Abstract
We aimed to investigate the effect of chronic radiation exposure associated with the Fukushima Daiichi Nuclear Plant accident on the testis from 2 bulls. Estimated dose of internal exposure in one bull was 0.7–1.2 mGy (134Cs) and 0.4–0.6 mGy (137Cs) and external exposure was 2.0 mGy (134Cs) and 0.8 mGy (137Cs) (196 days). Internal dose in the other was 3.2–6.1 mGy (134Cs) and 1.8–3.4 mGy (137Cs) and external dose was 1.3 mGy (134Cs) and 0.6 mGy (137Cs) (315 days). Sperm morphology and spermatogenesis were within normal ranges. 134, 137Cs radioactivity was detected but Cs was not detectable in the testis by electron probe microanalysis. Thus, adverse radiation-induced effects were not observed in bull testes following chronic exposure to the above levels of radiation for up to 10 months. Since we could analyse a limited number of testes, further investigation on the effects of ionizing radiation on spermatogenesis should be extended to more animals.
Radiation accidents can result in localized or whole-body exposure and both the internal and external deposition of radioactive materials. Clinical manifestations of radiation exposure depend on the extent of penetration and the dose absorbed by various tissues1. After the Great East Japan Earthquake on 11 March 2011, the Fukushima Daiichi Nuclear Power Plant (FNPP) accident led to a discharge of a tremendous amount of radioactive substances2,3. On 22 April 2011, the evacuation zone was set to a 20-km radius surrounding the FNPP, leaving approximately 3,400 cows, 31,500 pigs, and 630,000 chickens behind within the zone. On 12 May 2011, the Japanese government ordered Fukushima prefectural government to euthanize unleashed livestock within the evacuation zone. Abandoned animals now have formed an invaluable model for studying the effects of chronic radionuclide intake. A comprehensive assessment of the effect of long-term exposure to internally deposited radionuclides on surviving domestic animals in the evacuation area is therefore urgently needed for the benefit of the livestock industry, as well as for human health. Radiobiological data from the FNPP accident could help to develop a set of internationally harmonized measures to protect domestic animals in the event of a future nuclear or radiological emergency.
Exposure to a large dose of ionizing radiation can cause irreparable damage to multiple organ systems, particularly those with highly proliferative cells, such as the skin, the hematopoietic and gastrointestinal system4. The testis is a relatively radiosensitive organ5, composed of a series of spermatogenic cells such as stem cells, spermatogonia, spermatids, spermatocytes, and sperm. These different types of germ cells differ remarkably in their susceptibility to radiation-induced effects according to their level of reproductive activity6. The effect on reproductive organs and behaviour by chronic exposure to radionuclides is one of major concerns. Furthermore, radiation-induced genomic changes, occurring in germ cells may have hereditary effects, including carcinogenesis, congenital malformation and growth retardation in offspring. Data used for estimating the risk associated with exposure to ionizing radiation have been primarily obtained from epidemiological studies of survivors of the atomic bombing of Hiroshima and Nagasaki7,8, the Chernobyl nuclear accident9, and some complementary animal experiments10,11,12. However, reports of the effect of chronic low-dose radiation on livestock animals are limited.
We recently reported radionuclide deposition in organs of abandoned cattle following the FNPP accident. The deposition occurred in an individual radionuclide and in an organ-specific manner, and radioactive caesium (Cs) was detected in all the organs examined13. Discharge of 134Cs and 137Cs that emit γ- and β-rays is of primary concern, because they were released in a large amount and have a long half-life14. Thus, significant questions regarding the effect of long-term exposure to radioactive Cs on human health are now being raised. The current study focused on the effect of exposure to radioactive caesium on the reproductive organs of bulls that were abandoned in the 20-km FNPP evacuation zone from 12 March to 27 September 2011 and 24 January 2012. The aim of the present study was to investigate the effect of chronic radiation exposure on bull testes to 134Cs and 137Cs associated with the FNPP accident.
Results
Radioactivity concentration of 134Cs and 137Cs in male bull organs and blood for liquid
Organs, including testes, and peripheral blood (PB) were collected from 12 bulls and a male foetus at different sites within the 20-km FNPP evacuation zone on different dates. PB could not be obtained from any foetus examined because they were too small. The concentration of radionuclides in these tissues was then determined (Table 1). Radioactive concentrations of 134Cs and 137Cs were almost similar in all the organs and PB examined for liquid. We could measure the radioactivity concentration of 134Cs and 137Cs of the testis from 2 bulls and 1 foetus as listed in Table 2. Organ concentration of radioactive Cs was the highest in skeletal muscles among organs examined. Testicular concentrations of 134Cs and 137Cs for liquid were approximately 13- to 16-fold higher in bull 1 and 17- to 18-fold higher in bull 2 than PB concentrations for liquid. The foetal organs also showed deposits of both the two radionuclides.
Table 1. Radioactivity concentration of 134Cs and 137Cs of 12 bulls n organs and peripheral blood for liquid (Bq/kg).
| Organs | 134Cs | 137Cs |
|---|---|---|
| Longissimus thoracis muscle | 475 ± 175 | 480 ± 182 |
| Tensor fasciae late muscle | 382 ± 186 | 399 ± 195 |
| Diaphragm | 193 ± 18 | 186 ± 23 |
| Heart | 237 ± 104 | 233 ± 92 |
| Liver | 165 ± 66 | 175 ± 75 |
| Kidney | 253 ± 117 | 258 ± 118 |
| Lung | 279 ± 282 | 294 ± 297 |
| Spleen | 183 ± 46 | 185 ± 46 |
| Blood | 17 ± 9 | 18 ± 8 |
Data are presented as mean ± SD.
Table 2. Radioactivity concentration of 134Cs and 137Cs in organs and peripheral blood for liquid (Bq/kg).
| Bull | Organs | 134Cs | 137Cs |
|---|---|---|---|
| No.1 | Longissimus thoracis muscle | 284 ± 8 | 288 ± 7 |
| Tensor fasciae late muscle | 356 ± 7 | 370 ± 6 | |
| Diaphragm | 195 ± 5 | 205 ± 4 | |
| Liver | 171 ± 6 | 181 ± 5 | |
| Kidney | 251 ± 8 | 259 ± 7 | |
| Lung | 164 ± 7 | 173 ± 6 | |
| Spleen | 218 ± 7 | 210 ± 6 | |
| Testis | 195 ± 8 | 213 ± 6 | |
| Blood | 12 ± 1 | 16 ± 1 | |
| No.2 | Tensor fasciae late muscle | 999 ± 14 | 1050 ± 11 |
| Masseter muscle | 1177 ± 18 | 1234 ± 14 | |
| Tongue | 890 ± 9 | 948 ± 7 | |
| Heart | 656 ± 12 | 694 ± 10 | |
| Liver | 273 ± 8 | 276 ± 6 | |
| Kidney | 830 ± 15 | 897 ± 12 | |
| Lung | 537 ± 15 | 542 ± 11 | |
| Spleen | 257 ± 9 | 273 ± 7 | |
| Testis | 643 ± 23 | 661 ± 18 | |
| Blood | 38 ± 1 | 36 ± 1 | |
| Foetus | Longissimus thoracis muscle | 308 ± 8 | 336 ± 5 |
| Tensor fasciae late muscle | 383 ± 6 | 403 ± 5 | |
| Tongue | 322 ± 57 | 359 ± 28 | |
| Heart | 202 ± 9 | 232 ± 5 | |
| Liver | 163 ± 6 | 178 ± 7 | |
| Kidney | 166 ± 2 | 176 ± 6 | |
| Lung | 103 ± 6 | 111 ± 3 | |
| Spleen | 218 ± 6 | 203 ± 6 | |
| Submandibular gland | 187 ± 10 | 198 ± 10 | |
| Umbilical cord | 42 ± 2 | 44 ± 2 | |
| Testis | 187 ± 24 | 200 ± 20 | |
| Blood | ND* | ND* |
Data are presented as mean ± SD.
*ND: Not determined.
Calculated doses of 134Cs and 137Cs in bull
The average and maximum doses of internal exposure calculated are shown in Table 3. In bull 1, the estimate of the internal dose during 196 days from combined 134Cs and 137Cs was 1.1–1.8 mGy. In bull 2, the estimate of the internal dose for 315 days was 5.0–9.5 mGy. External exposure of bull 1 was 2.8 mGy and that of bull 2 was 1.9 mGy.
Table 3. Internal and external exposure from 134Cs and 137Cs in the bulls.
| Bull | Exposure | 134Cs | 137Cs |
|---|---|---|---|
| No.1 | Internal | 0.7–1.2* | 0.4–0.6* |
| (0.1–0.2**) | (0.09–0.1**) | ||
| External dose (mGy) | 2.0 | 0.8 | |
| No.2 | Internal | 3.2–6.1* | 1.8–3.4* |
| (0.4–0.8**) | (0.2–0.4**) | ||
| External dose (mGy) | 1.3 | 0.6 |
*Estimated average dose – maximal dose (mGy).
**Estimated average dose rate – maximal dose rate (μGy/h).
Spermatogenesis and sperm morphology are normal in irradiated bulls
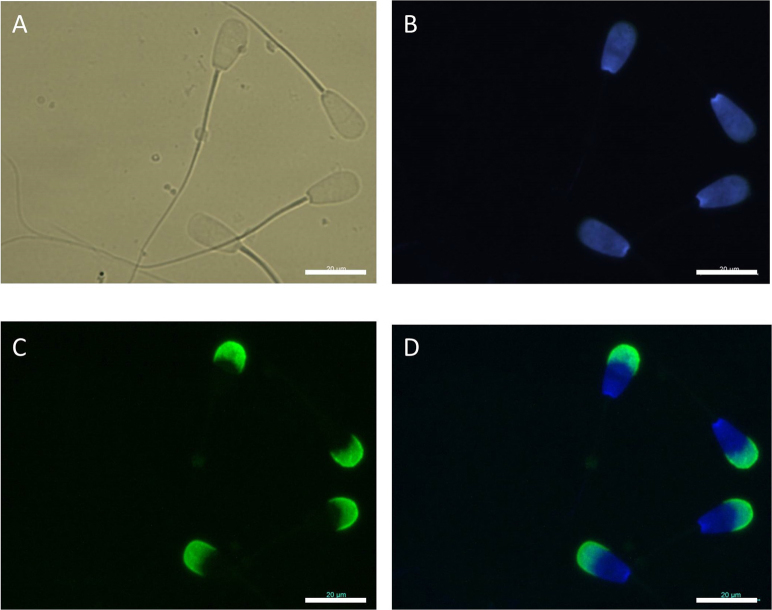
Effects of long-term radiation exposure on the number and morphology of several types of germ cells present in the testis were investigated. Nuclear and acrosome morphology of sperm was assessed by DAPI and FITC-PNA staining, respectively. The sperm acrosome was located between the nucleus and the plasma membrane, and in bull, sperm envelops two-thirds of the nucleus (Figure 1). We observed that the total number and morphology of epididymal sperm from irradiated bulls were normal. In addition, relative sizes of nuclei and acrosomes were normal in almost all sperm tested.
Figure 1. Bull epididymal sperm nuclei and acrosomes stained with 4′,6′-diamino-2′-phenylindole (DAPI) and fluorescein isothiocyanate (FITC-PNA).
A. Phase-contrast, B. DAPI-stained, C. FITC-PNA stained, and D. DAPI + FITC-PNA- stained images. Scale bar, 20 μm; magnification, 1000×.
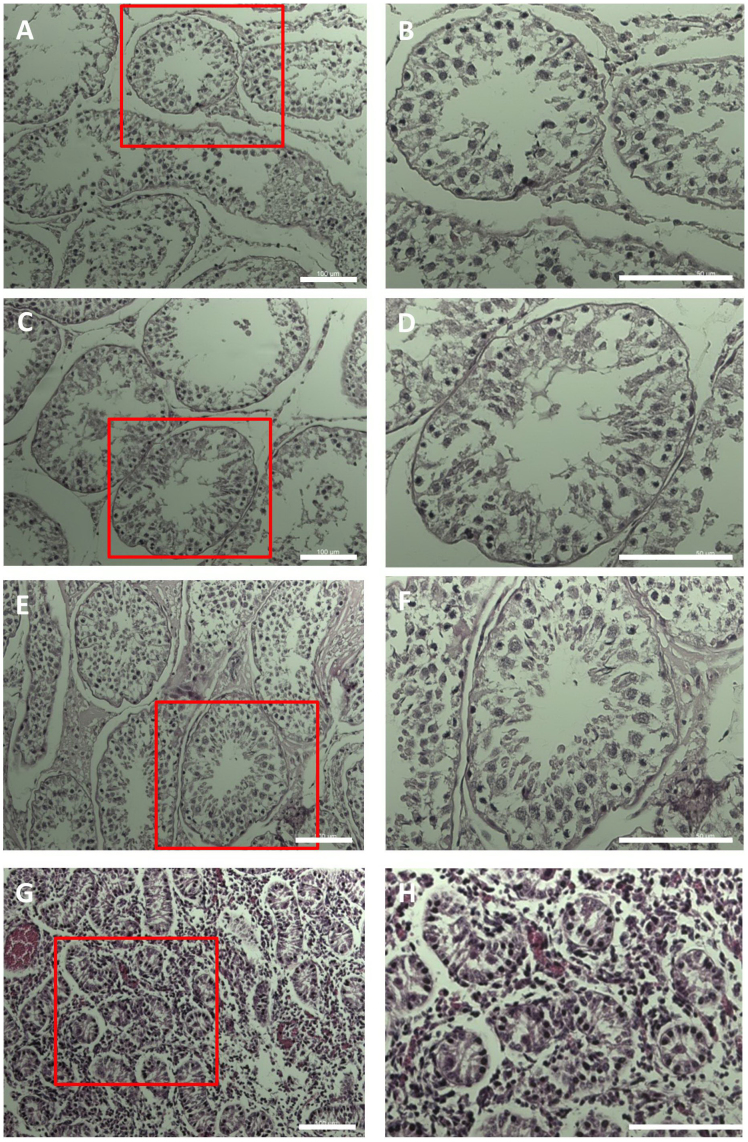
We next examined the morphology of a series of germ cells present in the testis under a microscope. Control seminiferous tubules showed normal spermatogenesis (Figure 2A, B). Interestingly, spermatogenesis was not disrupted in the testes from radiation-exposed animals compared to controls (Figure 2C, E), indicating that radiation exposure in the present study did not affect this process. In addition, there was no difference in the number of spermatogonia, spermatocytes, spermatids, and sperm in the testes of radiation-exposed animals compared with control testes (Figure 2D, F). HE staining of foetal testes confirmed that spermatogonial cells were present in the seminiferous tubules (Figure 2G, H).
Figure 2. Histological sections of the seminiferous tubules of bull testes.
A, B. Images of control bull testis. C–F. Images of bull exposed to radiation. G, H. Images of seminiferous tubules from foetal testes. Scale bar, 100 μm.
Alterations of micro-constituents in irradiated testes
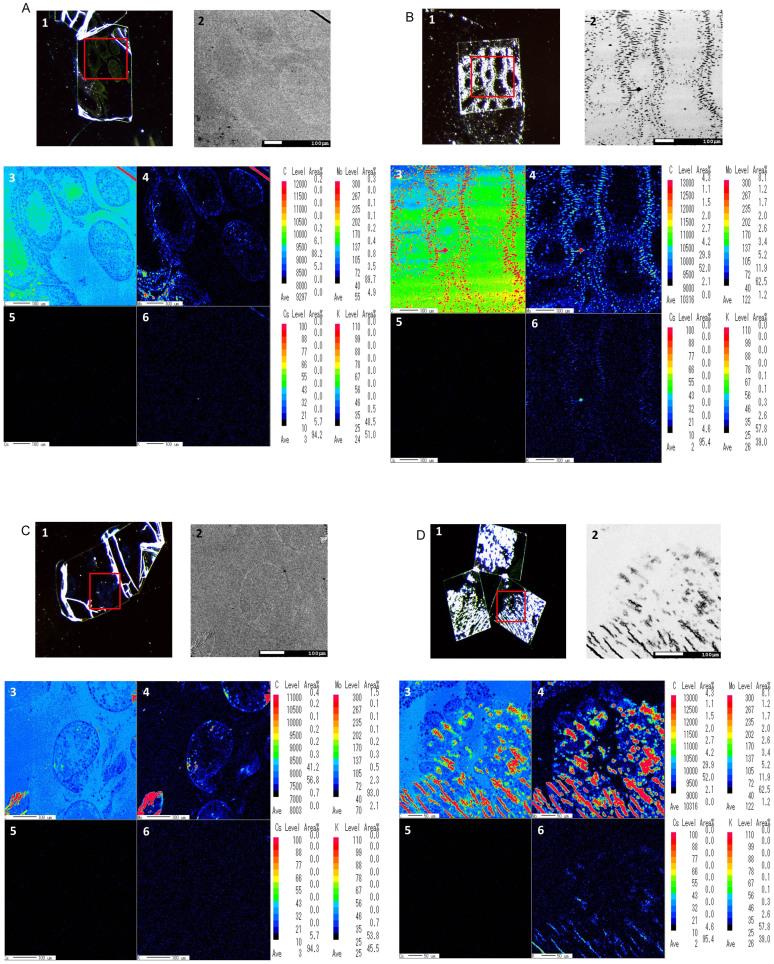
Figures 3A to 3D: 3,4,5, and 6 show phase maps indicating the concentrations of the micro-constituents carbon (C), molybdenum (Mo), potassium (K), and Cs, respectively. Colour imaging can rapidly and effectively facilitate the overall analysis of the composite structure: decreasing levels of metal distribution are indicated from red to black. In the control image, the C imaging result was shown as intermediate (light green) in the seminiferous tubules; however, Mo levels were low (blue) and K was not detected in germ cells (Figure 3A: 3, 4, and 6). Interestingly, in the testis of bull 1, C imaging result indicated high to intermediate levels (red and light green); however, it showed low levels of Mo (dark blue; Figure 3B: 3, 4). In contrast, similar expression patterns of C, Mo, and K were observed in the testes of both bull 2 and the control (Figure 3C: 3, 4, and 5). A comparison of foetal and control colour map imaging results revealed that C and Mo levels were higher expression patterns in the foetal testis (Figure 3D: 3, 4). However, Cs was not detected in any of the bull testes, including the foetal sample (Figure 3A–D: 5).
Figure 3. EPMA analysis of bull testis.
A. Control, B. Bull No. 1, C. Bull No. 2, D. Foetus. 1. Stereo-microscopy images of bull testis. 2. Composite backscattered electron microscopy images. 3. Secondary electron colour map image of C (carbon) regions. 4. Corresponding distribution of Mo (molybdenum) obtained for the same section. 5. X-ray colour-coded phase map of Cs (caesium). 6. Corresponding X-ray profiles for K (potassium). Scale bar, 100 μm (50 μm for Figure 3D: 3–6).
Since testicular Cs was not detectable by EPMA, we determined Cs concentration by ICP-MS in the control testis. We found that the mole ratio of radioactive Cs to stable Cs was at the order of 10−5 (Table 4).
Table 4. Testicular concentration of 133Cs, 134Cs and 137Cs.
| 134Cs | 137Cs | 133Cs | (134Cs + 137Cs)/133Cs | ||||
|---|---|---|---|---|---|---|---|
| mg/kg (×10−10) | mol/kg (×10−13) | mg/kg (×10−9) | mol/kg (×10−12) | mg/kg (×10−4) | mol/kg (×10−7) | (×10−5 fold) | |
| No. 1 | 40.6 | 0.30 | 66.6 | 0.49 | ND* | ND* | 1.6 |
| No. 2 | 134.0 | 1.00 | 206.6 | 1.51 | ND* | ND* | 5.1 |
| Foetus | 39.0 | 0.29 | 62.5 | 0.46 | ND* | ND* | 1.5 |
| Control | ND* | ND* | ND* | ND* | 41.7 | 0.31 | ND* |
Concentrations of radioactive Cs were calculated from the values in Table 2 using specific activity of 134Cs (4.8 × 10−10 Bq/mg) and of 137Cs (3.2 × 10−9 Bq/mg), respectively.
*ND: Not determined.
Discussion
The risk of external and internal radiation exposure to health is of great concern worldwide. Analysis of 14 species of birds common to Fukushima and Chernobyl revealed a negative effect of radiation on abundance, and the relationship was more strongly negative in Fukushima than in Chernobyl15. The effect of radiation on farm animals in the evacuation zone following the FNPP accident provides information about the health risks of livestock and can also be extrapolated to humans. Cattle are assumed to have received high doses of irradiation in radioactively contaminated environments, because they consume large amounts of vegetation and drinking water, resulting in accumulation of radionuclides in their bodies16 as well as external exposure from terrestrial radionuclides. This study was designed to provide insight into the biological response of bull testes to different doses of radiation following the FNPP accident.
Although various types of radionuclides were released into the environment, radioactive substances of major concern now are 134Cs and 137Cs because of their relatively long half-life. The internal dose varies in a more complex manner than the external dose depending on a number of factors such as soil-to-plant ratio, the distance to the nearest forest, dietary habits, water, air, and body burden17. These factors make it difficult to estimate chronic irradiation of wildlife under natural conditions18. We recently revealed that the levels of 134Cs and 137Cs were the same in all the organs examined and that concentration of radioactive Cs in each organ was significantly correlated with that in PB in an organ-specific manner; moreover, radionuclide deposition was strongly affected by the environment where the cattle were caught13. We calculated effective internal exposure from the concentration of radioactive caesium in organs and effective external dose from that in soil obtained in our study13. Since the testis is a relatively radiosensitive organ, we considered that radiation exposure would lead to changes in the morphology or the function of this organ. Activity concentration of radioactive caesium was 13- to 18-fold higher in the testis than in PB for liquid. Radioactivity concentration of caesium in the testis was about more than half of that in the skeletal muscle and the level was the same as in other organs. The ratio of external exposure to internal exposure in the testis of bull 1 was 1.6- to 2.5-fold and in that of bull 2 was 0.20- to 0.38-fold. This indicates that contribution of external exposure to total exposure is not negligible compared to internal exposure. Organ-specific concentration of radiocaesium in the foetus, compared with bull 1 and bull 2, of this study showed that radiocaesium is transferred to the foetus from its mother through the placenta and the deposition is organ dependent.
Spermatogenesis is a complex process of male germ cell proliferation and maturation from spermatogonia to spermatozoa in the seminiferous tubules of the testis. Spermatogonia are especially sensitive to irradiation; doses as low as 0.1 Gy are known to cause damage to these cells5. Carefully controlled small animal experiments have indicated that effects of low-dose radiation (LDR) and high-dose radiation (HDR) on the testis are different. Exposure to HDR has an inhibitory effect on genomic and cytological changes. However, exposure to LDR has a stimulatory effect on the metabolism, antioxidant capacity, proliferation, and maturation of male mouse germ cells. This phenomenon is termed hormesis19. Taki et al. analysed alterations in gene expression profiles in mouse testes continuously irradiated with HDR or LDR for 485 days. They reported that expression of genes categorized as ‘DNA metabolism’, ‘response to DNA damage’, and ‘DNA replication’ in the gene ontology are inversely correlated with dose rate to the testis20. Some studies have suggested that exposure to LDR leads to adaption to the subsequent exposure to HDR, known as adaptive responses24. Male mouse foetuses exposed to radiation at 5–6 weeks of pregnancy are at an increased risk of developing testicular cancer in mice21. The present study revealed that chronic exposure to radiation even from conception onwards did not affect foetal germ cell morphology.
EPMA is a powerful tool for detecting trace chemical elements in both single cells and tissues by measuring the characteristic X-ray spectra to specific elements within samples through the use of an accelerated electron beam22. However, we could not detect Cs by EPMA because the detection limit of EPMA is 100 ppm (0.1 g/kg)23. Therefore, we measured stable 133Cs in the testes of non-radio-contaminated control by inductively coupled plasma-mass spectrometer (ICP-MS) as a representative concentration of total Cs in the testis. The ratio of radioactive Cs to total Cs in the testis was calculated by (134Cs + 137Cs)/133Cs of control. The ratio was at 10−5 order, indicating that chemical toxicity of radioactive Cs is negligible. Considering that the bulls in this study were exposed to various radionuclides as well as 134Cs and 137Cs, we suggest that spermatogenesis occurred normally following long-term exposure to the levels of radioactive substances in the evacuation zone of the FNPP accident.
In conclusion, no adverse radiation-induced effects were observed in bull testes following chronic exposure to 3.6–4.6 mGy for bull 1 and 6.9–11.4 mGy for bull 2 for up to 10 months. These data improve our understanding of the effect of radiation on bull reproductive organs exposed to long-term radiation, and emphasize the importance of assessing the effect of nuclear accidents on reproduction. Dubrova et al. reported that the frequency of germ-line mini-satellite mutations among children born to parents who resided in constantly polluted areas (>250 kBq/m2) after the Chernobyl accident is about twice as high as in controls25. The air dose rate in the evacuation zone was the highest on March 15, 2011 and has been continuously declining mainly because the decay of 134Cs. The air dose rate now, 2 years after the FNPP accident, is three quarters of the highest levels26. Furthermore, marginal areas of the evacuation zone have been decontaminated. Therefore, it is now impossible to investigate more animals from the different annual dose field to cover the range up to the deterministic threshold for gonads. In order to develop a system of protection against radiation, the long-term impact of radiation on large animals within the FNPP evacuation zone needs to be continuously investigated. The study to compare whole genome sequences among bulls in the evacuation zone of FNPP, foetuses obtained by fertilization using sperm from bulls in the evacuation zone, and control bulls is underway in our laboratory. The negative results observed here may not represent the whole picture of radiation effects. Further investigation on the effect of ionizing radiation on spermatogenesis should be extended to more animals.
Methods
Ethics
This study is one of the national projects associated with the Great East Japan Earthquake and has been entirely endorsed and supported by the Japanese government through the Ministry of Education, Culture, Sports, Science and Technology, Japan. The Japanese government ordered Fukushima prefecture to euthanize cattle in the evacuation zone on 12 May 2011 to prevent radio-contaminated beef products from entering the human food chain. We obtained organs and testes from the euthanized bull collected by the combined unit of veterinary doctors belonging to the Livestock Hygiene Service Centre (LHSC) of Fukushima prefecture and those belonging to the Ministry of Agriculture, Forestry and Fisheries, Japan. These veterinarians euthanized the bulls by using the following method according to the Regulation for Animal Experiments and Related Activities at Tohoku University (Regulation No 122). The ear tag of the cattle identified the owner of each bull, and informed consent from the owner was obtained by the veterinary doctors of Fukushima prefecture. Control testes were obtained at a castration operation for fattening of a feeder. The procedure of euthanasia was entirely carried out by the veterinary doctors of LHSC. The Ethics Committee of Animal Experiments, Tohoku University approved this study.
Animals
We collected testes from 12 euthanized Japanese black beef bulls and 3 foetuses between 29 August 2011 and 24 January 2012. Those were 4 castrated and 2 calve and testes from 4 bulls were used for sperm collection. One foetus was female and another was too small. Therefore, we could analyse the testis samples from 2 euthanized bulls and 1 male foetus. Testes from bull 1 (date of birth, 21 October 2010) and a foetus (body length approximately 80 cm, approximately 8-month gestation) were collected in Kawauchi village located 15 km southwest of FNPP on 27 September 2011: the air dose rate was 0.5 μSv/h, radioactivity concentration in the soil was 134Cs 230 kBq/m2 and 137Cs 240 kBq/m2, and the time elapsed since the major release of radioactive caesium was 196 days. Testes from bull 2 (more than 1 year old) were collected in Naraha town located 17 km south of FNPP on 24 January 2012: the air dose rate was 2 μSv/h, radioactivity concentration in the soil was 134Cs 100 kBq/m2 and 137Cs 110 kBq/m2, and the time elapsed since the FNPP accident was 315 days. Testes from control Japanese black beef (11 months old) were collected from a non-radio-contaminated site in Miyagi prefecture on 7 March 2012.
Calculation method of internal and external dose rates
Dose rates of internal and external exposure to 134Cs and 137Cs were calculated according to the method of dosimetry assumption described in ICRP Publication 10827.
We adopted several assumptions for the estimation of internal dose rate. Average radioactivity concentration of the total body was calculated by the mean of radioactivity concentrations of organs determined. Legs were excluded from the object of evaluation. From the body length and the depth and width of the chest, we calculated the conversion coefficient of dose rate.
External dose rate was calculated based on the concentrations of 134Cs and 137Cs in soil, assuming certain distance between soil surface and the body mentioned above. The external exposures of 134Cs and 137Cs during certain duration were calculated as follows:
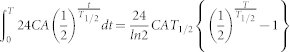 |
C; coefficient (μGy/h/(Bq/m2)), 2.04 × 10−6 for 134Cs and 7.3 × 10−7 for 137Cs.
A; radioactivity concentration in the soil (kBq/m2), 230 for 134Cs and 240 for 137C for bull 1, and 100 for 134Cs and 110 for 137C for bull 2 as of March 15, 2011.
T1/2; half-life (days), 754 for 134Cs and 11,016 for 137Cs.
T; period from March 15 to the day of capture (days), 196 for bull 1 and 315 for bull 2.
Measurements of radioactivity
Tissue radioactivity was determined by gamma-ray spectrometry using 3 HPGe detectors (ORTEC, USA). The relative efficiencies of the detectors were 30%, 20%, and 15% with a resolution at the 1.33 MeV line of 1.9 keV, 1.9 keV, and 1.8 keV, respectively. The time taken for the measurements varied from 3,600 s to 200,000 s, depending on the radioactivity of the samples. The detection efficiency was determined by measuring mixed sources of Eu-152 and 137Cs. An aliquot (200 μl) of the mixed source was diluted with appropriate amounts of water, and superabsorbent polymer was added to the mixture to obtain a gel standard source, which was used for mock samples that imitated organ tissues. Several different gel sources were prepared to cover a weight range of 0.5–130 g. Aqueous solutions of Eu-152 and 137Cs were used to determine the detection efficiency of liquid samples (e.g. blood cells, serum, and whole blood).
Blood samples
Although the blood samples collected were immediately placed into ice packs, on-site sample manipulation resulted in high variability. Most potassium is located within cells and caesium competes with potassium for active and passive membrane transport. In preliminary experiments using 6 bulls, the haematocrit was fairly constant (approximately 55%); however, the blood cell-to-plasma ratio for 137Cs varied from 1.5 to 5.2. We found that 137C radioactivity for liquid in whole blood reasonably reflected organ-specific radioactivity, and therefore adopted blood 137Cs radioactivity for liquid as a standard in this study.
Evaluation of epididymal sperm nucleus and acrosome integrity
The nucleus and acrosome of fresh epididymal sperm of bull were stained with 4′,6′-diamino-2′-phenylindole (DAPI; Invitrogen) and fluorescein isothiocyanate (FITC-PNA; Sigma), according to the procedure described by Yamashiro et al28.
Morphological assessment of testis cells
Testes were fixed in 10% paraformaldehyde, embedded in paraffin, and stained with haematoxylin and eosin (HE) according to standard protocols. Briefly, fixed testes were dehydrated in a series of different concentrations of alcohol, made transparent with dimethylbenzene, embedded in paraffin, and cut into 5-μm-thick sections, before staining.
Electron probe X-ray microanalysis (EPMA)
Chemical trace analysis of Cs (caesium), C (carbon), K (potassium), and Mo (molybdenum) in testicular structures was performed by JOEL (Tokyo, Japan) using a JXA-8230 SuperProbe Electron Probe Microanalyzer (JOEL) equipped for X-ray spectrometry and specially adapted for the examination of ultrathin sections. Specimens were loaded using a silicon wafer and focused by an electronic beam of 0.3 μm diameter. For analysis, the voltage of the electron microscope was set to 15 kV and the rate of the electron beam was 1 μA. The sections were viewed as secondary electron images, and mapping was performed using the chemical elemental mode.
Measurement of tissue 133Cs level
Tissue sample was digested with 2 ml of HNO3 (Wako) at 105°C for 6–18 h and dried. The processed samples were dissolved completely with 2% HNO3 to a final volume of 20 ml. An inductively coupled plasma-mass spectrometer (ICP-MS, ELAN DRC-e; PerkinElmer SCIEX, Concord, ON, Canada) was used for measurement of Cs.
Author Contributions
H.Y., Y.A., T.F., Y.K., Y.Ku., Mo.F., S.T., M.S., J.K., H.S., T.S., E.I., and M.F. collected specimens; H.Y., E.S., E.I., and M.F. designed the study; H.Y., Y.A., T.F., Y.K., I.K., S.Y., E.U., B.T., T.Y., and M.F. analysed the data; and H.Y. and M.F. wrote the paper.
Acknowledgments
We express our gratitude to the Iwaki Livestock Hygiene Service Centre in Fukushima Prefecture, especially to DVM. Yuji Kobayashi and livestock farmers in the 20-km FNPP evacuation zone. We would also like to thank Drs. M. Chiba, Y. Suzuki, A. Shumizu, A. Takahashi, H. Hotta, H. Tamura, and graduate and medical students of Tohoku University. This work was partly supported by a grant for Manabu Fukumoto of the Japan Society for the Promotion of Science, and the Emergency Budget for the Reconstruction of Northeastern Japan, MEXT, Japan, Discretionary Expense of the President of Tohoku University and Nippon Life Insurance Foundation supported this study. This work was also supported by the Programme for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry.
References
- Mettler F. A. & Voelz G. L. Major radiation exposure – What to expect and how to respond. Engl. J. Med. 346, 1554–1561 (2002). [DOI] [PubMed] [Google Scholar]
- Kinoshita N. et al. Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proc. Natl. Acad. Sci. 108, 19526–19529 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. et al. Isotopic evidence of plutonium release into the environment from the Fukushima DNPP accident. Sci. Rep. 2, 304 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute J. P. To survive radiation injury, remember your aPCs. Nat. Med. 18, 1013–1014 (2012). [DOI] [PubMed] [Google Scholar]
- Otala M. et al. Protection from Radiation-Induced Male Germ Cell Loss by Sphingosine-1-Phosphate. Biol. Reprod. 70, 759–767 (2004). [DOI] [PubMed] [Google Scholar]
- Liu G. et al. Effect of low-level radiation on the death of male germ cells. Radiat. Res. 165, 379–389 (2006). [DOI] [PubMed] [Google Scholar]
- Pierce D. A., Shimizu Y., Preston D. L., Vaeth M. & Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, part 1. Cancer: 1950–1990. Radiat. Res. 146, 1–27 (1996). [PubMed] [Google Scholar]
- Katayama H. et al. Reassessment of the cancer mortality risk among Hiroshima atomic-bomb survivors using a new dosimetry system, ABS2000D, compared with ABS93D. J. Radiat. Res. 43, 53–63 (2002). [DOI] [PubMed] [Google Scholar]
- Yablokv A. V., Nesterenko V. B. & Nesterenko A. V. Chernobyl: Consequences of the Catastrophe for People and the Environment. Annals of the New York academy of sciences Volume 1181, 1–327 (2009). [PubMed] [Google Scholar]
- Storer J. B., Mitchell T. J. & Fry R. J. M. Extrapolation of the relative risk of radiogenic neoplasms across mouse strains and to man. Radiat. Res. 114, 331–353 (1988). [PubMed] [Google Scholar]
- Shiragai A. et al. Estimation of the absorbed dose to mice in prolonged irradiation by low-dose rate gamma-rays from 137Cs sources. Radioisotopes. 46, 904–911 (1997). [Google Scholar]
- EPA. Cancer risk coefficients for environmental exposure to radionuclides. Federal Guidance Report No. 13, EPA 402–R–99–001, Office of Radiation and Indoor Air. (Environmental Protection Agency, Washington, DC (1999).
- Fukuda T. et al. Distribution of artificial radionuclides in the abandoned cattle in the evacuation zone of the Fukushima Daiichi Nuclear Power Plant. PloS One 8, e54312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E. Improving the scientific foundations for estimating health risks from the Fukushima incident. Proc. Natl. Acad. Sci. 108, 19447–19448 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller A. P. et al. Abundance of birds in Fukushima as judged from Chernobyl. Environ Pollut. 164, 36–39 (2012). [DOI] [PubMed] [Google Scholar]
- International Atomic Energy Agency (IAEA). Environmental consequences of the Chernobyl accident and their remediation: Twenty Years of experience. Report of the Chernobyl forum expert group ‘Environment’, Vienna, 2006.
- Thornberg C. et al. External and internal irradiation of a rural Bryansk (Russia) population from 1990 to 2000, following high deposition of radioactive caesium from the Chernobyl accident. Radiat. Environ. Biophys. 44, 97–106 (2005). [DOI] [PubMed] [Google Scholar]
- Vives I. et al. Inter-comparison of population models for the calculation of radiation dose effects on wildlife. Radiat. Environ. Biophys. 51, 399–410 (2005). [DOI] [PubMed] [Google Scholar]
- Liu G. et al. Apoptotic cell death induced by low-dose radiation in male germ cells: hormesis and adaptation. Crit. Rev. Toxicol. 37, 587–605 (2007). [DOI] [PubMed] [Google Scholar]
- Taki K. et al. Microarray analysis of differentially expressed genes in the kidneys and testes of mice after long-term irradiation with low-dose-rate γ–rays. J. Radiat. Res. 50, 241–252 (2009). [DOI] [PubMed] [Google Scholar]
- Shetty G., Comish P. B., Weng C. C., Matin A. & Meistrich M. L. Fetal radiation exposure induces testicular cancer in genetically susceptible mice. PLoS One 7, e32064 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorelov A., Budantsev G., Yu A. & Pogorelova V. N. Quantitative electron probe microanalysis of acetylcholinesterase activity in rat brain sections. J. Histochem. Cytochem. 41, 1795–1800 (1993). [DOI] [PubMed] [Google Scholar]
- Reed S. J. B. Quantitative trace analysis by wavelength dispersive EPMA. Mikrochim. Acta. 132, 145–151 (2000). [Google Scholar]
- ICRP. Recommendations of the international commission on radiological protection. ICRP Publication 60, Annals of the ICRP. Vol. 21, No. 1–3 (1991). [PubMed] [Google Scholar]
- Dubrova Y. E. et al. Further evidence for elevated human minisatellite mutation rate in Belarus eight years after Chernobyl accident. Mutant. Res. 281, 267–278 (1997). [DOI] [PubMed] [Google Scholar]
- Monitoring information of environmental radioactivity level. Nuclear Regulation Authority. http://radioactivity.nsr.go.jp/ja/contents/8000/7312/24/20130318air.pdf. [Google Scholar]
- ICRP. Environmental protection: the concept and use of reference animals and plants. ICRP publication 108 (2008). [Google Scholar]
- Yamashiro H. et al. Freezability of rat epididymal sperm induced by raffinose in modified Krebs-Ringer bicarbonate (mKRB) based extender solution. Cryobiology 55, 285–294 (2007). [DOI] [PubMed] [Google Scholar]