Abstract
Fundus-driven perimetry, commonly known as microperimetry is a technique for measuring visual field sensitivity, whilst simultaneously viewing the fundus. Here we review the technique, focussing on the MP-1 microperimeter (Nidek Instruments, Inc., Padua, Italy); we compare it to conventional static automated perimetry, emphasizing the importance of understanding the effects of the different stimulus conditions and data analyses, on the interpretation of microperimetry data. The clinical applications of the technique, in the evaluation of functional and structural changes that accompany retinal diseases, are illustrated by its use in patients with age-related macular degeneration, Stargardt disease, and retinitis pigmentosa. In addition, the advantages and limitations of the technique are summarised.
Keywords: microperimetry, perimetry, age-related macular degeneration, retinitis pigmentosa, Stargardt disease
INTRODUCTION
Perimetry, the quantitative evaluation of the visual field, was introduced into clinical medicine in 1856 by Albrecht von Graefe.1 Static automated perimetry has since been established as an important clinical tool in ophthalmology. However this form of conventional perimetry cannot be used to accurately evaluate function of the macular area of patients with retinal disease who have unstable and/or extrafoveal fixation. The technique of microperimetry (more appropriately termed fundus controlled or fundus-driven perimetry) in which perimetry is performed with simultaneous fundus viewing2–4 provides a solution. It allows for the quantification of visual sensitivity at any specific point on the retina with accurate test-retest reliability for the same point as well as accurate correlation to retinal pathology. These measurements have become increasingly important in patients with retinal disease due to the growing number of pharmacological and surgical interventions.
Early forms of microperimetry or fundus controlled perimetry were developed in the late 1970s. The scanning laser ophthalmoscope (SLO) was later implemented to enhance the imaging capability of fundus controlled perimetry5 and landmark-driven perimetry techniques.6, 7
The development of commercially available microperimeters, such as the MP-1 (Nidek Instruments, Inc., Padua, Italy), the OPKO Spectral OCT/SLO and Centervue Macular Integrity Assessment Technology (MAIA), has led to widespread use of microperimetry.8–13 These instruments allow for registration of fundus imaging with the visual field map, and make it possible to compare retinal morphology to visual function. The main advantage of these automated microperimeters is that they can be used to evaluate visual sensitivity in patients with eccentric and/or unsteady fixation; another advantage is that they can be used to quantify the location and stability of fixation, measurements that are relevant in the evaluation of disease progression.
Here we review the technique focussing on the MP-1 microperimeter, compare it to conventional static automated perimetry, emphasizing the importance of understanding the effects of the different stimulus conditions and data analyses, in the interpretation of microperimetry data. Additionally, we illustrate its utility in the evaluation of functional and structural changes that accompany retinal disease affecting the macula. It should be noted that in this review, we use the term “visual” rather than “retinal” when referring to function assessed with microperimetry, as it is a psychophysical technique, and the resulting measurements reflect the sensitivity of the visual system as a whole, rather than just the retina.
MICROPERIMETRY AND CONVENTIONAL STATIC PERIMETRY: SIMILARITIES AND DIFFERENCES
The MP-1 microperimeter presents stimuli on a liquid crystal display background. The mesopic background luminance, at 1.27cd/m2, allows for stimuli to be presented over a 2 log unit dynamic range (0 to 20dB), using a Goldmann III stimulus. The size and colour of the stimuli and fixation target may be varied and thresholds can be estimated using a “4-2 dB” or “4-2-1 dB” staircase strategy.
The MP-1 offers various stimulus patterns to test the central visual field. Clinicians however should be aware that the “10-2” pattern differs slightly from the “10-2” pattern available on the Humphrey Field Analyzer (HFA II 750, Carl Zeiss Meditec, Dublin, CA). For the HFA, the stimulus separation is fixed at 2°, whereas for the MP-1, the mean separation is 2.3° (±0.08°).14 However, a “10-2” pattern with a stimulus separation of 2° may be programmed into the MP-1, using the custom pattern option, which can include stimulus locations at retinal eccentricities between 0° and 22°.
The fundus image is viewed via an infrared fundus camera, and tracked throughout perimetric testing, whilst visual field data are mapped directly to the fundus image. Significant deviations in the patient’s fixation cause perimetric testing to be interrupted until fixation is restored. The MP-1 incorporates a colour fundus camera, the image from which may be registered to the infrared image, after perimetric testing. Alternative fundus images e.g. short-wavelength fundus autofluorescent images, may be imported to the instrument software for registration with the visual field data.
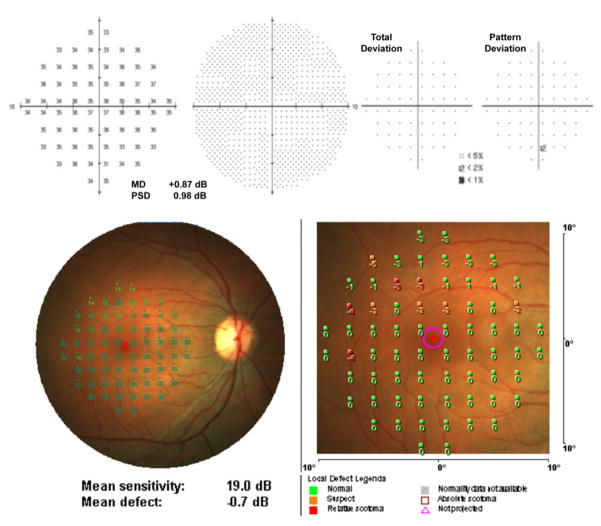
Sensitivity values can be displayed as numeric, symbolic or interpolated colour maps. The local defect map displays numeric deviations from the machine normative data and classifies abnormalities as “suspect”, “relative scotoma” or “absolute scotoma” (Figure 1). In addition to automated static perimetry assessments such as kinetic perimetry, fixation tasks and reading tasks, may also be performed on the MP-1.
Figure 1.
(Top) Humphrey Field Analyzer (10-2 pattern, SITA Standard) and (bottom) MP-1 microperimetry (10-2 pattern, 4-2 strategy) results from a normal individual (age 29).
In contrast, conventional static perimetry as implemented by the HFA presents stimuli upon a cupola background with a luminance of 10 cd/m2. The dynamic range is 5 log units. Gaze monitoring is performed via an infrared system that measures the distance between the first corneal reflex (Purkinje 1) and the pupil centre. Statistical analyses of visual field data include global indices, total and pattern deviation probability analyses, as well as disease progression analyses. On the HFA, thresholds can be estimated using the Swedish Interactive Threshold Algorithm (SITA). This group of algorithms minimises test duration whilst maintaining the quality of data compared to the Full Threshold strategy15 (similar to the “4-2” strategy), which uses smaller dB steps.
FACTORS THAT CAN AFFECT THE INTERPRETATION OF VISUAL FIELD RESULTS
Adaptation Levels
Despite similarities in spatial patterns, stimulus sizes and threshold procedures there are important differences between the MP-1 microperimeter and the HFA that need to be taken into account when interpreting the visual field results.14, 16, 17
A fundamental difference is the background luminance levels provided by the two instruments; stimuli are presented on a lower background level on the MP-1 than on the HFA. The systems mediating detection of the test lights may differ under the higher adapting background conditions of the HFA, from those mediating detection at the lower, mesopic background levels of the MP-1. For the MP-1, as the test lights are presented on a low mesopic background, thresholds may be mediated by a mixed rod-cone system response or by mainly a cone system response (Crossland MD, et al. IOVS 2012;53:ARVO E-Abstract 4822). The systems mediating detection will also depend on the extent and type of damage resulting from retinal disease, retinal eccentricity and on intricate photoreceptor interactions.18–20
We recently demonstrated that, in retinitis pigmentosa (RP), the MP-1 detected a greater sensitivity loss than the HFA, whereas in glaucoma the MP-1 detected less extensive loss.16 An explanation for this difference is due to the dimmer background luminance of the MP-1. In RP, a disease which primarily affects the photoreceptors, increment threshold measurements are dependent upon adaptation level, however in glaucoma, which primarily involves the inner retina or postreceptoral sites, increment thresholds are independent of adaptation level.21, 22 This suggests there should be improved performance of the MP-1 in the detection of defects due to RP.
Dynamic Range
Another key difference between the two instruments that can affect clinical interpretation of visual field results is the difference in dynamic range. The limited dynamic range of the MP-1, of 2 log units, revealed ceiling and floor effects in individuals with macular disease23–25 and in individuals with RP and glaucoma,16 thus restricting the ability to follow patients with defects either too severe or too subtle relative to the dynamic range of the MP-1. In healthy individuals, a flattened age-related sensitivity decline was found due to the significant ceiling effect in the MP-1 data.14, 26 Despite the ceiling effect in healthy individuals, a linear relationship was found between thresholds when comparing the MP-1 with the HFA and with the OPKO.17
Normative Data
In order to identify and quantify visual field defects normative data are essential. The MP-1 normative database was collected using a 77-point 10° circular grid in which the separation increases with eccentricity (4-2-1 threshold strategy).24 Data must be interpolated by the MP-1 local defect analysis, when stimulus patterns other than the 77-point grid are used. In a recent study, we applied a Bayesian model to the non-gaussian MP-1 data (10-2 pattern, 4-2 strategy, in healthy individuals) in order to derive the global indices and probability defects similar to those used by the HFA.14 We found that the local defect map of the MP-1, based on the machine normative data appeared to overestimate defects compared to those derived by the Bayesian model, when using the 10-2 pattern.14, 16
Another observation of MP-1 normal data that may affect interpretation is the increased variability and decreased sensitivities found in the superior retina.14, 26, 27 This effect is thought to be caused by an instrument artefact and can be corrected for mathematically (Woods RL et al. Apparent visual field defect found with Nidek MP-1 microperimeter is caused by an instrument artefact. Vision 2008 The 9th International Conference on Low Vision; Montreal, Canada).
ADVANTAGES AND LIMITATIONS OF THE MP-1
Microperimetry offers several advantages over conventional perimetry. The precise fundus tracking throughout perimetric testing is very useful in the study of subjects with unsteady or non-foveal fixation and the simultaneous quantification of fixation stability offers additional helpful information. The co-registration of the results to the fundus image allows for structural to functional comparisons, although these may be limited by the accuracy of potential mapping errors during image registration. The mesopic adapting background of the MP-1 may result in a greater sensitivity to defect detection in receptoral disease, however the dynamic range may limit the number of individuals that can be examined. In addition, the ceiling effect in visual sensitivities in normal individuals complicates the modelling of normative data in order to define abnormality. Additional possible confounders in the interpretation of abnormality are the increased variability in the superior retina and the potential for defocus, due to lack of provision for cylindrical refractive correction in patients with significant astigmatism. Further disadvantages are that the MP-1 does not allow for adaptive threshold-estimating strategies; in retinal disease, this lengthens examination time16, 28 and increases susceptibility to fatigue effects. Newer microperimeters have already addressed some of the limitations of the MP-1, such as providing a wider dynamic range and the ability to test dark adapted sensitivity, these and other improvements will serve to establish microperimetry as a very useful clinical instrument in ophthalmology.
ROLE OF MICROPERIMETRY IN EVALUATING FUNCTIONAL CHANGES ASSOCIATED WITH RETINAL DISEASE AFFECTING THE MACULA
To illustrate its role in a clinical setting we review the use of microperimetry in age-related macular degeneration (AMD), Stargardt disease, and RP.
Age-related Macular Degeneration
In early and late-stage AMD, microperimetry has proved to be a useful clinical tool. In early AMD it has been used to evaluate visual sensitivity over discrete areas of lesions such as drusen. For example, sensitivity was found to be reduced in early AMD;29 specifically it was decreased in areas of drusen and pigmentary change.30 The relationship between structural change using spectral domain optical coherence tomography (SD-OCT) and visual sensitivities assessed with microperimetry have also been examined.31, 32 The ability to co-register morphological features of neovascular AMD with microperimetric results has allowed for the evaluation of the differential impact of retinal changes on the corresponding sensitivity. Pigment epithelial detachments were associated with a greater variability in sensitivity than other morphological features of neovascular AMD, whilst locations of neovascular complex demonstrated sensitivities of 0dB.31 In a recent study of patients with early AMD we found that there was significant thinning of the outer segment layer and thickening and elevation of the retinal pigment epithelium and that there were significant relationships between outer segment layer thickness values and visual field loss (Figure 2).32 Thinning of the outer segment layer in early AMD is consistent with known structural changes associated with drusen, in terms of decreased photoreceptor density33 and deflected and shortened photoreceptor outer segments.34
Figure 2.
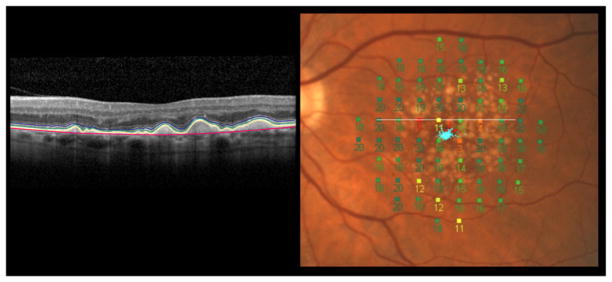
(Left) Segmented SD-OCT image from an eye with early AMD. A computer-aided manual segmentation procedure was used.45 The following layers have been demarcated (from outer to inner retina): Bruch’s membrane (red), retinal pigment epithelium (lower border: yellow and upper border: green) and inner segment ellipsoid band (blue). (Right) MP-1 microperimetry 10-2 visual field superimposed on a colour fundus photo. The white line corresponds to the SD-OCT scan.
In late-stage AMD features such as disrupted retinal pigment epithelium, absent photoreceptor integrity and macular oedema have been associated with severe sensitivity loss.31 Microperimetry has been used to quantify the loss in visual sensitivity over time, for example a longitudinal decline in mean sensitivity was found in individuals with geographic atrophy, when followed over a period of 2 years35 and in individuals with progressive atrophic macular disease with stable visual acuity, when followed over 1 year.25 Microperimetry has also been used as an outcome measure to demonstrate improved visual sensitivity following pharmaceutical therapy in AMD.36–38
Retinitis Pigmentosa
Microperimetry has been advocated as a potential outcome measure of macular function for clinical trials involving patients with RP.39 In a study which used red stimuli upon a red background (1 cd/m2) to avoid ceiling effects in patients with RP, near-normal sensitivities were observed in parafoveal regions while sensitivities decreased with increasing eccentricity.39
As in AMD, microperimetry has been used in studies comparing the relationships between retinal morphology and function.40–42 For example, in RP, rings of hyperautofluorescence are seen in short-wavelength fundus autofluorescence imaging. The inner border of the ring corresponds spatially to the lateral extent of the preserved inner segment ellipsoid (ISe) band aka the photoreceptor inner segment/outer segment junction. In the area outside the ring of hyperautofluorescence, visual sensitivity was reported to be markedly decreased or non-recordable.40, 41 It was relatively preserved inside the hyperfluorescent ring, and decreased within the region of hyperfluorescence (see examples in Figure 3).40 Mean sensitivity was significantly correlated with the extent of normal-appearing fundus autofluorescence inside the ring.40, 42
Figure 3.
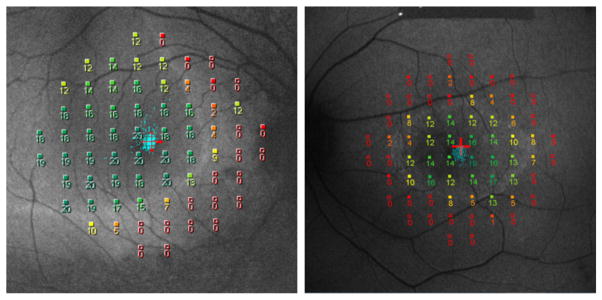
MP-1 microperimetry 10-2 results for 2 patients with retinitis pigmentosa superimposed on short-wavelength fundus autofluorescent images.
Stargardt Disease
In patients with ABCA4-associated retinal degeneration (STGD), microperimetry has also been recommended as a reliable outcome measure of macular function.39, 43, 44 Reduced visual sensitivity was noted in the parafovea and higher sensitivity in the perifovea, in patients with STGD, who underwent red-on-red microperimetry testing.39 No relationship was found between repeatability and sensitivity, and it was concluded that a single estimate of test-retest repeatability was appropriate to determine significant change in local visual function.39
In STGD, there is relative sparing of the peripapillary area and it has been suggested that this is a region of interest for monitoring the efficacy of treatment in this disease. A detailed structural and functional characterization of this retinal region is therefore of interest. A recent study compared the relationship between retinal structure and function of this region and the central macula, using SD-OCT and microperimetry.43 In patients with extramacular disease, there was a greater loss in visual sensitivity in the temporal compared to the nasal macula, which corresponded to photoreceptor layer thickness abnormalities; whereas in the peripapillary area in these patients, less abnormality was observed in the functional and structural findings.43 In another study comparing microperimetry results with SD-OCT imaging, STGD patients were classified into groups according to whether there was disorganisation or loss of the photoreceptor inner segment/outer segment junction at the fovea and it was found that visual sensitivity significantly differed between these groups.44
CONCLUSION
In summary there is an increasing amount of evidence supporting the usefulness of microperimetry both as a clinical instrument and as a research tool. The clinician should however be aware of the differences between microperimetry and conventional perimetry, differences in the manufacturer’s design that may affect testing conditions and therefore, the interpretation of results. The advantages over conventional perimetry include fundus tracking features and the co-registration of the perimetric results with fundus imaging.
Acknowledgments
Support: NIH Grants R01 EY02115 and R01 EY09076
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson CA, Wall M, Thompson HS. A history of perimetry and visual field testing. Optom Vis Sci. 2011;88(1):8–15. doi: 10.1097/OPX.0b013e3182004c3b. [DOI] [PubMed] [Google Scholar]
- 2.Inatomi A. A simple fundus perimetry with fundus camera. Doc Ophthalmol Proc Ser. 1979;19:359–62. [Google Scholar]
- 3.Kani K, Eno N, Abe K, Ono T. Perimetry under television ophthalmoscopy. Doc Ophthalmol Proc Ser. 1977;14:231–6. [Google Scholar]
- 4.Kani K, Ogita Y. Fundus controlled perimetry. Doc Ophthalmol Proc Ser. 1979;19:341–50. [Google Scholar]
- 5.Timberlake GT, Mainster MA, Webb RH, Hughes GW, Trempe CL. Retinal localization of scotomata by scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 1982;22(1):91–7. [PubMed] [Google Scholar]
- 6.Sunness JS, Schuchard RA, Shen NM, Rubin GS, Dagnelie G, Haselwood DM. Landmark-driven fundus perimetry using the scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 1995;36(9):1863–74. [PMC free article] [PubMed] [Google Scholar]
- 7.Rohrschneider K, Fendrich T, Becker M, Krastel H, Kruse FE, Volcker HE. Static fundus perimetry using the scanning laser ophthalmoscope with an automated threshold strategy. Graefes Arch Clin Exp Ophthalmol. 1995;233(12):743–9. doi: 10.1007/BF00184084. [DOI] [PubMed] [Google Scholar]
- 8.Cappello E, Virgili G, Tollot L, Del Borrello M, Menchini U, Zemella M. Reading ability and retinal sensitivity after surgery for macular hole and macular pucker. Retina. 2009;29(8):1111–8. doi: 10.1097/IAE.0b013e3181a3b832. [DOI] [PubMed] [Google Scholar]
- 9.Finger RP, Issa PC, Fimmers R, Holz FG, Rubin GS, Scholl HPN. Reading Performance Is Reduced by Parafoveal Scotomas in Patients with Macular Telangiectasia Type 2. Invest Ophthalmol Vis Sci. 2009;50(3):1366–70. doi: 10.1167/iovs.08-2032. [DOI] [PubMed] [Google Scholar]
- 10.Kiss CG, Barisani-Asenbauer T, Simader C, Maca S, Schmidt-Erfurth U. Central visual field impairment during and following cystoid macular oedema. Br J Ophthalmol. 2008;92(1):84–8. doi: 10.1136/bjo.2007.124016. [DOI] [PubMed] [Google Scholar]
- 11.Kriechbaum K, Prager F, Geitzenauer W, Benesch T, Schutze C, Simader C, et al. Association of Retinal Sensitivity and Morphology during Antiangiogenic Treatment of Retinal Vein Occlusion over One Year. Ophthalmology. 2009;116(12):2415–21. doi: 10.1016/j.ophtha.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Varano M, Tedeschi M, Oddone F, Perillo L, Coppe AM, Parravano M. Microperimetric retinal changes in myopic choroidal neovascularization treated with intravitreal ranibizumab. Retina. 2010;30(3):413–7. doi: 10.1097/IAE.0b013e3181bd2d23. [DOI] [PubMed] [Google Scholar]
- 13.Yodoi Y, Tsujikawa A, Nakanishi H, Otani A, Tamura H, Ojima Y, et al. Central Retinal Sensitivity After Intravitreal Injection of Bevacizumab for Myopic Choroidal Neovascularization. Am J Ophthalmol. 2009;147(5):816–24. doi: 10.1016/j.ajo.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Acton JH, Bartlett NS, Greenstein VC. Comparing the Nidek MP-1 and Humphrey Field Analyzer in normal subjects. Optom Vis Sci. 2011;88(11):1288–97. doi: 10.1097/OPX.0b013e31822b3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengtsson B, Olsson J, Heijl A, Rootzen H. A new generation of algorithms for computerized threshold perimetry, SITA. Acta Ophthalmol. 1997;75(4):368–75. doi: 10.1111/j.1600-0420.1997.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 16.Acton JH, Smith RT, Greenberg JP, Greenstein VC. Comparison between MP-1 and Humphrey visual field defects in glaucoma and retinitis pigmentosa. Optom Vis Sci. 2012;89(7):1050–8. doi: 10.1097/OPX.0b013e31825da18c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiple W, Lima VC, Prata TS, Greenstein VC, Rosen R. The physics and psychophysics of microperimetry. Optom Vis Sci. 2011;89(8):1182–91. doi: 10.1097/OPX.0b013e3182640c83. [DOI] [PubMed] [Google Scholar]
- 18.Stockman A, Sharpe LT. Into the twilight zone: the complexities of mesopic vision and luminous efficiency. Ophthalmic Physiol Opt. 2006;26(3):225–39. doi: 10.1111/j.1475-1313.2006.00325.x. [DOI] [PubMed] [Google Scholar]
- 19.Latch M, Lennie P. Rod-cone interaction in light adaptation. J Physiol. 1977;269(3):517–34. doi: 10.1113/jphysiol.1977.sp011912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frumkes TE, Sekuler MD, Barris MC, Reiss EH, Chalupa LM. Rod-cone interaction in human scotopic vision. 1. Temporal analysis. Vision Res. 1973;13(7):1269–82. doi: 10.1016/0042-6989(73)90202-2. [DOI] [PubMed] [Google Scholar]
- 21.Hood DC, Greenstein V. Models of the normal and abnormal rod system. Vision Res. 1990;30(1):51–68. doi: 10.1016/0042-6989(90)90127-7. [DOI] [PubMed] [Google Scholar]
- 22.Greenstein VC, Hood DC. The effects of light adaptation on L-cone sensitivity in retinal disease. Clin Vis Sci. 1992;7(1):1–7. [Google Scholar]
- 23.Chen FK, Patel PJ, Xing W, Bunce C, Egan C, Tufail AT, et al. Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. 2009;50(7):3464–72. doi: 10.1167/iovs.08-2926. [DOI] [PubMed] [Google Scholar]
- 24.Rohrschneider K, Bultmann S, Springer C. Use of fundus perimetry (microperimetry) to quantify macular sensitivity. Prog Retin Eye Res. 2008;27(5):536–48. doi: 10.1016/j.preteyeres.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Chen FK, Patel PJ, Webster AR, Coffey PJ, Tufail A, Da Cruz L. Nidek MP1 is able to detect subtle decline in function in inherited and age-related atrophic macular disease with stable visual acuity. Retina. 2011;31(2):371–9. doi: 10.1097/IAE.0b013e3181e46af3. [DOI] [PubMed] [Google Scholar]
- 26.Midena E, Vujosevic S, Cavarzeran F. Normal values for fundus perimetry with the microperimeter MP1. Ophthalmology. 2010;117(8):1571–6. doi: 10.1016/j.ophtha.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 27.Springer C, Bultmann S, Volcker HE, Rohrschneider K. Fundus perimetry with the micro perimeter 1 in normal individuals - Comparison with conventional threshold perimetry. Ophthalmology. 2005;112(5):848–54. doi: 10.1016/j.ophtha.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 28.Rohrschneider K, Springer C, Bultmann S, Volcker HE. Microperimetry - Comparison between the Micro Perimeter 1 and scanning laser ophthalmoscope - Fundus perimetry. Am J Ophthalmol. 2005;139(1):125–34. doi: 10.1016/j.ajo.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 29.Parisi V, Perillo L, Tedeschi M, Scassa C, Gallinaro G, Capaldo N, et al. Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina. 2007;27(7):879–90. doi: 10.1097/IAE.0b013e318042d6aa. [DOI] [PubMed] [Google Scholar]
- 30.Midena E, Vujosevic S, Convento E, Manfre A, Cavarzeran F, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91:1499–503. doi: 10.1136/bjo.2007.119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sulzbacher F, Kiss C, Kaider A, Eisenkoelbl S, Munk M, Roberts P, et al. Correlation of SD-OCT features and retinal sensitivity in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53(10):6448–55. doi: 10.1167/iovs.11-9162. [DOI] [PubMed] [Google Scholar]
- 32.Acton JH, Smith RT, Hood DC, Greenstein VC. The relationship between retinal layer thickness and the visual field in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53(12):7618–24. doi: 10.1167/iovs.12-10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson PT, Brown MN, Pulliam BC, Anderson DH, Johnson LV. Synaptic pathology, altered gene expression, and degeneration in photoreceptors impacted by drusen. Invest Ophthalmol Vis Sci. 2005;46(12):4788–95. doi: 10.1167/iovs.05-0767. [DOI] [PubMed] [Google Scholar]
- 34.Johnson PT, Lewis GP, Talaga KC, Brown MN, Kappel PJ, Fisher SK, et al. Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci. 2003;44(10):4481–8. doi: 10.1167/iovs.03-0436. [DOI] [PubMed] [Google Scholar]
- 35.Meleth AD, Mettu P, Agron E, Chew EY, Sadda SR, Ferris FL, et al. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol Vis Sci. 2011;52(2):1119–26. doi: 10.1167/iovs.10-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozdemir H, Karacorlu M, Senturk F, Karacorlu SA, Uysal O. Microperimetric changes after intravitreal bevacizumab injection for exudative age-related macular degeneration. Acta Ophthalmol. 2012;90(1):71–5. doi: 10.1111/j.1755-3768.2009.01838.x. [DOI] [PubMed] [Google Scholar]
- 37.Parravano M, Oddone F, Tedeschi M, Chiaravalloti A, Perillo L, Boccassini B, et al. Retinal functional changes measured by microperimetry in neovascular age-related macular degeneration treated with ranibizumab: 24-month results. Retina. 2010;30(7):1017–24. doi: 10.1097/IAE.0b013e3181cfd3c6. [DOI] [PubMed] [Google Scholar]
- 38.Prager F, Michels S, Simader C, Geitzenauer W, Schmidt-Erfurth U. Changes in retinal sensitivity in patients with neovascular age-related macular degeneration after systemic bevacizumab (avastin) therapy. Retina. 2008;28(5):682–8. doi: 10.1097/IAE.0b013e318161dc70. [DOI] [PubMed] [Google Scholar]
- 39.Cideciyan AV, Swider M, Aleman TS, Feuer WJ, Schwartz SB, Russell RC, et al. Macular function in macular degenerations: repeatability of microperimetry as a potential outcome measure for ABCA4-associated retinopathy trials. Invest Ophthalmol Vis Sci. 2012;53(2):841–52. doi: 10.1167/iovs.11-8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenstein VC, Duncker T, Holopigian K, Carr RE, Greenberg JP, Tsang SH, et al. Structural and functional changes associated with normal and abnormal fundus autofluorescence in patients with retinitis pigmentosa. Retina. 2012;32(2):349–57. doi: 10.1097/IAE.0b013e31821dfc17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popovic P, Jarc-Vidmar M, Hawlina M. Abnormal fundus autofluorescence in relation to retinal function in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2005;243(10):1018–27. doi: 10.1007/s00417-005-1186-x. [DOI] [PubMed] [Google Scholar]
- 42.Wakabayashi T, Sawa M, Gomi F, Tsujikawa M. Correlation of fundus autofluorescence with photoreceptor morphology and functional changes in eyes with retinitis pigmentosa. Acta Ophthalmol. 2010;88(5):e177–83. doi: 10.1111/j.1755-3768.2010.01926.x. [DOI] [PubMed] [Google Scholar]
- 43.Burke TR, Rhee DW, Smith RT, Tsang SH, Allikmets R, Chang S, et al. Quantification of peripapillary sparing and macular involvement in Stargardt disease (STGD1) Invest Ophthalmol Vis Sci. 2011;52(11):8006–15. doi: 10.1167/iovs.11-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Testa F, Rossi S, Sodi A, Passerini I, Di Iorio V, Della Corte M, et al. Correlation between photoreceptor layer integrity and visual function in patients with Stargardt disease: implications for gene therapy. Invest Ophthalmol Vis Sci. 2012;53(8):4409–15. doi: 10.1167/iovs.11-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of Receptor and Post-receptor Retinal Layers in Patients with Retinitis Pigmentosa Measured with Frequency-Domain Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2009;50(5):2328–36. doi: 10.1167/iovs.08-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]