Abstract
We recorded brain activity when 21 subjects judged the beauty (aesthetic or affective judgment) and brightness (perceptual or cognitive judgment) of simultaneously presented paintings. Aesthetic judgments engaged medial and lateral subdivisions of the orbitofrontal cortex as well as subcortical stations associated with affective motor planning (globus pallidus, putamen–claustrum, amygdala, and cerebellar vermis), whereas the motor, premotor and supplementary motor areas, as well as the anterior insula and the dorsolateral prefrontal cortex, were engaged by both kinds of judgment. The results lead us to conclude: (i) that there is a functional specialization for judgment, with aesthetic judgments engaging distinct systems, in addition to those that they share with perceptual judgments; (ii) that the systems engaged by affective judgments are those in which activity correlates with polar experiences (e.g. love–hate, beauty–ugliness, and attraction–repulsion); and (iii) that there is also a functional specialization in the motor pathways, with aesthetic judgments engaging motor systems not engaged by perceptual judgments, in addition to those engaged by both kinds of judgment.
Keywords: affective and perceptual judgment, affective motor planning, functional specialization, orbitofrontal cortex
Introduction
In his Critique of Judgment, Immanuel Kant (1790/1987) affirmed that aesthetic judgments are based on feelings of pleasure, and must be distinguished from cognitive judgments based on perception, e.g. about the brightness of objects. Kant’s distinction is a useful introduction to a neurobiological study of the difference between objective, cognitive judgments based on perception, and those based on feeling or pleasure. Kant made further distinctions within aesthetic judgments, considering some to have a cognitive basis and others not. His views stand in opposition to those of others who claimed that feelings associated with judgments of beauty have no cognitive content (Hume, 1757) or that beauty judgments are about objective properties (Baumgarten, 1783). In this study, we chose to use Kant’s distinction, and address the question of whether the neural systems engaged in aesthetic judgments about paintings can be distinguished from those concerning their brightness. This study is the inevitable consequence of our previous studies (Kawabata & Zeki, 2004; Ishizu & Zeki, 2011), which showed that activity in one area, field A1 of the medial orbitofrontal cortex (mOFC), always correlates with the experience of beauty, regardless of its source, i.e. whether it is visual or musical (Ishizu & Zeki, 2011).
The basis for making judgments is an inequality of one kind or another. This applies to aesthetic judgments (‘this painting is more beautiful than the other’) as much as to judgments based on low-level qualities such as size or brightness (‘this painting is brighter than the other’). The inequality is relatively easy to verify psychophysically and relate to brain activity in respect of judgments such as brightness; this is more difficult to do in relation to aesthetic judgments, except in terms of brain activity within an individual (Ishizu & Zeki, 2011). However, the fact that one can distinguish between the two kinds of judgment – even if the former may contribute to the latter – led us to use them both, to understand something about the brain activity involved in making two qualitatively different kinds of judgment about the same stimulus. This held the promise of showing us whether the two types of judgment can be separated neurobiologically.
To experience something as beautiful implies making a judgment about it. This raises the question, also addressed by Kant (1790), of whether the judgment precedes the experience of beauty or is subsequent to it, and indeed whether the two can be separated at all. We address that question obliquely here, by asking whether the area in the mOFC demarcated by us (Kawabata & Zeki, 2004; Ishizu & Zeki, 2011) and others (Vartanian & Goel, 2004; Tsukiura & Cabeza, 2011 inter alia) as one in which activity correlates with the experience of beauty, and facial attractiveness (O’Doherty et al., 2003; Ishai, 2007), is also engaged when aesthetic judgments are made, with or without other areas. To minimize the influence of past memories and experiences, we asked subjects to judge the brightness and beauty of simultaneously presented pairs of paintings.
Materials and methods
Subjects
Twenty-one healthy right-handed volunteers (11 males; 10 females; mean age, 28.8 years) participated. Except for one male volunteer, none was an artist or musician. All had normal or corrected-to-normal vision, and none had a history of neurological or psychiatric disorder. Written informed consent was obtained from all participants. The study was approved by the Ethics Committee of University College London, and conforms with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All data were anonymized.
Psychophysical testing
Prior to scanning, two psychophysical tests were conducted: the first to select paintings for pairing (see below), and the second to test whether the paired paintings had the same task difficulty for aesthetic and brightness judgments. In the first test, we tested 30 volunteers (15 males; 15 females; mean age, 21.9 years) who did not participate in the second test or in the scanning. Each subject viewed 510 paintings, consisting of 10th–20th-century paintings of landscapes, portraits and still lifes, mostly from Western art, but including some from Oriental art. All painting stimuli were acquired from an online database, Web Gallery of Art (http://www.wga.hu/), and were presented on a computer screen for 3 s with an inter-trial interval of 2 s. Each stimulus was given a score from 1 to 7, with scores of 1–3 being classified as ‘ugly’, 4 as ‘indifferent’, and 5–7 as ‘beautiful’. On the basis of the psychophysical testing, we selected 30 ‘beautiful’ pairs and 30 ‘ugly’ pairs (which constituted the pairings) from each painting category, so that the paired paintings belonged to the same category (e.g. landscape), and had the same beauty scores.
In the second psychophysical test, 12 volunteers (six males; six females; mean age, 23.2 years) who did not participate in the scanning, and none of whom was an artist, participated in judgment tests, which consisted of two tasks: an aesthetic judgment, and a brightness judgment. For the aesthetic judgment, volunteers were asked to judge which painting of a pair presented was more beautiful, by pressing the left or right button with the index or middle finger. For the brightness judgment, they were asked to judge which painting of the same pair appeared brighter. All paired stimuli were presented on a computer screen for 3 s with an inter-trial interval of 2 s. Participants were asked to press a button as soon as possible after they had made their judgment. The results of reaction times showed that there were no significant differences between judgment tasks or between categories in a two-way anova (2 tasks × 3 categories). This indicated that both judgment tasks had the same difficulty.
Stimuli
Stimuli were generated with Cogent 2000 (http://www.vislab.ucl.ac.uk/cogent_2000.php) running in matlab (MathWorks, Natick, MA, USA). They were back-projected onto a screen, by use of an LCD projector, through an angled mirror. The resolution of the screen was 1400 × 1050 pixels; the height of each stimulus pair was 19°, and the width varied.
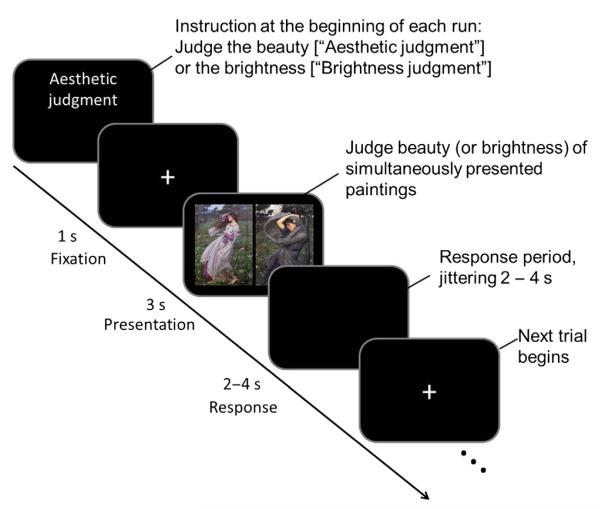
The session began with subjects viewing a flat black screen for 20 s to allow for T1 equilibration effects to subside (the corresponding first six brain volumes were discarded). After this 20-s blank period, an instruction, i.e. aesthetic judgment or brightness judgment, appeared on the screen to inform participants which judgment they would make in that session. A fixation point was then presented at the centre of the screen for 1 s against a black background. After this, visual stimuli were presented in a pseudorandom order for 3 s, and this was followed by an interval of 2–4 s. Following each stimulus presentation, participants were asked to judge which of the two paintings was more beautiful in the aesthetic judgment session, and which was brighter in the brightness judgment session, by pressing the left or right button with their right index or middle finger. The response period lasted for 2–4 s, and participants could make their rating at any time during that period (see Fig. 1 for an illustration of the paradigm). The session ended with a blank period of 20 s, during which the scanner continued to acquire blood oxygen level-dependent (BOLD) signals. The stimuli were presented in 12 blocks: six for the aesthetic judgment, and six for the brightness judgment. Each block consisted of 15 stimulus pairs and contained five pairs of each painting category, presented in pseudorandom order. Prior to the scanning, participants had a short practice session with different visual stimuli to those used in the scanning session. The same stimulus pairs were used in the aesthetic and brightness judgment blocks, so that participants viewed the same stimulus pairs twice separately in the scanner but not in contiguous blocks.
Fig. 1.
An illustration of the paradigm used in this study.
Functional magnetic resonance imaging (fMRI) scanning details
Scanning data were acquired in a 3-T Siemens Magnetom Trio magnetic resonance imaging scanner (Siemens, Erlangen, Germany) fitted with a 12-channel head-coil. An echo-planar imaging (EPI) sequence was applied for functional scans to obtain BOLD signals (echo time, 30 ms; repeat time, 3.36 s), using 48 slices to cover the whole brain. The voxel resolution was 3 × 3-mm in-plane resolution, with a 2-mm slice thickness and 1-mm inter-slice gap. Magnetic resonance imaging signal losses in the orbitofrontal cortex (OFC) and amygdala were reduced by applying a z shim gradient moment and slice tilt (Weiskopf et al., 2006). T1-weighted anatomical images were acquired at the end of experimental sessions for each subject (176 slices; resolution, 1 × 1 × 1 mm; echo time, 2.48 ms; repeat time, 7.92 ms). Field maps were also acquired with Siemens standard gradient-echo field map sequence for correcting geometric distortion of EPI images (Hutton et al., 2002). We also recorded the heart and respiration rates for each subject.
fMRI data analysis
All data were analysed with spm8 (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The EPI images for each subject were realigned and normalized into Montreal Neurological Institute (MNI) space, smoothed with a Gaussian smoothing kernel of 9 × 9 × 9 mm, and filtered with a high-pass cutoff (128 s) to remove drift terms. The stimulus for each subject was modelled as a set of regressors in a general linear model first-level (within subject) analysis. The stimulus was a block design, and box-car functions were used to define stimulus functions; these modelled the onsets and durations of the visual stimuli. Head movement parameters calculated from the realignment pre-processing step and physiological recordings were included as regressors of no interest. Stimulus functions were convolved with a canonical haemodynamic response function, with time and dispersion derivatives to provide regressors for the general linear model. We carried out categorical contrast analyses, encoding the same data in two ways. For the first analysis, we used separate stimulus functions for painting types (landscape, portrait, and still life), and for the second, main, analysis we used separate stimulus functions for aesthetic and brightness judgment tasks. Contrast images were taken to second-level (between subject) t-tests to produce summary t-statistical maps at the group level.
Conjunction analyses (Price & Friston, 1997) were used to characterize brain activations common to aesthetic and brightness judgments. We modelled button presses, regressed motor-related responses out, and carried out a conjunction analysis using aesthetic judgment > button press and brightness judgment > button press.
We report peak level activations significant at P < 0.05 family-wise error (FWE)-corrected, although some of these (indicated in the tables) were significant at the cluster level at P < 0.05 FWE-corrected.
Results
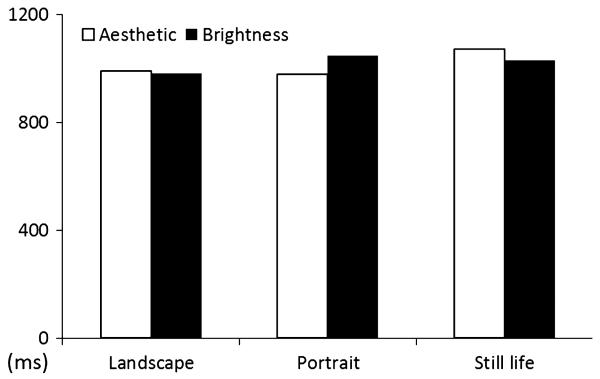
Figure 2 shows behavioural data collected during the scanning experiment, averaged by the reaction times needed to make judgments about the beauty and the brightness of simultaneously presented pairs of paintings belonging to three different categories – landscapes, portraits, and still lifes. There was no significant difference in reaction time needed to reach judgment about the beauty or brightness in a two-way anova (2 judgment types × 3 painting types), from which we conclude that there was no difference in task difficulty between the two kinds of judgment.
Fig. 2.
Behavioural data collected in the fMRI scanning session, showing averaged reaction times by judgment and stimulus type. There was no significant difference between judgment and category of painting in a two-way anova (2 judgments × 3 painting categories).
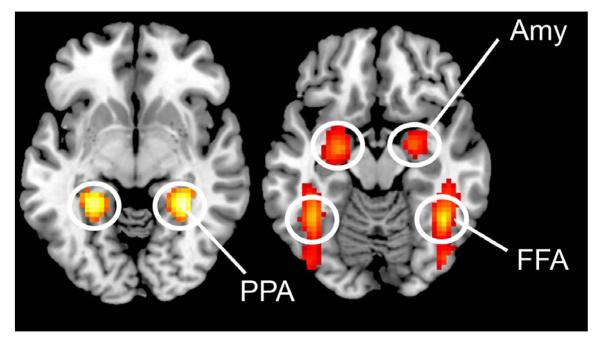
Figure 3 shows that different categories of paired paintings activated different brain areas, reflecting the functional specialization of the visual brain (Zeki, 1978; Zeki et al., 1991): bilateral parahippocampal gyrus at 30 −43 −5 and −30 −43 −2, and bilateral middle occipital gyrus at −42 −82 28 and 42 −79 28, for the contrast landscape > portrait and still life, showing activation within an area known to respond selectively to the viewing of scenes and landscapes (Epstein & Kanwisher, 1998 inter alia); bilateral activation of the fusiform gyrus at 42 −52 −17 and −39 −49 −14, of the middle temporal gyrus at 51 −70 10 and −48 −64 13, and of the inferior occipital gyrus at 48 −76 −5 and −42 −82 −2 for the contrast portrait > landscape and still life, regions that correspond to the fusiform face area and the occipital face area, both of which have been implicated in the recognition of faces (Sergent & Signoret, 1992; Kanwisher et al., 1997; Halgren et al., 2000), and the extrastriate body area associated with the recognition of human bodies (Downing et al., 2001). There was also bilateral amygdala activation at −24 −7 −14 and 24 −4 −14 (the results are summarized in Table 1). There was no activity at the corrected significance level in the contrast still life > landscape and portrait, possibly because the two contained many common elements and therefore cancelled out in the subtractions. To confirm this, we analysed the contrast portrait and landscape > baseline and the contrast still life > baseline, and compared the activation patterns. The activations produced by these two contrasts overlapped in the visual cortex, including the lateral occipital complex, which has been implicated in object and shape recognition (Grill-Spector et al., 1999). This is consistent with our explanation.
Fig. 3.
Sites activated by (left) landscape and (right) portrait paintings. Statistical parametric maps rendered onto canonical anatomical sections showing the t-statistic for the contrasts landscapes > portraits and still lifes (left), and portraits > landscapes and still lifes (right). Random effects analysis with 21 subjects. Display threshold P < 0.05 (FWE-corrected). The result of the contrast still lifes > landscapes and portraits is not shown, because it did not result in significant activation at this threshold. Amy, amygdala; FFA, fusiform face area; PPA, parahippocampal place area.
Table 1.
Location, MNI coordinates, cluster size and values for the activa-tions produced by the contrasts portraits > landscapes and still lifes, and landscapes > portraits and still lifes
| Brain regions | L/R | x | y | z | T | Z | kE |
|---|---|---|---|---|---|---|---|
| Portraits > landscapes and still lifes | |||||||
| Inferior occipital gyrus | R | 48 | −76 | −5 | 17.38 | Inf | 1097 |
| Fusiform gyrus | R | 42 | −52 | −17 | 13.69 | Inf | 1097 |
| Middle temporal gyrus | R | 51 | −70 | 10 | 13.14 | Inf | 1097 |
| Inferior occipital gyrus | L | 42 | −82 | −2 | 13.53 | Inf | 817 |
| Fusiform gyrus | L | 39 | −49 | −14 | 11.37 | Inf | 817 |
| Middle temporal gyrus | L | 48 | −64 | 13 | 10.75 | Inf | 817 |
| Amygdala | L | 24 | −7 | −14 | 10.1 | 7.69 | 190 |
| Amygdala | R | 24 | −4 | −14 | 9.02 | 7.15 | 99 |
| Cuneus | R | 6 | −88 | 16 | 8.59 | 6.91 | 288 |
| Landscapes > portraits and still lifes | |||||||
| Para-hippocampal gyrus | R | 38 | −43 | −5 | 10.68 | Inf | 248 |
| Para-hippocampal gyrus | L | 30 | −43 | −2 | 10.07 | 7.67 | 149 |
| Middle occipital gyrus | L | 42 | −82 | 28 | 5.81 | 5.15 | 27 |
| Middle occipital gyrus | R | 42 | −79 | 28 | 5.62 | 5.02 | 20 |
Inf, > 8; L, left; R, right. In this and Table 2, all activations are peak level significant at P < 0.05 (FWE-corrected), although some of these were signifi-cant at the cluster level (as indicated in the tables). The result of the contrast still lifes > landscapes and portraits is not shown here, because there was no significant activation at this threshold.
Aesthetic judgment and brightness judgment
Our main interest was to determine the neural correlates of aesthetic (affective) and brightness (perceptual or cognitive) judgments, and learn about the extent to which they differ and overlap. We therefore used the following contrasts: (i) aesthetic judgment > brightness judgment; and (b) brightness judgment > aesthetic judgment.
A number of areas were activated in the contrast aesthetic judgment > brightness judgment (Fig. 4). These included subcortical areas such as the left globus pallidus, left amygdala (encroaching upon the putamen), and right putamen (encroaching upon the claustrum). They also involved cortical areas, as follows: the lateral OFC (lOFC) and mOFC bilaterally (the latter at cluster level significance), and the right superior frontal gyrus.
Fig. 4.
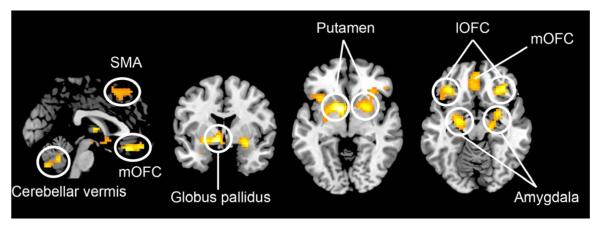
Sites active during aesthetic judgment alone. Statistical parametric maps rendered onto canonical anatomical sections showing the t-statistic for the contrast aesthetic judgment > brightness judgment. Random effects analysis with 21 subjects. Display threshold P < 0.001 (uncorrected).
In the contrast brightness judgment > aesthetic judgment, there was no activity at the corrected significance level, suggesting that, although the OFC and subcortical regions have a role in aesthetic judgment, they have none in brightness judgment.
We also conducted an anova with three picture types (landscape, portrait, and still life) and two judgments (aesthetic and brightness) separately from categorical contrast and conjunction analyses, to test interactions between the two factors. The results showed that there were main effects of judgment and painting type, as we have reported in the categorical contrasts, but no significant interaction was found.
Conjunction analysis of aesthetic judgment and brightness judgment
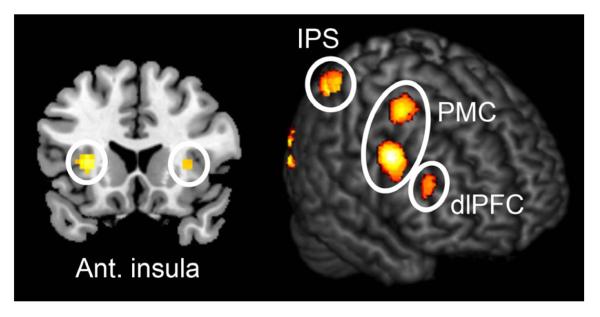
Next, we investigated which brain areas were commonly activated by both aesthetic and brightness judgments, through the application of a conjunction analysis (Price & Friston, 1997). We modelled button presses, regressed motor responses out, and carried out a conjunction analysis with the following contrast: aesthetic judgment > button press, and brightness judgment > button press. The results showed that activations were distributed over the occipital, parietal and frontal cortex, including the bilateral dorsolateral prefrontal cortex (dlPFC) (−42 5 28; 48 14 16) and bilateral anterior insula (−27 23 10; 36 23 13) (Fig. 5). There was also activation in the premotor cortex and supplementary motor area (SMA), as well as in the intraparietal sulcus (IPS) (Table 2).
Fig. 5.
Sites active during both aesthetic and brightness judgment. The results of a conjunction analysis for aesthetic judgment > button press, and brightness judgment > button press. Display threshold P < 0.05 (FWE-corrected). PMC, premotor cortex.
Table 2.
Location, MNI coordinates, cluster size and values for the activations produced by the contrasts aesthetic judgment > brightness judgment, and brightness judgment > aesthetic judgment. Also shown are activations produced by the conjunction analysis aesthetic judgment > button press and brightness judgment > button press
| Brain regions | L/R | x | y | z | T | Z | kE | |
|---|---|---|---|---|---|---|---|---|
| Aesthetic > brightness | ||||||||
| Globus pallidus | L | −9 | 5 | −5 | 5.53 | 5.21 | 333 | |
| Amygdala | L | −24 | −10 | −11 | 4.85 | 4.62 | ||
| Thalamus | Cluster | R | 6 | −7 | 7 | 4.45 | 4.27 | |
| Lateral OFC | L | −36 | 32 | −17 | 4.68 | 4.48 | 194 | |
| Inferior frontal gyrus | L | −42 | 17 | −23 | 4.06 | 3.92 | ||
| Anterior insula | L | −36 | 20 | −2 | 3.9 | 3.78 | ||
| Medial OFC | 0 | 32 | −20 | 4.64 | 4.44 | 107 | ||
| Superior frontal gyrus | Cluster | R | 24 | 47 | 43 | 4.55 | 4.36 | 279 |
| SMA | 6 | 20 | 64 | 4.03 | 3.89 | |||
| Lateral OFC | Cluster | R | 36 | 32 | −14 | 4.54 | 4.36 | 340 |
| Putamen | R | 30 | 5 | −8 | 4.28 | 4.12 | ||
| Putamen | R | 21 | 8 | −5 | 4.27 | 4.11 | ||
| Cerebellar vermis | Cluster | R | 6 | −64 | −38 | 4.22 | 4.07 | 121 |
|
| ||||||||
| Brightness > aesthetica | ||||||||
|
| ||||||||
| Conjunction analysis: aesthetic > button press, and brightness > button press | ||||||||
| Calcarine gyrus | L | −6 | −85 | −2 | 17.09 | Inf | 5803 | |
| Calcarine gyrus | R | 12 | −91 | 1 | 16.59 | Inf | ||
| Superior occipital gyrus | R | 21 | −97 | 7 | 16.1 | Inf | ||
| Premotor cortex | R | 45 | 5 | 31 | 10.25 | Inf | 173 | |
| dlPFC | R | 57 | 11 | 37 | 7.87 | 6.75 | ||
| Premotor cortex | R | 33 | −1 | 55 | 7.61 | 6.58 | 84 | |
| Premotor cortex | L | − 42 | 5 | 28 | 7.57 | 6.55 | 157 | |
| Intraparietal sulcus | L | −38 | −58 | 58 | 7.43 | 6.46 | 363 | |
| Anterior insula | L | −30 | 20 | 7 | 6.53 | 5.83 | 40 | |
| dlPFC | R | 39 | 29 | 19 | 5.85 | 5.32 | 20 | |
| Anterior insula | R | 33 | 20 | 4 | 5.28 | 4.87 | 15 | |
| SMA | Cluster | R | 6 | 17 | 49 | 4.39 | 4.14 | 109 |
| SMA | Cluster | L | −9 | 17 | 46 | 3.95 | 3.76 | 102 |
| Intraparietal sulcus | Cluster | R | 33 | −55 | 49 | 4.55 | 4.21 | 152 |
Inf, > 8; L, left; R, right.
There was no significant activation in Brightness > aesthetic at this threshold
Taken together, these results show that aesthetic and brightness judgments engage separate and also shared brain systems. Aesthetic judgments correlate with activity in additional cortical areas, notably the orbitofrontal regions and the subcortical nuclei mentioned above, which were not engaged by brightness judgments in our study.
Discussion
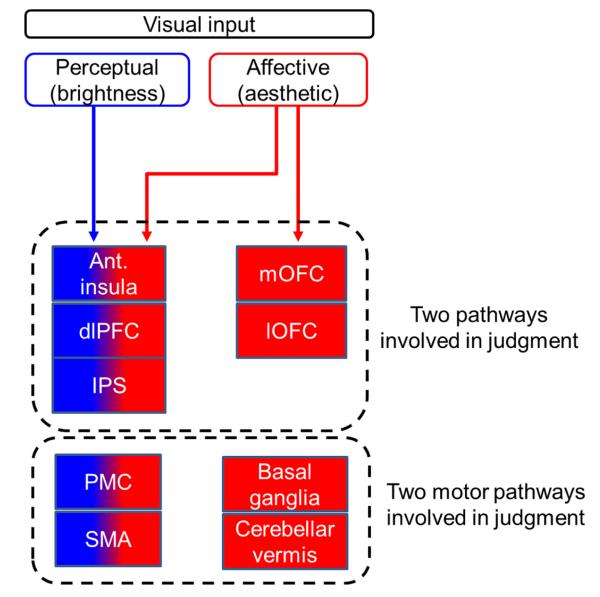
Implicit in the experience of a stimulus as beautiful (or ugly) is a judgment, in this instance an aesthetic one. Although aesthetic judgments have been subdivided into further categories, e.g. sublime and beautiful or teleological and non-teleological (Burke, 1757; Kant, 1790), we did not distinguish between these kinds of aesthetic judgment in this initial study. Instead, we pooled all aesthetic judgments and compared them with judgments based on brightness, asking subjects to judge the stimuli independently for both. We acknowledge that qualities such as brightness may contribute significantly to the overall aesthetic appeal, and therefore to the aesthetic judgment, or could be unrelated to it. To study the nature of the contribution that one makes to the other would require a different experimental paradigm. Here, we simply wanted to learn whether the two different kinds of judgment applied to the same simultaneously presented pictures have different neural correlates. Collectively, our results show that aesthetic judgments engage areas over and above those that are also engaged in brightness judgments of the same stimuli, and hence that aesthetic judgments can be separated from brightness judgments on neural grounds (see Fig. 6 for a summary diagram).
Fig. 6.
Summary diagram. A proposed hypothetical scheme to illustrate the separation between brain systems involved in perceptual (brightness) judgment and those involved in affective (aesthetic) judgments, without implying that the two systems are not linked, either directly or indirectly. The system to the left (anterior insula, dlPFC, and IPS) is engaged during judgment of brightness (perceptual–cognitive) and of beauty (affective–aesthetic), whereas that to the right (mOFC and lOFC) is engaged in aesthetic judgments alone. The two motor pathways engaged in both kinds of judgment [premotor cortex (PMC) and SMA] to the left and the cortical motor systems involved in affective judgments alone (basal ganglia and cerebellar vermis) are shown to the right.
Cortical sites within the OFC that are active during aesthetic judgment
Of all of the areas that were active in the contrast aesthetic judgment > brightness judgment, perhaps the most interesting were the mOFC and lOFC, as many previous studies have shown field A1 of the mOFC to also be active during the experience of beauty (Kawabata & Zeki, 2004; Vartanian & Goel, 2004; Ishizu & Zeki, 2011; Tsukiura & Cabeza, 2011 inter alia), thus highlighting the closeness of the cortical relationship between aesthetic experience and judgment. mOFC activation here overlapped field A1 only partially (Fig. S1), suggesting that separate subdivisions within the mOFC may be active when judgments are made and beauty is experienced (see below). The mOFC is heavily connected with the lOFC (Kringelbach, 2005), which was also engaged in aesthetic judgment in this study. What role each of these two subdivisions of the OFC plays in such judgments is not clear. Whereas almost all studies of the experience of beauty have implicated the mOFC [see Ishizu & Zeki (2011) for a review], the status of the lOFC is less clear. Our results do not sit easily with the two contradictory suggestions of Kringelbach (2005) and of Elliott et al. (2000), the former that the lOFC is related to the evaluation of ‘punishers’, and the latter that it is related to over-riding previously rewarded stimuli. Although these may be among the functions of the lOFC, we suggest that it must have more general functions, into which our results, as well as previous ones, can more easily fit. Such a general function might be that of judgment, especially as judgment of something as being beautiful, for example, also implies a concurrent judgment of it not being ugly. Indeed, as we discuss below, many of the areas engaged during affective, aesthetic judgment are engaged during the experience of polar affective states.
Subcortical motor planning sites that are active during aesthetic judgment
The sites active in the contrast aesthetic judgment > brightness judgment share the common property that they: (i) have been implicated in motor planning involving affective judgments; and (ii) have been implicated in both positive and negative affective judgments (Schmidt et al., 2008). The globus pallidus, which has been reported to be involved in moral judgments (Eslinger et al., 2009), has also been implicated in negative emotional experiences such as disgust (Mataix-Cols et al., 2008); the putamen has been implicated in the polar emotional states of disgust (Schurmann et al., 2011), hate (Zeki & Romaya, 2008), and unattractiveness (Liang et al., 2010), but also of love (Bartels & Zeki, 2004) and attractiveness (Liang et al., 2010); and the amygdala, which has been found to be active during affective judgment and to modulate motor control circuits (Wagar & Thagard, 2004; Sagaspe et al., 2011), has also been implicated in experiences of both pleasure and disgust, e.g. attraction and repulsion (Winston et al., 2007), and its activity is seemingly modulated by viewing emotional stimuli [see Büchel & Dolan (2000) for a review]. Recent studies have reported that quadratic activation is also characteristic of the anterior insula, which was active more in aesthetic than in brightness judgment in our current study (see also Viinikainen et al., 2010; Bensafi et al., 2012). To this list, we add the cerebellar vermis, whose clear-cut involvement in aesthetic judgment adds to the growing evidence of cerebellar involvement in polar affective states, such as sadness and happiness (Baumann & Mattingley, 2012).
The activity that we observed in the putamen was difficult to separate from that in the claustrum, which we also assume to have been active. The claustrum, as part of the basal ganglia, may also be involved in motor planning (Crick & Koch, 2005), but it has also been described as a critical station for cross-model processing (Calvert, 2001) and integration of information from different perceptual modalities and sources, e.g. colour and motion in vision (Crick & Koch, 2005). Judging the beauty of two similar stimuli may be a complicated integrative process that may involve many different features, such as colours, forms, proportion, or facial expression. However, as the claustrum and the rest of the striatum was not involved in brightness judgment, we conclude that, if brightness does contribute to overall aesthetic judgment, it is not through activity in the striatum.
Cortical motor systems that are engaged during aesthetic judgment and perceptual judgment
In addition to the above subcortical motor planning sites, there were two cortical areas that have also been implicated in motor planning which, however, were engaged during both aesthetic and brightness judgment, namely the SMA and premotor cortex. Thus, our study suggests that the two kinds of judgment share a common cortical motor system, but not a common subcortical affective motor planning system. We may therefore be able to think of a motor system that is common, apparently lacks specialization, and is largely cortically based, and another one that is specialized for the planning and execution of emotionally determined actions and engages many subcortical stations.
Polar affective states engaging the same areas and the push–pull mechanism
What our study highlights, therefore, is the extent to which the same structures and motor pathways (or subdivisions within them) are engaged during the experience of polar affective states related to the same types of emotion; this is true for both subcortical motor-related areas and brain areas without any obvious direct motor function, and is no doubt aided by antagonistic neurotransmitters (Graybiel, 1990). This point, which has not been highlighted in previous studies, should hardly be surprising, as these states are really opposites of the same emotion. To account for the pattern of activation–deactivation of the same areas during the experience of romantic love, we posited the operation of a push–pull mechanism that regulates their activity (Bartels & Zeki, 2004). This can now be extended to include other areas with polar activations, such as those mentioned above and possibly others yet to be charted. This push–pull mechanism can become apparent in two ways. Areas (or subdivisions within them) can become active during polar emotional states, with a proportional relationship between the intensity of a given declared experience, e.g. beauty or ugliness, and the intensity of the BOLD signal in them, as in the mOFC (Kawabata & Zeki, 2004; Ishizu & Zeki, 2011 inter alia). Alternatively, areas can be active during the experience of, for example, desire and no-desire, and show a lower activity for a state of indifference, and thus display a quadratic relationship when the BOLD signal is related to the declared intensity of the experience, as is the case with the amygdala and the experience of facial attractiveness (Winston et al., 2005), or the mid-cingulate cortex and the experience of desire (Kawabata & Zeki, 2008). No doubt judgments about reward and punishment fall into the same push–pull category, which is able to accommodate apparently contradictory findings, as with the lOFC referred to above. Finally, the push–pull mechanism can work by regulating activity reciprocally in two separate areas. A good example of this is to be found in a study of moral beauty (Tsukiura & Cabeza, 2011), which showed that activity in the mOFC has a reciprocal relationship with that in the insula; as activity in the former increases during the experience of moral beauty, so the activity in the latter decreases, with the reverse relationship being seen when subjects experience moral repugnance.
A functional specialization for judgment
The fact that each of the affective subcortical motor stations mentioned above is involved in aesthetic but not brightness judgment points to a functional specialization in the pathways mediating the two kinds of judgment. This is not to say that the motor pathways involved in the two kinds of judgment are entirely separate, because they share the cortical motor system (see above). There may indeed be further specializations within the subcortical affective motor planning system, as it is hard to suppose that all of the subcortical stations active in aesthetic judgment in our study undertake the same motor planning tasks. Moreover, the motor specialization that we discuss here is not one restricted to aesthetic judgment, as the subcortical areas involved in the aesthetic judgments in our study have also been reported to be active during many other emotional states; hence, the specialization is a more general one relating to affective states on the one hand and non-affective ones on the other.
Sites active during both aesthetic and brightness judgment
In addition to structures that were active during aesthetic judgment alone, there were areas that were active during both aesthetic and brightness judgment. Of these, the anterior insula has been implicated in a variety of functions, including emotional and cognitive affective processes, and its overall role (or roles) is far from clear. That it may be involved in both affective and cognitive judgments is suggested by previous studies showing that activation in it (and in the dlPFC) plays a role in making choices (Sanfey et al., 2003; Ernst & Paulus, 2005), which itself must also be related to judgment. In our study, anterior insular activity was more intense when subjects were making aesthetic rather than brightness judgments, thus reflecting the results of Sanfey et al. (2003), who also found it to be more involved in affective judgment. Recent findings also suggest that the anterior insula plays a role in cognitive–affective integration (Gu et al., 2012), implying that it is important in cognitive and affective processes.
The dlPFC has also been found to be active in both reward conditions and aversive ones (Plassmann et al., 2010), another example of polar involvement. To that extent, it must, like the anterior insula, have multiple functions, of which an involvement in judgment is only one. Although we are unable to speculate as to what role it plays in judgment, we note that the study of Sanfey et al. (2003) also implicated the dlPFC in both cognitive and affective judgment, with more pronounced activity during cognitive judgment, although, in our studies, there was no difference between the two types of judgment.
The IPS was also active in both types of judgment. Previous work on brightness judgment showed activation of this region, which is also activated by judgments of number, size, and luminance (Pinel et al., 2004; Cohen Kadosh et al., 2008). In addition to such a range of perceptual judgments, our results suggest that the IPS is also recruited when aesthetic, and therefore affective, judgments are made.
Although we did not have a passive viewing condition to use in a contrast, beyond what is described above, there may be other brain areas that are engaged during both kinds of judgment. Among these is the occipital visual cortex, which we assume, and which previous studies have shown (Pinel et al., 2004; Cohen Kadosh et al., 2008), to be involved in visual judgment. Given the range of visual stimuli that we used, the activation would be expected to be extensive, as indeed it was in the contrasts aesthetic judgment > button press and brightness judgment > button press. Thus, the areas constituting the visual occipital brain may contribute in an undetermined way to the judgment of both the aesthetic quality and the brightness of visual stimuli.
Judgment, decision, and experience
Our results highlight the relationship between judgment, experience, and decision, and their relationship to motor planning, a relationship that is all the more imposing given that many studies have shown the OFC in general, and the mOFC in particular, to be engaged in decision-making [see Grabenhorst & Rolls (2011) for a review] and aesthetic experience [see Ishizu & Zeki (2011) for a review]. It is difficult to know whether judgment, aesthetic experience and decision can be easily separated, and whether one precedes the other, a difficulty experienced in philosophical discourse (Kant, 1790) and in dictionary definitions, which often combine them (e.g. in Webster’s Dictionary and the Oxford English Dictionary). In the absence of a clear demarcation, there is a compelling neurobiological reason to consider them together, as indeed has been done in the neurobiological literature, as almost none of the decision-making studies addresses the question of judgment.
Part of the reason for the difficulty in separating them lies in the activation pattern produced by decision-making and aesthetic judgment or experience; both involve the OFC. Another, equally compelling, reason may be the ubiquitous involvement of areas that are implicated in motor planning in our study and in previous studies that have explored decision-making in relation to reward. Among these, we include the putamen, the globus pallidus, the cerebellar vermis, the premotor cortex, and the SMA. The experience of any stimulus as agreeable or disagreeable on the one hand, or as rewarding or non-rewarding on the other, must inevitably mobilize the motor system for appropriate action. Hence, the involvement of all of these motor areas in decision-making related to affective judgment is not surprising. Even less surprising is the fact, outlined above, that both the striatal motor areas and the cerebellar vermis, both of which call for action, either immediate or deferred, are engaged during polar affective states.
Even in spite of the difficulty – in common experience, in definition, in philosophical debate, and in experimental study – of separating judgment and experience from decision and action, and even in spite of the common involvement of brain areas during decision-making, judgment, and aesthetic experience, the relationship remains an interesting one, and is worthy of further study. Our present demonstration of functional specialization within the domain of judgment, with aesthetic judgment mobilizing cortical and subcortical pathways that are not engaged during perceptual judgment, raises important questions for future study within a field – that of judgment – that is crucial in our daily lives. One of these relates to whether there are further differences in the neural pathways engaged during the making of different affective judgments, including different aesthetic judgments, as was posited in more general terms by Kant and others. The other relates to the same problem that every demonstration of functional specialization entails – namely, how the many neural stations that are involved in judgment interact to give us the apparently seamless ability to take decisions.
Supplementary Material
Acknowledgements
This work was supported by the Wellcome Trust, London. We thank John Romaya for his help during this study.
Abbreviations
- BOLD
blood oxygen level-dependent
- dlPFC
dorsolateral prefrontal cortex
- EPI
echo-planar imaging
- fMRI
functional magnetic resonance imaging
- FWE
family-wise error
- IPS
intraparietal sulcus
- lOFC
lateral orbitofrontal cortex
- MNI
Montreal Neurological Institute
- mOFC
medial orbitofrontal cortex
- OFC
orbitofrontal cortex
- SMA
supplementary motor area
Footnotes
Conflict of interest: There is no conflict of interest with regard to this study.
References
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Baumann O, Mattingley JB. Functional topography of primary emotion processing in the human cerebellum. NeuroImage. 2012;61:805–811. doi: 10.1016/j.neuroimage.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Baumgarten AG. Metaphysik. 2nd Edn. Frommann-Holzboog; Stuttgart: 1783. translated into English by Meier, G.M., with notes by Eberhard, J.A. [Google Scholar]
- Bensafi M, Iannilli E, Poncelet J, Seo HS, Gerber J, Rouby C, Hummel T. Dissociated representations of pleasant and unpleasant olfacto-trigeminal mixtures: an FMRI study. PLoS ONE. 2012;7:e38358. doi: 10.1371/journal.pone.0038358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr. Opin. Neurobiol. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Burke E. A Philosophical Enquiry into the Origin of Our Ideas of the Sublime and Beautiful. R. and J. Dodsley; London: 1757. [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb. Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Cohen Kadosh K, Henik A. When brightness counts: the neuronal correlate of numerical-luminance interference. Cereb. Cortex. 2008;18:337–343. doi: 10.1093/cercor/bhm058. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C. What is the function of the claustrum? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1271–1279. doi: 10.1098/rstb.2005.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb. Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol. Psychiat. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Robinson-Long M, Realmuto J, Moll J, deOliveira-Souza R, Tovar-Moll F, Wang J, Yang QX. Developmental frontal lobe imaging in moral judgment: Arthur Benton’s enduring influence 60 years later. J. Clin. Exp. Neuropsychol. 2009;31:158–169. doi: 10.1080/13803390802298064. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends. Cogn. Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends. Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Gu X, Liu X, Van Dam NT, Hof PR, Fan J. Cognition–emotion integration in the anterior insular cortex. Cereb. Cortex. 2012;23:20–27. doi: 10.1093/cercor/bhr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Raij T, Marinkovic K, Jousmaki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cereb. Cortex. 2000;10:69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- Hume D. Four Dissertations. A. Millar; London: 1757. [Google Scholar]
- Hutton C, Bork A, Josephs O, Deichmann R, Ashburner J, Turner R. Image distortion correction in fMRI: a quantitative evaluation. NeuroImage. 2002;16:217–240. doi: 10.1006/nimg.2001.1054. [DOI] [PubMed] [Google Scholar]
- Ishai A. Sex, beauty and the orbitofrontal cortex. Int. J. Psychophysiol. 2007;63:181–185. doi: 10.1016/j.ijpsycho.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Ishizu T, Zeki S. Toward a brain-based theory of beauty. PLoS ONE. 2011;6:e21852. doi: 10.1371/journal.pone.0021852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant I. Kritik der Urteilskraft, translated into English by Pluhar, W.S. as Critique of Judgment. Hacket Publishing Co; Indianapolis: 1790/1987. [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata H, Zeki S. Neural correlates of beauty. J. Neurophysiol. 2004;91:1699–1705. doi: 10.1152/jn.00696.2003. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Zeki S. The neural correlates of desire. PLoS ONE. 2008;3:e3027. doi: 10.1371/journal.pone.0003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Liang X, Zebrowitz LA, Zhang Y. Neural activation in the ‘reward circuit’ shows a nonlinear response to facial attractiveness. Soc. Neurosci. 2010;5:320–334. doi: 10.1080/17470911003619916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-Cols D, An SK, Lawrence NS, Caseras X, Speckens A, Giam-pietro V, Brammer MJ, Phillips ML. Individual differences in disgust sensitivity modulate neural responses to aversive/disgusting stimuli. Eur. J. Neurosci. 2008;27:3050–3058. doi: 10.1111/j.1460-9568.2008.06311.x. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Pinel P, Piazza M, Le Bihan D, Dehaene S. Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron. 2004;41:983–993. doi: 10.1016/s0896-6273(04)00107-2. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J. Neurosci. 2010;30:10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. NeuroImage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Sagaspe P, Schwartz S, Vuilleumier P. Fear and stop: a role for the amygdala in motor inhibition by emotional signals. NeuroImage. 2011;55:1825–1835. doi: 10.1016/j.neuroimage.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schmidt L, d’Arc BF, Lafargue G, Galanaud D, Czernecki V, Grabli D, Schupbach M, Hartmann A, Levy R, Dubois B, Pessiglione M. Disconnecting force from money: effects of basal ganglia damage on incentive motivation. Brain. 2008;131:1303–1310. doi: 10.1093/brain/awn045. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Hlushchuk Y, Hari R. Embodied visual perception of distorted finger postures. Hum. Brain Mapp. 2011;32:612–623. doi: 10.1002/hbm.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent J, Signoret JL. Functional and anatomical decomposition of face processing: evidence from prosopagnosia and PET study of normal subjects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1992;335:55–61. doi: 10.1098/rstb.1992.0007. discussion 61-62. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Cabeza R. Shared brain activity for aesthetic and moral judgments: implications for the Beauty-is-Good stereotype. Soc. Cogn. Affect. Neurosci. 2011;6:138–148. doi: 10.1093/scan/nsq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian O, Goel V. Neuroanatomical correlates of aesthetic preference for paintings. NeuroReport. 2004;15:893–897. doi: 10.1097/00001756-200404090-00032. [DOI] [PubMed] [Google Scholar]
- Viinikainen M, Jaaskelainen IP, Alexandrov Y, Balk MH, Autti T, Sams M. Nonlinear relationship between emotional valence and brain activity: evidence of separate negative and positive valence dimensions. Hum. Brain Mapp. 2010;31:1030–1040. doi: 10.1002/hbm.20915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar BM, Thagard P. Spiking Phineas Gage: a neurocomputational theory of cognitive–affective integration in decision making. Psychol. Rev. 2004;111:67–79. doi: 10.1037/0033-295X.111.1.67. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Deichmann R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: a whole-brain analysis at 3 T and 1.5 T. NeuroImage. 2006;33:493–504. doi: 10.1016/j.neuroimage.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Winston JS, Gottfried JA, Kilner JM, Dolan RJ. Integrated neural representations of odor intensity and affective valence in human amygdala. J. Neurosci. 2005;25:8903–8907. doi: 10.1523/JNEUROSCI.1569-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Functional specialisation in the visual cortex of the rhesus monkey. Nature. 1978;274:423–428. doi: 10.1038/274423a0. [DOI] [PubMed] [Google Scholar]
- Zeki S, Romaya JP. Neural correlates of hate. PLoS ONE. 2008;3:e3556. doi: 10.1371/journal.pone.0003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.