Abstract
Ionizing radiation therapy is a crucial treatment for cancer, but can damage surrounding normal tissues. Damage to articular cartilage leading to arthropathy can occur at irradiated sites. It is unclear whether this response is due to damaging surrounding skeletal structures or direct effects on cartilage. In this study, we showed that irradiation with 2 Gy of X-rays causes a significant reduction in the stiffness of porcine explants 1 week post-irradiation. By using both microindentation and indentation-type atomic force microscopy, ionizing radiation reduces stiffness in both the superficial zone and throughout the entire thickness of the tissue. Young’s modulus values were 75% and 60% lower in 2 Gy irradiated samples when compared with controls using microindentation and nanoindentation, respectively. Glycosaminoglycans (GAGs) released into the culture media of irradiated samples was nearly 100% greater at 24 hours after exposure. While collagen content in the tissue is similar between groups, GAG content is 55% lower in irradiated explants compared with controls by one week. Therefore, the irradiated explants are unable to recover from the initial loss of GAGs by one week. This acute loss of GAGs is a likely contributor to the reduction in modulus seen after exposure to ionizing radiation.
Keywords: articular cartilage, radiation exposure, cartilage mechanics, atomic force microscopy, glycosaminoglycans
INTRODUCTION
Ionizing radiation has developed into a key treatment option to prevent tumor growth and metastasis in cancer patients. However, radiation can cause both acute and chronic damage to normal tissues through a dynamic process involving both cell death and altered cell and tissue function independent of reduced viability.1, 2 Musculoskeletal tissues have historically been considered late-responding tissues,3, 4 with bone damage and fractures of irradiated sites a well documented response to exposure for treatment of malignancies, especially in the femoral neck or sacrum for pelvic malignancy5, 6 or ribs following stereotactic body radiation therapy.7 However, recent studies have shed new light onto the sensitivity of skeletal tissues to low doses of radiation, with early skeletal deficits occurring after exposure resulting from elevated osteoclast activity.4, 8 Joint injury, including degenerative arthritis or arthropathy within synovial joints, are also considered late consequences of radiation exposure.9 The arthropathy observed in the hip and knee (commonly termed “post-irradiation osteoarthritis”) are generally attributed to osteonecrosis rather than chondrocyte-induced cartilage degradation.10, 11 Little information is known regarding early effects on articular cartilage metabolism or mechanical properties following exposure to radiation.
While the radiation response of articular cartilage from embryonic or very young animal models remain inconsistent,12–14 articular cartilage from adult humans or large animal species appears to degrade following exposure.12, 15 This response is characterized by an active degradation of cartilage matrix and reduced proteoglycan production in pigs,15 dogs,12 and from human donors.15 Collagen II synthesis following radiation exposure has been shown to be lowered in articular chondrocytes harvested from a large animal species (bovine).16 If radiation alters cartilage matrix metabolism, including active degradation of proteoglycans or lowered proteoglycan or collagen II synthesis, a reduction compressive modulus of the irradiated cartilage is imminent. In our pilot study, we showed a significant decrease in Young’s modulus of articular cartilage in mice one week after a 2Gy, whole-body X-ray irradiation.17
The goal of this study was to characterize the alterations in mechanical properties and matrix composition in porcine articular cartilage explants following direct exposure to X-rays, a model that has been shown to respond similarly to human tissues.15 No studies have addressed the possible mechanical alterations that might occur as a result of the direct damage to cartilage following radiation exposure. While our prior studies showed a marked decrease in modulus following radiation exposure, the scope of these results is limited. Due to the small sample size of murine articular cartilage, indentation-type atomic force microscopy (IT AFM) was used to measure the Young’s modulus of the tissue. However, the limitations of IT AFM only allow for the tissue to be indented 1–2µm, while the measured thickness of our articular cartilage samples was found to be ~70µm. Therefore, in this study, we ran mechanical tests at multiple length scales to determine if radiation exposure reduces the stiffness of articular cartilage only in the superficial zone, or if it affects the bulk properties of the tissue.
MATERIALS AND METHODS
Tissue Harvest and Culture
Articular cartilage was excised from fresh tibiofemoral condyles of 4–6 month old swine using aseptic techniques (Snow Creek Meat Processing, Seneca, SC). Explants were cut into disks 5 mm in diameter and ~2 mm thick using a dermal biopsy punch. All samples were measured for thickness using a digital caliper (Mitutoyo, Aurora, IL) and only explants 2.0 ± 0.1 mm in thickness were used. Care was taken to avoid collecting subchondral bone with the cartilage samples. Cartilage disks were rinsed in Hank’s balanced salt solution (HBSS) supplemented with 1% penicillin/streptomycin and amphotericin B (Gibco, Carlsbad, CA). After 3 rinses, explants were individually placed in 24-well plates and separated into control and irradiated groups. All samples were cultured at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA), 1% nonessential amino acids, 1% penicillin G, 1% streptomycin, and 1% amphoticerin B (Gibco), 20 mM ascorbic acid, 10 mM HEPES buffer, and 0.4 mM proline (Sigma-Aldrich, St. Louis, MO).18 Culture media was replaced every other day. For all studies, each animal was represented with 2 explants, 1 for the control group and 1 for the treatment group (2Gy X-ray irradiation).
X-Ray Irradiation
Cartilage disks were equilibrated for 24h in tissue culture conditions before irradiation. The covers of the 24-well plates were removed and the plates were placed in sterile plastic bags to prevent attenuation of the X-rays (the X-ray source was above the samples). Explants in the irradiated group were exposed to 2 Gray (Gy) of 125 peak kilovoltage (kVp) X-rays using a 150kV industrial portable X-ray unit (Philips Medical Systems, Bothell, WA). Control samples were placed in plastic bags and transported to the X-ray facility (but not irradiated) to prevent discrepancies in culture conditions.
AFM Nanoindentation Testing
Samples from both groups (n = 5) were cultured for either 24h or 168h after radiation exposure (or sham irradiation for control group). Each explant was placed on a glass slide, hydrated with culture media, and securely mounted on the atomic force microscope (AFM). Standard indentation testing in fluid was performed on an Asylum Research MFP-3D (Goleta, CA). A borosilicate glass cantilever with a 0.12 N/m nominal spring constant and a 5µm diameter spherical indenter was utilized for the indentation testing. Samples were indented 1µm at a speed of 1µm/s.
Microindentation Testing
Control and irradiated samples (n = 5) were cultured and tested using microindentation at the same time points as the AFM indentation. Each samples was placed in a Petri dish and covered in culture media. Cartilage disks were indented using a CETR Universal Mechanical Tester with a 20N load cell and a 1mm diameter stainless steel spherical indenter (Campbell, CA). Explants were pre-loaded to a force of 0.15N for 20s, then indented 300µm at a speed of 5µm/s.
Indentation Data Analysis
Both the nanoindentation and the microindentation curves were fit the to Hertz model, which assumes an infinitely hard sphere indents a flat, linear elastic, infinite half-space: where F is the measured force (N), E is apparent Young’s modulus (Pa), υ is Poisson’s ratio, and R is the spherical indenter radius (R = 2.5 µm), and δ is the indentation depth (m).19 Since biological tissue is nearly incompressible at the indentation rates used in the present study, the Poisson’s ratio (υ) was assumed to be 0.5.20 Since cartilage is viscoelastic in nature and the Hertz model assumes linear elastic behavior, only the first 250nm and 100µm of indentation were used to fit the Hertz model to the nanoindentation and microindentation curves, respectively. Each explant was indented at 3 different locations and AFM samples were indented 5 times at each location.
Quantification of GAG Content
Glycosaminoglycan (GAG) degradation and synthesis was analyzed by measuring the sulfated GAG (sGAG) quantity in the cartilage explants and in culture media. Conditioned media was collected immediately before irradiation (Day 0) and 1, 3, and 7 days after radiation exposure. Articular cartilage tissue explants were digested 7 days post-irradiation using previously described methods.21 Briefly, explants were individually placed in 500µL papain solution consisting of 125µg/mL papain, 0.1M sodium acetate, 5mM EDTA, 5mM L-cysteine-HCl and heated at 60°C for 12h (Sigma-Aldrich). Media and digests were tested for sGAG content using the dimethylmethylene blue colorimetric assay.22, 23 Using a 96-well plate, 180µL of dimethymethylene blue was added to 20µL of each sample. The standard curve was created using increasing concentrations of chondroitin sulfate (Sigma-Aldrich). Absorbance was read immediately at 525nm using a Synergy 3 microplate reader and each reaction was performed in triplicate (BioTek, Winooski, VT). Sulfated GAG content for each sample was determined by substituting its absorbance measurement into the linear regression equation determined using the standard curve, then normalized to each cartilage explant’s wet weight.
Quantification of Collagen Content
Collagen concentrations were assessed in the explants 7 days after radiation exposure using the hydroxyproline (HYP) colorimetric method.24, 25 Following papain digestion as described before, 100µL of the tissue lysates were hydrolyzed by adding 900µL of 6M HCl and heated at 110°C for 18h. Following hydrolysis, the lysates were neutralized using NaOH and diluted to 5mL to minimize salt concentrations. In a 96-well plate, 100µL of Chloramine T was added to 10µL of each sample and incubated for 5 minutes at room temperature. Then, 100µL of Ehrlick’s reagent (DMAB) was added to each well and incubated for 90m at 60°C. Trans-4-hydroxy-L-proline in increasing concentrations was used to create the standard curve (Sigma-Aldrich). All reactions were performed in triplicate. The absorbance was read at 560nm on the microplate reader. HYP content was calculated by using the measured absorbance and the linear regression equation fit to the standard curve, then normalized to tissue wet weight.
Histology
Histological techniques were used to examine alterations to cell morphology and matrix composition in the explants following radiation exposure. Control and irradiated cartilage samples were fixed with 10% neutral buffered formalin 1 and 7 days after radiation exposure. Cartilage disks were embedded in paraffin wax and cut into 6µm cross sections using a microtome. Explants were then stained with 1 of 3 stains. Hematoxylin and Eosin (H&E), Safranin O-Fast Green, and Masson’s Trichrome were used to assess cell viability, GAG integrity, and collagen content, respectively.
Statistics
All data are shown as mean ± standard deviation. Significance was determined using SigmaStat version 3.5 (Systat Software, Inc.; Richmond, CA). For both the microindentation and nanoindentation data, a Student’s t-test was used to compare Young’s modulus between control and irradiated groups (n=15 per group). One-way analyses of variance (ANOVAs) were used to test for inter-animal variability within each group (n=3 per animal). Student’s t-tests were used to test for significance in GAG content between control and irradiated samples for the DMB assay on the culture media (n=6 per group) and tissue (n=9 per group). Additionally, one-way ANOVAs followed by Tukey’s post hoc tests were used to compare GAG content in conditioned culture media between different days within the same treatment group (n=6 per day). For the hydroxyproline assay, a Student’s t-test was used to test for significance in collagen content control and irradiated cartilage explants (n=9 per group).
RESULTS
Mechanical Testing
One week after radiation exposure, the average Young’s modulus in the articular cartilage calculated from both the microindentation and AFM curves was significantly lower in the irradiated groups when compared to the non-irradiated groups (p < 0.001, Figures 1 & 2). The Young’s modulus values were ~75% and 60% lower in the irradiated cartilage when compared with the control cartilage for microindentation and nanoindentation, respectively. For both mechanical testing modalities, there were no significant differences in Young’s modulus between animals within each group (p > 0.05).
Figure 1.
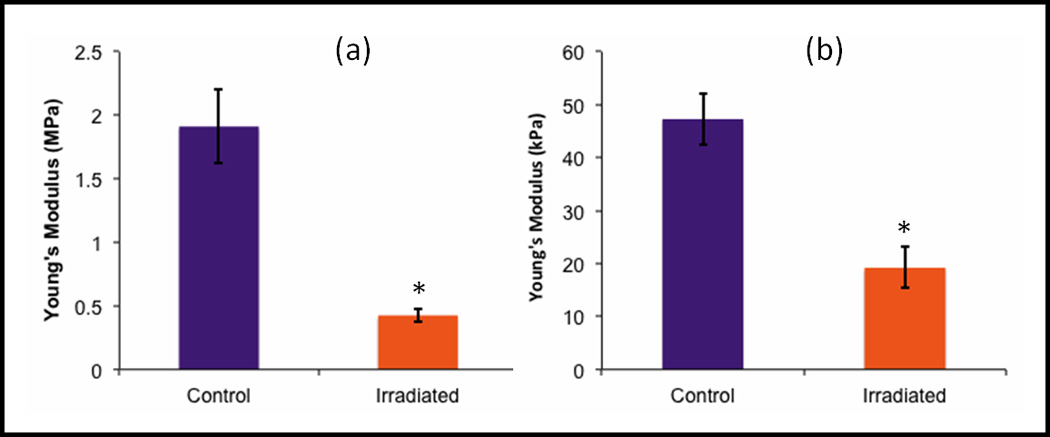
Young’s modulus values for control (purple) and 2Gy-irradiated samples estimated using the Hertz model tested by a) microindentation and b) nanoindentation. The modulus values for irradiated samples were significantly lower than control samples using both mechanical tests (*, p < 0.001, n = 15). Error bars show ±standard deviation.
Figure 2.
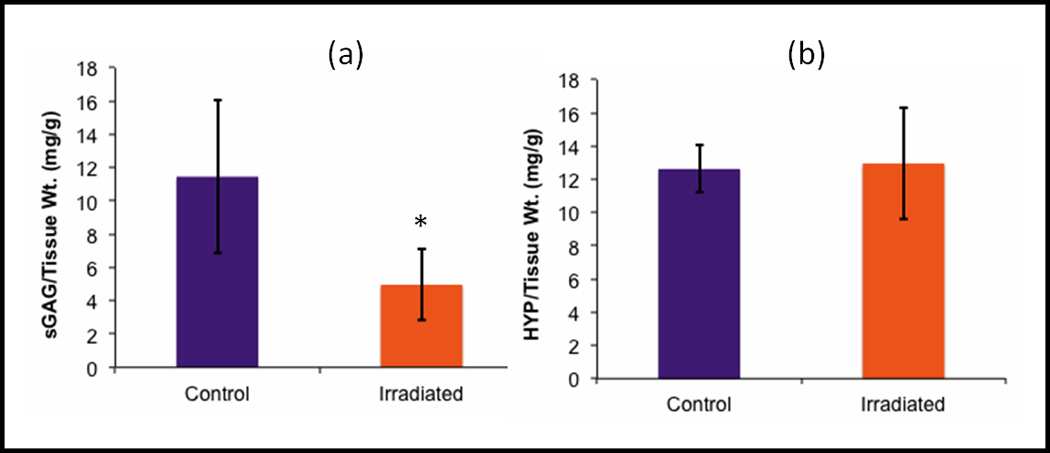
Normalized a) sGAG and b) HYP content in the tissue at Day 7, the time point used for mechanical testing. Irradiated samples had significantly lower sGAG content when compared to control groups (*, p < 0.05, n = 9). HYP content was similar between groups. Error bars indicate ±standard deviation.
Dimethylmethylene Blue Assay
Using the DMB assay, normalized sulfated GAG (sGAG) content was significantly lower in the 2 Gy irradiated cartilage tissue when compared with the control cartilage 7 days after radiation exposure (p < 0.001, Figure 3). The DMB assay also showed significantly higher sGAG released into the culture media in irradiated samples when compared to control samples at Day 1 (p < 0.001), while there was no significant difference in sGAG content released between treatment groups at the Day 0, 3, or 7 time points (p > 0.05) (Figure 4). Sulfated GAG concentrations in the conditioned culture media at Day 1 were approximately 100% greater in the irradiated group when compared with the control group. Moreover, GAG concentrations in the culture media of the irradiated cartilage samples were significantly higher at Day 1 than Days 0, 3, and 7 (p < 0.001). Sulfated GAG content in the media of the control group was significantly higher at Day 1 compared to Day 3 (p < 0.01).
Figure 3.
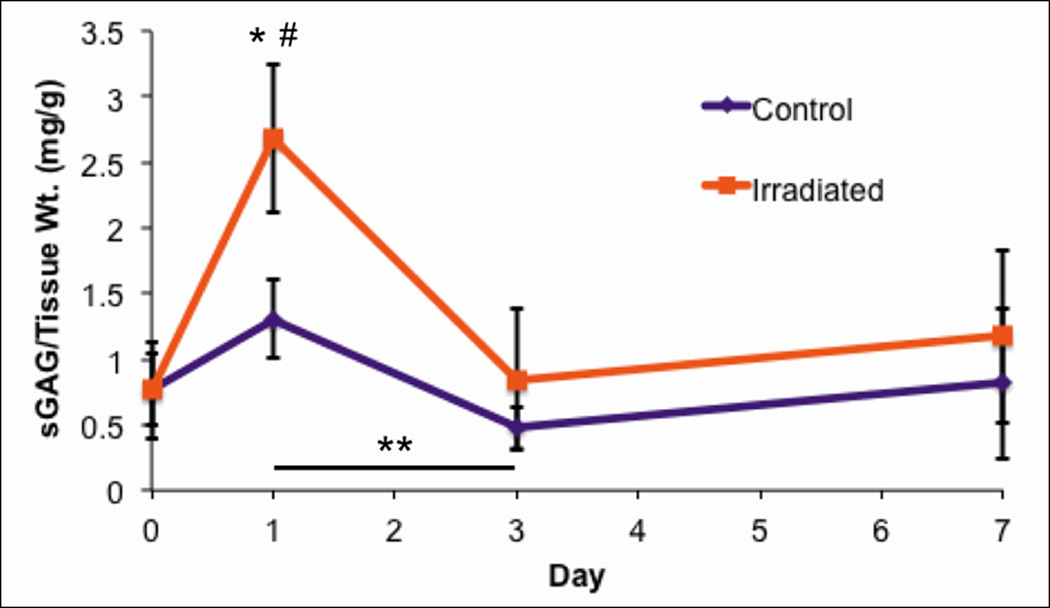
Normalized sGAG content in the culture media over time (n = 6, error bars ±standard deviation). Significantly higher sGAG was released into the media in irradiated samples when compared to control samples at Day 1 (*, p < 0.001), while there was no significant difference in sGAG released between treatment groups at the Day 0, 3, or 7 time points. Concentrations of sGAG in the media of the irradiated samples were significantly higher at Day 1 when compared to Days 0, 3, and 7 (#, p < 0.001). Additionally, sGAG content in the media of the control samples was significantly greater at Day 1 when compared with Day 3 (**, p < 0.01).
Figure 4.
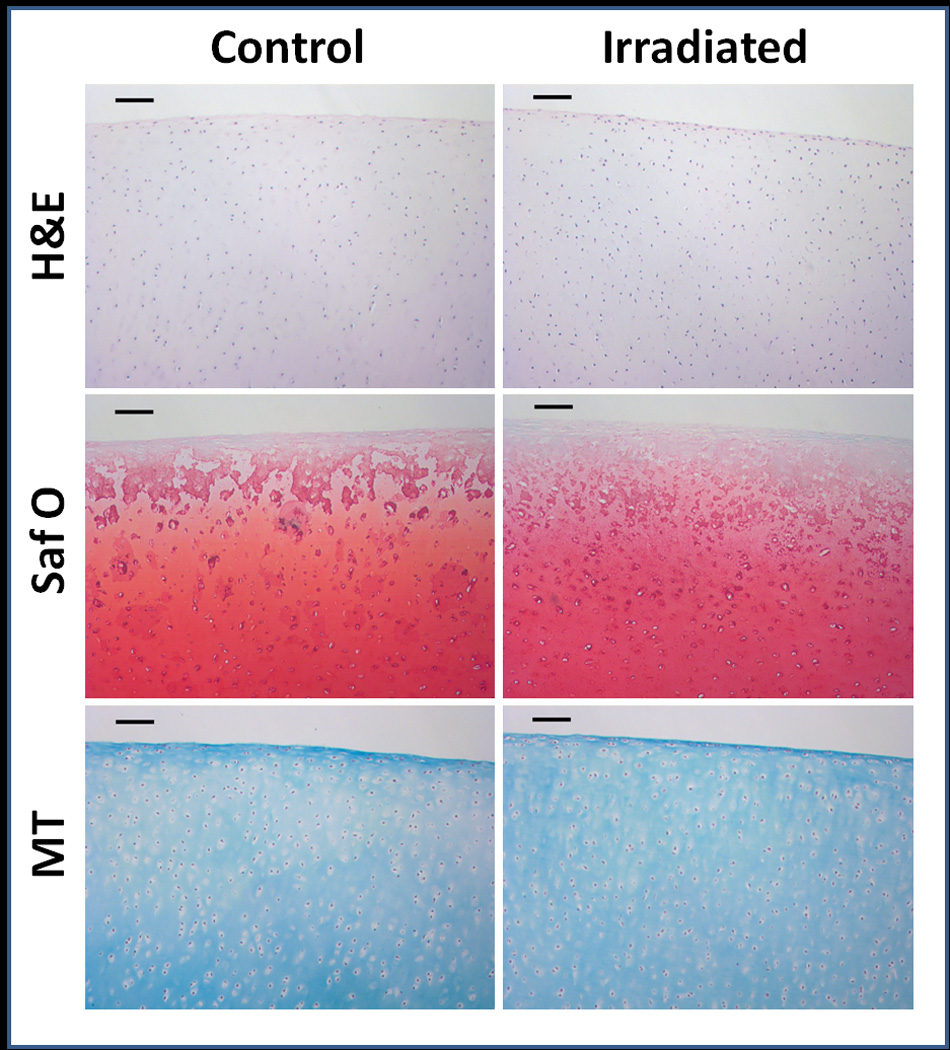
Histological cross sections of articular cartilage explants stained with H&E, Safranin O, and Masson’s trichrome. Qualitatively, there were no differences seen between control and irradiated samples. Scale bars represent 100µm.
Hydroxyproline Assay
Normalized HYP concentrations in the cartilage tissue 1 week post-irradiation averaged at 12.62 ± 1.43 and 12.96 ± 3.35 mg HYP/g total tissue for control and irradiated samples, respectively (Figure 5). HYP content between control and irradiated cartilages explants was similar.
Histology
Qualitatively, no differences were seen between control and irradiated samples for all 3 histological stains (Figure 6). For Hematoxylin and Eosin stained sections, there were no signs of significant apoptosis or necrosis in either the control or irradiated samples, and chondrocyte density and morphology were similar between groups. No signs of osteoarthritis were observed histologically using using the Osteoarthritis Research Society International (OARSI) scoring system.26. Collagen content as determined histologically was similar between control and irradiated explants.
DISCUSSION
Joint degradation following exposure to ionizing radiation is an understudied issue that may face radiation therapy patients, though evidence indicates late arthropathy or joint failure following cancer treatment at irradiated sites.9, 27 Arthopathy in the hip and knee have often been attributed to osteonecrosis rather than chondrocyte-induced cartilage degradation.10, 11 Recent work of explanted cartilage and cells from adult humans and large animal (pig) models indicate that radiation of cartilage in isolation can induce an active and early degradation of proteoglycans coincident with reduced proteoglycan synthesis and IGF-1 sensitivity, all characteristic of an osteoarthritic phenotype.15 However, the effect of radiation on cartilage mechanical properties was previously unknown.
In this study, we demonstrated that low doses of radiation causes a significant decrease in the compressive stiffness of articular cartilage at multiple length scales (Figures 1 and 2). In addition, the Young’s modulus values for our control tissue are comparable to those found in the literature using similar testing parameters for both microindentation and IT AFM.28, 29 With Young’s modulus values around 75% and 60% less in irradiated samples compared with controls using microindentation and nanoindentation, respectively, we believe that radiation affects the entire thickness of articular cartilage, not just the superficial zone. Such a drastic reduction in mechanical properties following irradiation suggests that alterations are occurring in the matrix.
Results from the DMB assay suggest that an acute release of GAGs occurs early after radiation exposure, in agreement with others.15 Samples exposed to radiation showed significantly higher sGAG levels in the culture media at Days 1 when compared with Days 0, 3, and 7. Also, sGAG release in the control samples was significantly higher at Day 1 when compared with Day 3, but this is usually seen in cartilage explants cultures.30 At Day 1, irradiated samples released around twice as many sGAGs in the media when compared with controls. In both control and irradiated samples, sGAG concentrations in the media decrease to around Day 0 levels by Day 3 and maintain this concentration through Day 7. However, the concentration of sGAGs in the irradiated tissue is over 50% less than the concentration found in control tissue. Thus, GAGs present in the tissue at exposure were likely degraded and released. A direct reduction of proteoglycan synthesis has been shown from pig and human chondrocytes early after direct exposure at similar doses.15 New GAG synthesis may have been insufficient to replenish these degraded and released GAGs after 1 week post-irradiation, though direct GAG synthesis was not measured in this study.
We observe 75% and 60% decreases in Young’s modulus after radiation exposure with microindentation and nanoindentation, respectively; it is unlikely that this large decrease is solely due to the observed loss of proteoglycan. Proteoglycans and their associated GAGs are only estimated to be responsible for about 50% of the compressive stiffness of articular cartilage.31 Therefore, we believe GAG loss is not the only factor affecting the modulus post-irradiation, though other causes remain undefined. While we observe no significant difference in collagen content between control and irradiated groups, the hydroxyproline assay is unable to detect the integrity of collagen in the tissue. As a result, it was not possible to determine if changes in cross-linking or other structural damage are contributing to the reduction in modulus post-irradiation. However, there is no observable fibrillation in our irradiated histology samples (Figure 6). Additionally, reduced cell viability or necrosis is unlikely to have contributed to the observed results. Our histological data indicate no evidence of necrosis or apoptosis after radiation exposure. These observations are in in agreement with others who have examined cell viability in pig, human, and growing rabbit chondrocytes at similar time points following 10 Gy exposure.15, 32 Thus, while reduction in GAGs within pig articular cartilage occurs after exposure and likely contributes to our observed lowering of stiffness, the entirety of the cause remains unclear.
Explant culture is a commonly used method to observe the metabolic activity of articular cartilage. However, interactions between individual tissues within the joint (e.g., synovium, synovial fluid, subchondral bone) can affect cartilage morphology, as observed during conditions such as rheumatoid arthritis.33 The entire joint would absorb dose during cancer therapy and thus would likely affect several joint tissues. Direct irradiation of synovial joints in vivo is necessary to identify whether the degradation of articular cartilage occurs in a similar manner after exposure, and identify possible tissue interactions. Furthermore, determining the nature of the cartilage degradation following exposure was not a component of this study. Further research (both in vivo and ex vivo) will identify potential molecular targets for the functional deficits in cartilage after exposure.
In conclusion, the Young’s modulus of articular cartilage was found to decrease after exposure to low doses of ionizing radiation, regardless of mechanical testing length scale. Therefore, we believe that radiation affects the bulk mechanical properties of the cartilage, not only the superficial zone. The acute release of GAGs is a likely contributor to this change in stiffness, as irradiated samples had a significantly higher release of GAGs 24 hours after irradiation. Therefore, further investigation should be performed to determine if radiotherapy causes long-term damage to articular cartilage.
ACKNOWLEDGEMENTS
The authors would like to thank Linda Jenkins and Stephen Price for assistance with histological analysis and radiation exposure, respectively. Funding was provided by SC Space Grant Palmetto Academy (DD), NIH K25 HL09228 (DD), and T32 CA113267 (JSW).
REFERENCES
- 1.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 2.Coleman CN, Stone HB, Moulder JE, Pellmar TC. Modulation of radiation injury. Science. 2004;304:693–694. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 3.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003;41:208–211. doi: 10.1002/mpo.10338. [DOI] [PubMed] [Google Scholar]
- 4.Willey JS, Livingston EW, Robbins ME, et al. Risedronate prevents early radiation-induced osteoporosis in mice at multiple skeletal locations. Bone. 2010;46:101–111. doi: 10.1016/j.bone.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294:2587–2593. doi: 10.1001/jama.294.20.2587. [DOI] [PubMed] [Google Scholar]
- 6.Tokumaru S, Toita T, Oguchi M, et al. Insufficiency fractures after pelvic radiation therapy for uterine cervical cancer: An analysis of subjects in a prospective multi-institutional trial, and cooperative study of the japan radiation oncology group (JAROG) and japanese radiation oncology study group (JROSG) Int J Radiat Oncol Biol Phys. 2012;84:e195–e200. doi: 10.1016/j.ijrobp.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Asai K, Shioyama Y, Nakamura K, et al. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy: Risk factors and dose-volume relationship. Int J Radiat Oncol Biol Phys. 2012;84:768–773. doi: 10.1016/j.ijrobp.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Kondo H, Searby ND, Mojarrab R, et al. Total-body irradiation of postpubertal mice with (137)cs acutely compromises the microarchitecture of cancellous bone and increases osteoclasts. Radiat Res. 2009;171:283–289. doi: 10.1667/RR1463.1. [DOI] [PubMed] [Google Scholar]
- 9.Kolár J, Vrabec R, Chyba J. Arthropathies after irradiation. J Bone Joint Surg Am. 1967;49:1157–1166. [PubMed] [Google Scholar]
- 10.Massin P, Duparc J. Total hip replacement in irradiated hips. A retrospective study of 71 cases. J Bone Joint Surg Br. 1995;77:847–852. [PubMed] [Google Scholar]
- 11.Chen E, Sethi S, Lee A, Sethi A, Vaidya R. Knee pain in patients with cancer after chemotherapy, radiotherapy, and bone marrow transplantation. Orthopedics. 2012;35:e1177–e1183. doi: 10.3928/01477447-20120725-16. [DOI] [PubMed] [Google Scholar]
- 12.Hugenberg ST, Myers SL, Brandt KD. Suppression of glycosaminoglycan synthesis by articular cartilage, but not of hyaluronic acid synthesis by synovium, after exposure to radiation. Arthritis Rheum. 1989;32:468–474. doi: 10.1002/anr.1780320417. [DOI] [PubMed] [Google Scholar]
- 13.Jikko A, Hiranuma H, Iwamoto M, et al. Effects of X irradiation on metabolism of proteoglycans. Radiat Res. 1996;146:93–99. [PubMed] [Google Scholar]
- 14.Cornelissen M, Thierens H, De Ridder L. Effects of ionizing radiation on cartilage: Emphasis on effects on the extracellular matrix. Scanning Microsc. 1996;10:833–840. [PubMed] [Google Scholar]
- 15.Willey JS, Long DL, Vanderman KS, Loeser RF. Ionizing radiation causes active degradation and reduces matrix synthesis in articular cartilage. Int J Radiat Biol. 2013;89(4):268–277. doi: 10.3109/09553002.2013.747015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ailland J, Kampen W, Schünke M, Trentmann J, Kurz B. Beta irradiation decreases collagen type II synthesis and increases nitric oxide production and cell death in articular chondrocytes. Ann Rheum Dis. 2003;62:1054–1060. doi: 10.1136/ard.62.11.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindburg A, Elpers M, Dean D. Effect of radiation on articular cartilage mechanical properties. Trans Soc Biomater. 2010;35:106. [Google Scholar]
- 18.Jin M, Frank EH, Quinn TM, Hunziker EB, Grodzinsky AJ. Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch Biochem Biophys. 2001;395:41–48. doi: 10.1006/abbi.2001.2543. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Costa KD, Ateshian GA. Microscale frictional response of bovine articular cartilage from atomic force microscopy. J Biomech. 2004;37:1679–1687. doi: 10.1016/j.jbiomech.2004.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker KJ, Huang SR, Musulin RA, Lerner RM. Tissue response to mechanical vibrations for sonoelasticity imaging. Ultrasound Med Biol. 1990;16:241–246. doi: 10.1016/0301-5629(90)90003-u. [DOI] [PubMed] [Google Scholar]
- 21.Stanton H, Golub SB, Rogerson FM, et al. Investigating ADAMTS-mediated aggrecanolysis in mouse cartilage. Nat Protoc. 2011;6:388–404. doi: 10.1038/nprot.2010.179. [DOI] [PubMed] [Google Scholar]
- 22.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 23.Liebman J, Goldberg RL. Chondrocyte culture and assay. Curr Protoc Pharmacol. 2001;12(Unit 12.2) doi: 10.1002/0471141755.ph1202s12. [DOI] [PubMed] [Google Scholar]
- 24.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 25.Hwang NS, Varghese S, Elisseeff J. Cartilage tissue engineering: Directed differentiation of embryonic stem cells in three-dimensional hydrogel culture. Methods Mol Biol. 2007;407:351–373. doi: 10.1007/978-1-59745-536-7_24. [DOI] [PubMed] [Google Scholar]
- 26.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 28.Simha NK, Jin H, Hall ML, Chiravarambath S, Lewis JL. Effect of indenter size on elastic modulus of cartilage measured by indentation. J Biomech Eng. 2007;129:767–775. doi: 10.1115/1.2768110. [DOI] [PubMed] [Google Scholar]
- 29.Park S, Costa KD, Ateshian GA, Hong KS. Mechanical properties of bovine articular cartilage under microscale indentation loading from atomic force microscopy. Proc Inst Mech Eng H. 2009;223:339–347. doi: 10.1243/09544119JEIM516. [DOI] [PubMed] [Google Scholar]
- 30.Morales TI, Wahl LM, Hascall VC. The effect of bacterial lipopolysaccharides on the biosynthesis and release of proteoglycans from calf articular cartilage cultures. J Biol Chem. 1984;259:6720–6729. [PubMed] [Google Scholar]
- 31.Buschmann MD, Grodzinsky AJ. A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J Biomech Eng. 1995;117:179–192. doi: 10.1115/1.2796000. [DOI] [PubMed] [Google Scholar]
- 32.Hong EH, Lee SJ, Kim JS, et al. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010;285:1283–1295. doi: 10.1074/jbc.M109.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]


