Abstract
Study Objectives:
Electroencephalographic slow wave activity (SWA) during non-rapid eye movement (NREM) sleep results from the synchronous oscillation of cortical neurons and is the standard measurement of sleep homeostasis. SWA is not a direct measure of sleep pressure accumulation, but rather a measure of the NREM-sleep response to accumulated sleep pressure. Currently, no practical standard for the direct measurement of sleep pressure accumulation exists. Recently, it was demonstrated that rat cortical neurons undergo oscillations during wake that are similar to the cortical oscillations responsible for SWA. Furthermore, these oscillations increase in number as time awake increases. Here we hypothesize that period-amplitude analysis of the electroencephalogram (EEG), which treats the EEG as a series of discrete waves, can measure these cortical oscillations, and thus, is a measure of sleep-pressure accumulation during extended wake.
Design:
Mice were sleep deprived for 24 h by confinement to a slowly rotating wheel in order to assess wake-dependent changes in EEG wave incidence.
Measurements and Results:
Continuous period-amplitude analysis of the waking EEG across 24 h of sleep deprivation revealed that the incidence of 2 to 6 Hz waves increased exponentially over the deprivation period. This increase in wave incidence appeared to occur in two phases with exponential time constants of approximately 0.12 h and 3 h. Further analysis revealed that the changes in wave incidence were significantly correlated with two established markers of sleep pressure, SWA and NREM sleep latency.
Conclusions:
The data suggest that wave incidence is an effective method of measuring sleep homeostasis in the waking EEG that provides better temporal resolution than spectral power analysis.
Citation:
Ehlen JC; Jefferson F; Brager AJ; Benveniste M; Paul KN. Period-amplitude analysis reveals wake-dependent changes in the electroencephalogram during sleep deprivation. SLEEP 2013;36(11):1723-1735.
Keywords: Sleep deprivation, waking, homeostasis, sleep propensity, mice
INTRODUCTION
Sleep is regulated by the interaction of a circadian and a homeostatic process. The homeostatic process regulates sleep pressure which accumulates with wake duration and dissipates during subsequent sleep. Slow wave activity (SWA) during non-rapid eye movement (NREM) sleep is the standard measurement for sleep homeostasis in mammals.1–3 Spectral power in this 0.5-4 Hz band of the electroencephalogram (EEG) is highest at the onset of the sleep period and declines across NREM sleep.4 SWA also increases following sustained wakefulness and decreases across subsequent sleep episodes with the intensity of SWA proportional to the duration of prior wakefulness.5,6 Further, local activation of cortical areas during wake is proportional to SWA over these areas during subsequent NREM sleep episodes.7,8 Thus, SWA is currently the most reliable EEG measure of the homeostatic process. However, it is indirect and measures the dissipation not the active accumulation of sleep pressure.
EEG correlates of the homeostatic process are not limited to NREM sleep. Several human studies have reported increased power in EEG frequencies below 9 Hz during prolonged wakefulness.9–13 Some of these changes in spectral power are also correlated with non-spectral markers of sleep homeostasis. Specifically, spectral power in the 1-7 Hz band of the waking EEG correlates with subjective and objective measures of sleepiness during 32 h to 40 h of continuous wake.14,15 This broad frequency range marking sleep homeostasis has been further delineated by several reports; however, the results of these studies are not in complete agreement.11,13,16 For example, analysis of the waking EEG in 1-Hz bands has revealed that objective measures of sleep homeostasis correlate with EEG power in the 5.25-6 Hz range, whereas subjective measures of sleep homeostasis are better represented by higher (10.25-13 Hz) frequencies.16 Another study demonstrated that the rise rate in theta power (5-8 Hz) during sustained wakefulness is correlated with an increase in SWA occurring during the first 2 h of recovery sleep. These changes in theta power and SWA were centered around similar areas of the frontal cortex.11 Recently, the results of a study using a forced desynchrony protocol indicated that power in the 1-4.5 Hz range, and not the 4.5-8 Hz range, was the best measure of human sleep homeostasis during 28 h of continued wakefulness.13 Thus, despite strong evidence that the homeostatic process in humans is reflected in EEG power during wake, the exact frequencies reflecting this relationship are not entirely clear.
Rodents have been invaluable in understanding the homeo-static regulation of sleep in mammals; however, the frequency bands reflecting sleep homeostasis in rodents during wakefulness are also not entirely clear. EEG power density below 5 Hz and greater than 9 Hz increase gradually over 24 h of sleep deprivation in rats.17,18 In a more recent report, however, 6 h of sleep deprivation in rats significantly increased EEG power in the theta band (5-7 Hz) in both active and quiet wake. This change during quiet wake was significantly correlated with increased SWA in NREM sleep that followed the deprivation.19 In another study of three inbred strains of mice, spectral power in the 7-15 Hz range increased over 6 h of sleep deprivation.20 Differences below 7 Hz were found in this study, but varied significantly between strains. Thus, the frequency band reflecting sleep homeostasis during wakefulness in rodents, as in humans, remains undefined.
Spectral power in the waking EEG is also influenced by the circadian system. Several human studies have addressed this issue, and provided valuable information regarding the interacting effects of wake duration and circadian modulation on spectral power. A sequential fitting procedure applied to EEG spectral power obtained during 40 h of continuous wake indicates that both homeostatic and circadian factors influence EEG frequencies below 9 Hz.12 In this study and a follow-up study, circadian influences were found to be greatest in the theta band (4.25-7 Hz).12,16 A third study found a significant interaction between circadian phase and elapsed time awake in both the delta and theta bands of the waking EEG using a forced desynchrony protocol.13 The findings of these studies suggest that circadian modulation and wake duration interact to alter the rise in theta power and, as a result, theta power is not a monotonically increasing function.11–13,16,21 Circadian influences are most prominent in the first 12 to 18 h of continuous wake.11–14,21 These reports suggest that theta power reflects sleep homeostasis during durations greater than 24 h, but may be influenced by the circadian system in shorter durations.
The intrinsic, bistable, membrane properties of cortical neurons result in synchronous oscillations during NREM sleep. These oscillations include active firing ‘up’ states and hyperpolarized ‘down’ states with virtually no firing. These oscillations are the source of EEG SWA.22–25 Recently, it was demonstrated that rat cortical neurons undergo similar oscillations between states during wake26,27 and the occurrence of these oscillations increases with wake duration.26 Spectral analysis may not be efficient in resolving changes in the occurrence of these oscillations because spectral power represents the average across a window of time.28 This averaging that occurs in the transformation of EEG data from the time domain to the frequency domain may result in the loss of temporal resolution. Period-amplitude analysis, which treats the EEG as a series of discrete waves with individual periods and amplitudes, provides an alternate way to analyze the EEG and may allow better visualization of these underlying cortical events.28,29 In the current study, we apply period-amplitude analysis to the waking EEG across a 24-h period of sleep deprivation and measure changes in the incidence of EEG waves. Furthermore, we demonstrate that wave incidence may serve as a measure of sleep homeostasis with better time resolution than spectral power.
METHODS
Animals
Adult male C57BL/6J mice were maintained on a 12-h light:12-h dark schedule throughout the study. Food and water were available ad libitum, and animals (10-12 weeks of age) were individually housed for at least 2 weeks prior to experimental use. All protocols and procedures were approved by the Morehouse School of Medicine Institutional Animal Care and Use Committee.
Surgery
EEG and electromyography (EMG) electrodes were implanted in anesthetized mice. A prefabricated head mount (Pinnacle Technologies, Lawrence, KS) was used to position three stainless-steel epidural screw electrodes. The first electrode (frontal—located over the frontal cortex) was placed 1.5 mm anterior to bregma and 1.5 mm lateral to the central suture, whereas the second two electrodes (interparietal— located over the visual cortex and common reference) were placed 2.5 mm posterior to bregma and 1.5 mm on either side of the central suture. The resulting two leads (frontal-interparietal and interparietal-interparietal) were referenced contralaterally. A fourth screw served as a ground. Electrical continuity between the screw electrode and head mount was aided by silver epoxy. EMG activity was monitored using stainless-steel Teflon-coated wires that were inserted bilaterally into the nuchal muscle. The head mount (integrated 2 × 3 pin grid array) was secured to the skull with dental acrylic. Mice were allowed to recover for at least 14 days before sleep recording.
EEG/EMG Recordings
One week after surgery, mice were moved to the sleep-recording chamber and connected to a lightweight tether attached to a low-resistance commutator mounted over the cage (Pinnacle Technologies). This enabled complete freedom of movement throughout the cage. Except for the recording tether, conditions in the recording chamber were identical to those in the home cage. Mice were allowed a minimum of seven additional days to acclimate to the tether and recording chamber. Recording of EEG and EMG waveforms began at zeitgeber time (ZT) 0 (light onset). Data acquisition was performed on a personal computer running Sirenia Acquisition software (Pinnacle Technologies), a software system designed specifically for polysomnographic recording in rodents. EEG signals were low-pass filtered with a 40-Hz cutoff and collected continuously at a sampling rate of 400 Hz. After collection, all waveforms were classified by a trained observer (using both EEG leads and EMG) as wake (low-voltage, high-frequency EEG; high-amplitude EMG; Figures 1C and 1D), NREM sleep (high-voltage, mixed-frequency EEG; low- amplitude EMG; Figures 1C and 1D) or rapid eye movement (REM) sleep (low-voltage EEG with a predominance of theta activity [6-10 Hz]; very low amplitude EMG). EEG epochs determined to have artifact (interference caused by scratching, movement, eating, or drinking) were excluded from analysis. Artifact comprised less than 5% of all recordings used for analysis. All recordings were scored in 2-sec epochs except the 6-h gentle handling group—scored in 10-sec epochs. NREM bout length (= total NREM sleep time / number of NREM bouts) was calculated without excluding brief arousals.
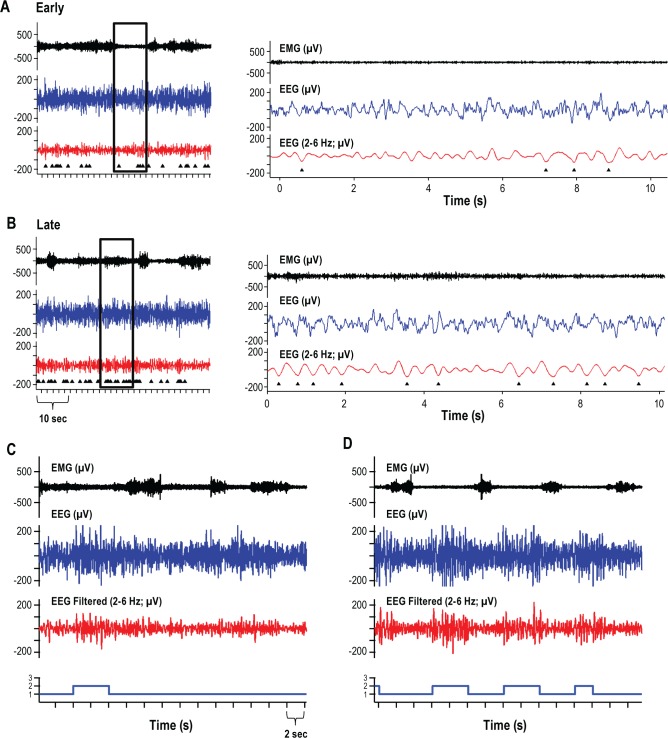
Figure 1.
Representative electroencephalogram (EEG) traces. EEG waveforms from time points both early (A, C) and late (B, D) in the 24 hours of sleep deprivation are shown from one representative mouse. Detected waves are identified below the filtered EEG data (triangles; 2-6 Hz) (A, B). Right panels of A and B are enlargements of the areas identified in the left panels of A and B. Hypnograms below C and D identify epochs hand scored as awake (1) or non-rapid eye movement (NREM) sleep (2). Brief episodes of NREM sleep (6.9 ± 1.6 sec, mean ± standard error of the mean) occurred during the automated forced wakefulness procedure; no rapid eye movement sleep occurred during sleep deprivation.
Forced Wakefulness
Following a 24-h baseline recording, mice were moved to a slowly rotating wheel (9 inches in diameter; 1 rpm) adjacent to the recording cage. Mice were confined to this wheel for 24 hours beginning at ZT 0 (lights on) during which time they had free access to food and water. Following sleep deprivation, animals were returned to the baseline recording cage and EEG acquisition was continued for a 24-h recovery opportunity. As a control for exposure to a novel environment, one group of mice were sleep deprived in their home cage by gentle handling. Trained observers monitored these mice over a 6-h period, beginning at lights off, and intervened if the animals exhibited behaviors indicating attempts at sleep. Interventions included tapping on the cage, disturbing the bedding, and, if necessary, gently nudging the animal's flank.
Signal Analysis
Analysis was performed on signals obtained from the frontal-interparietal lead unless otherwise noted. Analysis was performed using custom written functions in IGOR Pro 6.2 (WaveMetrics Inc., Lake Oswego, OR). Raw EEG signals from the frontal-parietal derivation were band pass-filtered in the frequency range indicated using a Butterworth fourth-order band-pass filter (IGOR Pro routine FilterIIR; WaveMetrics Inc., Lake Oswego, OR). Peaks in the filtered data were detected as negative deflections between two zero crossings. The amplitudes (maximum negative deflection from zero crossing) of all peaks detected in an entire 24-h period were then determined. The upper 30% (upper 60% and no cutoff were also examined) of peak amplitudes that occurred in epochs scored as wake were then counted (Figures 1A and 1B) and expressed as peaks per minute (wave incidence). The wave incidence data were binned in both 2-minute and 10-minute bins for graphing and statistical analysis. Some variability was found in short durations of wake. These short durations, less than 10 epochs, were excluded from analysis. A similar 24-h pattern of wave incidence was observed when these shorter wake epochs were included in the analysis.
A two-component exponential curve (y = A1e-t/τ 1 + A2e-t/τ 2 + offset) or one-component exponential curve (y = Ae-t/τ 1 + offset) was fit to the wave incidence data as indicated. The certainty of data points utilized in the fits was weighted by 1/standard deviation (SD).
All spectral analysis was performed on the frontal-interparietal lead. Power spectral analysis was accomplished by applying a fast Fourier transform (FFT, 0.5 Hz frequency resolution) to 1-h, 10-minute, and 2-minute blocks of the EEG recording. Only epochs classified as wake were included in this analysis. Spectral power in each frequency interval was then normalized to 24-h baseline values for each animal or expressed as a percentage of the first hour or half hour of sleep deprivation for 1-h intervals. Two- and 10-minute intervals were expressed as a percentage of 24 hour total power in the corresponding frequency band. SWA in NREM sleep was determined by FFT of all epochs scored as NREM sleep during the first 6-hours following sleep deprivation. Power in the 0.5-4 Hz range was then averaged for all NREM epochs in the 6-h period.
Statistics
Sleep data were analyzed using repeated-measures analysis of variance (ANOVA). Significance was defined as P ≤ 0.05. Post hoc analysis was conducted using the Holm-Sidak method (versus control when appropriate). Student's t-test was also used where indicated. Eleven animals began this study. Two had EEG artifact greater than 5% while being sleep deprived and were removed from analysis. One animal had NREM onset (first epoch scored as NREM sleep during forced wakefulness) that was greater than 2 SDs from the mean (NREM onset of eight animals = 75.0 ± 24 min, mean ± standard error of the mean (SEM); NREM onset of subject animal = 702 min) and was removed from the analysis.
RESULTS
Confinement to a slowly rotating wheel resulted in substantial sleep deprivation with mice awake for approximately 82% of the 24-h period (277 ± 56 min NREM sleep during deprivation versus 537 ± 17 min NREM sleep during baseline; mean ± SEM; paired student's t-test, t(7) = 4.36, P = 0.003). The NREM sleep that did occur during sleep deprivation was fragmented with an average NREM bout length of 6.9 ± 1.6 seconds versus 56.6 ± 10 seconds for baseline sleep (Figures 1C and 1D; paired student's t-test, t(7) = 5.64, P = 0.001). Further evidence of this fragmentation can be seen in the total number of NREM sleep bouts (≥ 2 sec), which were increased in animals undergoing sleep deprivation (2408 ± 273 bouts versus 576 ± 85 bouts; t(7) = 6.46, P < 0.001). NREM onset (the first epoch scored as NREM sleep) occurred 75 ± 24 minutes after the onset of wheel confinement. No REM sleep was observed during sleep deprivation.
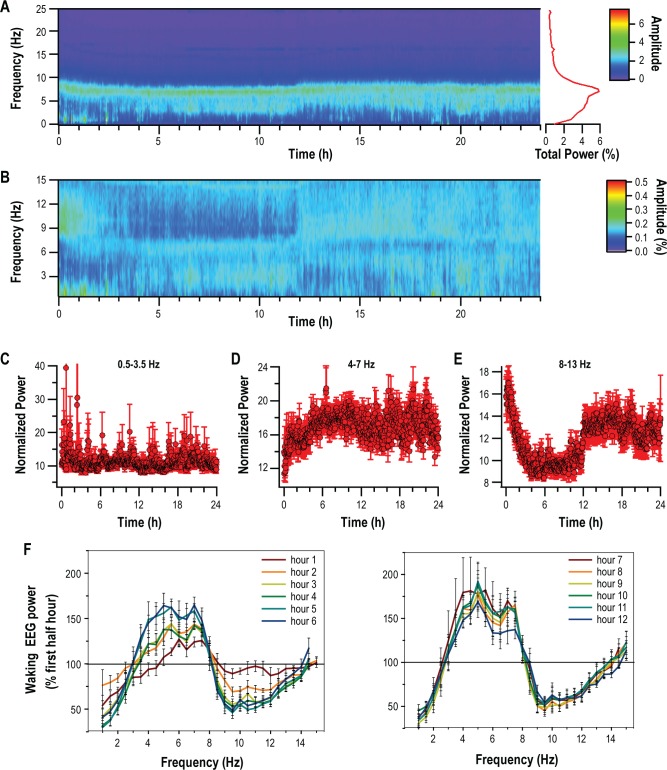
We first examined total spectral power in 0.5 Hz bands across the 24-h sleep deprivation period (Figure 2A) in 2-minute intervals. A subset of these data (0.5 to 15 Hz), which represented 90% of total power in the wake EEG, were then chosen for subsequent analysis. For each animal, normalized total power in 0.5 Hz frequency bands (% 24 h total power in each band) was calculated at 2-min intervals. These values were then averaged and are presented in Figure 2B. From Figure 2B we identified three frequency bands of interest: 0.5 to 3.5 Hz, 4 to 7 Hz, and 8 to 13 Hz. A 24-h time course of normalized power in each of these bands is presented in Figures 2C, 2D, and 2E. Only power in the 4-7 Hz band elicited a continuous increase in normalized power over the 24-h period that could be correlated with sleep pressure. When the first 2 h were examined in 2-minute intervals, this increase was significant within the first 52 minutes of sleep deprivation (one-way repeated-measures ANOVA, F(7,59) = 5.13, P < 0.001; Holm-Sidak post hoc versus first 2 min, P < 0.0004). FFT of the EEG data in 1-h intervals yielded similar frequency bands of interest (Figure 2F). Sleep deprivation was associated with an increase in 4-7 Hz waking spectral power that occurred gradually over the first 6 hours of sleep deprivation. Across the remaining 24 hours, values did not appear to differ from the sixth hour of sleep deprivation (Figure 2F).
Figure 2.
Changes in spectral power over 24 hours of forced wakefulness. (A) Heat map of total power over the 24-hour sleep-deprivation period averaged across eight animals and presented in 2-min bins. Average total power for all mice in each frequency band is plotted to the right. (B) Heat map of average (n = 8) normalized total power over 24 hours of sleep deprivation in the 0.5-15 Hz frequency range. Three frequency ranges were identified from this heat map, 0.5-3.5 Hz (C), 4-7 Hz (D), and 8-13 Hz (E), and are presented in 2-min bins. For each plot, mean ± standard error of the mean (SEM) for each mouse is presented separately. (F) Fast Fourier transform applied at 1-h intervals over the 24-h sleep-deprivation period presented as % change over the first half-hour (mean ± SEM; n = 8).
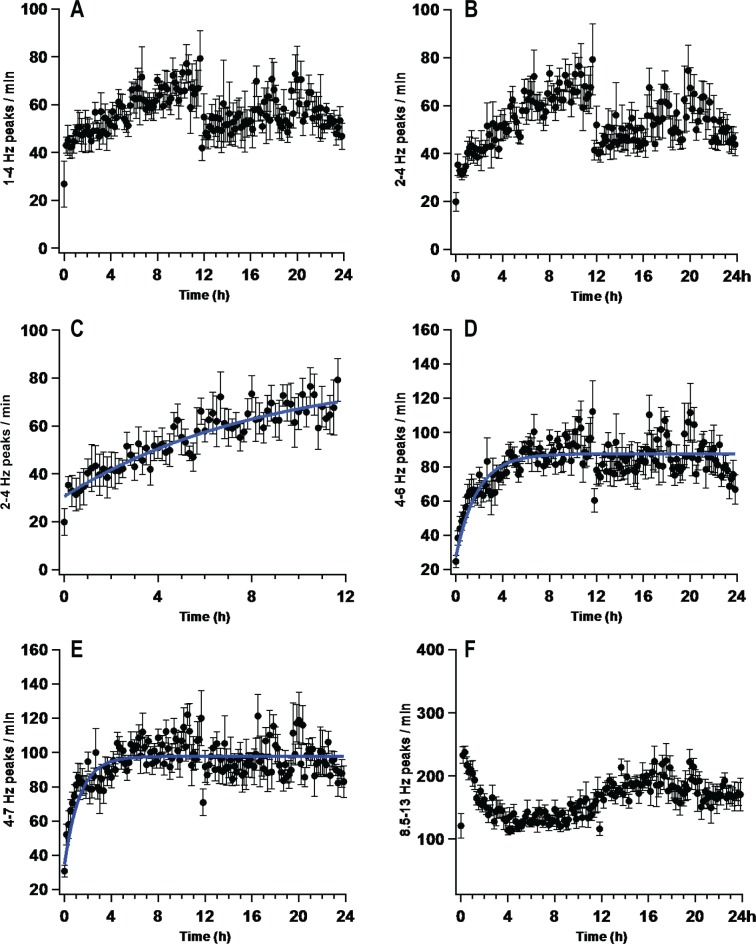
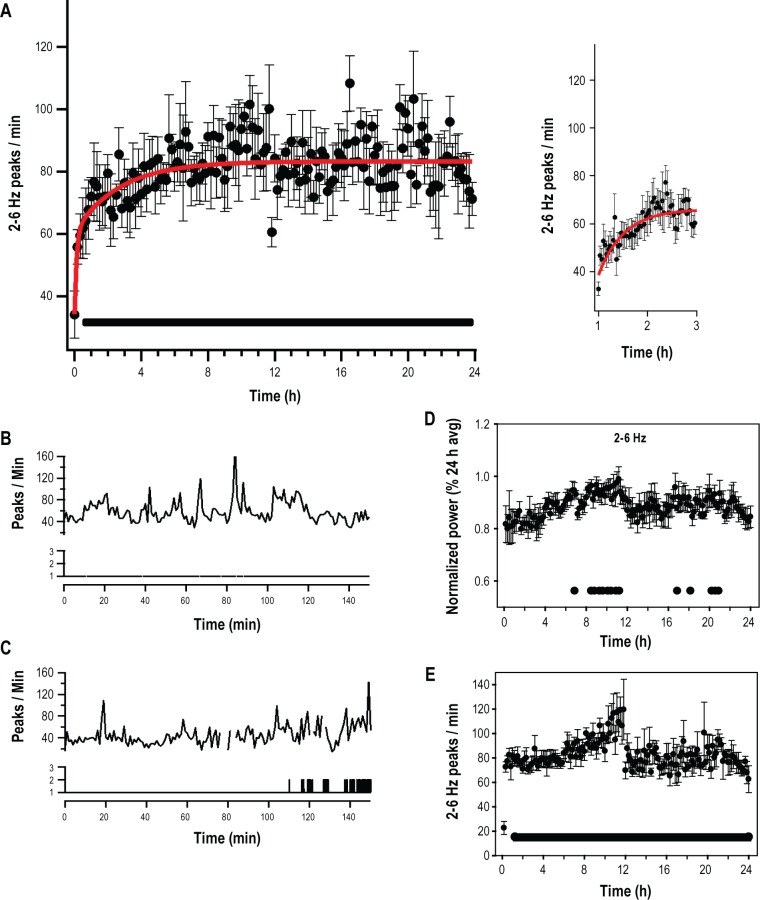
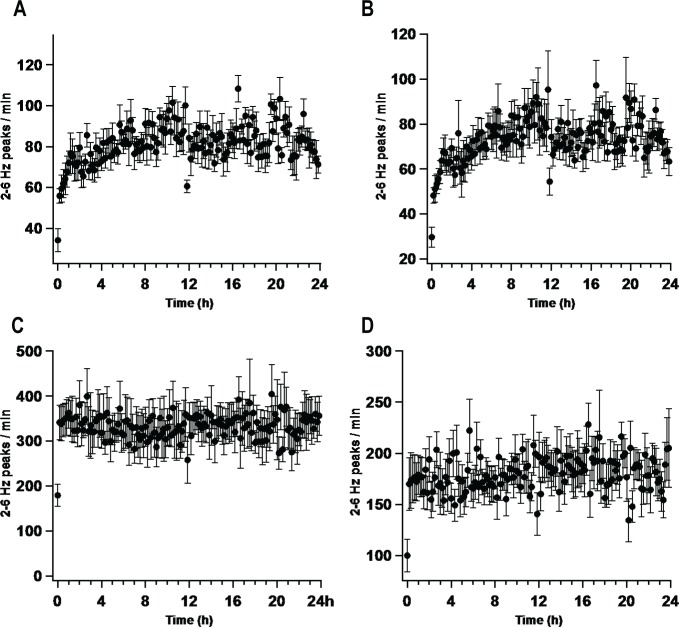
We next calculated wave incidence using frequency bands identified in the analysis of spectral power. These data are presented in Figure 3. Frequency bands filtered below 4 Hz were not monotonically increasing functions (Figures 3A and 3B). Frequencies above 8 Hz also did not display a monotonic increase (Figure 3F). However, wave incidence between these two ranges, 4-7 Hz (I4-7), was best fit by an exponential function (Figure 3E; I4-7, τ = 78 ± 21 min; χ2 (139) = 69.78; P < 0.001). During the first half of the sleep deprivation period, the patterns of wave incidence in frequency bands below 4 Hz appeared to have relatively slow time constants (Figures 3A and 3B) when compared to I4-7. Wave incidence in the 2-6 Hz range, which increases with short durations of wake in the rat,26,27 overlapped both the 1-4 Hz and 4-7 Hz range and was also examined. I2-6 of the waking EEG (I2-6) significantly increased during sleep deprivation (Figure 4A, one-way repeated-measures ANOVA, F(7,143) = 2.03, P < 0.001). This increase was statistically significant 34 minutes into the deprivation period when the first 2 hours were examined in 2-minute intervals (Figure 4A inset; one-way repeated measures ANOVA, F(7,59) = 2.14, P < 0.001; Holm-Sidak post hoc versus first 2 min, P < 0.0009). The time course of this increase was best fit by a two-exponential function (τ1 = 7.3 ± 11.9 min, A1 = 53.9 ± 36.8%; τ2 = 162.5 ± 119.9 min, A2 = 46.1 ± 24.9%;. χ2 (139) = 21.2; P < 0.001; Figure 4A). This fit was found to be significantly better than a single-exponential function (τ = 101.8 ± 44.5 min; χ2 (139) = 23.5; P < 0.001; Nested model comparison F(3,139) = 3.14; P < 0.05). Because the fast time-constant of the I2-6 two-exponential fit represents the equivalent of less than one data point (τ1 = 7.3 ± 11.9 min; 10-min bins) we reanalyzed I2-6 during the first 12 hours of sleep deprivation in 2-minute bins. A two-exponential equation fit to the first 12 hours (2-min bins) of the sleep deprivation resulted in a decrease in the fast component of the two-exponential when compared to the fit performed on 10-min bins (Figure 4A inset; τ1 = 2.4 ± 3.5 min, A1 = 52.0 ± 36.7%; τ2 = 280.9 ± 92.5 min, A2 = 48.0 ± 25.6%;. χ2 (360) = 49.3; P < 0.001). We then split this 2-6 Hz frequency range for further examination. A curve fit to the first 12 h of I2-4 had a time constant that was slower than a curve fit to I4-6 (I2-4, τ = 540 ± 420 min; χ2 (67) = 8.88; P < 0.001; I4-6, τ = 101 ± 22 min; χ2 (139) = 30.7; P < 0.001; Figures 3C and 3D).
Figure 3.
The rate of change in wave incidence over multiple frequency ranges. Average wave incidence was calculated from electroencephalogram (EEG) recordings filtered in the following frequency ranges: 1-4 Hz (A); 2-4 Hz (B, C); 4-6 Hz (D); 4-7 Hz (E); 8.5-13 Hz (F). Each plot represents the average (n = 8) wave incidence in 10-min bins over 12 or 24 hours. Curves providing the best fit to the data are plotted for each frequency range. Frequencies above 8 Hz (F) and below 4 Hz (A, B) did not produce wave incidence patterns that could be fit with a monotonic exponential, or linear, function. Note: 2-4 Hz is presented twice, panel C provides analysis of the first 12 hours of panel B.
Figure 4.
The 2-6 Hz waves in the waking electroencephalogram (EEG) over 24 h. (A) 2-6 Hz wave incidence during 24 hours of forced wakefulness was calculated in 10-min intervals and expressed as peaks per min wake time then averaged across individuals (frontal derivation; n = 8; mean ± standard error of the mean). A rapid increase occurred in the first 2 hours of forced wakefulness (inset) and was followed by a slow increase for the remaining 24-h period. Lower markers, significantly different from first 10-min period (P < 0.0004, Holm-Sidak post hoc analysis). (B, D) Wave incidence (peaks/min) in the first 2 hours of forced wakefulness in two representative animals. (Lower trace, sleep state; 1, wake; 2, non-rapid eye movement; 3, rapid eye movement). (C) Average normalized spectral power for the same eight animals in Figure 4A over 24 hours of forced wakefulness (2-6 Hz; lower markers = P < 0.0004, Holm-Sidak post hoc analysis). (E) Average 2-6 Hz wave incidence during 24 hours of forced wakefulness recorded over the visual cortex (lower markers = P < 0.0004, Holm-Sidak post hoc analysis).
In order to control for changes in the EEG that may be caused by a novel environment upon transfer to the rotating wheel, we also examined the pattern of I2-6 utilizing another method of sleep deprivation, gentle handling, where exposure to a novel environment could be avoided. Mice were awake 94.1 ± 1.2% of this 6-h sleep deprivation procedure. During this time I2-6 was significantly increased after 20 minutes and remained elevated for the entire sleep deprivation period (Figure 5G; n = 6; repeated- measures ANOVA, F(5,35) = 4.95, P < 0.001; Holm-Sidak post hoc versus first 10 min, P < 0.0003). I2-6 was also calculated using the interparietal lead (repeated measures ANOVA, F(5,143) = 1.78, P < 0.001). The pattern of change in I2-6 from this lead is shown in Figure 4E.
Figure 5.
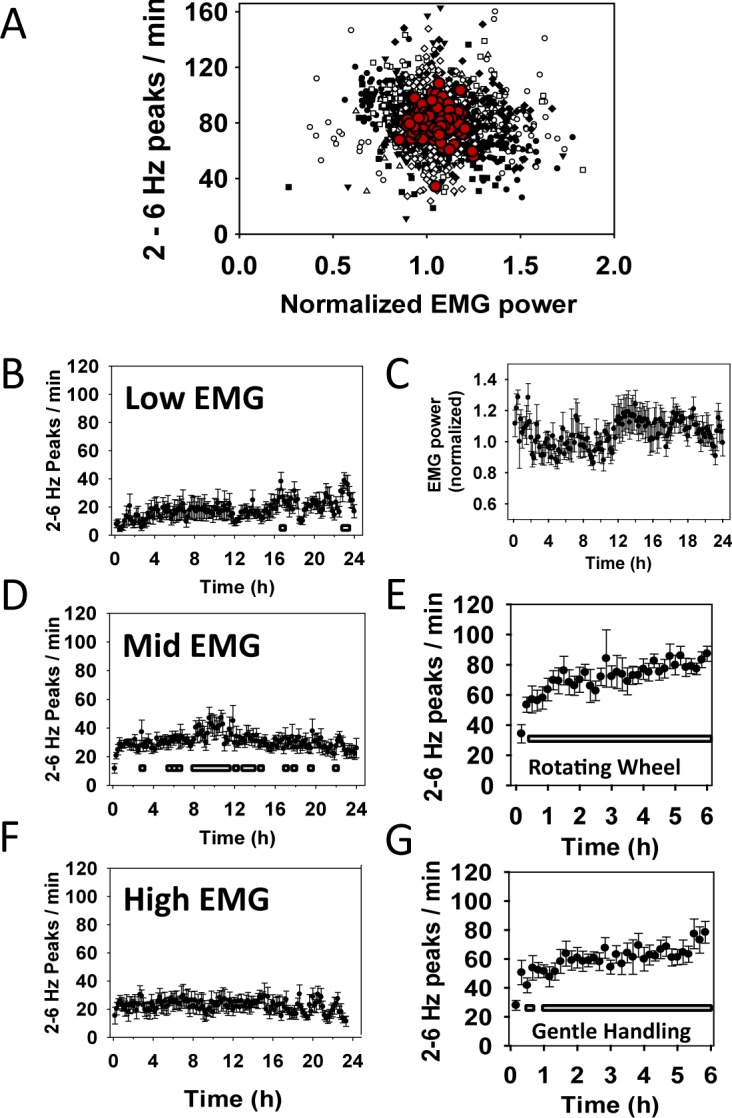
The 2-6 Hz wave incidence (I2-6) during waking substates. (A) Correlation of I2-6 with electromyograph (EMG) activity over the 24-h sleep deprivation period (Rsqr = 0.021, P < 0.001). Ten-min bins for each of eight mice are plotted separately. Red symbols represent means of each 10-min bin. (C) Normalized average total power in the EMG (repeated- measures analysis of variance did not detect a significant effect of time on EMG power). (B) I2-6 in the lower third, (D) middle third, and (F) upper third of EMG activity. Lower markers B, D, and F, P > 0.0004 Holm-Sidak post hoc analysis. (E) I2-6 during the first 6 hours of forced wakefulness by a slowly rotating wheel. Lower markers, P > 0.0014 Holm-Sidak post hoc analysis (replotted from Figure 4A to allow comparison). (G) I2-6 6 h of force wakefulness by gentle handling. Lower markers, P > 0.0014 Holm-Sidak post hoc analysis.
The influence of waking substates on wave incidence is shown in Figure 5. Across the 24-h sleep deprivation period, no significant change in EMG activity was found (Figure 5C; repeated-measures ANOVA, F(7,143) = 1.2, not significant). In addition, we found little correlation between I2-6 and EMG activity (Rsqr = 0.021; Figure 5A). Further analysis of I2-6 during low, mid and high EMG activity are shown in Figure 5B, 5D, and 5F.
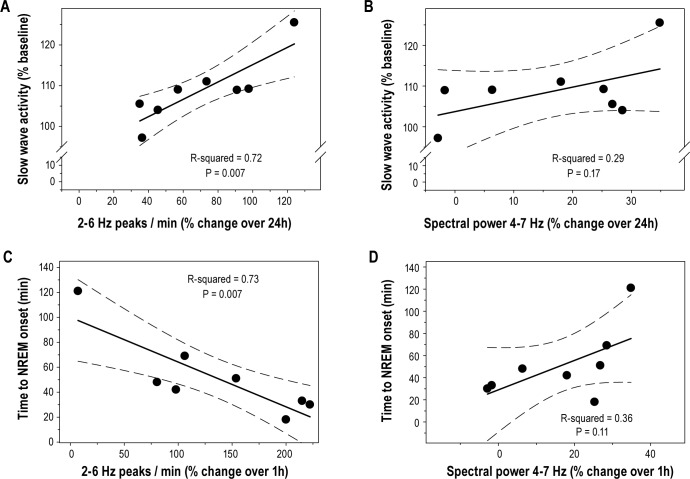
The increase in I2-6 after 24 h of sleep deprivation (average I2-6 during the first 20 min of the 24th h expressed as a percentage of the first 20 min) was significantly correlated with the increase in SWA for the first 6 h of recovery sleep (SWA expressed as a percentage of corresponding baseline average) that followed sleep deprivation (Figure 6A; Rsqr = 0.72; ANOVA F(1,6) = 15.76, P = 0.007). Time to NREM onset (latency to the first epoch scored as NREM sleep) was negatively correlated with the change in I2-6 during the first hour of sleep deprivation (Figure 6C; Rsqr = 0.73; ANOVA F(1,6) = 15.89, P = 0.007). Correlations with spectral power in the 4-7 Hz range were also evaluated. Over 24 h, the change in 4-7 Hz power (average normalized power in the 4-7 Hz band during the first 20 min of hour 24 expressed as a percentage of the first 20 min) indicated a trend toward correlation with SWA in recovery sleep (Figure 6B; Rsqr = 0.29; ANOVA F(1,6) = 2.5, P = 0.17). The change in 4-7 Hz spectral power during the first hour of sleep deprivation also showed a trend toward correlation with time to NREM onset (Figure 6D; Rsqr = 0.36; ANOVA F(1,6) = 3.42, P = 0.11). However, this correlation was positive whereas the correlation of I2-6 with time to NREM onset was negative.
Figure 6.
Wave incidence (2-6 Hz) is correlated with known markers of sleep pressure. (A) The increase in wave incidence (2-6 Hz) over 24 hours of forced wakefulness was significantly correlated with the increase in NREM slow wave activity (% change over baseline average) occurring across the first 6 hours of recovery sleep that followed the deprivation. (C) The percent change in wave incidence (2-6 Hz) over the first hour of forced wakefulness also showed a significant negative correlation with the time to NREM sleep onset (a shorter-term marker of sleep pressure). (B) The percent change in normalized (% 24 h average) spectral power in the 4-7 Hz range over 24 hours trended toward correlation with the increase in NREM SWA during recovery sleep; however, this correlation was not significant. (D) Change in normalized spectral power (4-7 Hz) during the first hour of sleep deprivation showed no significant negative correlation with latency to NREM onset.
Several peak amplitude cutoffs were evaluated prior to arriving at the final peak selection criterion (upper 30% of peak amplitudes) used for these analyses. Among those evaluated were the upper 60% of peak amplitudes (Figure 7D) and the upper 100% peak amplitudes (Figure 7C). Only the upper 30% selection criterion provided a wake-dependent increase in wave incidence over the 24-h deprivation period. In addition, we found that short wake durations (< 20 sec) produced some increases in variability. This increased variability resulted from our calculation method, which expressed values in peaks per minute (ppm). Short wake durations would, therefore, occasionally produce artificially high ppm values. To eliminate this problem, we excluded durations of wake that were shorter than 10 epochs (20 sec). Figure 6 presents these I2-6 results with (Figure 7B) and without (Figure 7A) waking bouts less than 20 seconds.
Figure 7.
Period-amplitude analysis was performed by band-pass filtering (2-6 Hz) EEG signals from the frontal-interparietal derivation. Peaks in the filtered data were detected as negative deflections between two zero crossings. The amplitude (maximum negative deflection from zero crossing) of all peaks detected in an entire 24-h period were then determined. (A, B) The upper 30% of peak amplitudes that occurred in epochs scored as wake were then counted and expressed as peaks per min (wave incidence). Other peak-amplitude cutoffs did not result in wake-dependent increases in wave incidence. (C) No amplitude cutoff. (D) Upper 60% of peaks. (A) High variability was found in short durations of wake. These short durations, less than 10 epochs, were excluded from analysis. (B) A similar 24-h pattern of wave incidence was observed when these shorter wake epochs were included in the analysis.
During the undisturbed baseline conditions, I2-6 exhibited a 24-h pattern with high values during the early light phase that decreased to low values around the light—dark transition (Figure 8A). I2-6 then gradually increased from these low values throughout the dark phase (one-way repeated measures ANOVA, F(7,23) = 2.2, P = 0.004). During the 24-hours following sleep deprivation, however, there was no significant variation in I2-6 detected (Figure 8B; one-way repeated measures ANOVA, F(7,23) = 2.2, P = 0.52). That is, I2-6 values remained relatively consistent throughout the 24-h recovery period.
Figure 8.
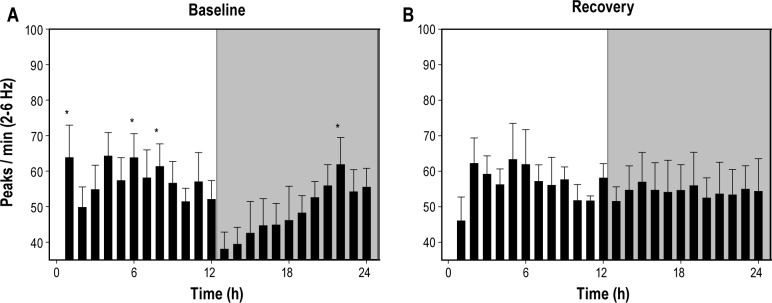
Baseline and recovery sleep. (A) During undisturbed baseline sleep the occurrence of 2-6 Hz waves exhibited a day/night difference (peaks/min awake, mean ± standard error of the mean). Values were high during the first half of the light (rest) period and then decreased to low values at the onset of dark. The waves then exhibited a gradual increase to light-like values over the remaining dark period. (B) During the period that followed 24 hours of forced wakefulness (recovery), no rhythm was observed in the incidence of 2-6 Hz waves. Values remained relatively high for the entire 24-h period. *Significantly different from lowest value (P < 0.05, Holm-Sidak post hoc).
DISCUSSION
In this study, we obtained EEG recordings continuously throughout 24 hours of forced wakefulness, which allowed us to assess changes under conditions of increasing homeostatic pressure. We hypothesized that subjecting EEG data to period amplitude analysis, which evaluates EEG data in the time domain, may allow the visualization of cortical events that are masked through averaging in power spectral analysis. The main result of this study demonstrated an increase in the incidence of 2-6 Hz waves (I2-6) during waking. The increase in I2-6 had a two-component rise; a rapid rise in the first 10 minutes was followed by a slower rise across the remaining 24-h period (Figures 4A, 4B, and 4D). Furthermore, the percent change in I2-6 across the sleep deprivation period was significantly correlated with two established markers of sleep homeostasis, slow wave activity (SWA) and latency to NREM sleep onset (Figures 6A and 6C).
Several frequency bands were chosen for the analyses in this study. These bands were chosen based on spectral-power data obtained in the current study and reported in the literature. Normalized spectral power across the 24-h sleep deprivation period is shown in Figures 2A, 2B, and 2F. Based on these data, we identified three frequency bands for further analysis (Figures 2C, 2D, and 2E). Only spectral power in the 4-7 Hz range appeared to be a monotonically increasing function. This frequency range is consistent with several reports in the literature investigating markers of sleep homeostasis in rats and mice.17–20 Notably, spectral power in the 5-7 Hz range during sleep deprivation was found to correlate with NREM SWA during recovery sleep.19 We found a similar trend in 4-7 Hz spectral power (Figure 6B); however, this correlation did not reach statistical significance. We also evaluated the correlation of 4-7 Hz spectral power with latency to NREM onset (Figure 6D). The change in spectral power after one hour of sleep deprivation was not correlated with this marker of sleep homeostasis.
Wave incidence analysis was applied to the frequency bands identified in our spectral analysis. A pattern emerged upon examining wave-incidence rate-constants across this range of frequencies (Figure 3). Frequency bands below 8 Hz were best fit by single exponential curves. Furthermore, these curves had time constants that varied with frequency (Figures 3A and 3E). During the first 12 hours of the sleep deprivation period, the patterns of wave incidence in frequency bands below 4 Hz were best fit by curves with relatively slow exponential time constants (Figure 3A). In frequencies between 4 Hz and 7 Hz, wave incidence patterns were best fit by curves with much faster exponential time constants (Figure 3E). Intracortical neuronal activity in the awake rat indicates that wave incidence in the 2-6 Hz band, a frequency range that includes a portion of both the 1-4 and 4-7 Hz band, increases during forced wakefulness.
Cortical neurons can exhibit brief periods of near-synchronous quiescence in multiunit activity (MUA) while rats are awake.26,27 Furthermore, the quiescent periods occur more frequently after the rat is forced to be awake for several hours.26 Simultaneous measurement of local field potential (LFP) and MUA reveals that the transitions to this quiescent state during waking are associated with LFP waves in the 2-6 Hz frequency band.26 This same relationship between MUA and LFP also exists with slow waves (0.5-4 Hz) during NREM sleep.22,24 Notably, these transitions between states measured by MUA and LFP recordings during NREM sleep have also been associated with slow waves in the EEG.30 Thus, it is highly likely that the cortical events observed in MUA and LFP recordings during wake are also reflected in EEG recordings.
Wave incidence in the 2-6 Hz range was best fit by a two-exponential function (Figure 4A). Further analysis of smaller frequency bands within this range revealed a fast and slow component of wave incidence. The time constant of a curve fit to I4-6 was relatively fast when compared to the first 12 hours of the 2-4 Hz band (Figure 3C, I2-4, τ = 540 ± 420 min; χ2 (67) = 8.88; P < 0.001; Figure 3D, I4-6, τ = 101 ± 22 min; χ2 (139) = 30.7; P < 0.001). These data suggest that a slower component of the curve fit to I2-6 may predominate in the lower frequencies whereas a faster component of this curve predominates in the higher frequencies. This change to lower frequencies with increasing durations of wake may reflect an increase in the duration of quiescent periods among cortical neurons as sleep pressure accumulates. This is supported by wave incidence data that demonstrate a decrease in cortical off (quiescent) periods in MUA, and a decrease in LFP slow waves, as NREM sleep duration increases.30
Mice were exposed to a novel environment upon transfer to the slowly rotating wheel. In order to control for this novel environment, we measured I2-6 under a different method of sleep deprivation, gentle handling. This procedure was carried out in the animals' home cage and minimized exposure to novel conditions. Over these 6 hours of sleep deprivation there was an increase in I2-6 that was significant after 20 minutes of sleep deprivation and remained significantly elevated, with the exception of one 10-minute period, for the remaining 6 hours (Figure 5G). These gentle handling data demonstrate that sleep deprivation also induces a rapid and sustained increase in I2-6 in the absence of a novel environment. The ability of sleep deprivation to increase I2-6 in both the presence and absence of a novel environment is evidence that novel conditions are not a significant contributor to the I2-6 increases observed on the rotating wheel.
We also examined I2-6 using the interparietal lead located over the visual cortex. The pattern observed from this lead did not have the exponential shape observed in the frontal lead (Figures 4A and 4E). These results indicate that changes in I2-6 are either localized to, or more easily detected in, more frontal brain areas.
After 24 hours of forced wakefulness, the increase in I2-6 is significantly and inversely correlated with the increase in NREM SWA that occurred immediately following forced wakefulness (Figure 6A). Although NREM SWA is the most established marker of sleep homeostasis, it cannot be measured during the deprivation procedure. Thus, NREM SWA is not sufficient to evaluate the fast component of the I2-6 change that was revealed in smaller frequency ranges within 2-6 Hz (4-6 Hz, Figure 3D). Accordingly, we chose latency to NREM sleep onset as a marker of sleep homeostasis that would effectively assess this fast component. The change in I2-6 during the first hour of forced wakefulness was inversely correlated with latency to NREM sleep onset (Figure 6C). These data suggest that I2-6 is a marker of sleep homeostasis during time periods on the order of 1 hour to 1 day.
Spectral power in the 4-7 Hz range was also correlated with NREM SWA; however, this correlation did not reach statistical significance (Figure 6B). Power in this frequency band was also found to have a positive, but nonsignificant, correlation with latency to NREM onset (Figure 6D). This relationship indicates that power in the 4-7 Hz band is not a strong marker of sleep homeostasis after one hour. Thus, under our experimental conditions, spectral power showed a trend indicating that it is a potential marker of sleep homeostasis over 24 hours, but not over one hour.
During baseline recording, I2-6 exhibited a pattern of change not observed in either forced wakefulness or recovery. I2-6 was relatively high during the early light period then decreased to its lowest levels at the light-dark transition. From these low values I2-6 underwent a gradual increase throughout the dark period (Figure 8A). This contrasts with the pattern observed during recovery (Figure 8B). No significant change in I2-6 was observed during the 24-h recovery period. Notably, the decrease in I2-6 seen at the light-dark transition was absent. It is possible that the dissipation of sleep pressure occurring during recovery is not complete by the light-dark transition. Thus, the decrease in I2-6 occurring in baseline sleep may be masked during recovery by increased sleep pressure. This is supported by other studies demonstrating that sleep pressure (NREM SWA) continues to be increased at the light-dark transition following prolonged forced wakefulness.31,32 However, further investigation is required to examine the relationship between I2-6 and sleep pressure in recovery and baseline sleep.
In previous studies examining the electrophysiological correlates of sleep pressure during waking, meticulous attention was paid to the waking substate used in analysis. That is, these studies carefully differentiated between active waking wherein the animal is moving (grooming, eating, etc.) and quiet waking wherein the animal is still, but alert with eyes open. Quiet waking was found to provide the best markers of sleep pressure in these studies.19,26 A rotating wheel was not used to deprive sleep in these experiments. In the current study, quiet waking may be reduced by a continuous level of locomotor activity caused by the rotating wheel. This prediction is supported by our assessment of EMG activity; there were no significant changes in total EMG power across the 24 hours of forced wakefulness (Figure 5C). In order to further assess the contribution of quiet waking, we identified waking substates using EMG power. Overall, EMG power was not correlated with I2-6 across the sleep deprivation period (Figure 5A). Furthermore, when I2-6 was determined for epochs assigned to one of three waking substates (Figures 5B, 5D and 5F), no single substate appeared to be responsible for the pattern of wave incidence that we observed for all waking epochs (Figure 4A). Epochs in low (Figure 5B) and mid (Figure 5D) EMG levels did show significant increases in I2-6; however, these increases were slower than the I2-6 increase during all wake epochs and they were not sustained for the entire 24-h sleep deprivation period. These data indicate that I2-6 provides a putative marker for sleep pressure even when most of the period is active waking.
Existing evidence suggests that increasing durations of waking lead to quiescent periods in MUA that can be detected in the 2-6 Hz range of LFP recordings. These quiescent periods increase in number and grow to include more and larger cortical areas as the duration of wake increases.26 The current study provides evidence that I2-6 in the waking EEG also increases with the duration of wake. We also demonstrate a significant correlation between I2-6 and two established markers of sleep pressure, latency to NREM sleep onset and SWA. These data are strong evidence that I2-6 in the waking EEG reflects the synchronous transitions between states in cortical neurons. Thus, I2-6 may be an alternate method to measure sleep homeostasis and provide a less invasive method of measuring these synchronous transitions between states. Notably, the two-process model33,34 predicts, and empirical evidence from human studies suggests,11,35 that the homeo-static drive for sleep rises exponentially during waking. The exponential increase of I2-6 observed in this study seems to further support our hypothesis that I2-6 is a measure of sleep homeostasis. Furthermore, I2-6 may provide substantially better time resolution than spectral power. In future studies, this enhanced temporal resolution may allow a better understanding of the rates and patterns by which sleep pressure accumulates during waking. It is our hope that these enhancements will aid in the discovery of practical biomarkers of sleep pressure and insufficient daily sleep. Efforts to develop a “sobriety test” for sleepiness may benefit from a better understanding of the dynamics of sleep pressure accumulation, and potentially dissipation, during extended wakefulness.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by National Institutes of Health awards NS078410 (to Dr. Paul), NS055883 (to Dr. Benveniste), NS060659; Research Centers in Minority Institutions Grant G12-RR03034; National Center on Minority Health, Health Disparities Grant 5S21MD000101-09, and the National Science Foundation Center for Behavioral Neuroscience. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful for the expert technical assistance of Lennisha Pinckney and Nicole Williams.
REFERENCES
- 1.Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–95. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 2.Dijk DJ, Beersma DG, Daan S, Bloem GM, van den Hoofdakker RH. Quantitative analysis of the effects of slow wave sleep deprivation during the first 3 h of sleep on subsequent EEG power density. Eur Arch Psychiatry Neurol Sci. 1987;236:323–8. doi: 10.1007/BF00377420. [DOI] [PubMed] [Google Scholar]
- 3.Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207–19. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- 4.Dijk DJ, Brunner DP, Borbely AA. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990;258:R650–61. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- 5.Friedman L, Bergmann BM, Rechtschaffen A. Effects of sleep deprivation on sleepiness, sleep intensity, and subsequent sleep in the rat. Sleep. 1979;1:369–91. doi: 10.1093/sleep/1.4.369. [DOI] [PubMed] [Google Scholar]
- 6.Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993;626:190–9. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 7.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 8.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 9.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 10.Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–4. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- 11.Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–9. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 12.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: evidence for frequency-specific circadian and homeostatic influences. Neurosci Lett. 1997;239:121–4. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- 13.Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience. 2002;114:1047–60. doi: 10.1016/s0306-4522(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 14.Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 15.Cajochen C, Knoblauch V, Krauchi K, Renz C, Wirz-Justice A. Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. Neuroreport. 2001;12:2277–81. doi: 10.1097/00001756-200107200-00046. [DOI] [PubMed] [Google Scholar]
- 16.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. Am J Physiol. 1999;277:R1771–9. doi: 10.1152/ajpregu.1999.277.6.R1771. [DOI] [PubMed] [Google Scholar]
- 17.Borbely AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14:171–82. doi: 10.1016/0166-4328(84)90186-4. [DOI] [PubMed] [Google Scholar]
- 18.Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 19.Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Huber R, Deboer T, Tobler I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 2000;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- 21.Landolt HP, Retey JV, Tonz K, et al. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology. 2004;29:1933–9. doi: 10.1038/sj.npp.1300526. [DOI] [PubMed] [Google Scholar]
- 22.Contreras DS. Cellular basis of EEG slow rhythms: A study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–22. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noda H, Adey WR. Neuronal activity in the association cortex of the cat during sleep, wakefulness and anesthesia. Brain Res. 1973;54:243–59. doi: 10.1016/0006-8993(73)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Mukovski MC. Detection of active and silent states in neocortical neurons from the field potential signal during slow-wave sleep. Cereb Cortex. 2007;17:400–14. doi: 10.1093/cercor/bhj157. [DOI] [PubMed] [Google Scholar]
- 25.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–85. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 26.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–7. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen CC, Brecht M, Hahn TT, Sakmann B. Synaptic changes in layer 2/3 underlying map plasticity of developing barrel cortex. Science. 2004;304:739–42. doi: 10.1126/science.1096750. [DOI] [PubMed] [Google Scholar]
- 28.Ktonas PY, Gosalia AP. Spectral analysis vs. period-amplitude analysis of narrowband EEG activity: a comparison based on the sleep delta-frequency band. Sleep. 1981;4:193–206. doi: 10.1093/sleep/4.2.193. [DOI] [PubMed] [Google Scholar]
- 29.Bergmann BM, Mistlberger RE, Rechtschaffen A. Period-amplitude analysis of rat electroencephalogram: stage and diurnal variations and effects of suprachiasmatic nuclei lesions. Sleep. 1987;10:523–36. [PubMed] [Google Scholar]
- 30.Vyazovskiy VV, Olcese U, Lazimy YM, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–78. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. 2010;107:15939–44. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007;104:10697–702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 34.Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–83. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 35.Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207–19. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]