Abstract
Ipilimumab is a fully human monoclonal antibody which blocks cytotoxic T-lymphocyte antigen-4, an immune checkpoint molecule that down-regulates pathways of T-cell activation. Ipilimumab has demonstrated a statistically significant improvement in overall survival in two randomized controlled phase III trials of patients with metastatic melanoma. A main complication of ipilimumab therapy is the development of inflammatory events which can occur in various organs, including the liver (i.e., hepatitis). Hepatic injury is a concern because it can develop with little warning and may potentially be severe. We analyzed liver biopsy findings in 4 cases of ipilimumab treatment-related hepatitis and compared them to a fifth, previously reported case. All 5 patients had a histologic pattern of injury that was similar to what is observed with acute viral and autoimmune hepatitis; however, the findings are not specific and require clinicopathologic correlation. Pathologic evidence of hepatitis resolved in all 5 patients with appropriate immunosuppressive therapy. Although a relatively uncommon adverse event with ipilimumab, patients should be monitored at regular intervals for biochemical/pathological evidence of hepatitis.
Keywords: Ipilimumab, Drug-induced hepatitis, CTLA-4, Immunosuppressive therapy, Metastatic melanoma
Introduction
Ipilimumab is a fully human, monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 (CTLA-4), an immune checkpoint molecule that negatively regulates T-cell activation [1]. It is hypothesized that CTLA-4 blockade can break peripheral tolerance to tumor antigens, promoting an antitumor immune response [2]. Ipilimumab has shown durable objective responses and encouraging long-term survival in phase II trials involving patients with metastatic melanoma [3–6]. In a phase III, randomized controlled trial, ipilimumab monotherapy demonstrated a statistically significant improvement in overall survival in previously treated patients with metastatic melanoma [7]. Recently, the results of another phase III trial with ipilimumab were reported for previously untreated patients with metastatic melanoma, which showed a statistically significant improvement in overall survival for ipilimumab plus dacarbazine compared with dacarbazine alone [8]. The treatment-related adverse events (AEs) with ipilimumab therapy can be severe and life-threatening, but most are reversible with appropriate treatment [7,8]. Treatment guidelines, which involve vigilant follow-up and early corticosteroid use, were used to manage AEs in ipilimumab clinical trials [9,10].
The most common treatment-related AEs with ipilimumab in clinical studies were inflammatory in nature [3–8]. The inflammatory AEs that occurred with ipilimumab monotherapy primarily affected the skin and gastrointestinal tract, but to a lesser extent affected the liver and endocrine system as well [3–7]. When ipilimumab was used in combination with dacarbazine in a phase III trial [8], much higher rates of elevated aminotransferases were observed compared with prior studies. Severe liver AEs (grade ≥3) were uncommon with ipilimumab monotherapy in clinical studies [3–7], but occurred at higher rates when ipilimumab was combined with dacarbazine in the phase III trial [8]. There are limited clinical descriptions of ipilimumab-related liver inflammation and only one report with biopsy findings to date [11]. We report 4 new cases of hepatitis in patients who received ipilimumab in clinical studies, along with a more complete histologic description of the previously reported case from the US National Cancer Institute [11].
Case Descriptions
The patients in this report had unresectable or metastatic melanoma and participated in clinical trials of ipilimumab at 10 mg/kg (CA184-007) [3] or 3 mg/kg (MDX010-05) [12] administered intravenously every 3 weeks for 4 doses. Four cases were from study CA184-007, a phase 2 trial which evaluated the effect of prophylactic oral budesonide on the rate of grade ≥2 diarrhea in previously treated and treatment-naïve patients who received ipilimumab [3]. The remaining case was from MDX010-05, a study involving the administration of ipilimumab with two melanoma-specific gp100 antigen peptides [12]. Eligibility criteria for both studies excluded viral hepatitis; all patients were negative for hepatitis B surface antigen and hepatitis C, and had baseline aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2.5 times upper limit of normal (ULN). Total bilirubin was ≤1.5 times ULN. In some cases, an autoimmune panel was tested, including rheumatoid factor, anti-dsDNA antibody, autoantibodies (SSA/RO IGG and SSB/LA IGG), and anticardiolipins (IGG-GPL and IGM-MPL). Table 1 gives a summary of the pertinent details of each case, and hepatic laboratory and histologic data are summarized in Tables 2 and 3.
Table 1.
Summary of case details
| Case # | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Study | CA184-007 | CA184-007 | CA184-007 | CA184-007 | MDX010-05 | |
| Location of treatment | USA | USA | USA | USA | NIH-NCI | |
| Age, gender | 63, male | 76, female | 59, male | 46, female | 43, male | |
| Timing of events , from study Day 1 | Ipilimumab treatments | 1, 21, 42, 64 | 1, 23, 43 | 1, 22, 43 | 1, 22 | 0, 21, 42 |
| Severe hepatic AEs (≥grade 3) | 71–225 | 43–92 | 63–116 | 39–42 | 69? | |
| Liver biopsy | 77 | 69 | Btwn 82 & 116 | Btwn 40 & 42 | 69 | |
| Viral serologies, all negative (type) | 7 (Viral hepatitis) | 6 (Viral hepatitus) | 7 (Viral hepatitis); 65 (Viral serologies) | 16 (Viral hepatitus) | (Viral serology) | |
| Autoimmune panels, all normala | 7,b 42 | 1,c 6,d 43, 78, 162 | −7, 40, 78, 162e | 6,d 43,f 78, 162 | at time of event | |
| Steroid treatment | 76–138 | 49–92, 107–135 | 65-83-C | 39–100, 107-C | yes | |
| Treatment with immune-suppressants | 85–225 | 97-C | 89-C | none | none | |
| Hospitalization | Yes | Yes | Yes | Yes | Yes | |
| Time of re-challenge | N/A | N/A | 385 | 175 | N/A | |
| Death | 351 | 300 | Alive at last follow-up | Alive at last follow-up | Died 1 year later | |
| Non-hepatic AEs |
Not drug-related, inflammatory:
|
Not drug-related, inflammatory:
|
Not drug-related, inflammatory:
Drug-related and inflammatory:
|
Drug-related and inflammatory:
|
Drug-related, inflammatory:
|
|
Autoimmune panels included rheumatoid factor, Ch50 kinetic assay, anti-dsDNA antibody, SSA and SSB/RO IgG autoantibodies, anticardiolipin IGG GPL and anticardiolipin IGM MPL
Reported only rheumatoid factor
Reported only erythrocyte sedimentation rate (ESR)
Reported only rheumatoid factor only
Only reported rheumatoid factor on day −7, 40, and 78; both rheumatoid factor and CH50 kinetic assay on day 162
CH50 kinetic assay results lower than normal
C=continuing (no resolution at last follow-up)
Table 2.
Summary of clinical and laboratory features.
| Case # |
Age/Sex | Doses of Ipilimumab Received |
Days Between Last Cycle and DILI Onset |
Laboratory Values at Onset |
Laboratory Values at the Peak ALT Elevation |
Maximum Bilirubin Elevation |
Days to Biochemical Resolution |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALT (IU/L) |
AP (IU/L) |
R* | ALT (IU/L) |
AP (IU/L) |
R* | ||||||
| 1 | 63/M | 4 | 7 | 1360 | 194 | 20.4 | 3070 | 367 | 22.9 | 5.1 | – |
| 2 | 76/F | 3 | 0 | 804 | 257 | 12.9 | 804 | 257 | 12.9 | 3.1 | 35 |
| 3 | 60/M | 3 | 20 | 438 | 146 | 6.7 | 1459 | 206 | 14.9 | 1.8 | 118 |
| 4 | 46/F | 2 | 17 | 320 | 396 | 1.8 | 326 | 427 | 1.7 | 1.5 | 46 |
| 5 (NCI case) | 43/M | 3 | 173 | 131 | 3.7 | 2860 | 401 | 20.2 | 2.2 | 134 | |
R, the ratio of ALT to AP, normalized to the upper limit of normal.
ALT, alanine aminotransferase; AP, alkaline phosphatase; DILI, drug-induced liver injury; NCI, National Cancer Institute.
Table 3.
Summary of pathologic findings.
| Case # |
Ishak Grading of Necroinflammation and Fibrosis* | Plasma Cells |
Eosinophils | Duct Injury | Pattern of Injury | ||||
|---|---|---|---|---|---|---|---|---|---|
| Periportal Inflammation |
Confluent Necrosis |
Focal Necrosis |
Portal Inflammation |
Fibrosis Stage |
|||||
| 1 | 4 | 3 | 4 | 3 | 0 | + | + | Present, mild | Acute hepatitis |
| 2 | 2 | 4 | 4 | 1 | 3 | − | − | Absent | Acute hepatitis |
| 3 | 1 | 1 | 4 | 1 | 0 | − | − | Absent | Acute hepatitis |
| 4 | 3 | 3 | 4 | 3 | 0 | − | + | Present | Acute hepatitis and Granulomatous hepatitis |
| 5 (NCI case) | 4 | 2 | 4 | 3 | 2 | − | + | Absent | Acute hepatitis and steatohepatitis |
Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699.
NCI, National Cancer Institute.
Case 1 (Study CA184-007)
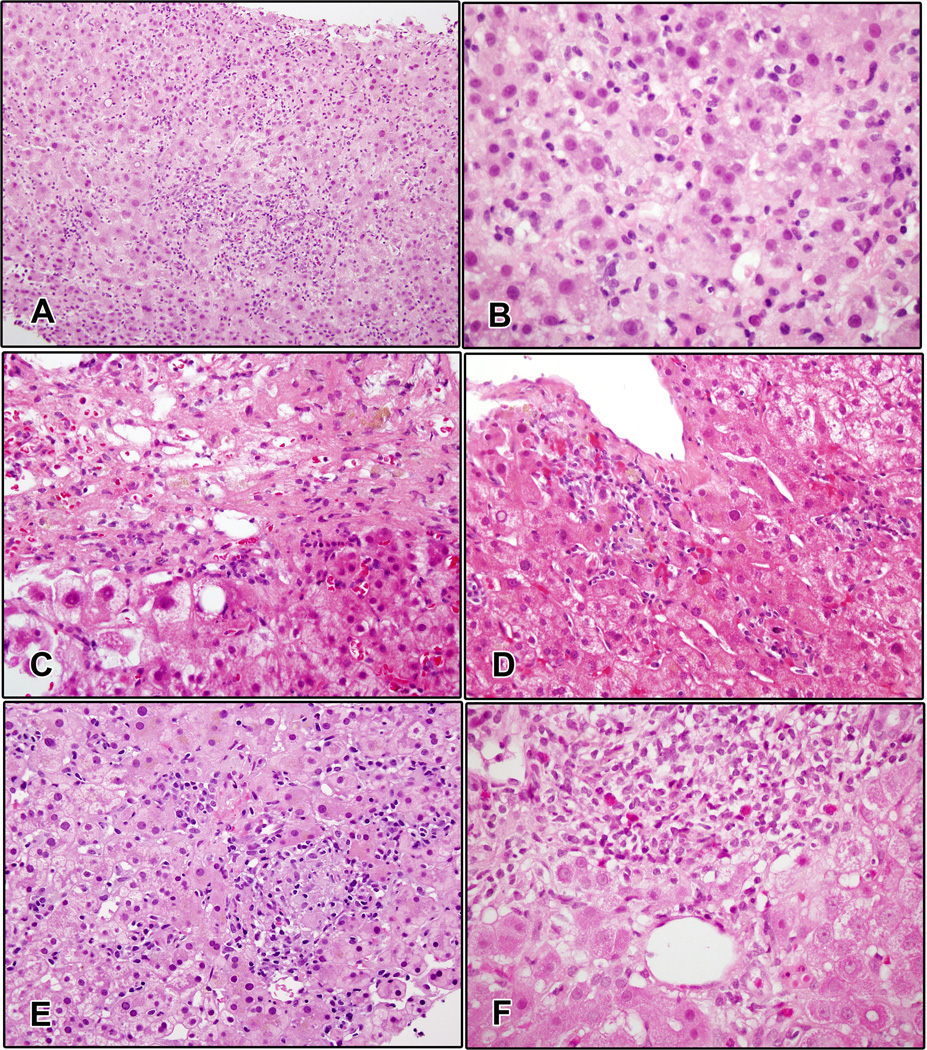
Patient 1 was a 63-year-old man who developed liver function abnormalities after the fourth dose of ipilimumab. The day he received his fourth dose (Day 64), ALT was 58 IU/L and AST was 44 IU/L. Eleven days later, ALT peaked at 3070 IU/L, AST was 1888 IU/L, alkaline phosphatase (AP) was 367 IU/L, total bilirubin was 3.5 mg/dL, and eosinophils rose to 1000 cells/µL. A liver biopsy performed on Day 77, 2 days after the peak of the ALT, showed acute hepatitis characterized by lobular disarray with numerous foci of lobular inflammation and scattered acidophil bodies, with accentuation of injury around central veins and endotheliitis (Fig. 1A and 1B). Portal areas exhibited moderate to marked lymphohistiocytic inflammation associated with marked interface hepatitis and increased numbers of eosinophils. Plasma cells were present but not prominent. There were no areas of multi-acinar or bridging necrosis. Hepatitis resolved by Day 225 with administration of corticosteroids and tacrolimus. Prior to the development of liver abnormalities, the patient’s autoimmune panel was normal (Day 42), indicating the absence of autoimmune disease. In addition, the patient experienced rectal hemorrhage (Day 49–51) prior to the hepatic events, although the investigator did not consider this to be drug-related. The patient died approximately from progression of melanoma 4 months after the hepatitis resolved(Day 351).
Figure 1. Representative photomicrographs of ipilimumab-related hepatitis.
A and B. Case 1. There is diffuse inflammation throughout portal areas and parenchyma, with lobular disarray and hepatocyte rosette formation. C. Case 2. There is confluent necrosis and early fibrosis with milder inflammation and ballooning injury. D. Case 3. Numerous foci of spotty lobular inflammation are seen near a large central vein. E. Case 4. The lobular inflammation in this case was mostly granulomatous, with large and small granulomas throughout the parenchyma. F. Case 5. There is portal inflammation with increased eosinophils and interface hepatitis.
Case 2 (Study CA184-007)
Patient 2 was a 76-year-old woman who had elevated liver enzymes on the day she received her third dose of ipilimumab (Day 43), and had elevated eosinophils (700 cells/µL) and monocytes (1500 cells/µL). Liver biopsy performed on Day 69, 9 days after ALT peak (Fig. 1C) revealed injury most suggestive of the postnecrotic phase of acute hepatitis. There was evidence of recent portal-central bridging necrosis, with accentuation of hepatocyte loss around veins. The predominantly lymphocytic inflammation was concentrated along the portal or septal interfaces. The parenchyma showed numerous foci of lymphocytic spotty inflammation and occasional hepatocyte rosettes. Some hepatocytes near the areas of central necrosis showed ballooning changes but no Mallory-Denk bodies were observed. Masson trichrome staining showed early bridging fibrosis. Liver function tests (LFTs) resolved after a month of treatment with corticosteroids and tacrolimus (Day 92). On day 43, 78, and 162 the patients’ immune panel was normal, suggesting that the hepatitis was not due to idiopathic autoimmune disease. The patient experienced several other inflammatory adverse events believed to be therapy-related: pruritis prior, rash during and after, and adrenal insufficiency sometime after the hepatic adverse events. Other drug-related but non-inflammatory symptoms included fatigue, hyperhiderosis, sweating, hot flashes, and nausea. The patient died from progression of melanoma approximately 7 months after LFTs resolved.
Case 3 (Study CA184-007)
Patient 3 was a 60-year-old man who developed liver AEs after 3 doses of ipilimumab. He had a prolonged course of hepatitis (Day 63–116), with ALT rising slowly from 438 IU/L at onset to 1459 IU/L approximately 4 weeks later. Although peripheral eosinophilia was noted on Day 40, 23 days prior to the onset of hepatitis, eosinophil levels were normal during the hepatic AEs. Monocyte levels were elevated on Day 64, however, viral serologies were negative. A liver biopsy, performed 6 days before the peak of the ALT, showed an acute hepatitis pattern with mild to moderate lymphocytic inflammatory activity (Fig. 1D). Most of the inflammation was in the form of lymphocytic spotty inflammation, concentrated in zone 3. Acidophil bodies were present, and there was mild perivenular hemorrhage. There was no confluent or bridging necrosis and no cholestasis. Immunophenotyping studies showed that the vast majority of the infiltrating lymphocytes were CD8+ T cells, with only rare CD4+ T cells and B cells found mainly in the portal areas. Scattered lymphocytes within the lobular infiltrate were positive for perforin and granzyme B. The patient was treated with corticosteroids, with gradual resolution of ALT. Rheumatoid factor was measured and was normal on Days −7, 40, 78 and 162, consistent with the absence of autoimmune disease before, during, and after the hepatic events. Prior to the hepatic adverse events, the patient had experienced therapy-related inflammatory events of the skin, including macular rash and pigmented macule; the latter did not resolve. He also experienced stomatitus prior to the hepatic events, which was inflammatory in nature but not considered drug-related. Non-inflammatory drug-related symptoms included headache and a nail disorder. Approximately 9 months after the resolution of the hepatitis, and 55 days after LFTs had returned to normal, he was rechallenged with ipilimumab at 10 mg/kg. His liver biochemistries became abnormal after 7 days, with AP peaking at 442 IU/L (day 14) and ALT peaking at 790 IU/L (day 22). His bilirubin rose to only 1.3 mg/dL. He was treated with corticosteroids and mycophenolate with gradual resolution of ALT. The patient was alive at last follow-up.
Case 4 (Study CA184-007)
Patient 4 was a 47-year-old woman who developed liver AEs after 2 doses of ipilimumab (beginning on day 39). Her injury was initially cholestatic, with AP elevated to 427 IU/L, AST 329 IU/L, and ALT 326 IU/L, although the bilirubin rose to only 1.5 mg/dL. Her eosinophil count rose to 530 cells/µL. A liver biopsy performed on the day of peak ALT, showed a pattern of acute and granulomatous hepatitis (Fig. 1E). There were numerous foci of lobular inflammation with accentuation in zone 3 and venular endotheliitis. Many of these were microgranulomas but larger nonnecrotizing epithelioid granulomas were also present. Acidophil bodies were frequent. Portal areas showed a mixed infiltrate with eosinophils associated with moderate interface hepatitis and duct injury. She was treated with corticosteroids, with hepatitis resolving on Day 42, and gradual resolution of AST and ALT to normal levels on Day 78. The autoimmune panel was taken on day 43, 78, and 162, and all results were normal, consistent with the absence of autoimmune disease after the hepatotoxic events. Prior to, during, and after the hepatic AEs the patient experienced diarrhea that was considered to be inflammatory and treatment-related. She also experienced pyrexia, fever, nausea, headache, abdominal pain, and fatigue shortly before the onset of hepatitis. On Day 175, a little over 3 months after full resolution of LFTs, and over 1 month after resolution of the diarrhea, she was rechallenged with ipilimumab at 10 mg/kg. Six days after receiving a single dose, she developed a cholestatic liver injury with an ALT of 143 IU/L, AST of 104 IU/L, AP of 184 IU/L, and a total bilirubin of 3.2 mg/dL (Fig. 2). The AP peaked first at 301 IU/L 11 days after the dose, and ALT peaked at 304 IU/L after 4 weeks. The patient was treated with corticosteroids and mycophenolate, with resolution of the elevated liver function tests 5 months after the ipilimumab dose. At last follow-up, the patient was alive.
Figure 2.
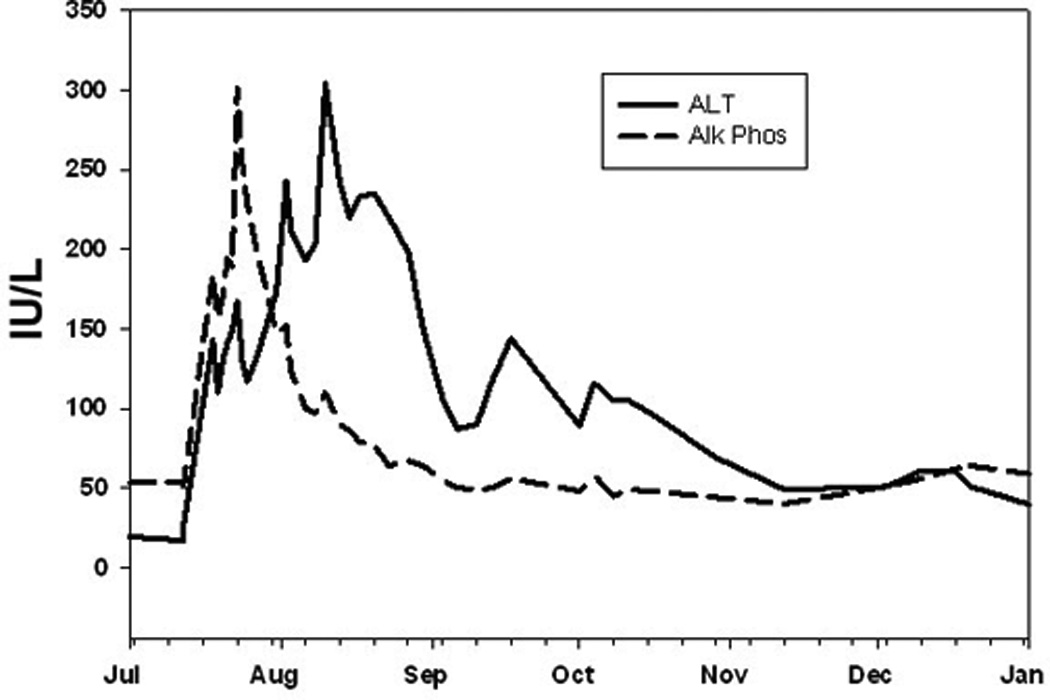
Enzyme profile seen after rechallenge (arrow) of case 4 with one dose of ipilimumab at 10 mg/kg. Note the early rise of alkaline phosphatase (AP) followed by alanine aminotransferase (ALT). Minor tick-marks denote weeks.
Case 5 (MDX010-05)
This patient was previously described in a review of inflammatory AEs following ipilimumab therapy (3 mg/kg) for metastatic melanoma [11]. He was a 43-year-old man who developed abnormal liver enzyme levels after 3 doses of ipilimumab, first detected on Day 43, which rose to grade 4 by Day 64, and which peaked at an ALT of 2860 IU/L. At the time of the event, repeat viral serologies for hepatitis B and C were negative, antinuclear antibody was positive at 2.4 enzyme-linked immunosorbent assay units, and antibodies to liver and kidney microsomes and anti-smooth muscle antibodies were negative. Serum immunoglobulin G was within normal limits. The absolute eosinophil count rose to 889 cells/µL. A liver biopsy, which was performed on Day 69, the day after the ALT peaked, showed a mixed injury with features of both acute hepatitis and steatohepatitis (Fig. 1F). There were numerous foci of lymphohistiocytic lobular inflammation with scattered acidophil bodies. Mild steatosis was present in zone 3, along with ballooning hepatocellular injury and rare Mallory-Denk bodies. The portal areas showed moderate lymphocytic and eosinophilic inflammation with marked interface hepatitis. Masson trichrome stain revealed both central perisinusoidal fibrosis and periportal fibrosis. The patient was treated with low dose oral prednisone, with gradual normalization of liver function tests. This patient also presented with vitiligo, macropapular rash, and erythema at the same time as the hepatic inflammatory events. The patient died approximately 1 year later from progression of melanoma.
Discussion
Inflammatory hepatic AEs related to ipilimumab monotherapy were uncommon in clinical studies. Of any grade, these AEs were reported in 3.8% (5/131) of patients treated with ipilimumab monotherapy at 3 mg/kg in a phase III trial [7], and in 14.8% (17/115), 2.8% (2/71) and 9.0% (14/155) of patients who received ipilimumab monotherapy at 10 mg/kg in the phase II studies CA184-007 [3], CA184-022 [4], and CA184-008 [5], respectively. Although uncommon with ipilimumab monotherapy, elevations of ALT and AST (regardless of causality) occurred at 33.2% and 29.1%, respectively, with ipilimumab plus dacarbazine compared with 5.6% for dacarbazine alone in a phase III trial [8]. Elevated aminotransferases were among the most common immune-related AEs in this study, likely due to a synergistic effect between the two agents [8]. Notably, most cases of liver AEs responded to corticosteroids in this trial, as did the patients in our present study [8]. Hepatic injury is of concern because liver failure can develop with little warning and liver enzyme elevations only loosely correlate with the degree of inflammation and necrosis. While the exact mechanism of hepatic AEs related to ipilimumab therapy remain unclear, these AEs can be managed with corticosteroid administration.
We present 4 new cases of ipilimumab treatment-related hepatitis, and a more complete histologic description of a fifth, previously reported case [11]. These 5 patients had a similar histologic pattern of injury consistent with acute hepatitis; 4 of the 5 patients had hepatocellular injury diagnosed by elevated liver function tests, with a normalized ratio of ALT to AP >5 at the onset of injury. Based on these data, the differential diagnosis includes drug-induced liver injury (DILI), idiopathic autoimmune hepatitis (AIH), acute viral hepatitis, and acute alcoholic liver disease. Correct diagnosis of liver injury etiology is difficult, and the evidence required to distinguish between causalities is widely debated, although it is clear that using a combination of lab tests and histology improves the accuracy of diagnosis [13, 14]. DILI is especially difficult to diagnose because the characteristics can be highly drug-specific [15]. Although more data would be necessary to strictly rule out causes other than DILI, circumstantial evidence suggests that the hepatic AEs observed were caused by DILI by ipilimumab, and that other etiologies are less likely.
Acute viral hepatitis may develop from any of the hepatitis viruses [16]. The diagnosis of acute viral hepatitis is based on clinical presentation and serologic findings, and the distinction of DILI or AIH from acute viral hepatitis is not possible based solely on liver biopsy. All patients had negative viral serology prior to initiation of therapy, and two patients (case 3 and 5) had negative viral serology at the time of the hepatic event, suggesting that the hepatitis observed was not viral. The inflammatory features seen in these patients, such as immune-cell infiltration, interface hepatitis, and serum eosinophilia, as well as biliary changes, can occur in viral hepatitis, AIH or DILI [13]. Therefore these features do not rule out viral causality. DILI is best distinguished from viral hepatitis based on temporal relationships to administration of drug therapy and corticosteroid or immunosuppressant treatment. Consistent with DILI and inconsistent with viral causality, the hepatotoxicities described here had onsets that correlated with ipilimumab treatment, showed responsiveness to corticosteroid immunosuppressants and cessation of ipilimumab, did not relapse upon removal of treatment with corticosteroids or immunosuppressants, and rapidly recurred when re-challenged with ipilimumab in a companion study (2 patients). Clinically, acute alcoholic hepatitis may mimic acute viral or drug-induced hepatitis, but the histologic changes are very different, often having features of steatohepatitis [17]. The absence of Mallory-denk bodies in the biopsies described suggests against cirrhosis, which is rare in DILI [15], consistent with the hypothesis that the liver injuries seen were not due to alcohol.
Distinguishing between AIH and DILI is particularly challenging as these two forms of liver injury share many features, and AIH can be triggered or unmasked by drug-usage [14, 15, 18]. The distinction becomes even more difficult when the drug under consideration is an immunotherapy. Both AIH and DILI can be asymptomatic, usually show dramatically elevate LFTs (even more so than other types of liver injury), commonly cause serum eosinophilia and elevate serum IgG, respond to corticosteroids, can be accompanied by other inflammatory events, such as rash or fever, and share many histologic features, such as portal and peri-portal immune cell infiltrates, focal necrosis, interface hepatitis, fibrosis, and rosette formation [13–15, 19]. Two recent retrospective analyses identified subtle histologic differences between AIH and DILI [13, 19]. Specifically, a number of histological features are seen in both AIH and DILI, but are more common or more pronounced in AIH: interface hepatitis, portal inflammation, confluent necrosis, focal necrosis, emperipolesis, rosette formation, and fibrosis [13, 19]. Cholestasis can also be seen in both AIH and DILI, but is more prevalent in DILI. Both AIH and DILI can present with a variety of immune cell infiltrates, but the types and distribution of these infiltrates may distinguish between the two causalities. Portal plasma cells are significantly more prevalent in AIH, whereas prominent portal neutrophils may be a distinguishing marker of DILI, and portal eosinophils and lymphocytes have similar prevalence in both forms of liver injury [15, 19]. Intra-acinar plasma cells and eosinophils are also more likely in AIH, while prominent intra-acinar lymphocytes are more likely in DILI, and intra-acinar neutrophils are equally uncommon for both DILI and AIH [15, 19]. In the 5 cases described here, the acute hepatitis pattern that mostly lacked infiltrating plasma cells favors diagnosis of DILI over AIH. Classic AIH usually presents with advanced fibrosis [18]. Three of the patients had no fibrosis at the time of biopsy (cases 1, 3, 4), consistent with the acute presentation. Case 5 had both periportal and perisinusoidal fibrosis, which may have been due to underlying steatohepatitis unrelated to ipilimumab. In case 2, the pattern of fibrosis and inflammation was consistent with the postnecrotic fibrosis that sometimes follows severe acute hepatitis. Three cases had eosinophils and 1 had granulomas, suggesting a hypersensitivity reaction.
Although once thought to be a distinguishing feature, serum auto-antibodies have been observed in both AIH and DILI, but are more common in AIH, present in up to 95% of AIH cases [13–15]. Autoimmune serologies were determined at the time of the event for cases 2, 3, and 5, including tests of rheumatoid factor, Ch50 kinetic assay, anti-dsDNA antibody, SSA and SSB/RO IgG autoantibodies, anticardiolipin IGG GPL and anticardiolipin IGM MPL, and all came out normal (negative for autoantibodies). Although the presence of autoantibodies cannot be used to distinguish AIH from DILI, the high incidence of elevated serum autoantibodies in AIH observed in some studies suggests that the absence of serum autoantibodies could be informative. The absence of these antibodies in the patients that were tested suggests that the hepatotoxicities observed were due to DILI rather than AIH, and that ipilimumab-induced livery injury, although possibly an immune-mediated phenomenon, nonetheless differs from AIH on the molecular level. Given that for other organ systems ipilimumab-related immune-related adverse reactions (irAEs) are dissimilar from autoimmune disease (e.g. gastrointestinal irAEs differ from inflammatory bowel disease (IBD) [20], it is not surprising that ipilimumab-associated hepatitis is dissimilar from autoimmune hepatitis.
Given the controversy over interpretation of histology results and lab tests, it is not surprising that the Roussel Uclaf Causality Assessment Method (RUCAM) for determining the likelihood of DILI (over other causes) focuses on other forms of circumstantial evidence. Namely, the criteria relevant to these case studies are as follows: the relationship between the start of therapy and onset of hepatotoxicity, time to resolution after drug cessation, time to recurrence upon re-challenge, and previous evidence of hepatotoxicity of the drug in question [14]. Consistent with these criteria, the hepatotoxicities described here were similar to those seen in ipilimumab-treated patients in phase 3 trials [7, 8], had a temporal relationship to therapy, and for the 2 patients who were well enough to be rechallenged with ipilimumab, liver inflammation recurred within a week of a single dose of ipilimumab. Furthermore, all patients had other inflammatory adverse events either before, during, or after the hepatic adverse events, supporting the hypothesis that these toxicities were due to ipilimumab. Another key difference between idiopathic AIH and drug-induced liver injury is that patients with drug-induced forms are can achieve lasting remission even when taken off immunosuppressive therapy shortly after resolution of symptoms, while those with idiopathic disease usually require 2–4 years maintenance therapy in order to achieve lasting remission [21]. The patients described here responded promptly to immunosuppression and did not relapse upon cessation of corticosteroids or immunosuppressant treatment.
In summary, the histologic changes observed with ipilimumab-related hepatitis are similar to those with acute viral and autoimmune hepatitis. As in other forms of hepatitis, the diagnosis of ipilimumab-related hepatitis will require clinicopathologic correlation since the pathologic findings are not specific. Hepatic inflammation in the 5 patients reported here resolved with appropriate immunosuppressive therapy. Thus, patients who receive ipilimumab therapy should be monitored at regular intervals for biochemical and pathological evidence of hepatitis so that appropriate treatment can be promptly administered.
Acknowledgments
Grant support: This research was supported in part by the Intramural Research Program of the US National Institutes of Health (National Cancer Institute).
Editorial and writing assistance was provided by StemScientific, funded by Bristol-Myers Squibb Co.
Abbreviations used in this paper
- AE
adverse event
- ALT
alanine aminotransferase
- AP
alkaline phosphatase
- AST
aspartate aminotransferase
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- ULN
upper limit of normal
Footnotes
Disclosures:
David E. Kleiner: No financial, professional or personal conflicts of interest exist with respect to this work.
David Berman is an employee of Bristol-Myers Squibb Company and discloses ownership of equity in the company.
References
- 1.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 2.Wolchok JD, Saenger Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist. 2008;13(suppl 4):2–9. doi: 10.1634/theoncologist.13-S4-2. [DOI] [PubMed] [Google Scholar]
- 3.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 4.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 5.O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with previously treated, advanced melanoma: a multicenter, single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 6.Maio M, Lebbé C, Neyns B, et al. Three-year survival rates for patients with advanced melanoma who received ipilimumab at 10 mg/kg in phase II trials [Abstract]. Presented at the XIV Annual Meeting of the Perspectives in Melanoma; 2010 September 17–18; Amsterdam, The Netherlands. [Google Scholar]
- 7.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011 Jun 5; doi: 10.1056/NEJMoa1104621. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 10.Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu D, Wang Q, Wang H, et al. Validation of the simplified criteria for diagnosis of autoimmune hepatitis in Chinese patients. J Hepatol. 2011;54:340–347. doi: 10.1016/j.jhep.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver Injury. Clin Pharmacol Ther. 2011;89:806–815. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- 15.Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011;56:958–976. doi: 10.1007/s10620-011-1611-4. [DOI] [PubMed] [Google Scholar]
- 16.Suriawinata AA, Thung SN. Acute and chronic hepatitis. Semin Diagn Pathol. 2006;23:132–148. doi: 10.1053/j.semdp.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 18.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, Brunt EM, Kleiner DE, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931–939. doi: 10.1002/hep.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11–21. [PMC free article] [PubMed] [Google Scholar]
- 21.Sulz MC, Gerlach TJ. Autoimmune hepatitis. Ther Umsch. 2011;68:189–194. doi: 10.1024/0040-5930/a000149. [DOI] [PubMed] [Google Scholar]