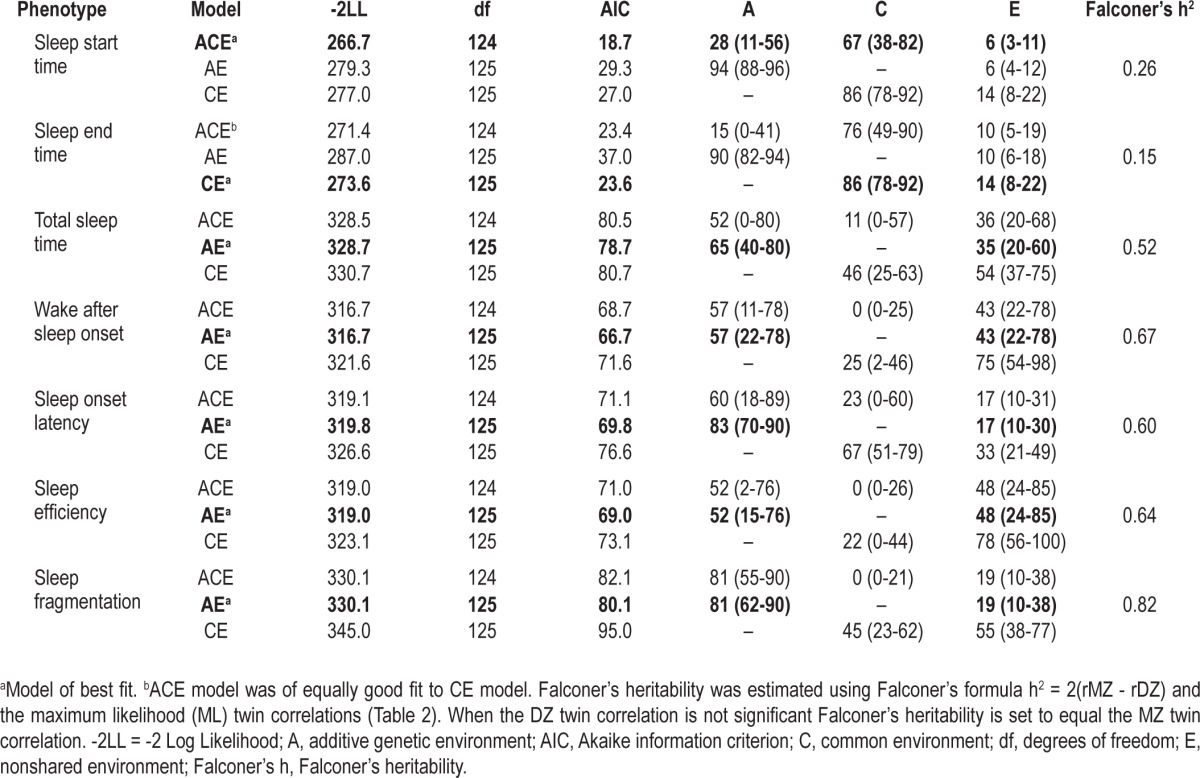
For total sleep time, sleep onset latency, wake after sleep onset, sleep efficiency, and sleep fragmentation an AE model provided the best fit, with additive genetic factors accounting for a significant proportion of the variability in sleep. 83% of the variance in sleep onset latency was due to additive genetic factors (A) and 17% due to unique environmental factors (E). For sleep fragmentation, 81% of the variance was due to genetic factors and 19% due to unique environment. Additive genetic factors contributed to 65% of the variance in total sleep time, 57% of the variance in wake after sleep onset, and 52% of the variance in sleep efficiency.
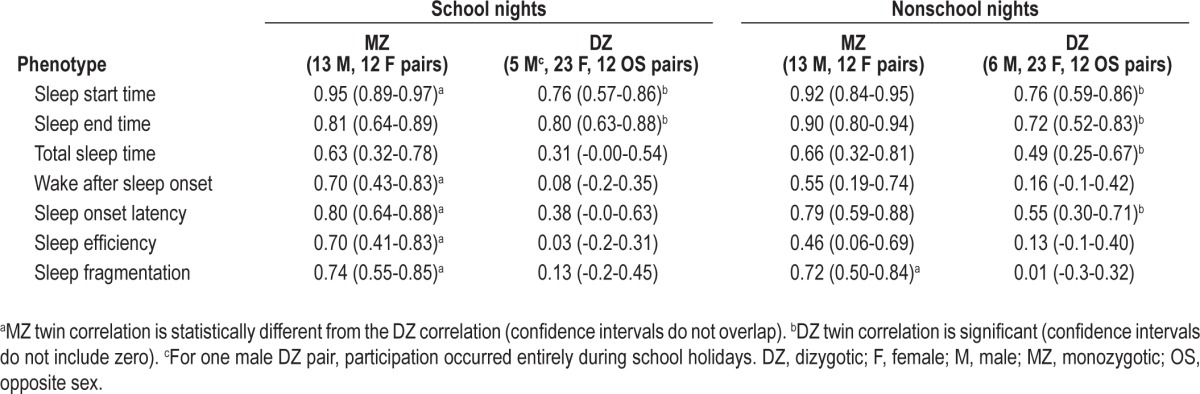
For school nights alone, correlation coefficients for MZ twin pairs remained significantly larger than DZ pairs for wake after sleep onset, sleep efficiency, and sleep fragmentation (Table 4). On nonschool nights the DZ correlations for wake after sleep onset, sleep efficiency, and sleep fragmentation were not significant and the MZ pair correlation was significantly higher than the DZ correlation for sleep fragmentation. DZ correlations for total sleep time and sleep onset latency were significant on nonschool nights (r = 0.49 and r = 0.55, respectively) but not on school nights (r = 0.31 and r = 0.38, respectively). For non-school nights, the sleep end time correlation for DZ twins was slightly lower (r = 0.72) than school nights (r = 0.80) and all nights (r = 0.83).
Table 4.
Maximum likelihood monozygotic and dizygotic twin correlations (with 95% confidence intervals) for school nights and nonschool nights (nights prior to weekends, school holidays, and public holidays).
DISCUSSION
This study examined the sleep habits of 12-year-old adolescents in the community and estimated the role of genetic and environmental factors on sleep behavior. The current study demonstrates an overall stronger association between the sleep habits of MZ twin pairs compared to DZ twin pairs. Maximum likelihood correlations indicated that the sleep phenotypes between MZ twins were similar. MZ twins were more closely matched than DZ twins for indicators of sleep quality including wake after sleep onset, sleep efficiency, and sleep fragmentation, indicating that the phenotypes of sleep quality are strongly attributable to common genetic influences in this population. The correlations between MZ twins being several times that of DZ twins denotes that genetic effects are not operating in an additive manner. For timing of sleep (sleep start and end time), sleep duration (total sleep time), and time taken to fall asleep, an insignificant trend for stronger associations between MZ than DZ twins indicates a strong influence of environment.
Parameter estimates for genetic, shared, and nonshared environment indicate that both genetic and environmental factors influence most sleep phenotypes but the magnitude differs between variables. Sleep start and end time were most significantly influenced by environmental factors common between the twins with a minor role of genetic factors on sleep start time and end time. This indicates that sleep timing is likely to be determined by parental decisions of bedtimes and rise times for their children. Other examples of shared environment that may play a role in sleep patterns include school start time, family schedules, and sport and social activities. In contrast, the influence of genetic factors is much greater for the time taken to fall asleep and for measures of sleep quality (e.g., sleep fragmentation). Although parents are most likely to dictate the timing of adolescent sleep periods and thereby explains the high environmental influence on these variables, genetic factors may account for individual differences in the ability to initiate and maintain sleep at a specific time of day.
Analyses were conducted for school and nonschool nights separately to confirm that biological tendencies were not masked by the commitment of school schedules. The pattern of results remained relatively consistent when school nights and nonschool nights were separated. Measures of sleep quality (wake after sleep onset, sleep efficiency, and sleep fragmentation) were more highly correlated for MZ pairs than DZ pairs on school nights, indicating a strong role of genetics on the quality of sleep. In DZ pairs, correlations for total sleep time and sleep onset latency were significant on nonschool nights, but were not significant on school nights. This apparent difference is difficult to explain and should be interpreted with caution due to the small number of vacation nights or total nonschool nights during the collection interval. Regardless of night type (school versus nonschool), significant correlations between DZ pairs for sleep start and end times suggest a robust role of environment on the timing of sleep in this population, despite daytime commitments. Sleep times were later on nonschool nights compared to school nights in this sample. One explanation for this pattern is that on weekends, children revert to the sleep time that is dictated by the circadian system. However, as suggested by others,33 it is likely that sleep-wake times of young adolescents is largely determined by their parents both on weekdays and weekends.
The role of genetics in determining the duration and quality of sleep may be associated with chronotype (i.e., morningness-eveningness). Variability in the timing of sleep between individuals can arise from differences in morningness-eveningness and differential preferences for early or late sleep. Morningness-Eveningness assessed in 238 MZ pairs revealed higher correlations in the MZ twins compared to DZ twin pairs, indicating a larger influence of genetics than environment on timing of sleep.34 The heritability of morningness-eveningness has been reported to range from 44-54% in adults and children.24,26,35 Similarly, the 24-h profile of cortisol in MZ and DZ twin pairs and the timing of the cortisol nadir have been demonstrated to be genetically influenced, although the peak was primarily controlled by environmental influences.36,37 Specific genes have also been shown to have a distinct role in our circadian tendency. For instance, association between circadian tendencies assessed with the Horne Ostberg questionnaire, and the CLOCK gene polymorphism38 and PER3 polymorphism11 have been revealed but have not been found by all.39–41 A role of genetics in morningness-eveningness may not have had a strong influence on the timing of sleep in the current sample relative to environmental factors, but total sleep time and sleep quality is likely to have varied based on the relationship between the timing of sleep and circadian phase.
The current study indicates a more substantial role of genetics in determining sleep behavior than has been reported previously in adults for usual bedtime (22-46%),23 sleep quality (32-44%),21,22 sleep duration (9-31%),20,21,23,42 number of nocturnal awakenings (23%),20 and sleep latency (32-39%).20,43 We propose that adults are more likely than adolescents to vary their sleep behavior due to environmental factors including social activities and work commitments, thereby limiting the opportunity for genetics to influence the duration and quality of habitual sleep. One study of adolescents aged 10-17 y revealed that genes accounted for a relatively small amount of variance (30%) in sleep complaints. These sleep complaints were assessed by a subjective sleep report checklist, which may not have sufficient sensitivity for demonstrating the influence of genetic factors.27
Mean total sleep time revealed for all participating adolescents (7.8 h) is considerably less than has been reported for objective estimates of sleep length during the school year for 10- and 14-year-old Polish children (9.7 h and 8.7 h, respectively).44 Actigraphy can underestimate total sleep time and overestimate wake after sleep onset compared to overnight polysomnography.45 An 8.8-h period from sleep onset to wake time in the current study indicates a high detection of awakenings during sleep with actigraphy. Sleep time in the current study is more similar to subjective reports of the nightly sleep duration of sixth grade children in the United States (8.4 h).46
This research extends that conducted in adolescent samples previously. Past studies have predominantly relied on subjective reporting from child or parent, which may be considerably affected by the individual rater. For example, children often report more sleep difficulties than their parents, and parents rate a higher genetic influence than children.47 Rather than relying on self-report, we objectively assessed sleep behavior over 2 complete weeks. Actigraphic methods of assessment involve minimal interference of normal sleep patterns and permit recording over a longer period. In the current study the proportion of data loss was considerably less than has been reported previously for actigraphic assessments in adolescents aged 11 to 16 y (5% versus 28% in the previous study).48
It is possible that the behavior of one twin influences the behavior of the other, particularly when the same bedroom is shared between the pair, which was more likely for MZ pairs in the current dataset. Similar correlations between MZ and DZ twin pairs suggest that shared environment has a strong influence. For all sleep phenotypes the MZ correlations were less than 1, implicating an influence of nonshared environment (plus measurement error) onto the sleep variable. Nonshared environmental factors may include accidents resulting in injuries that influence sleep or friends not shared by both twins of the pairs or individual preferences for social activities that may influence time dedicated to sleep.
Some limitations to the current study should be acknowledged. The sample size is modest for establishing the importance of genetics and environment for phenotype as indicated by relatively wide confidence intervals, particularly for measures of sleep quality in DZ twins where the confidence intervals spanned zero. Further research should extend the objective assessment of sleep behavior in a larger sample. In particular, additional data should be collected for nonschool nights so that the role of genetics and environment on sleep behavior can be more closely examined for school nights and nonschool nights separately. The 2-week assessment period in this study included sleep on school days and weekends and, for some participants, school holidays, which are likely to increase day-to-day variability in sleep behavior. For each pair of twins, however, data were collected over the same interval so the same influence of school days and free days is anticipated. It should be acknowledged that the use of twin participants may not be representative of nontwin populations who may have reduced influence on their siblings' sleep-wake patterns, particularly when siblings are of different ages. Variance in sleep duration within our population, however, is not significantly different from those revealed with actigraphy in nontwin adolescents.49,50 Finally, it is recognized that MZ twins may be more likely to influence each other's activities than DZ twins and thereby indirectly demonstrate similar sleep behavior not as a result of genetics per se. Although in our study we accounted for potential social influences within pairs by controlling for bedroom sharing, the probability of which is increased among MZ twins, future research may consider examining sleep during nonsimultaneous time intervals to address the possibility that MZ twins may be more inclined to influence each other's behavior.
Genetic factors have been found to play a strong role in sleep difficulties and in the stability of sleep complaints over time. In one study the proportion of variance in sleep complaints accounted for by genetic and nonshared environment at age 8 y (63% genetic, 32% non-shared environment) was similar to that at 10 y of age (66% genetic, 27% nonshared environment, 7% shared environment).51 It has been suggested that sleep problems are more strongly affected by genetics at preschool/school age (63-69%)21,27,52 and that the effect of shared environment follows a reverse U-shaped pattern21,51,52 such that environment plays its most significant role during adolescence. Further, in twins of mean age 17 y, genetic factors explained 44% of the variance in morningness-eveningness but this reduced to only 4% of the variance in parents of mean age 47 y.24 Therefore, the role of genetic factors in determining sleep patterns, in particular sleep complaints, appears to change with age. The current analyses will form the basis of a longitudinal study to compare the role of genetics in sleep-wake behavior of the twin pairs at age 14 and 16 y.
CONCLUSIONS
This study indicates that genetic, shared, and nonshared environmental factors interact to contribute to sleep-wake behavior. In the 12-year-old children, genetic factors play a primary role in determining sleep duration and sleep quality, whereas family environment influences contribute more to the timing of the major sleep episode.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Sletten has indicated that her institution has received equipment donations or other support from Philips Lighting, Philips Respironics, Optalert and Compumedics. Dr. Rajaratnam has served as a consultant through his institution to Vanda Pharmaceuticals, Philips Respironics, EdanSafe, The Australian Workers' Union, Rail, Bus and Tram Union, and National Transport Commission, and has through his institution received research grants and/or unrestricted educational grants from Vanda Pharmaceuticals, Takeda Pharmaceuticals North America, Philips Lighting, Philips Respironics, Cephalon and ResMed Foundation, and reimbursements for conference travel expenses from Vanda Pharmaceuticals. His institution has received equipment donations or other support from Optalert, Compumedics, and Tyco Healthcare. He has also served as an expert witness and/or consultant to shift work organizations. Dr. Hickie is supported by a National Health and Medical Research Council Australia Fellowship (No. 464914). He was a director of headspace: the national youth mental health foundation until January 2012. He is the executive director of the Brain and Mind Research Institute. He has received research support from Servier and Pfizer. He has received honoraria from Servier, Pfizer, AstraZeneca, and Eli Lilly. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Kerrie McAloney at the Queensland Institute of Medical Research for collation and transfer of diary and actigraphic data. Work for this study was performed at Monash University, Victoria, Australia and Queensland Institute of Medical Research, Queensland, Australia.
REFERENCES
- 1.Sletten TL, Vincenzi S, Redman JR, Lockley SW, Rajaratnam SM. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:12. doi: 10.3389/fneur.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–96. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 3.Aeschbach D, Cajochen C, Landolt H, Borbely AA. Homeostatic sleep regulation in habitual short sleepers and long sleepers. Am J Physiol. 1996;270:R41–53. doi: 10.1152/ajpregu.1996.270.1.R41. [DOI] [PubMed] [Google Scholar]
- 4.Monk TH, Buysse DJ, Welsh DK, Kennedy KS, Rose LR. A sleep diary and questionnaire study of naturally short sleepers. J Sleep Res. 2001;10:173–9. doi: 10.1046/j.1365-2869.2001.00254.x. [DOI] [PubMed] [Google Scholar]
- 5.Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab. 2003;88:26–30. doi: 10.1210/jc.2002-020827. [DOI] [PubMed] [Google Scholar]
- 6.Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Invest Med. 1999;47:141–50. [PMC free article] [PubMed] [Google Scholar]
- 7.Kerkhof GA, Van Dongen HPA. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neurosci Lett. 1996;218:153–6. doi: 10.1016/s0304-3940(96)13140-2. [DOI] [PubMed] [Google Scholar]
- 8.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 9.Buhr A, Bianchi MT, Baur R, et al. Functional characterization of the new human GABA(A) receptor mutation beta3(R192H) Hum Genet. 2002;111:154–60. doi: 10.1007/s00439-002-0766-7. [DOI] [PubMed] [Google Scholar]
- 10.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–3. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 11.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 12.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3:872–82. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 13.Martin N, Boomsma D, Machin G. A twin-pronged attack on complex traits. Nat Genet. 1997;17:387–92. [Google Scholar]
- 14.Hori A. Sleep characteristics in twins. Jpn J Psychiatry Neurol. 1986;40:35–46. doi: 10.1111/j.1440-1819.1986.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuna ST, Maislin G, Pack FM, et al. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep. 2012;35:1223–33. doi: 10.5665/sleep.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linkowski P. EEG sleep patterns in twins. J Sleep Res. 1999;8:11–3. doi: 10.1046/j.1365-2869.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 17.Linkowski P, Kerkhofs M, Hauspie R, Susanne C, Mendlewicz J. EEG sleep patterns in man: a twin study. Electroencephalogr Clin Neurophysiol. 1989;73:279–84. doi: 10.1016/0013-4694(89)90106-5. [DOI] [PubMed] [Google Scholar]
- 18.Webb WB, Campbell SS. Relationships in sleep characteristics of identical and fraternal twins. Arch Gen Psychiatry. 1983;40:1093–5. doi: 10.1001/archpsyc.1983.01790090055008. [DOI] [PubMed] [Google Scholar]
- 19.van Someren EJ. Improving actigraphic sleep estimates in insomnia and dementia: how many nights? J Sleep Res. 2007;16:269–75. doi: 10.1111/j.1365-2869.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 20.de Castro JM. The influence of heredity on self-reported sleep patterns in free-living humans. Physiol Behav. 2002;76:479–86. doi: 10.1016/s0031-9384(02)00699-6. [DOI] [PubMed] [Google Scholar]
- 21.Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990;13:318–35. doi: 10.1093/sleep/13.4.318. [DOI] [PubMed] [Google Scholar]
- 22.Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–85. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb DJ, O'Connor GT, Wilk JB. Genome-wide association of sleep and circadian phenotypes. BMC Med Genet. 2007;8:S9. doi: 10.1186/1471-2350-8-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vink JM, Groot AS, Kerkhof GA, Boomsma DI. Genetic analysis of morningness and eveningness. Chronobiol Int. 2001;18:809–22. doi: 10.1081/cbi-100107516. [DOI] [PubMed] [Google Scholar]
- 25.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 26.Hur YM, Bouchard TJ, Lykken DT. Genetic and environmental influence on morningness-eveningness. Pers Individ Diff. 1998;25:917–25. [Google Scholar]
- 27.Moore M, Slane J, Mindell JA, Burt SA, Klump KL. Genetic and environmental influences on sleep problems: a study of preadolescent and adolescent twins. Child Care Health Dev. 2011;37:638–41. doi: 10.1111/j.1365-2214.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boomsma DI, van Someren EJ, Beem AL, de Geus EJ, Willemsen G. Sleep during a regular week night: a twin-sibling study. Twin Res Hum Genet. 2008;11:538–45. doi: 10.1375/twin.11.5.538. [DOI] [PubMed] [Google Scholar]
- 29.Heath AC, Eaves LJ, Kirk KM, Martin NG. Effects of lifestyle, personality, symptoms of anxiety and depression, and genetic predisposition on subjective sleep disturbance and sleep pattern. Twin Res. 1998;1:176–88. doi: 10.1375/136905298320566140. [DOI] [PubMed] [Google Scholar]
- 30.Wright MJ, Martin NG. Brisbane adolescent twin study: Outline of study methods and research projects. Aust J Psychol. 2004;56:65–78. [Google Scholar]
- 31.Heath AC, Nyholt DR, Neuman R, et al. Zygosity diagnosis in the absence of genotypic data: an approach using latent class analysis. Twin Res. 2003;6:22–6. doi: 10.1375/136905203762687861. [DOI] [PubMed] [Google Scholar]
- 32.Neale MC, Boker SM, Xie G, Maes HH. 2006 Mx: Statistical Modeling. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry. 7th Edition. [Google Scholar]
- 33.Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- 34.Drennan M, Shelby J, Kripke D, Kelsoe J, Gillin J. Morningness/ eveningness is heritable. Soc Neurosci Abstract Book. 1992;8:196. [Google Scholar]
- 35.Hur YM. Stability of genetic influence on morningness-eveningness: a cross-sectional examination of South Korean twins from preadolescence to young adulthood. J Sleep Res. 2007;16:17–23. doi: 10.1111/j.1365-2869.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 36.Linkowski P, Kerkhofs M, Van Cauter E. Sleep and biological rhythms in man: a twin study. Clin Neuropharmacol. 1992;15:42A–3A. doi: 10.1097/00002826-199201001-00024. [DOI] [PubMed] [Google Scholar]
- 37.Linkowski P, Van Onderbergen A, Kerkhofs M, Bosson D, Mendlewicz J, Van Cauter E. Twin study of the 24-h cortisol profile: evidence for genetic control of the human circadian clock. Am J Physiol. 1993;264:E173–81. doi: 10.1152/ajpendo.1993.264.2.E173. [DOI] [PubMed] [Google Scholar]
- 38.Katzenberg D, Young T, Finn L, et al. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–76. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- 39.Katzenberg D, Young T, Lin L, Finn L, Mignot E. A human period gene (HPER1) polymorphism is not associated with diurnal preference in normal adults. Psychiatric Genet. 1999;9:107–9. doi: 10.1097/00041444-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Pedrazzoli M, Ling L, Finn L, et al. A polymorphism in the human timeless gene is not associated with diurnal preferences in normal adults. Sleep Res Online. 2000;3:73–6. [PubMed] [Google Scholar]
- 41.Robilliard DL, Archer SN, Arendt J, et al. The 3111 Clock gene polymorphism is not associated with sleep and circadian rhythmicity in phenotypically characterized human subjects. J Sleep Res. 2002;11:305–12. doi: 10.1046/j.1365-2869.2002.00320.x. [DOI] [PubMed] [Google Scholar]
- 42.Watson NF, Buchwald D, Vitiello MV, Noonan C, Goldberg J. A twin study of sleep duration and body mass index. J Clin Sleep Med. 2010;6:11–7. [PMC free article] [PubMed] [Google Scholar]
- 43.Ando J, Nonaka K, Ozaki K, et al. The Tokyo Twin Cohort Project: overview and initial findings. Twin Res Hum Genet. 2006;9:817–26. doi: 10.1375/183242706779462480. [DOI] [PubMed] [Google Scholar]
- 44.Szymczak JT, Jasinska M, Pawlak E, Zwierzykowska M. Annual and weekly changes in the sleep-wake rhythm of school children. Sleep. 1993;16:433–5. doi: 10.1093/sleep/16.5.433. [DOI] [PubMed] [Google Scholar]
- 45.Spruyt K, Gozal D, Dayyat E, Roman A, Molfese DL. Sleep assessments in healthy school-aged children using actigraphy: concordance with polysomnography. J Sleep Res. 2011;20:223–32. doi: 10.1111/j.1365-2869.2010.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Sleep Foundation. Washington, USA: 2006. 2006 Sleep in America Poll. National Sleep Foundation Website. Available at www.sleepfoundation.org. [Google Scholar]
- 47.Gregory AM, Rijsdijk FV, Eley TC. A twin-study of sleep difficulties in school-aged children. Child Dev. 2006;77:1668–79. doi: 10.1111/j.1467-8624.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 48.Acebo C, Sadeh A, Seifer R, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- 49.Short MA, Gradisar M, Lack LC, Wright H, Carskadon MA. The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Med. 2012;13:378–84. doi: 10.1016/j.sleep.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: the role of individual and health related characteristics. Sleep Med. 2011;12:239–45. doi: 10.1016/j.sleep.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregory AM, Rijsdijk FV, Lau JY, Dahl RE, Eley TC. The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. Sleep. 2009;32:189–99. doi: 10.1093/sleep/32.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Oord EJ, Verhulst FC, Boomsma DI. A genetic study of maternal and paternal ratings of problem behaviors in 3-year-old twins. J Abnorm Psychol. 1996;105:349–57. doi: 10.1037//0021-843x.105.3.349. [DOI] [PubMed] [Google Scholar]


