Abstract
T cell migration is crucial for an effective adaptive immune response to invading pathogens. Naive and memory T cells encounter pathogen antigens, become activated, and differentiate into effector cells in secondary lymphoid tissues, and then migrate to the site(s) of infection where they exert effector activities that control and eliminate pathogens. To achieve activation, efficient effector function, and good memory formation, T cells must traffic between lymphoid and non-lymphoid tissues within the body. This complex process is facilitated by chemokine receptors, selectins, CD44, and integrins that mediate the interactions of T cells with the environment. The expression patterns of these migration receptors (MR) dictate the tissues into which the effector T cells migrate and enable them to occupy specific niches within the tissue. While MR have been considered primarily to facilitate cell movement, we highlight how the heterogeneity of signaling through these receptors influences the function and fate of T cells in situ. We explore what drives MR expression heterogeneity, how this affects migration, and how this impacts T cell effector function and memory formation.
Keywords: T cell, heterogeneity, subset, migration, memory, cellular, immunity, motility
Introduction
In this review, we focus on T cell heterogeneity defined as the variation in the expression of migration receptors (MR), including chemokine receptors (CCRs), selectins, CD44, and integrins. The heterogeneity of effector T cells is evident during clonal expansion, differentiation, functional development, and transition to memory and is influenced by interactions with dendritic cells (DC), the tissue environment, and the inflammatory status. MR expression heterogeneity not only governs T cell migration to specific niches in the lymphoid tissues or in non-lymphoid sites of infection and inflammation, but also allows for contextual communication through engagement of the microenvironment at these sites to facilitate T cell differentiation and effector function.
Setting the Scene: Motility Enables the Initiation of the T Cell Response
For pathogens that enter and infect sites that interface with the external environment, such as the lung, skin, and intestinal tract, a complex interplay of innate and adaptive immune responses is required to achieve pathogen control and/or clearance. A functional T cell response is a crucial component of effective immunity to infection with pathogens and is influenced by a multitude of factors that include the microbe-specific mechanisms of host engagement, the site(s) of entry, and the virulence of the pathogen. Migration of T cells is crucial for an effective effector response to occur. For many intracellular pathogens, control and/or clearance depends upon the effector activities of CD8+ and CD4+ T cells that are programed to mediate type I responses characterized by cytolytic activity and production of cytokines, such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α. These responses are initiated primarily by antigen-presenting DC that have migrated from the site of infection to the secondary lymphoid organs (SLO). In lymph nodes (LN), DC compartmentalize into the T cell zone along fibroblastic reticular cells that express the CCR7 ligands, CCL19 and CCL21 (1, 2). In systemic infections, responses are also initiated in the spleen, where DC enter from the blood into the marginal zone of the red pulp, and from there migrate into the T cell zone of the white pulp in response to CCR7 ligands. DC become functional as antigen-presenting cells (APC) by upregulating MHC molecules and co-stimulatory molecules in response to innate signals that include pathogen-induced toll-like receptors and type I IFN, as well as by signaling via MR in response to migration (3). Many pathogen-specific aspects dictate and influence the innate response (4), which in turn impacts the extent of effector development in the adaptive response through effects on macrophages, DC, and other innate immune cells.
Highly motile naïve T cells that themselves are continuously recirculating interact with DC after entry into SLO. T cell migration from the blood into different tissues is regulated by a general cascade of events that is initiated by engagement of endothelial cells. This interaction consists of rolling and tethering, followed by firm adhesion, spreading/crawling, and finally extravasation (5). For naïve T cells, it is well-established that L-selectin (CD62L), CCR7, and LFA (CD18, β2) are the key molecules that regulate entry into LNs, which occurs through high endothelial venules that present their respective ligands, PNAd, CCL21, and ICAM-1. Within the LN, naïve T cells undergo cytoskeletal rearrangements that support motility, which in combination with CCR signaling facilitates directional motility toward DC (6). Naïve antigen-specific T cells engage antigen-bearing DC in a progression of serial encounters that result in the upregulation of activation markers such as CD69, CD25, and CD44. To ensure maximal activation and early retention in the LN, expression of the sphingosine-1-phosphate receptor 1 (S1P1) is initially reduced thereby lowering the ability of the T cell to be responsive to blood S1P levels [reviewed in Ref. (7)]. When T cells engage DC, their motility is greatly reduced and extended contacts between T cells and DC facilitate optimal activation and differentiation. During this process, MR expression profiles change dramatically. The impact of MR heterogeneity on T cell migrational capacity and effector function will be discussed in more detail below.
Induction of Migratory Heterogeneity during Effector T Cell Development
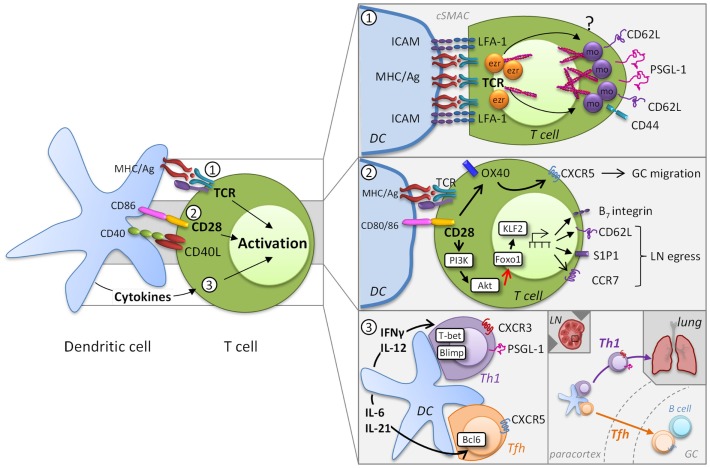
MR expression and ligation have important roles in T cell activation processes and thereby control effector T cell development in lymphoid tissues and non-lymphoid sites of infection and inflammation. At early stages in the development of effectors, MR heterogeneity is introduced by the differences in activation states of responding T cells that are largely determined by the degree of access to highly stimulatory APC. Here, we will explore the contribution of T cell receptor (TCR) affinity, the level of co-stimulation, and the cytokine milieu to MR expression and how they can influence MR heterogeneity during priming (Figure 1).
Figure 1.
Induction of migratory heterogeneity during priming. MR phenotype is impacted by TCR engagement, the level of co-stimulation, and the cytokine milieu (left). MR play a direct role in the formation of the immunological synapse, but TCR signaling subsequently impacts MR expression [panel 1, adapted from Ref. (8)]. Cytoskeletal rearrangements that involve the actin-binding ezrin (ezr), radixin, and moesin (mo) proteins are necessary for TCR signaling complex polarization. The integrin LFA-1 forms a ring surrounding the cSMAC that supports prolonged T cell-DC engagement, while other MR become excluded from the cSMAC. This process is possibly due to differential polarization of ezrin and moesin. Co-stimulatory signaling through molecules also contributes to the migratory heterogeneity of T cells (panel 2). For example, CD28 controls migration through upregulation of OX40, which is instrumental for CXCR5 expression and T cell localization to germinal centers (GC). In addition CD28/TCR signaling activates the PI3K/AKT pathway, which inhibits Foxo1 leading to decreased KLF2 expression. Differential co-stimulation can impact the levels of CD62L, CCR7, and S1P1 and thereby regulate the egress of T cells into the circulation. Cytokines released by DC promote specific transcriptional profiles that introduce further MR heterogeneity (panel 3). DC-derived IL-12 induces expression of the transcription factor T-bet and determines a CD4+ Th1 or CD8+ effector transcriptional program that results in part in the expression of CXCR3 and PSGL-1, which contribute to homing to peripheral sites. Alternatively, induction of the Tfh-associated transcription factor Bcl6 by IL-6 and IL-21 results in the downregulation of PSGL-1 and increased expression of CXCR5, which allows these cells to migrate from the T cell zones in the paracortex into GC.
TCR Signaling Induces MR Redistribution and De Novo MR Expression
Effective T cell activation depends on a dynamic interplay between TCR and peptide-MHC binding kinetics and the epitope density on the DC. MR play a direct role in the formation of the immunological synapse when engaging APC through actin rearrangement [reviewed in Ref. (8)]. Cytoskeletal rearrangements that involve the actin-binding ezrin, radixin, and moesin (ERM) proteins are necessary for T cell activation and IL-2 production (9, 10). To achieve TCR signaling complex polarization, MR that include CD44, CD62L, P-selectin glycoprotein (PSGL)-1, and ICAMs 1–3, become excluded from the central immunological synapse where the TCR and associated signaling molecules coalesce to form the central supramolecular activation cluster (cSMAC). The aforementioned MR become cross-linked to the actin cytoskeleton at the back of the cell, whereas the integrin LFA-1 forms a ring surrounding the cSMAC that supports prolonged T cell-DC engagement (11). Although little is known regarding the mechanisms by which T cells disengage from APC, once this occurs, T cells can interact with other cells via MR. For example, a recent study demonstrates that reciprocal ICAM-LFA interactions facilitate antigen-independent T cell–T cell synapses, which are required for the optimal generation of CD8+ effector T cell responses (12). These findings underscore that proper distribution and coordinated interplay of molecules in the TCR complex and MR are critical for full T cell activation.
The strength of TCR signaling represents a key checkpoint in the development of heterogeneous effector T cells. Strong stimulatory conditions lead to modulation of MR including upregulation of various integrins, CD44, and PSGL-1, with downregulation of CD62L and CCR7, a phenotype associated with the most highly functional effectors. This can, to some extent, be achieved by activating T cells with high affinity TCRs that can engage greater or distinct downstream signaling compared to low affinity TCRs (13, 14), and can result in proliferation versus cytokine production (13). However, for both CD4+ and CD8+ T cells, even individual naïve cell clones can give rise to a whole spectrum of heterogeneous effector phenotypes that can be influenced by antigen-dose and the duration of peptide-MHC binding for CD4+ T cells (15–17).
Co-Stimulation during Priming Impacts MR Heterogeneity
Another major contributor to T cell activation and modulation of MR expression is the availability of co-stimulatory signaling through molecules such as CD28 that are not only essential for T cell proliferation, differentiation, and survival, but also impact T cell migration (Figure 1, panel 2). The amount of co-stimulation received and the individual co-stimulatory receptor(s) involved in T cell activation can also contribute to the migratory heterogeneity of T cells responding to a pathogen. For example, while CD28 and CTLA4 engagement both increase β1 integrin-mediated adhesion (18, 19), ligation of these co-stimulatory markers has markedly different effects on T cell migration. Engagement of CD28 enhances the migrational capacity of T cells into inflamed tissue whereas ligation of CTLA4 inhibits T cell recruitment (20). However, the underlying mechanisms of these opposing effects are unknown. CD28 controls migration through upregulation of OX40, which is instrumental for CXCR5 expression and T cell localization to germinal centers (21). Co-stimulation by CD28 in combination with strong TCR signaling activates the PI3K/AKT pathway, a key regulator of glucose metabolism, which together with the mammalian target of rapamycin (mTOR) orchestrates the energy demands necessary for effector development (22). The PI3K/AKT and mTOR pathways not only regulate the necessary metabolic changes to the T cell, but also regulate their migratory capacity. Specifically, mTOR and Akt activation inhibits the Foxo family of transcription factors leading to decreased expression of kruppel-like factor 2 (KLF2), which in turn leads to the reduced expression of CD62L, the IL-7 receptor, and CCR7 (23–26). Importantly for the ability of cells to leave the LN, KLF2 also regulates the expression of S1P1 promoting the egress of T cells into the circulation (27).
CCR7 and CD62L expression may also be impacted by signaling via the co-stimulatory molecule ICOS, which is a member of the CD28-superfamily and expressed on activated T cells. Ligation of ICOS was demonstrated to down-regulate CCR7 and CD62L after activation, leading to more efficient migration of CD4+ T cells into the lungs and a reduced return to the LN (28).
Whether other co-stimulatory pathways link migratory capacity and T cell activation has not yet been defined, but activated T cells express high levels of multiple MR, and engagement of MR themselves can provide co-stimulation. Early participation of T cells in a response, when antigen levels and co-stimulatory signals are high, leads to loss of CD62L and increased expression of CD44, PSGL-1, S1P1, and the integrins LFA-1 and/or α4β1 (VLA-4) as well as other integrins that engage the extracellular matrix (ECM) (29). LFA-1 contributes to T cell responses by enhancing TCR signaling, production of IL-2, and proliferation, but also modulates T cell polarization and motility (11). α4β1 can also contribute to Th1 development by acting as a co-stimulatory molecule (30). In addition, pro-inflammatory cytokines can rapidly upregulate CD44 and its ability to bind its ligand, the ECM component hyaluronic acid (HA) (31). DC can synthesize and bind HA and during naïve T cell-DC interactions, ligation of CD44 by HA can enhance T cell cytokine production and proliferation by T cells that have received signals through the TCR (32). Similarly, ligand binding capacity is induced on PSGL-1 in response to T cell activation. In our own work, we find that CD4+ Th1 effector cells are heterogeneous with respect to expression of functional PSGL-1. Specifically, those cells with the highest levels of functional PSGL-1 are the most proliferative effectors with the greatest capacity for effector cytokine secretion and for cytotoxic activity (Bradley and Swain, unpublished observations).
DC-Derived Cytokines Drive Distinct MR Expression Profiles in T Cell Subsets
DC further influence T cell heterogeneity by virtue of the cytokines they secrete (3). As the immune response progresses, exposure of activated CD4+ T cells to polarizing cytokines leads to the development of subsets variously defined by function and transcription factor expression. These include the well-defined Th1, Th2, Th17, Tfh, and Treg subsets and the less understood Th9 and Th22 subsets, as well as CD4+ cells with cytotoxic activity, ThCTL (33). Cytokines released by DC work in part by inducing particular transcriptional profiles in T cells that promote expression of effector cytokines, but they also induce the expression of MR that allow for microanatomical localization (Figure 1, panel 2). For example, T cell activation is associated with the expression of the transcription factors Blimp-1 as well as T-bet. T-bet expression is sustained by DC-derived IL-12 and determines a CD4+ Th1 and CD8+ transcriptional program, which allows effector T cells to produce IFN-γ (33). T-bet induction is accompanied by the expression of CXCR3 (34) that binds the chemokines CXCL9, CXCL10, and CXCL11, which are frequently associated with inflammation. Asymmetric division can be associated with differential partitioning of T-bet (35, 36) and might also contribute to effector cell heterogeneity. This concept has been predominantly studied in terms of the generation of effector T cells that give rise to subsets memory cells that differentially express CD62L/CCR7 (see Role of Location and MR Signaling on the Effector to Memory T Cell Transition). Tfh cells develop concurrently with Th1 and CD8+ effector cells, regulated by induction of the transcription factor Bcl6 (with loss of Blimp-1) (37), which results in the downregulation of PSGL-1 (38), loss of CCR7, and increased expression of CTLA4 and CXCR5. The expression of CXCR5 results in increased responsiveness to CXCL13 and allows Tfh cells to migrate into B cell follicles in SLO (39).
Thus, changes in MR expression define unique phenotypes that can play critical roles in the migration and retention of effector cells. The combined outcome of TCR engagement of MHC in the context of peptide, signaling via co-stimulatory molecules, and access to polarizing cytokines determines in part the heterogeneity in MR expression profiles on T cell, which has a significant effect on their ability to exert their effector function.
Distinct MR Expression Profiles Determine Tissue-Specific Migration and Effector Function
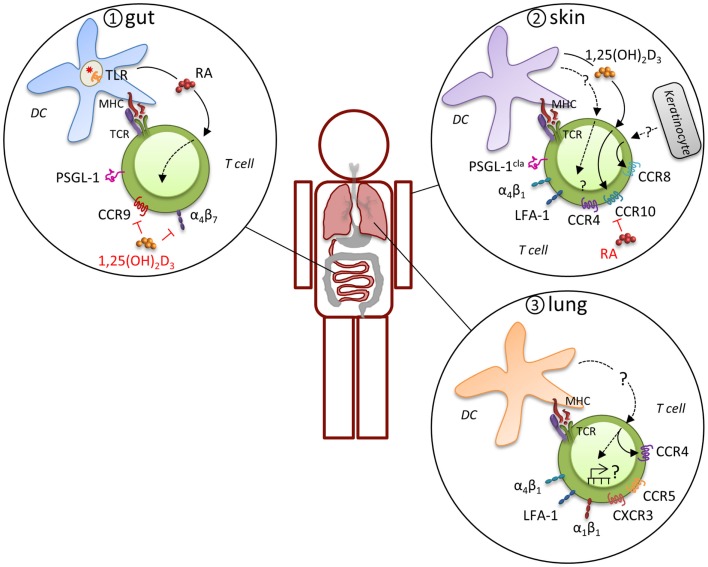
In general, effector T cells express a variety of MR that may be used alternatively or in combinations for migration and retention at sites of inflammation, which has led to the concept that expression of distinct MR combinations results in tissue-specific migration (Figure 2). For example, there is considerable evidence that a specialized program develops in T cells that are primed in gut-associated lymphoid tissues (mesenteric LN and Peyer’s patches) that directs migration into the gut mucosa. Effector T cells in these sites preferentially express functional PSGL-1, α4β7, and CCR9, which support migration via P-selectin, MAdCAM-1, and CCL25, respectively, through the post capillary venules of the small intestine to enter the lamina propria and intraepithelial compartment (40, 41). CD103 (integrin αE)-expressing DC impart the intestinal homing signature on T cells during priming, although CD103-positive and -negative DC populations can both prime T cells (42, 43). The induction of α4β7 and CCR9 is driven by retinoic acid, which is specifically synthesized by gut-associated CD103-positive DC, but not by CD103-negative or extra-intestinal DC (44). This capacity of intestinal CD103-positive DC to produce retinoic acid is acquired via toll-like receptor signaling, presumably in response to interactions with the microbiota (45). In addition to CD103-positive DC, the stroma of the mesenteric LN also expresses high levels of retinoic acid-producing enzymes and may have a considerable effect on the intestinal homing signature of T cells (46). T cell migration to the gut epithelium leads to TGF-β-dependent induction of αEβ7 on the T cells, which contributes to the retention of both CD4+ and CD8+ effectors by engagement of epithelial cell-expressed E-cadherin. Once an effector response subsides, residual CD8+ T cells can remain in the epithelial compartment as memory cells by this mechanism. CD4+ T cells are primarily maintained in the lamina propria, where they may require the continued local exposure to antigen for persistence.
Figure 2.
Induction of tissue-specific MR profile. Expression of distinct MR combinations results in tissue-specific migration. T cells that are primed in gut-associated lymphoid tissues preferentially express functional PSGL-1, α4β7, and CCR9, which support migration through the post capillary venules of the small intestine to enter the lamina propria and intraepithelial compartment (panel 1). CD103-expressing DC impart the intestinal homing signature on T cells during priming by expression of retinoic acid (RA) in response to microbiota-driven toll-like receptor (TLR) signaling. A different program of MR usage is induced during priming of naïve T cells in skin-draining LN (panel 2). T cells in the skin express the cutaneous lymphocyte antigen (CLA), an inducible carbohydrate modification of PSGL-1, CCR4, CCR8, CCR10, α4β1, and LFA-1, which mitigates their migration into the skin. Analogous to intestinal imprinting, CCR10, but not CCR4, expression is regulated by skin-draining DCs that synthesize the vitamin D3 metabolite, 1,25(OH)2D3. 1,25(OH)2D3 suppresses α4β7 and CCR9 expression and RA inhibits CCR4 and CCR10 expression. In addition, CCR8 expression is imprinted by epidermal keratinocytes, although the skin-specific factors that induce CCR8 remain unknown. Evidence for imprinting of T cells in the lung is limited, but recent evidence suggests that lung DC-activated T cell migrate more efficiently into the lung, which was attributed to CCR4, although other MR are likely to contribute (panel 3). Many lymphocytes in the lung express high levels of α4β1, α1β1, and LFA-1 and CD8+ T cells primed in the mediastinal LN are enriched for CCR5 and CXCR3 expression, suggesting a pulmonary profile driven by distinct molecular mechanisms.
A different program of MR usage is induced during priming of naïve T cells in skin-draining LN under inflammatory conditions. Both migrating Langerhans cells and conventional DC can present antigens to naïve T cells (47). Upon activation, these T cells express the cutaneous lymphocyte antigen, an inducible carbohydrate modification of PSGL-1 that preferentially binds to E-selectin on the endothelium of inflamed skin (48, 49). CD43 and CD44 expressed by T cells can also act to engage E-selectin. Although many chemokines can participate in migration of effector T cells to the skin depending on the characteristics of the infection or inflammation (50), the capacity for recruitment of effector T cells to the skin is primarily associated with expression of CCR8 and CCR10. While skin T cells express high levels of CCR4 and its ligands CCL17 and CCL22 are produced by epidermal keratinocytes and dermal fibroblasts, skin DC have not been shown to imprint CCR4 expression, which suggests that this MR is not exclusively a skin-homing molecule. Conversely, CCR8 expression on T cells is imprinted by epidermal keratinocytes, although the skin-specific factors that induce CCR8 remain unknown (51). CCR10 is also acquired by effector CD8+ T cells that migrate to skin in response to the epidermal chemokine CCL27. Analogous to intestinal imprinting, CCR10 expression is regulated by skin-draining DCs that synthesize the vitamin D3 metabolite, 1,25(OH)2D3 (52). While1,25(OH)2D3 suppresses α4β7 and CCR9 expression, it is not sufficient to induce a bona fide skin-homing program (i.e., other skin-homing receptors are not induced). Interestingly, retinoic acid inhibits the generation of T cells expressing these skin-homing receptors. The integrins LFA-1 and α4β1 can both mediate transmigration from the blood into the skin through engagement of their respective ligands, ICAM-1 and VCAM-1 (53). Expression of the αEβ7 integrin is also highly associated with skin-homing effector T cells and is thought to account for retention of effector cells that can make a transition to memory in situ [(54), see below].
While specific MR phenotypes that allow directed migration to the intestinal mucosa or skin have been identified, evidence for distinct MR expression patterns for the respiratory system has been lacking. In a recent paper, CD4+ and CD8+ T cells that were activated specifically by lung DC were shown to home more efficiently into the lung in response to inhaled antigen (55). CCR4 contributed to the lung-homing advantage of lung DC-activated CD4+ T cells and to reduced morbidity in vivo in response to influenza virus infection. However, this was a partial effect and it is likely that other MR are induced by lung DC. For example, we demonstrated that numerous genes involved in cell migration and motility are differentially expressed between lymphocytes responding in the SLO versus the lung (56) and many lymphocytes in the lung express high levels of α4β1, α1β1 (CD49a, VLA-1), and LFA-1 (57–61). In addition, CD8+ T cells primed in the lung-draining mediastinal LN by DC are enriched for CCR5 and CXCR3 expression compared to T cells primed in other sites (62) and these receptors regulate T cell effector responses and their contraction after infections with influenza viruses or Mycobacterium tuberculosis (Mtb) (63). Thus, it is tempting to speculate that some MR contribute to a specific pulmonary profile driven by distinct molecular mechanisms (see also Role of Location and MR Signaling on the Effector to Memory T Cell Transition).
A critical factor that can also impact T cell migration to particular sites is the changes to the site mediated by the pathogen and the inflammation initiated by the pathogen. Inflammation can alter the functional activity of the stromal cells thereby impacting recruitment from the circulation. After influenza virus infection, the ThCTL subset and ThCTL-associated genes are observed almost exclusively in the lung (56, 64, 65) suggesting a role for virus-specific inflammation in coordinating migration and function. For effective immunity to influenza virus, activated CD8+ T cells need to interact with pulmonary DC in an antigen-specific manner at the site of inflammation and while the impact on MR phenotype was not studied, this interaction could introduce additional heterogeneity (66). Following infection with Mtb, the infected site becomes dominated by macrophages before the arrival of T cells, thereby creating an environment very different from the normal lung (67). Indeed as disease, develops T cell migration into the lung and within the inflamed site appears to be regulated by the development of B cell follicles and the expression of CXCL13 (68, 69). We have demonstrated that within the chronically inflamed Mtb-infected tissue, activated effector T cells express high levels of α4β1 and that this is regulated by the presence of nitric oxide (70). This brings to prominence the idea that the inflammatory site, particularly the presence of inflammatory macrophages, can influence the expression of MR and thereby influence function and persistence of T cells.
It is important to bear in mind that tissue-specific responses can be re-programed by the exposure of T cells to alternative environments. For example, in a tumor model in which the site of implantation led to CD8+ T cell priming in different LN, different patterns of expression of integrins and selectin ligands were acquired by dividing cells in different sites that were associated with differential homing (71). Another study showed that CD4+ T cells primed in the gut-associated mesenteric LN by oral immunization acquired a skin-homing phenotype when transferred to recipients immunized subcutaneously (72). In addition to the significant role of DC imprinting on the acquisition of tissue-specific homing, DC trafficking itself has a substantial impact on T cell migratory heterogeneity [reviewed in Ref. (73)]. In conclusion, the function and location of effector T cells is governed by the expression of distinct MR combinations that are regulated by the environment. The combination of cell types and chemokines that are induced in response to inflammation can dramatically alter the environment within the tissue, which can significantly alter the MR phenotype and influence T cell migration. This suggests that phenotypes with respect to MR expression can be dynamically modulated for therapeutic purposes at particular sites to maximize vaccine efficacy and protection from invading pathogens.
MR Signaling Directs Movement of T Cells into and within Peripheral Tissues
MR-mediated localization of cells from blood into tissue is well-studied. Once activated in SLO, T cells are released into the circulation, a process that is regulated by the presence of high concentrations of S1P, in the lymphatic vessels (29). Once in the circulation, effector T cells are directed to sites of inflammation by activated endothelium. Pro-inflammatory mediators including TNF-α, IL-1, and IL-6 can rapidly activate endothelial cells to upregulate expression of selectins and CD44 (5). The latter binds to its ligand HA that is liberated as fragments from the ECM in response to inflammation [reviewed in Ref. (74)]. These molecules can be functionally redundant with respect to migration and individually regulate T cell encounters with the luminal surface of the vasculature through engagement of PSGL-1 or E-Selectin ligand. Chemokines, presented or secreted by endothelial cells as a consequence of the innate response, induce signaling via G protein-coupled receptors that upregulate the adhesiveness of integrins for their counter receptors that include ICAM-1, VCAM-1, and MadCAM-1 [reviewed in Ref. (5)].
Much less is known regarding the mechanisms that are engaged to mediate movement of cells within tissues. Upon extravasation, T cells must traverse the basement membrane, which is achieved by activation of matrix metalloproteases (MMPs). MMPs facilitate migration into and within the tissue interstitium by proteolytic degradation of ECM components. T cells predominantly produce the gelatinases MMP2 and MMP9 that cleave type IV collagen (75). We and others find that different T cell subsets express different amounts of MMP2 or MMP9 in vitro [Baaten, unpublished observation, (76, 77)]. It is unclear what induces activity in vivo and what causes their differential induction in these subsets, but the numerous cell–cell contact-dependent interactions via selectins, CCRs, and integrins during the extravasation process could impact MMP expression and/or activity. Initial interactions with the endothelial layer could upregulate MMP expression in T cells during transmigration (78–81). MMP2 and MMP9 production in T cells can be induced by ligation of integrins (αLβ2, α4β1, α5β1, αVβ1) following interaction with the endothelial layer (e.g., ICAM, VCAM) or constituents of the basement membrane (e.g., fibronectin) (80–86). Depending on the type of integrin, differential MMP expression can be induced in T cells (80). Thus, MR heterogeneity could impact T cell motility by regulating MMP activity. In addition, integrins are able to bind MMP2 and MMP9, which has been proposed to target catalytic activity to specific substrates within the pericellular space and assist cellular invasion (87, 88). However, the in vivo function of gelatinase activity for basement membrane degradation remains controversial and proteolytic cleavage of both ECM and non-ECM substrates can regulate migration and motility through other mechanisms (89). For example, chemotactic factors or MR expression could be altered by MMP-mediated proteolysis (90) thereby modulating T cell migration. More studies are required to identify how the molecular events that regulate rolling and tethering affect the phenotype and function of migrating T cells during transmigration.
To achieve directed migration, T cells undergo cytoskeletal rearrangements that allow for the formation of a polarized shape with a leading edge and a uropod that remains in contact with the ECM (91, 92). This process requires multiple signaling events that translate signals from membrane proteins into cell movement. Actin polymerization, regulated by members of the Rho GTPase family (93), controls forward movement of the cell at the leading edge where CCRs (94), integrins, and the TCR are located. The cell organelles, microtubule organizing center, and Golgi apparatus, as well as most MR, including PSGL-1, CD44, CD62L, and ICAM-1, become localized in the uropod. These receptors contribute to the signaling processes that regulate cell migration by binding ezrin and moesin, which anchor MR to the actin cytoskeleton and support the formation of clusters of MR. ERM proteins are activated through PI3K signaling to associate with the cytoplasmic domains of MR in the uropod, where they can serve as adaptor proteins for signal transduction. There can be considerable crosstalk between MR in response to signaling. For example, ligation of either PSGL-1 or CD44 can be associated with PI3K activation. Functional PSGL-1 also requires recruitment of ERM proteins to transmit signals necessary for adhesion (95). The activation status of ERM proteins is positively regulated by RhoA (93) that signals through the Rho-associated kinase, ROCK, which has critical roles in cell division and cell survival (96). Rho activation is associated contraction of the uropod that enables the forward movement of the cells (97).
The signaling pathways engaged by MR to mediate cell movement can profoundly modulate effector T cell responses and fitness for proliferation and survival. During T cell movement within tissues, signaling pathways continue to be dynamically regulated by engagement of the ECM via CD44 and integrins. For example, in a tumor model, Cd44-deficient CD8+ effector T cells could not maintain polarity and as a consequence had impaired cytotoxic activity (98). Recently, the integrin αV was demonstrated to be involved in context-dependent motility of Th1 cells in inflamed skin, and its expression was crucial for pathogen clearance (99). During migration within tissues T cells interact with other migrating and non-migrating cells including DC and tissue macrophages, which can present antigens and produce cytokines to enhance the effector T cell response. For instance, after influenza virus infection, DC elicit both cytotoxic activity and cytokine production that was dependent upon co-stimulation via CD80 and CD86, whereas engagement of epithelial cells that lack these receptors selectively stimulated cytotoxicity (100), revealing that effector cells can display heterogeneity depending on contextual cues.
Role of Location and MR Signaling on the Effector to Memory T Cell Transition
The hallmark of immunological memory is a quicker and more effective response upon re-encountering a pathogen. Migratory capacity can be intimately associated with effector function of responding cells at sites of inflammation that have the potential to become memory cells. MR contribute significantly to the functionality of memory T cells by enabling migration for surveillance, motility for in situ positioning, and signal interpretation from the ECM that enable cell survival. The timing of migration of naïve T cells into an inflamed LN can impact the formation of memory. Naïve T cells that enter LN later during the immune response are more likely to become central memory T cells (101, 102). These T cells receive less stimulation and consequently are less likely to adopt the migratory phenotype characterized by loss of CD62L and CCR7, and upregulation of integrins and PSGL-1. Strength of signaling also impacts the PI3K/Akt-mTOR pathway (see above) that can also influence the development of memory. Inhibition of this pathway limits differentiation of effector CD8+ T cells and results in greater generation of central memory CD8+ T cells during acute lymphocytic choriomeningitis virus (LCMV) infection (23). Memory T cells that preserve expression of CD62L and CCR7 maintain the migration patterns of naïve cells through SLO, allowing them to maximize the chance of finding antigen-specific DC early during a re-infection. These T cells also maintain expression of IL-2, a key cytokine for optimal memory cell responses [Swain, unpublished observations, (103, 104)].
Although the majority of effector T cells responding to pathogens in non-lymphoid tissues lack naïve T cell MR expression, CCR7 distinguishes T cells with the capacity to egress from non-lymphoid organs, such as the skin and lung (105, 106). While most effector cells in the lung die by apoptosis, some responding T cells can undergo regulated egress via the lymphatics and return to the circulation by engagement of the CCR7 ligand CCL21, which is expressed on lymphatic endothelium. Since effector CD4+ T cells responding to lung inflammation can be partitioned into subsets by expression of CCR7 (107), it is possible that these represent functionally distinct effectors with those capable of egress having a greater potential for survival and effector memory formation in SLO. Interestingly, the absence of CCR7 is associated with effector cell accumulation in the lung in allergic inflammation, suggesting that CCR7-regulated egress from the lungs may be an important factor in terminating the effector response (108). In addition, S1P may contribute to the regulation of egress from non-lymphoid tissues (109). However, some effector T cells express CD103 and are programed to persist in non-lymphoid sites. These effector memory T cells are known as tissue resident memory cells that are thought to provide site-specific protection against repeat infections (110). Their localization and persistence is mediated by MR to facilitate the accelerated response to re-encounter with an antigen.
Although MR expression predicts differences in migration of memory T cells to SLO and peripheral tissues, it is now becoming clear that this can have important functional consequences. In the skin, the anatomical localization and migration pattern of CD4+ and CD8+ memory T cells are substantially different following epicutaneous infection with herpes simplex virus as a result of distinct migratory programing in these two subpopulations (111). Whereas CD103+ CD8+ memory T cells remained in the epidermis as tissue resident memory cells, CD4+ effector memory T cells resided in the dermis, but were highly mobile and able to recirculate due to expression of E/P-selectin ligands. Whether this migration-linked T cell subset specialization is further impacted by T cell heterogeneity within the subsets is unknown. Nevertheless, signals received from the ECM via MR in situ can modulate the effector program to increase the potential for surviving the contraction phase of an immune response to enable transition to memory. For example, CD8+ and CD4+ T cells infiltrating the lung can differentially express α1β1 and α2β1 (CD49b, VLA-2), respectively (112). As a result, the different subsets localize to different areas within the lungs: CD8+ T cells that preferentially express α1β1 locate near the basement membranes of either the airways or blood vessels, whereas α2β1 CD4+ T cells primarily localize within the interstitial spaces. After influenza virus infection, α1β1-binding of collagen allows memory T cells to persist and function in the lungs (59, 113, 114). Similarly, signaling via CD44 is necessary for the survival of Th1 effector cells in lungs and the transition to memory (115). We and others find that acquisition of HA binding capacity by CD8+ T cells distinguishes effector cells with a greater potential to form memory cells [Bradley, unpublished observations, (116)].
It is becoming increasingly appreciated that microanatomical location can be critical in determining the signals that T cells receive during activation (86), but perhaps also during homeostasis and transition to memory. For example, T cells that are in proximity to the lymphatic vessels in the LN have ready access to antigens and pro-inflammatory cytokines that drain from sites of infection (117). In some viral and bacterial infections, notably systemic infections with LCMV (118) or Listeria monocytogenes (119), potent, but short-lived CD8+ effector T cells are distinguished by high expression of KLRG1 and low levels of IL-7Rα, whereas effectors with the reciprocal phenotype are better able to form memory cells. During acute infection with LCMV, short-lived effector CD8+ T cells are localized in the red pulp, whereas memory precursors preferentially locate to the white pulp in contact with stromal cells that produce IL-7 (120). During chronic LCMV infection, which leads to T cell exhaustion with sustained expression of the inhibitory receptor, programed death-1 (PD-1), both CD4+ and CD8+ effector T cells locate in the red pulp where their motility is impaired by PD-1 engagement of PD-L1 on macrophages and DC (121). However, in acute infections PD-1 plays a role in preventing terminal Th1 and CD8+ effector cell differentiation following pathogen clearance (122), highlighting that the integration of positive and negative signals is crucial for both the development of functional effector cells and memory. These examples underscore that a coordinated interplay of signaling via MR and the cytoskeleton is integral to T cell responses and survival within tissues and demonstrate the complexity and differences of CD4+ and CD8+ memory T cell responses and suggest that interactions with the ECM at the site of infection could have a significant impact on T cell function. By extension it is likely that MR signals play roles in the micro-location of effector and memory subsets that enable them to receive optimum homeostatic signals.
Conclusion
The ability of T cells to move around the body is crucial for their effector function and immunity to infection with pathogens. CD4+ and CD8+ T cells are heterogeneous and subsets have been defined based on the expression of CCRs, selectins, and integrins that are able to engage inflamed endothelium, the ECM, and cells of the innate immune system. For effector T cells that relocate to sites of inflammation, considerable plasticity in their responses can occur during the priming process with DC that imparts a tissue-specific MR phenotype to ensure efficient homing of activated T cells to the correct tissue. There are many mechanisms that contribute to control of T cell migration, motility, and egress within tissues and the coordinated interplay of these signals are only now becoming appreciated as crucial regulators of T cell function. MR are able to provide important contextual information from the ECM that allow for distinct transcriptional profiles that dictates not only cell polarity and interstitial motility, but also effector function, cell survival, and transition to, and maintenance of memory. Thus, MR heterogeneity has a direct impact on T cell immunity and protection from invading pathogens. The identification of additional distinct tissue profiles and the molecular mechanisms that control them have direct therapeutic relevance.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Koning JJ, Mebius RE. Interdependence of stromal and immune cells for lymph node function. Trends Immunol (2012) 33(6):264–70 10.1016/j.it.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 2.Forster R, Braun A, Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol (2012) 33(6):271–80 10.1016/j.it.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 3.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol (2013) 31:563–604 10.1146/annurev-immunol-020711-074950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tate MD, Schilter HC, Brooks AG, Reading PC. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol (2011) 24(2):77–88 10.1089/vim.2010.0118 [DOI] [PubMed] [Google Scholar]
- 5.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol (2007) 7(9):678–89 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- 6.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature (2006) 440(7086):890–5 10.1038/nature04651 [DOI] [PubMed] [Google Scholar]
- 7.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol (2005) 23:127–59 10.1146/annurev.immunol.23.021704.115628 [DOI] [PubMed] [Google Scholar]
- 8.Gordon-Alonso M, Veiga E, Sanchez-Madrid F. Actin dynamics at the immunological synapse. Cell Health Cytoskelet (2010) 2:33–47 [Google Scholar]
- 9.Ilani T, Khanna C, Zhou M, Veenstra TD, Bretscher A. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. J Cell Biol (2007) 179(4):733–46 10.1083/jcb.200707199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaffer MH, Dupree RS, Zhu P, Saotome I, Schmidt RF, McClatchey AI, et al. Ezrin and moesin function together to promote T cell activation. J Immunol (2009) 182(2):1021–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montoya MC, Sancho D, Vicente-Manzanares M, Sanchez-Madrid F. Cell adhesion and polarity during immune interactions. Immunol Rev (2002) 186:68–82 10.1034/j.1600-065X.2002.18607.x [DOI] [PubMed] [Google Scholar]
- 12.Gerard A, Khan O, Beemiller P, Oswald E, Hu J, Matloubian M, et al. Secondary T cell-T cell synaptic interactions drive the differentiation of protective CD8+ T cells. Nat Immunol (2013) 14(4):356–63 10.1038/ni.2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guy CS, Vignali KM, Temirov J, Bettini ML, Overacre AE, Smeltzer M, et al. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat Immunol (2013) 14(3):262–70 10.1038/ni.2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol (2012) 13(2):121–8 10.1038/ni.2190 [DOI] [PubMed] [Google Scholar]
- 15.Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, et al. Single naive CD4(+) T cells from a diverse repertoire produce different effector cell types during infection. Cell (2013) 153(4):785–96 10.1016/j.cell.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerlach C, Rohr JC, Perie L, van Rooij N, van Heijst JW, Velds A, et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science (2013) 340(6132):635–9 10.1126/science.1235487 [DOI] [PubMed] [Google Scholar]
- 17.Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity (2007) 27(6):985–97 10.1016/j.immuni.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 18.Schneider H, Valk E, da Rocha Dias S, Wei B, Rudd CE. CTLA-4 up-regulation of lymphocyte function-associated antigen 1 adhesion and clustering as an alternate basis for coreceptor function. Proc Natl Acad Sci U S A (2005) 102(36):12861–6 10.1073/pnas.0505802102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu Y, van Seventer GA, Ennis E, Newman W, Horgan KJ, Shaw S. Crosslinking of the T cell-specific accessory molecules CD7 and CD28 modulates T cell adhesion. J Exp Med (1992) 175(2):577–82 10.1084/jem.175.2.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirenda V, Jarmin SJ, David R, Dyson J, Scott D, Gu Y, et al. Physiologic and aberrant regulation of memory T-cell trafficking by the costimulatory molecule CD28. Blood (2007) 109(7):2968–77 [DOI] [PubMed] [Google Scholar]
- 21.Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med (1999) 190(8):1115–22 10.1084/jem.190.8.1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity (2009) 30(6):832–44 10.1016/j.immuni.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature (2009) 460(7251):108–12 10.1038/nature08155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol (2011) 12(4):295–303 10.1038/ni.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity (2009) 30(3):358–71 10.1016/j.immuni.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol (2009) 10(2):176–84 10.1038/ni.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature (2006) 442(7100):299–302 10.1038/nature04882 [DOI] [PubMed] [Google Scholar]
- 28.Moore TV, Clay BS, Cannon JL, Histed A, Shilling RA, Sperling AI. Inducible costimulator controls migration of T cells to the lungs via down-regulation of CCR7 and CD62L. Am J Respir Cell Mol Biol (2011) 45(4):843–50 10.1165/rcmb.2010-0466OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity (2008) 28(1):122–33 10.1016/j.immuni.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittelbrunn M, Molina A, Escribese MM, Yanez-Mo M, Escudero E, Ursa A, et al. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc Natl Acad Sci U S A (2004) 101(30):11058–63 10.1073/pnas.0307927101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ariel A, Lider O, Brill A, Cahalon L, Savion N, Varon D, et al. Induction of interactions between CD44 and hyaluronic acid by a short exposure of human T cells to diverse pro-inflammatory mediators. Immunology (2000) 100(3):345–51 10.1046/j.1365-2567.2000.00059.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mummert ME, Mummert D, Edelbaum D, Hui F, Matsue H, Takashima A. Synthesis and surface expression of hyaluronan by dendritic cells and its potential role in antigen presentation. J Immunol (2002) 169(8):4322–31 [DOI] [PubMed] [Google Scholar]
- 33.Jiang S, Dong C. A complex issue on CD4(+) T-cell subsets. Immunol Rev (2013) 252(1):5–11 10.1111/imr.12041 [DOI] [PubMed] [Google Scholar]
- 34.Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, et al. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity (2012) 37(6):1091–103 10.1016/j.immuni.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang JT, Ciocca ML, Kinjyo I, Palanivel VR, McClurkin CE, Dejong CS, et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity (2011) 34(4):492–504 10.1016/j.immuni.2011.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matheu MP, Teijaro JR, Walsh KB, Greenberg ML, Marsolais D, Parker I, et al. Three phases of CD8 T cell response in the lung following H1N1 influenza infection and sphingosine 1 phosphate agonist therapy. PLoS One (2013) 8(3):e58033. 10.1371/journal.pone.0058033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science (2009) 325(5943):1006–10 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol (2010) 185(1):313–26 10.4049/jimmunol.0904023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol (2011) 29:621–63 10.1146/annurev-immunol-031210-101400 [DOI] [PubMed] [Google Scholar]
- 40.Haddad W, Cooper CJ, Zhang Z, Brown JB, Zhu Y, Issekutz A, et al. P-selectin and P-selectin glycoprotein ligand 1 are major determinants for Th1 cell recruitment to nonlymphoid effector sites in the intestinal lamina propria. J Exp Med (2003) 198(3):369–77 10.1084/jem.20020691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature (2003) 424(6944):88–93 10.1038/nature01726 [DOI] [PubMed] [Google Scholar]
- 42.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med (2005) 202(8):1051–61 10.1084/jem.20040662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med (2005) 202(8):1063–73 10.1084/jem.20051100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity (2004) 21(4):527–38 10.1016/j.immuni.2004.08.011 [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Villablanca EJ, De Calisto J, Gomes DC, Nguyen DD, Mizoguchi E, et al. MyD88-dependent TLR1/2 signals educate dendritic cells with gut-specific imprinting properties. J Immunol (2011) 187(1):141–50 10.4049/jimmunol.1003740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med (2008) 205(11):2483–90 10.1084/jem.20080039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Igyarto BZ, Kaplan DH. Antigen presentation by Langerhans cells. Curr Opin Immunol (2013) 25(1):115–9 10.1016/j.coi.2012.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature (1997) 389(6654):978–81 10.1038/40166 [DOI] [PubMed] [Google Scholar]
- 49.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood (2011) 118(26):6743–51 10.1182/blood-2011-07-343566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCully ML, Moser B. The human cutaneous chemokine system. Front Immunol (2011) 2:33. 10.3389/fimmu.2011.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCully ML, Ladell K, Hakobyan S, Mansel RE, Price DA, Moser B. Epidermis instructs skin homing receptor expression in human T cells. Blood (2012) 120(23):4591–8 10.1182/blood-2012-05-433037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol (2007) 8(3):285–93 10.1038/ni1433 [DOI] [PubMed] [Google Scholar]
- 53.Grabbe S, Varga G, Beissert S, Steinert M, Pendl G, Seeliger S, et al. Beta2 integrins are required for skin homing of primed T cells but not for priming naive T cells. J Clin Invest (2002) 109(2):183–92 10.1172/JCI11703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A (2012) 109(18):7037–42 10.1073/pnas.1202288109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mikhak Z, Strassner JP, Luster AD. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J Exp Med (2013) 210(9):1855–69 10.1084/jem.20130091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strutt TM, McKinstry KK, Kuang Y, Bradley LM, Swain SL. Memory CD4+ T-cell-mediated protection depends on secondary effectors that are distinct from and superior to primary effectors. Proc Natl Acad Sci U S A (2012) 109(38):E2551–60 10.1073/pnas.1205894109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell JJ, Brightling CE, Symon FA, Qin S, Murphy KE, Hodge M, et al. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol (2001) 166(4):2842–8 [DOI] [PubMed] [Google Scholar]
- 58.Clark JG, Mandac-Dy JB, Dixon AE, Madtes DK, Burkhart KM, Harlan JM, et al. Trafficking of Th1 cells to lung: a role for selectins and a P-selectin glycoprotein-1-independent ligand. Am J Respir Cell Mol Biol (2004) 30(2):220–7 10.1165/rcmb.2003-0208OC [DOI] [PubMed] [Google Scholar]
- 59.Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity (2004) 20(2):167–79 10.1016/S1074-7613(04)00021-4 [DOI] [PubMed] [Google Scholar]
- 60.Thatte J, Dabak V, Williams MB, Braciale TJ, Ley K. LFA-1 is required for retention of effector CD8 T cells in mouse lungs. Blood (2003) 101(12):4916–22 10.1182/blood-2002-10-3159 [DOI] [PubMed] [Google Scholar]
- 61.Wolber FM, Curtis JL, Maly P, Kelly RJ, Smith P, Yednock TA, et al. Endothelial selectins and alpha4 integrins regulate independent pathways of T lymphocyte recruitment in the pulmonary immune response. J Immunol (1998) 161(8):4396–403 [PubMed] [Google Scholar]
- 62.Ferguson AR, Engelhard VH. CD8 T cells activated in distinct lymphoid organs differentially express adhesion proteins and coexpress multiple chemokine receptors. J Immunol (2010) 184(8):4079–86 10.4049/jimmunol.0901903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohlmeier JE, Reiley WW, Perona-Wright G, Freeman ML, Yager EJ, Connor LM, et al. Inflammatory chemokine receptors regulate CD8(+) T cell contraction and memory generation following infection. J Exp Med (2011) 208(8):1621–34 10.1084/jem.20102110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol (2012) 86(12):6792–803 10.1128/JVI.07172-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strutt TM, McKinstry KK, Marshall NB, Vong AM, Dutton RW, Swain SL. Multipronged CD4(+) T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol Rev (2013) 255(1):149–64 10.1111/imr.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med (2008) 205(7):1635–46 10.1084/jem.20080314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol (2009) 27:393–422 10.1146/annurev.immunol.021908.132703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BA, Fountain JJ, et al. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol (2011) 187(10):5402–7 10.4049/jimmunol.1101377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khader SA, Rangel-Moreno J, Fountain JJ, Martino CA, Reiley WW, Pearl JE, et al. In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J Immunol (2009) 183(12):8004–14 10.4049/jimmunol.0901937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pearl JE, Torrado E, Tighe M, Fountain JJ, Solache A, Strutt T, et al. Nitric oxide inhibits the accumulation of CD4+CD44hiTbet+CD69lo T cells in mycobacterial infection. Eur J Immunol (2012) 42(12):3267–79 10.1002/eji.201142158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, et al. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity (2005) 22(2):175–84 10.1016/j.immuni.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 72.Oyoshi MK, Elkhal A, Scott JE, Wurbel MA, Hornick JL, Campbell JJ, et al. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J Clin Invest (2011) 121(6):2210–20 10.1172/JCI43586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Villablanca EJ, Russo V, Mora JR. Dendritic cell migration and lymphocyte homing imprinting. Histol Histopathol (2008) 23(7):897–910 [DOI] [PubMed] [Google Scholar]
- 74.Baaten BJ, Tinoco R, Chen AT, Bradley LM. Regulation of antigen-experienced T cells: lessons from the quintessential memory marker CD44. Front Immunol (2012) 3:23. 10.3389/fimmu.2012.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leppert D, Waubant E, Galardy R, Bunnett NW, Hauser SL. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol (1995) 154(9):4379–89 [PubMed] [Google Scholar]
- 76.Abraham M, Shapiro S, Karni A, Weiner HL, Miller A. Gelatinases (MMP-2 and MMP-9) are preferentially expressed by Th1 vs. Th2 cells. J Neuroimmunol (2005) 163(1–2):157–64 10.1016/j.jneuroim.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 77.Oviedo-Orta E, Bermudez-Fajardo A, Karanam S, Benbow U, Newby AC. Comparison of MMP-2 and MMP-9 secretion from T helper 0, 1 and 2 lymphocytes alone and in coculture with macrophages. Immunology (2008) 124(1):42–50 10.1111/j.1365-2567.2007.02728.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aoudjit F, Potworowski EF, St-Pierre Y. Bi-directional induction of matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 during T lymphoma/endothelial cell contact: implication of ICAM-1. J Immunol (1998) 160(6):2967–73 [PubMed] [Google Scholar]
- 79.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity (2007) 26(2):227–39 10.1016/j.immuni.2006.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yakubenko VP, Lobb RR, Plow EF, Ugarova TP. Differential induction of gelatinase B (MMP-9) and gelatinase A (MMP-2) in T lymphocytes upon alpha(4)beta(1)-mediated adhesion to VCAM-1 and the CS-1 peptide of fibronectin. Exp Cell Res (2000) 260(1):73–84 10.1006/excr.2000.5002 [DOI] [PubMed] [Google Scholar]
- 81.Moore C, Shen XD, Gao F, Busuttil RW, Coito AJ. Fibronectin-alpha4beta1 integrin interactions regulate metalloproteinase-9 expression in steatotic liver ischemia and reperfusion injury. Am J Pathol (2007) 170(2):567–77 10.2353/ajpath.2007.060456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano-Marquez A, et al. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood (1999) 94(8):2754–66 [PubMed] [Google Scholar]
- 83.Kambara C, Nakamura T, Furuya T, Migita K, Ida H, Kawakami A, et al. Vascular cell adhesion molecule-1-mediated matrix metalloproteinase-2 induction in peripheral blood T cells is up-regulated in patients with HTLV-I-associated myelopathy. J Neuroimmunol (1999) 99(2):242–7 10.1016/S0165-5728(99)00110-1 [DOI] [PubMed] [Google Scholar]
- 84.Romanic AM, Madri JA. The induction of 72-kD gelatinase in T cells upon adhesion to endothelial cells is VCAM-1 dependent. J Cell Biol (1994) 125(5):1165–78 10.1083/jcb.125.5.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xia M, Leppert D, Hauser SL, Sreedharan SP, Nelson PJ, Krensky AM, et al. Stimulus specificity of matrix metalloproteinase dependence of human T cell migration through a model basement membrane. J Immunol (1996) 156(1):160–7 [PubMed] [Google Scholar]
- 86.Xia M, Sreedharan SP, Dazin P, Damsky CH, Goetzl EJ. Integrin-dependent role of human T cell matrix metalloproteinase activity in chemotaxis through a model basement membrane. J Cell Biochem (1996) 61(3):452–8 [DOI] [PubMed] [Google Scholar]
- 87.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood (2006) 108(5):1441–50 10.1182/blood-2006-02-005363 [DOI] [PubMed] [Google Scholar]
- 88.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell (1996) 85(5):683–93 10.1016/S0092-8674(00)81235-0 [DOI] [PubMed] [Google Scholar]
- 89.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol (2008) 18(11):560–74 10.1016/j.tcb.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 90.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol (2004) 4(8):617–29 10.1038/nri1418 [DOI] [PubMed] [Google Scholar]
- 91.Sanchez-Madrid F, Serrador JM. Bringing up the rear: defining the roles of the uropod. Nat Rev Mol Cell Biol (2009) 10(5):353–9 10.1038/nrm2680 [DOI] [PubMed] [Google Scholar]
- 92.del Pozo MA, Sanchez-Mateos P, Nieto M, Sanchez-Madrid F. Chemokines regulate cellular polarization and adhesion receptor redistribution during lymphocyte interaction with endothelium and extracellular matrix. Involvement of cAMP signaling pathway. J Cell Biol (1995) 131(2):495–508 10.1083/jcb.131.2.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Curtis I, Meldolesi J. Cell surface dynamics – how Rho GTPases orchestrate the interplay between the plasma membrane and the cortical cytoskeleton. J Cell Sci (2012) 125(Pt 19):4435–44 10.1242/jcs.108266 [DOI] [PubMed] [Google Scholar]
- 94.Nieto M, Frade JM, Sancho D, Mellado M, Martinez AC, Sanchez-Madrid F. Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J Exp Med (1997) 186(1):153–8 10.1084/jem.186.1.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urzainqui A, Serrador JM, Viedma F, Yanez-Mo M, Rodriguez A, Corbi AL, et al. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity (2002) 17(4):401–12 10.1016/S1074-7613(02)00420-X [DOI] [PubMed] [Google Scholar]
- 96.Rougerie P, Delon J. Rho GTPases: masters of T lymphocyte migration and activation. Immunol Lett (2012) 142(1–2):1–13 10.1016/j.imlet.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 97.Martinelli S, Chen EJ, Clarke F, Lyck R, Affentranger S, Burkhardt JK, et al. Ezrin/Radixin/Moesin proteins and flotillins cooperate to promote uropod formation in T cells. Front Immunol (2013) 4:84. 10.3389/fimmu.2013.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mrass P, Kinjyo I, Ng LG, Reiner SL, Pure E, Weninger W. CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity. Immunity (2008) 29(6):971–85 10.1016/j.immuni.2008.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun YM, Lambert K, et al. Inflammation-induced interstitial migration of effector CD4(+) T cells is dependent on integrin alphaV. Nat Immunol (2013) 14(9):949–58 10.1038/ni.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp Med (2011) 208(1):167–80 10.1084/jem.20101850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med (2006) 203(4):1045–54 10.1084/jem.20051954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D’Souza WN, Hedrick SM. Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol (2006) 177(2):777–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med (2007) 13(7):843–50 10.1038/nm1592 [DOI] [PubMed] [Google Scholar]
- 104.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med (2007) 204(3):547–57 10.1084/jem.20062381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol (2005) 6(9):889–94 10.1038/ni1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jennrich S, Lee MH, Lynn RC, Dewberry K, Debes GF. Tissue exit: a novel control point in the accumulation of antigen-specific CD8 T cells in the influenza a virus-infected lung. J Virol (2012) 86(7):3436–45 10.1128/JVI.07025-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Debes GF, Bonhagen K, Wolff T, Kretschmer U, Krautwald S, Kamradt T, et al. CC chemokine receptor 7 expression by effector/memory CD4+ T cells depends on antigen specificity and tissue localization during influenza A virus infection. J Virol (2004) 78(14):7528–35 10.1128/JVI.78.14.7528-7535.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol (2005) 6(9):895–901 10.1038/ni1240 [DOI] [PubMed] [Google Scholar]
- 109.Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol (2008) 9(1):42–53 10.1038/ni1534 [DOI] [PubMed] [Google Scholar]
- 110.Carbone FR, Mackay LK, Heath WR, Gebhardt T. Distinct resident and recirculating memory T cell subsets in non-lymphoid tissues. Curr Opin Immunol (2013) 25(3):329–33 10.1016/j.coi.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 111.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature (2011) 477(7363):216–9 10.1038/nature10339 [DOI] [PubMed] [Google Scholar]
- 112.Richter M, Ray SJ, Chapman TJ, Austin SJ, Rebhahn J, Mosmann TR, et al. Collagen distribution and expression of collagen-binding alpha1beta1 (VLA-1) and alpha2beta1 (VLA-2) integrins on CD4 and CD8 T cells during influenza infection. J Immunol (2007) 178(7):4506–16 [DOI] [PubMed] [Google Scholar]
- 113.Chapman TJ, Topham DJ. Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1. J Immunol (2010) 184(7):3841–9 10.4049/jimmunol.0902281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richter MV, Topham DJ. The {alpha}1beta1 integrin and TNF receptor II protect airway CD8+ effector T cells from apoptosis during influenza infection. J Immunol (2007) 179(8):5054–63 [DOI] [PubMed] [Google Scholar]
- 115.Baaten BJ, Li CR, Deiro MF, Lin MM, Linton PJ, Bradley LM. CD44 regulates survival and memory development in Th1 cells. Immunity (2010) 32(1):104–15 10.1016/j.immuni.2009.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maeshima N, Poon GF, Dosanjh M, Felberg J, Lee SS, Cross JL, et al. Hyaluronan binding identifies the most proliferative activated and memory T cells. Eur J Immunol (2011) 41(4):1108–19 10.1002/eji.201040870 [DOI] [PubMed] [Google Scholar]
- 117.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med (2000) 192(10):1425–40 10.1084/jem.192.10.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity (2007) 27(3):393–405 10.1016/j.immuni.2007.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham QM, Zickovich JM, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol (2011) 187(10):4967–78 10.4049/jimmunol.1102335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J Immunol (2010) 185(9):5315–25 10.4049/jimmunol.1001948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zinselmeyer BH, Heydari S, Sacristán C, Nayak D, Cammer M, Herz J, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med (2013) 210(4):757–74 10.1084/jem.20121416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity (2007) 27(1):111–22 10.1016/j.immuni.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]