Summary
The individuals carrying melanocortin-1-receptor (MC1R) variants, especially those associated with red hair color, fair skin and poor tanning ability (RHC-trait), are more prone to melanoma while the underlying mechanism is poorly defined. Here, we report that UVB exposure triggers PTEN interaction with wild-type (WT), but not RHC-associated MC1R variants, which protects PTEN from WWP2-mediated degradation, leading to AKT inactivation. Strikingly, the biological consequences of the failure of MC1R variants to suppress PI3K/AKT signaling are highly context dependent. In primary melanocytes, hyperactivation of PI3K/AKT signaling leads to premature senescence; in the presence of BRAFV600E, MC1R deficiency-induced elevated PI3K/AKT signaling drives oncogenic transformation. These studies establish the MC1R-PTEN axis as a central regulator for melanocytes’ response to UVB exposure, and reveal the molecular basis underlying the association between MC1R variants and melanomagenesis.
Introduction
Environmental UV radiation exposure, fair skin, family history of melanoma and a high number of melanocytic nevi are all well-characterized risk factors for developing melanoma (Miller and Mihm, 2006). Notably, molecular and genetic data indicate that variations in the coding region of the melanocortin-1-receptor (MC1R) play important roles in tanning, as well as pigmentation and melanoma development (Raimondi et al., 2008). Consistent with its critical role in melanocyte biological processes, MC1R is mainly expressed in melanocytes and activated by its physiological ligand, α-melanocyte-stimulating hormone (α-MSH) upon UVB exposure (D'Orazio et al., 2006).
Melanoma risk, attributable to MC1R genetic status, is associated with the tanning response of skin to ultraviolet (UV) light (Thomas et al., 2010). Specific MC1R variants, such as V60L, I40T, R142H, R151C, R162P, R160W and D294H, cannot stimulate cAMP production as strongly as WT-MC1R in response to α-MSH, thereby resulting in decreased pigmentation (Schioth et al., 1999). To this end, the MC1R-RHC variants are found to be associated with phenotypes such as red hair, fair skin, poor tanning ability, and skin sensitivity to UV (Goldstein et al., 2005). Recent studies revealed that several MC1R-RHC variants are also associated with severity of melanocytic nevi (Kinsler et al., 2012) and increased melanoma risk (Goldstein et al., 2005). These findings suggest that UV-induced MC1R signaling might play an important role in melanoma development, possibly via both the pigmentary and non-pigmentary pathways.
Phosphatase and tensin homolog (PTEN) is a well-characterized tumor suppressor (Hollander et al., 2011) that inhibits the phosphatidylinositol 3-kinase (PI3K) oncogenic pathway (Maehama and Dixon, 1998). Decreased PTEN expression is observed in 30–50% of melanoma cell lines and in 5–20% of primary melanoma tumors (Wu et al., 2003). Recent studies revealed PTEN as a key player in mediating the signaling pathways of both DNA repair and melanocyte viability in the context of UV exposure (Ming et al., 2010). More importantly, loss of PTEN leads to the onset of premature senescence presumably by super-physiological activation of the AKT oncoprotein (Chen et al., 2005), a phenotype termed oncogene-induced senescence (OIS) (Braig et al., 2005). Similarly, overexpression of BRAFV600E also triggers premature senescence that is tightly associated with nevi formation (Michaloglou et al., 2005). Therefore, OIS has been recently proposed to be a natural barrier to oncogene-induced cellular transformation and bypass of this protection mechanism results in tumorigenesis (Peeper, 2011). Here, we further examine if α-MSH/MC1R participates in regulating the PI3K/PTEN/AKT signaling pathway after UV exposure and its potential contribution to the development of melanoma.
Results
The Involvement of MC1R in UVB-induced PTEN and AKT Phosphorylation in vivo
To define the molecular mechanism by which α-MSH/MC1R maintains melanocyte viability, we evaluated the phosphorylation status of several GPCR-regulated proteins, including p-ERK1/2, p-MEK, p-p38/MAPK, p-JNK, p-AKT and p-PTEN (Garcia-Borron et al., 2005) (Figure S1A) in UV-irradiated mouse skin samples derived from the engineered K14/SCF transgenic mice (Kunisada et al., 1998) expressing either wild-type MC1R or the loss-of-function frameshift MC1R mutation.
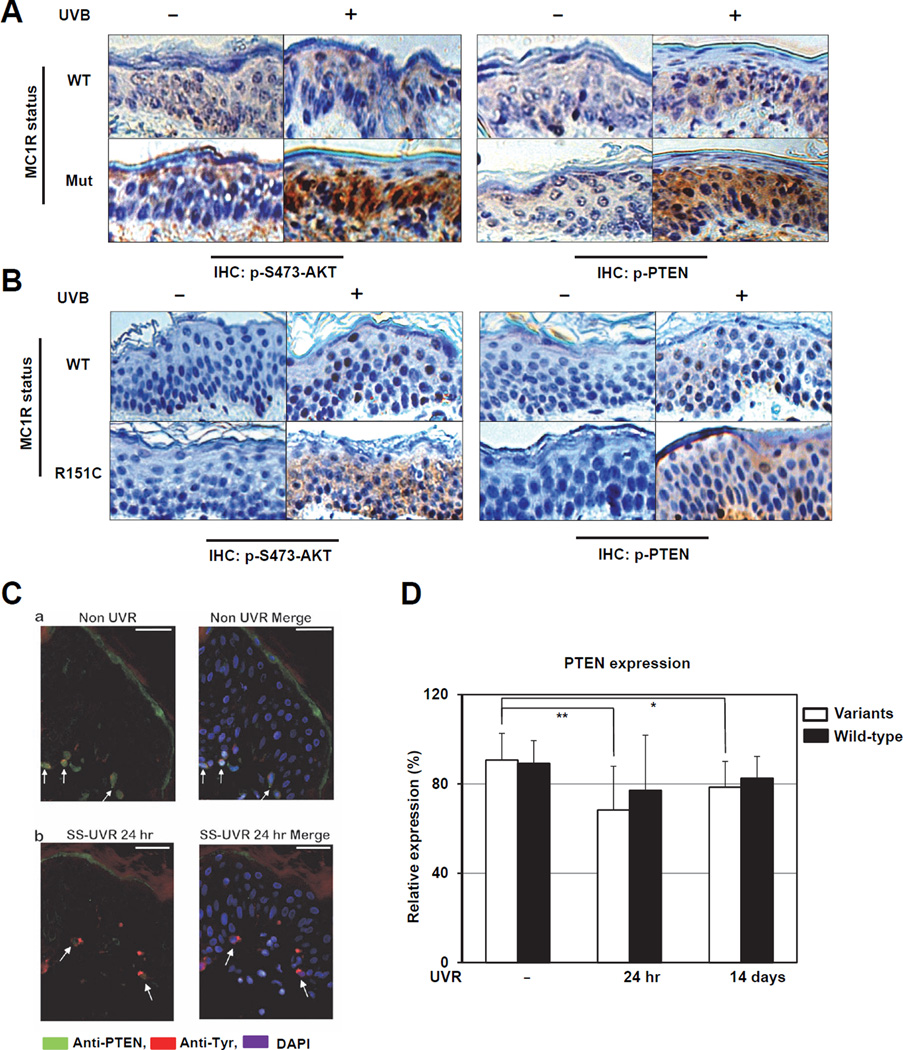
Notably, phosphorylation of PTEN at Ser380 and Thr382/383 (indicators of inactive PTEN), and phosphorylation of AKT at Ser473 (indicator of active AKT), were much more significantly induced by UVB in epidermal cells expressing the MC1R mutant, compared to cells expressing wild-type (WT) MC1R (Figure 1A). However, no significant difference was detected for other examined proteins (Figure S1A) (data not shown). Consistently, pSer380/pThr382/383-PTEN and pSer473-AKT were significantly induced by UVB in human epidermal cells expressing R151C-, but not WT-MC1R (Figure 1A). However, no significant changes in AKT abundance were observed before and after UVB exposure regardless of the MC1R genetic status (Figures S1B–C). On the other hand, compared to WT-MC1R expressing melanocytes, there was a significant reduction of PTEN expression following UVB exposure in melanocytes with mutant MC1R (Figures S1B–C). Therefore, these results collectively indicate that MC1R signaling may regulate UV-induced PTEN/AKT actions in vivo.
Figure 1. PTEN Expression is Reduced in Human Skin Carrying MC1R Variants Following UV Exposure.
A–B. Immunohistochemistry (IHC) staining of phospho-PTEN (Ser380/Thr382/383) and phospho-AKT (Ser473) in Albino mouse skin (wild-type [WT] MC1R or loss-of-function MC1R frameshift mutation [Mut]) (A) or human foreskin (collected from Caucasian with wild-type MC1R [WT] or MC1R [R151C]) (B) with or without UVB irradiation.
C. Immunofluorescent staining (IF) identifying PTEN expression (green), melanocytes (tyrosinase, red) and nuclei (DAPI, blue). Arrows indicate PTEN expressing melanocytes. (a) Non-UVR; (b) SS-UVR.
D. The expression of PTEN was determined by IF in melanocytes of human volunteers harboring MC1R variants (n=10) or wild-type MC1R (n=10) at 24 hr and 14 days after UV irradiation. (* p = 0.03 and ** p = 0.008 by paired t test. Data are represented as mean ± SEM, n=10).
(See also Figure S1 and Supplemental Table 1–3)
To further confirm these results in melanocytes in vivo, twenty healthy volunteers with Fitzpatrick skin type Classification I-III, whose MC1R and PTEN genetic status have been determined (Supplemental Table 1), were exposed to 2 MED dose of UVR at the AusSun Laboratory in Australia. Notably, immunofluorescence staining for PTEN and tyrosinase, a reliable marker for melanocytes, revealed that 24 hours after SS-UVR exposure, there was a reduction in the number of melanocytes expressing PTEN in human skin (Figure 1C and Supplemental Table 2). On the other hand, compared to baseline pre-treatment levels, the expression of PTEN in melanocytes at both 24 hr and 14 days following SS-UVR exposure was lower among subjects carrying MC1R variants (Figures 1D and Supplemental Table 3). These in vivo results confirm our IHC results (Figures 1A–B), further revealing that MC1R genetic status impacts PTEN expression in melanocytes at the physiological setting after UVR exposure.
MC1R Regulates UVB-induced PTEN Inactivation and AKT Phosphorylation in vitro
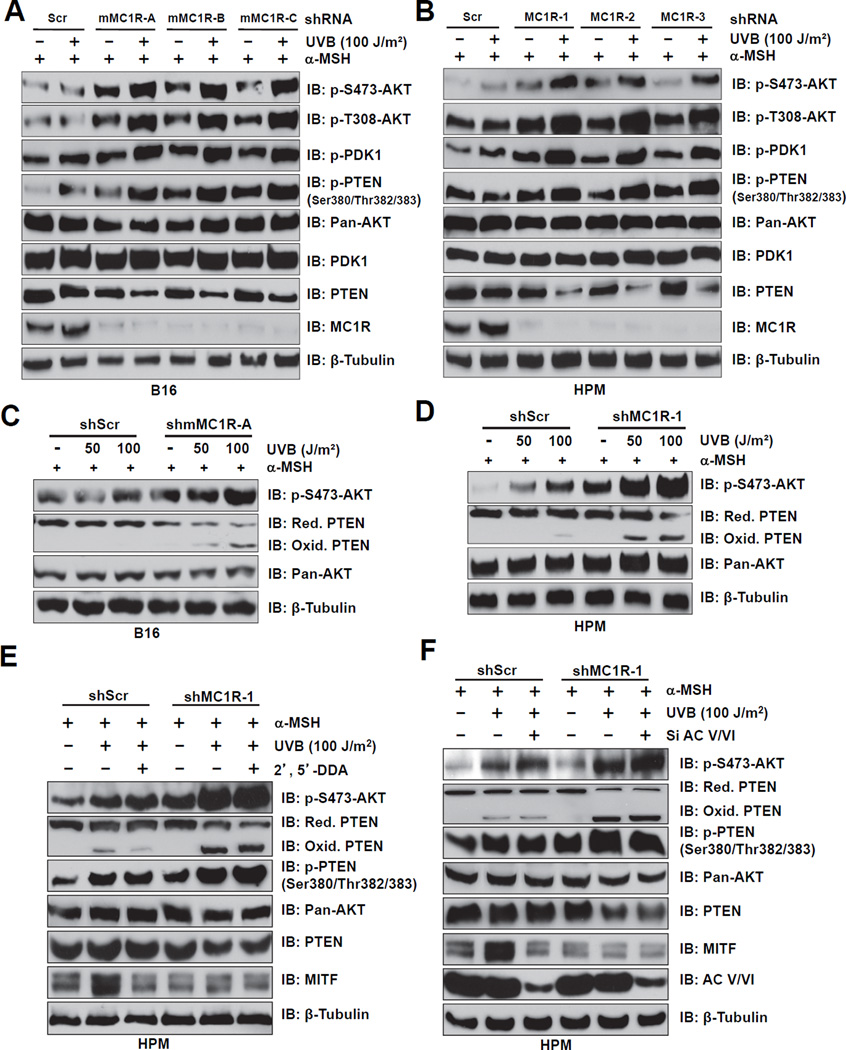
Importantly, elevation of both pS473-AKT and pS380/pT382/383-PTEN were observed after UVB exposure, especially in MC1R-depleted melanocytes (Figures 2A–B and S2C–D). Moreover, in MC1R-depleted cells, we observed a dose-dependent decrease of PTEN abundance and elevated levels of PTEN oxidation that correlates with a concomitant elevation of pS473-AKT (Figures 2C–D and S2E). These findings suggest that MC1R might regulate PTEN phosphatase activity or PTEN stability to influence UVB-induced AKT activation in melanocytes.
Figure 2. MC1R Depletion Augments UVB-induced AKT Phosphorylation by Inactivating PTEN.
A–B. B16 cells (A) and human primary melanocytes (HPMs) (B) stably expressing control shRNA (shScr) or multiple independent shMC1Rs (mouse or human MC1R-specific, respectively) were pre-incubated with 1 µM α-MSH for 30 min, exposed to 100 J/m2 UVB as indicated, and then harvested at 3 hr after UVB exposure for immunoblot (IB) analysis.
C–D. Whole cell lysates (WCL) derived from the B16 cells (C) and HPMs (D) generated in A and B with UVB treatment at the doses of 0, 50 and 100 J/m2 were used to detect PTEN oxidation by performing non-reducing SDS-PAGE followed by IB analysis.
E–F. MC1R silencing induced PTEN oxidation and PTEN phosphorylation following UVB exposure are independent of the cAMP pathway. HPMs were infected with shMC1R (with shScr as a negative control) to deplete endogenous MC1R. The resulting cells were treated with 1 µM α-MSH for 30 min followed by 100 J/m2 UVB exposure before harvesting for IB analysis. The cAMP pathway was inhibited by using either 2’, 5’-dideoxyadenosine (2’,5’-DDA, 2.5 mM) (E) or siRNA against endogenous adenylyl cyclase (F) as indicated.
(See also Figure S2)
Furthermore, UVB-exposure in the presence of MC1R silencing augmented AKT phosphorylation even when the cAMP pathway was inhibited by 2’, 5’-DDA (Figure 2E) or by depletion of endogenous adenylyl cyclase (Figure 2F) in melanocytes. Thus, these results indicate that MC1R silencing might augment UV-induced AKT phosphorylation independent of the downstream cAMP pathway.
MC1R Interacts with PTEN after UVB Exposure
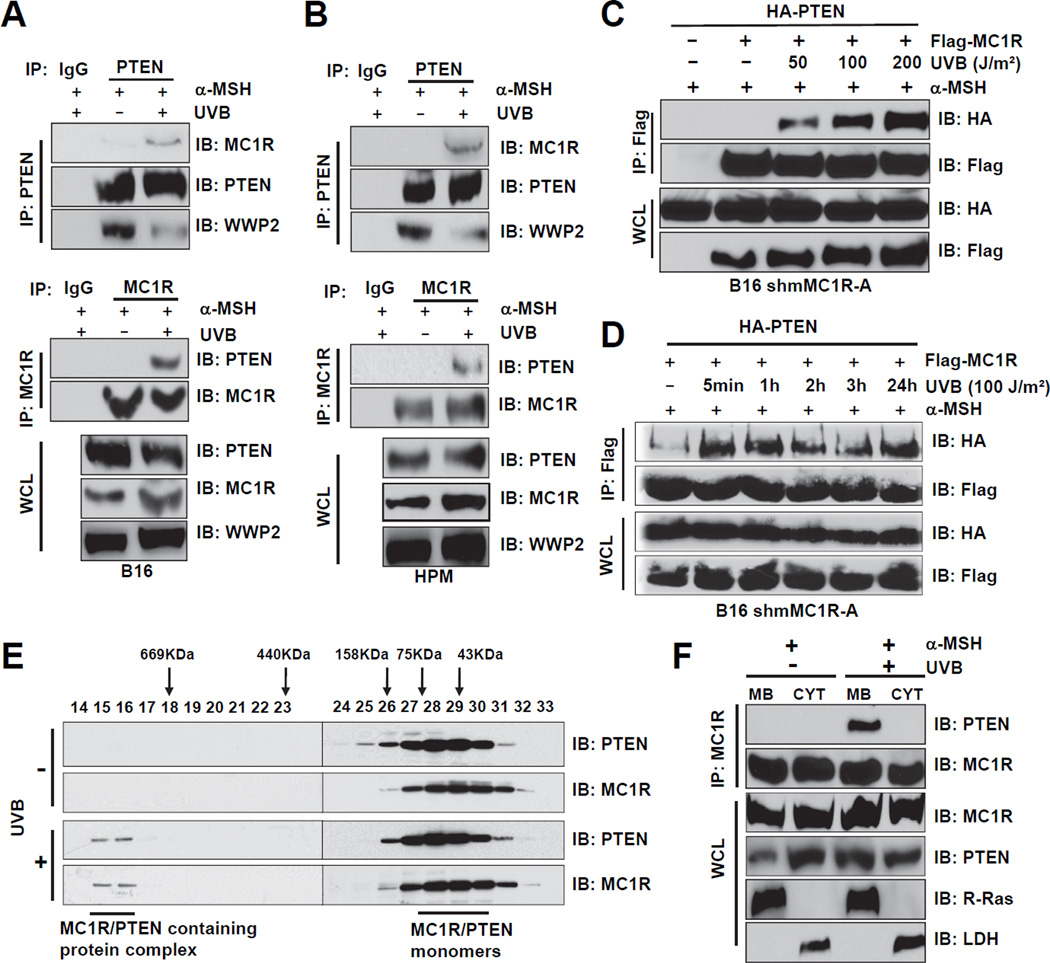
Given the critical role of PTEN in tumorigenesis, its function and stability are tightly controlled in vivo (Song et al., 2012). Therefore, next we explored whether MC1R is a novel upstream PTEN regulator. To this end, we found that endogenous PTEN complexes with endogenous MC1R after UV exposure (Figures 3A–B). Notably, both α-MSH addition and UVB exposure are required for induced MC1R/PTEN interaction in melanocytes (Figures S3A–B). For simplification, both UVB exposure and α-MSH addition were used in the following experiments to induce MC1R/PTEN interaction.
Figure 3. UVB Irradiation Promotes the Physical Interaction Between MC1R and PTEN in Melanocytes.
A–B. B16 cells (A) or HPMs (B) were pre-incubated with 1 µM α-MSH for 30 min, irradiated with 100 J/m2 UVB, and harvested 3 hr post UVB exposure. WCL were then subjected to immunoprecipitation (IP) and IB analysis.
C. MC1R interacts with PTEN in a UVB dose-dependent manner. MC1R-depleted B16 cells were infected with retrovirus encoding Flag-MC1R WT. The resulting cells were transfected with HAPTEN, then pretreated with the proteasomal inhibitor MG132 (25 µM) for 3 hr and with 1 µM α-MSH for 30 min followed by irradiation at the indicated doses. Cells were then harvested for IP and IB analysis at 3 hr after UVB exposure.
D. MC1R and PTEN interactions were detected after UVB exposure for up to 24 hr. B16 cells were generated and treated with MG132 and α-MSH as described in C before harvesting for IP and IB analysis at the indicated time points post UVB exposure.
E. B16 cells were treated with 1 µM α-MSH for 30 min followed by irradiation with 100 J/m2 UVB. WCL were subjected to the Superdex 200 size exclusion column to separate proteins at different sizes. The lysates collected at various fractions were separated by SDS-PAGE before IB analysis.
F. B16 cells were pretreated with MG132 (25 µM) for 3 hours and with 1 µM α-MSH for 30 min followed by irradiation with 100 J/m2 UVB. Cells were then harvested and subjected to subcellular fractionation at 3 hr after UV exposure. The fractionated lysates were subjected to IP and IB analysis. MB indicates membrane-associated fractions, CYT indicates cytosolic fractions.
(See also Figure S3)
In support of this finding, we also detected the interaction of exogenous PTEN and MC1R upon UV exposure (Figures S3C–D) in a dose-dependent manner (Figure 3C). Strikingly, the PTEN-MC1R complexes formed 5 min after low dose UVB (100 J/m2) exposure and persisted up to 24 hr (Figure 3D). Moreover, gel filtration results indicated that in the absence of UV exposure, both MC1R and PTEN mainly migrate as monomers while UV exposure led to the formation of a large complex composed of MC1R and PTEN as well as other unidentified components (Rabinovsky et al., 2009) (Figure 3E). We further showed that following UV exposure, there was more detectable membrane-bound fraction of PTEN (Figure 3F). Moreover, we found that the PTEN C2-domain (amino acids 186–274) was necessary for mediating the interaction with full-length MC1R (Figures S3E–F), and the two MC1R intra-membrane domains (amino acids 144–160 and 277–297, respectively) were largely necessary for mediating the interaction with full-length PTEN (Figures S3G–I).
MC1R Variants Impair Their Ability to Interact with PTEN
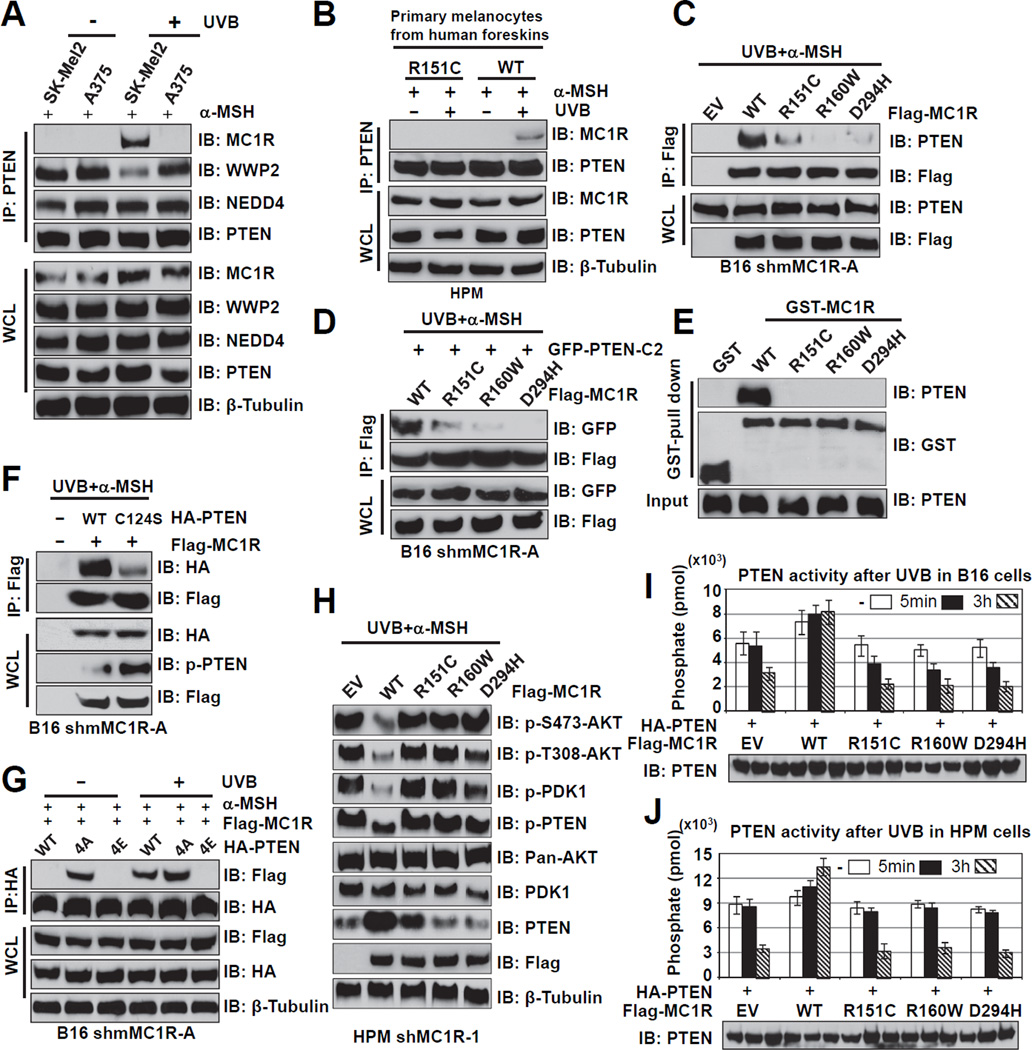
Next, we explored whether RHC variants in MC1R might affect its physical interaction with PTEN. In support of this notion, compared to SK-Mel2 cells with WT-MC1R (Tap et al., 2010), the strong interaction between endogenous WT-MC1R and PTEN following UV exposure was completely abolished in A375 cells harboring MC1R-R151C (Tap et al., 2010) (Figures 4A and S4A–B). Consistently, an interaction was observed between endogenous PTEN and MC1R in human primary melanocytes (HPMs) derived from discarded foreskin with WT-MC1R, but not MC1R-R151C (Figure 4B). These findings were further supported by co-immunoprecipitation and GST-pull down experiments showing that various MC1R-RHC variants (R151C, R160W and D294H) are defective in binding to PTEN both in vivo and in vitro (Figures 4C–E).
Figure 4. MC1R Variants are Defective in Association with PTEN Following UVB Irradiation.
A. IP and IB analysis of the WCL derived from SK-Mel2 (WT-MC1R) or A375 (R151C–MC1R) cells with or without UVB exposure (100 J/m2). Cells were pretreated with the MG132 (25 µM) for 3 hours and with 1 µM α-MSH for 30 min before UV exposure and harvested at 3 hr after UVB exposure.
B. IP and IB analysis of HPMs derived from discarded foreskin with WT-MC1R, or the R151C MC1R variant. The melanocytes were treated with 1 µM α-MSH for 30 min before UVB exposure (100 J/m2).
C. IP and IB analysis of WCL derived from shMC1R-B16 cells transfected with the indicated Flag-MC1R plasmids. Cells were pretreated with the MG132 (25 µM) for 3 hours and with 1 µM α-MSH for 30 min before UVB exposure (100 J/m2).
D. IP and IB analysis of WCL derived from MC1R-depleted B16 cells co-transfected with the indicated Flag-MC1R plasmids and GFP-PTEN-C2. Cells were pretreated with MG132 (25 µM) for 3 hours and with 1 µM α-MSH for 30 min before UVB exposure (100 J/m2).
E. Direct physical interactions between MC1R and PTEN were detected by GST pull-down assays. Recombinant His-PTEN (100 nM) was incubated with GST alone or the indicated GST-MC1R fusion proteins overnight. The bound PTEN protein was detected by IB analysis. Input represents 10% of the total amount of protein subjected to the GST pull-down assays.
F. IP and IB analysis of WCL derived from MC1R-depleted B16 cells transfected with Flag-MC1R and the indicated HA-PTEN plasmids. Cells were pretreated with MG132 (25 µM) for 3 hours and with 1 µM α-MSH for 30 min before UVB exposure (100 J/m2).
G. IP and IB analysis of WCL derived from MC1R-depleted B16 cells transfected with Flag-MC1R and the indicated HA-PTEN plasmids. Where indicated, cells were pretreated with MG132 (25 µM) for 3 hours and with 1 µM α-MSH for 30 min before UVB exposure (100 J/m2).
H. IP and IB analysis of WCL derived from MC1R-depleted HPMs infected with the indicated Flag-MC1R encoding retro-viral constructs. Cells were treated with 1 µM α-MSH for 30 min before UVB exposure (100 J/m2).
I–J. MC1R-depleted B16 (I) or HPMs (J) were reintroduced with retrovirus encoding Flag-tagged WT-MC1R or the indicated MC1R variants. These generated cell lines were transfected with HA-PTEN. 48 hr post-transfection, cells were treated with 1µM α-MSH for 30 min, irradiated with 100 J/m2 UVB, and then harvested 5 min or at 3 hr after UV irradiation. PTEN was then immuno-purified from the harvested lysates, and PTEN activity was measured by using a malachite green assay kit. The lower panel shows the amount of PTEN loaded for the ELISA analysis (Data are represented as mean ± SEM, n=3, the p values are listed in Supplemental Table 4).
(See also Figure S4 and Supplemental Table 4)
Interestingly, MC1R interaction with the C124S phosphatase-deficient PTEN was weaker than with wild-type PTEN (Figure 4F). Furthermore, even without UV treatment, 4A-PTEN, a non-phosphorylatable and constantly active form of PTEN (Rabinovsky et al., 2009) could efficiently interact with MC1R (Figure 4G). On the other hand, the phospho-mimetic mutant (4E) form of PTEN failed to interact with MC1R even following UV exposure. These results strongly suggest that MC1R preferentially interacts with, and protects the non-phosphorylated, active form of PTEN from destruction to suppress AKT activation. In keeping with this notion, following UV exposure, compared to cells expressing WT-MC1R, cells expressing various variants of MC1R that failed to interact with PTEN (Figures 4A–D) at levels comparable to WT-MC1R (Figures S4C–D), exhibited a rapid destruction of PTEN and a corresponding elevation of p-AKT (Figures 4H and S4E). Unlike the impaired interaction between PTEN and the MC1R-RHC variants such as R151C, R160W and D294H, there was no detectable reduction in the interaction between PTEN and non-RHC variants we examined, including F76Y, V174I and P230L (Figures S4F–G). These results support a unique feature associated with MC1R-RHC variants in their deficiency to protect PTEN stability, thereby predisposing melanocytes to melanomagenesis.
MC1R Regulates PTEN Activity after UVB Exposure
Notably, PTEN phosphatase activity was repressed by UVB exposure, especially in MC1R-depleted melanocytes infected with the EV control (Figures 4I–J). Importantly, re-introducing wild-type MC1R prevented UVB-induced repression of PTEN phosphatase activity, suggesting that MC1R protects PTEN activity after UVB exposure. However, PTEN phosphatase activity was still repressed in cells expressing MC1R-RHC variants defective in associating with PTEN (Figures 4I–J), indicating that these MC1R variants lost protective activity for PTEN following UVB exposure.
MC1R Protects PTEN from Proteolysis under UVB Exposure Conditions
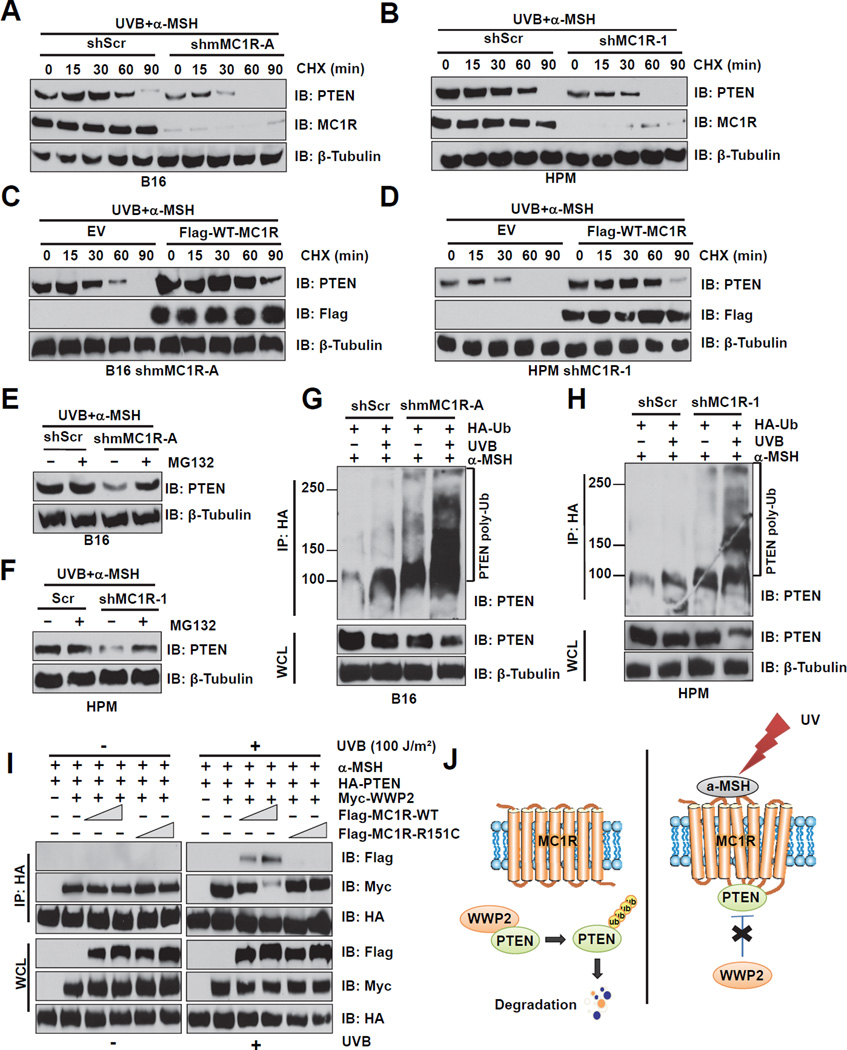
Notably, compared to control cells, the half-life of PTEN protein was much shorter in MC1R-depleted cells (Figures 5A–B and S5A–B). Re-introduction of WT-MC1R in MC1R-depleted cells stabilized PTEN (Figures 5C–D), indicating that MC1R protects UVB-induced PTEN degradation in melanocytes. Consistently, compared to shScr-treated control cells, there is a significant decrease of PTEN abundance in MC1R-depleted cells, which could be reversed by MG132 (Figures 5E–F). To further evaluate the contribution of the ubiquitination pathway in this process, PTEN ubiquitination was examined in B16 cells or human primary melanocytes before and after depletion of endogenous MC1R. Compared to control cells, ubiquitinated PTEN was much more readily detected in MC1R-depleted cells, especially after UVB exposure (Figures 5G–H and S5C–D). Furthermore, reintroducing WT-, but not MC1R-RHC variants, could suppress in vivo PTEN ubiquitination following UVB exposure (Figures S5E–F). These results suggest that MC1R might protect PTEN from UV-induced ubiquitination.
Figure 5. MC1R Protects PTEN from Ubiquitination and Subsequent Proteosome-mediated Destruction Following UVB Exposure.
A–B. B16 cells (A) or HPMs (B) were infected with the shMC1R lentiviral construct (with shScr as a negative control) to deplete endogenous MC1R. The resulting cells were pretreated with 1 µM α-MSH for 30 min before UVB irradiation (100 J/m2), and then cultured in media containing 50 µg/ml cycloheximide (CHX). The cells were then harvested at the indicated time points for IB analysis.
C–D. MC1R-depleted B16 cells (C) or HPMs (D) were infected with Flag-WT-MC1R encoding retrovirus. The resulting B16 cells (C) or human primary melanocytes (D) were pretreated with 1 µM α-MSH for 30 min followed by treatment with 50 µg/ml CHX before and during UVB irradiation (100 J/m2). The cells were then harvested at the indicated time points for IB analysis.
E–F. B16 cells (E) or HPMs (F) were infected with the shMC1R lentiviral construct (with shScr as a negative control) to deplete endogenous MC1R. The resulting cells were pretreated with 1 µM α-MSH for 30 min before UVB exposure (100 J/m2), followed by treatment with 25 µM MG132 during UVB irradiation, and then cultured in DMEM with MG132 for 4 hr. WCL were then collected for IB analysis.
G–H. B16 cells (A) or HPMs (B) were infected with the shMC1R lentiviral construct (with shScr as a negative control) to deplete endogenous MC1R. The resulting cells were transfected with HA-ubiquitin, 40 hr post-transfection, cells were pretreated with 25 µM MG132 for 6 hr and 1 µM α-MSH for 30 min prior to UVB irradiation (100 J/m2). The cells were harvested 3 hr after UVR and then immunoprecipitated with HA-conjugated agarose beads. Ubiquitin bound PTEN was analyzed by SDS-PAGE and IB analysis using anti-PTEN antibody.
I. IP and IB analysis of WCL derived from MC1R-depleted B16 cells transfected with HAPTEN, Myc-WWP2 and increasing amount of Flag-MC1R WT or R151C mutant. 48 hours post-transfection, cells were pretreated with MG132 (25 µM) for 3 hours and with 1 µM α-MSH for 30 min before UVB exposure (100 J/m2) and harvested 3 hr post UVB exposure.
J. Schematic diagram of the mechanism through which MC1R protects PTEN from WWP2-mediated degradation upon UVB exposure in the presence of α-MSH.
(See also Figure S5)
MC1R Competes with WWP2 to Interact with PTEN after UVB Exposure
Next, we sought to further pinpoint the exact molecular mechanisms underlying MC1R-mediated protection of PTEN ubiquitination following UVB exposure. We reasoned that UVB-induced MC1R/PTEN interaction might affect the interaction between PTEN and its physiological E3 ligases such as NEDD4 (Wang et al., 2007) and WWP2 (Maddika et al., 2011) in melanocytes. As both NEDD4 and WWP2 belong to the NEDD4 family of E3 ligases, we explored the specificity among NEDD4 family members that interact with PTEN. Consistent with previous reports (Maddika et al., 2011; Trotman et al., 2007; Wang et al., 2007), PTEN strongly interacted with WWP2, but weakly with NEDD4 (Figure S5G). More importantly, in both B16 and human primary melanocytes, UV exposure dramatically reduced the interaction between endogenous PTEN and WWP2 (Figures 3A–B), concomitantly inducing the interaction between endogenous PTEN and MC1R (Figures 3A–B).
As both WWP2 (Maddika et al., 2011) and MC1R (Figures S3E–F) interact with the C2-domain of PTEN, as well as preferentially bind to the non-phosphorylated, active form of PTEN (Figures 4G and S5H), these results suggest a model by which MC1R competes with WWP2 to interact with PTEN, thereby protecting PTEN from WWP2-mediated ubiquitination and subsequent destruction (Figure 5J). In support of this model, following UVB exposure, WT, but not MC1R variants, competes with WWP2 to interact with PTEN (Figures 4A and Figure 5I). Intriguingly, no significant reduction in PTEN/NEDD4 interaction was observed either under ectopic expression (Figure S5I) or physiological (Figure 4A) conditions. These results indicate that at least in melanocytes following UVB exposure, WWP2, but not NEDD4 is the physiological E3 ligase for PTEN, and UVB-triggered MC1R/PTEN interaction, in part by elevated secretion of α-MSH, protects PTEN from WWP2-mediated ubiquitination (Figure 5J).
MC1R Deficiency Triggers the Onset of UVB-induced Premature Senescence Largely via the PTEN/AKT Signaling Pathway
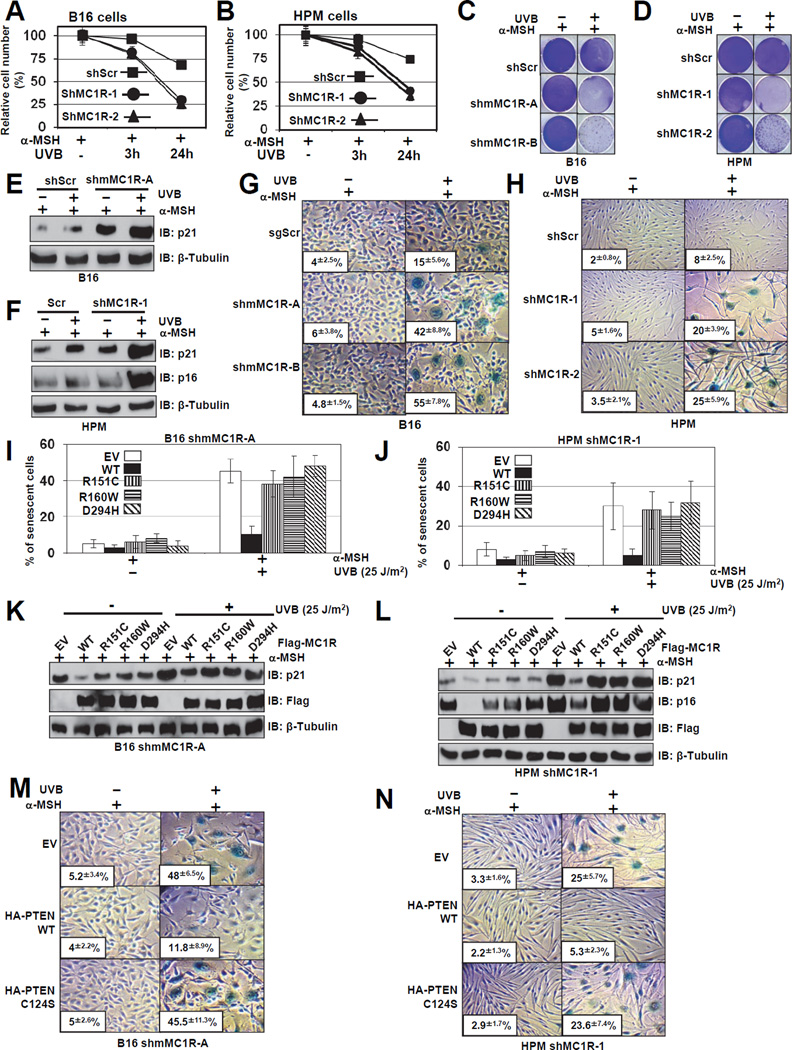
In keeping with the notion that melanocyte senescence is prevalent in melanocytic nevus, which is frequently found in individuals with MC1R variants (Ha et al., 2008), upon UVB exposure, depletion of MC1R severly reduced cell proliferation (Figures 6A–D and S6A–B) that correlated with elevated expression of cell cycle inhibitors, p16INK4a and p21 (Figures 6E–F). Consistently, compared to control cells, we observed a significant increase of the G1 cell population in MC1R-depleted cells following UVB exposure (Figures S6C). Further studies revealed no significant difference in apoptosis between control and MC1R-depleted cells following UVB exposure (Figure S6D–H). To eradicate possible off-target effects of shMC1R, we reintroduced WT-MC1R or various MC1R variants in MC1R-depleted cells and found that WT, but not MC1R-RHC variants could rescue UVB-induced cell growth retardation (Figures S6I–K). These results argue for a causal relationship between depletion of MC1R and reduced cell proliferation following UVB exposure to cause G1 cell cycle arrest.
Figure 6. MC1R Deficiency Triggers Premature Senescence Following UVB Exposure in Primary Human Melanocytes.
A–B. Control of MC1R depleted B16 cells (A) or HPMs (B) were treated with 1 µM α-MSH for 30min, and irradiated with 25 J/m2 UVB followed by MTT assays to evaluate the relative cell numbers at different time points. Data shown represent mean ± SEM, n=3.
C–F. The cells generated in A-B were pre-treated with 1 µM α-MSH for 30 min and then irradiated with or without UVB (25 J/m2). Cells were then seeded at equal densities after UVR and fixed and stained with crystal violet after 10 days (C–D). The pictures shown are representative of three experiments. IB analyses were also performed 10 days after UVR with the indicated antibodies (E–F).
G–H. The cells generated in A–B were subjected to SA-β-gal staining assay 6 days after UVR. The quantification of the percentage of SA-β-gal positive cells was indicated within the pictures. The pictures show one representative experiment out of three independent experiments. Data are represented as mean ± SEM, n=3.
I–J. MC1R-depleted B16 cells (I) or HPMs (J) were infected with retro-viruses encoding wild-type MC1R or MC1R variants (R151C, R160W or D294H). The resulting cells were exposed to 25 J/m2 UVB, and stained for SA-β-gal activity at day 6 after UVR. The quantification of the percentage of SA-β-gal positive cells was indicated within the pictures. The pictures show one representative experiment out of three independent experiments. Data are represented as mean ± SEM, n=3.
K-L. The cells generated in I–J were subjected to IB analysis.
M–N. MC1R-depleted B16 cells (M) or HPMs (N) were infected with lenti-viruses encoding wild-type PTEN or phosphatase dead, C124S PTEN as indicated. The resulting cells were exposed to 25 J/m2 UVB, and stained for SA-β-gal activity at day 6 after UVR. The quantification of the percentage of SA-β-gal positive cells was indicated within the pictures. The pictures show one representative experiment out of three independent experiments. Data are represented as mean ± SEM, n=3.
(See also Figure S6)
In keeping with the correlation between melanocyte senescence prevalent in melanocytic nevus and individuals with MC1R-RHC variants (Ha et al., 2008), depletion of endogenous MC1R induced the accumulation of SA-β-gal-positive cells (Figures 6G–H) that correlated with reduction in colony formation (Figures 6C–D). To further validate the role of MC1R in UV-induced melanocyte senescence, we reintroduced WT and MC1R-RHC variants into MC1R-depleted B16 cells and human primary melanocytes. Notably, there was a 75% reduction in UVB-induced SA-β-gal positive cells in those expressing WT-MC1R, compared to empty vector controls (Figures 6I–J). In contrast, cells expressing MC1R-RHC variants did not obviously repress the onset of premature senescence (Figures 6I–J), in part due to failure to suppress p16INK4a and p21 induction following UVB (Figures 6K–L). These results provide molecular insights into the observation that reintroduction of WT-MC1R, but not MC1R-RHC variants, could accelerate cell growth in MC1R-depleted cells as judged by MTT (Figures S6I) or colony formation (Figures S6J) assays.
Importantly, consistent with the Pandolfi group (Chen et al., 2005), we found that phenocopying MC1R depletion, depletion of endogenous PTEN in human primary melanocytes (Figure S6L) or mouse B16 melanocytes (Figure S6M) led to a significant increase in SA-β-gal-positive cells as well as p16INK4a and p21 expression levels. These findings indicate that depletion of MC1R-induced premature senescence after UVB exposure is largely via the PTEN pathway. Consistently, re-introducing WT-PTEN, but not the inactive, C124S–PTEN (Figures S6N–O), into MC1R-depleted melanocytes efficiently rescued premature senescence (Figures 6M–N). However, depletion of p16INK4a itself was not sufficient to bypass UVB-induced premature senescence in MC1R-depleted cells (Figure S6P), indicating that besides the p16INK4a/Rb pathway, other tumor suppressor pathways such as the p53/p21 pathway (Ben-Porath and Weinberg, 2005) might be also involved. Intriguingly, depletion of p21 alone (Figures S6R–T) or in combination with p16INK4a depletion (Figures S6U–W) could not rescue the premature senescence phenotype induced by depletion of MC1R. As simultaneous inhibition of Rb/p53 pathway has been reported to facilitate cellular transformation by bypassing oncogene-induced premature senescence (OIS) in various cellular contexts including melanocytes (Garraway et al., 2005), these results suggest that besides p16INK4a and p21 CDK inhibitors, other downstream targets of the Rb/p53 tumor suppressor pathways (Sherr and McCormick, 2002) might participate in OIS. Nonetheless, in the following studies, rather than co-depletion of p21 and p16INK4a, we chose to use ectopic expression of p53DD and R24C–Cdk4 to inactivate both the Rb and p53 tumor suppressor pathways in melanocytes (Garraway et al., 2005).
BRAFV600E Cooperates with MC1R Deficiency to Drive Melanomagenesis
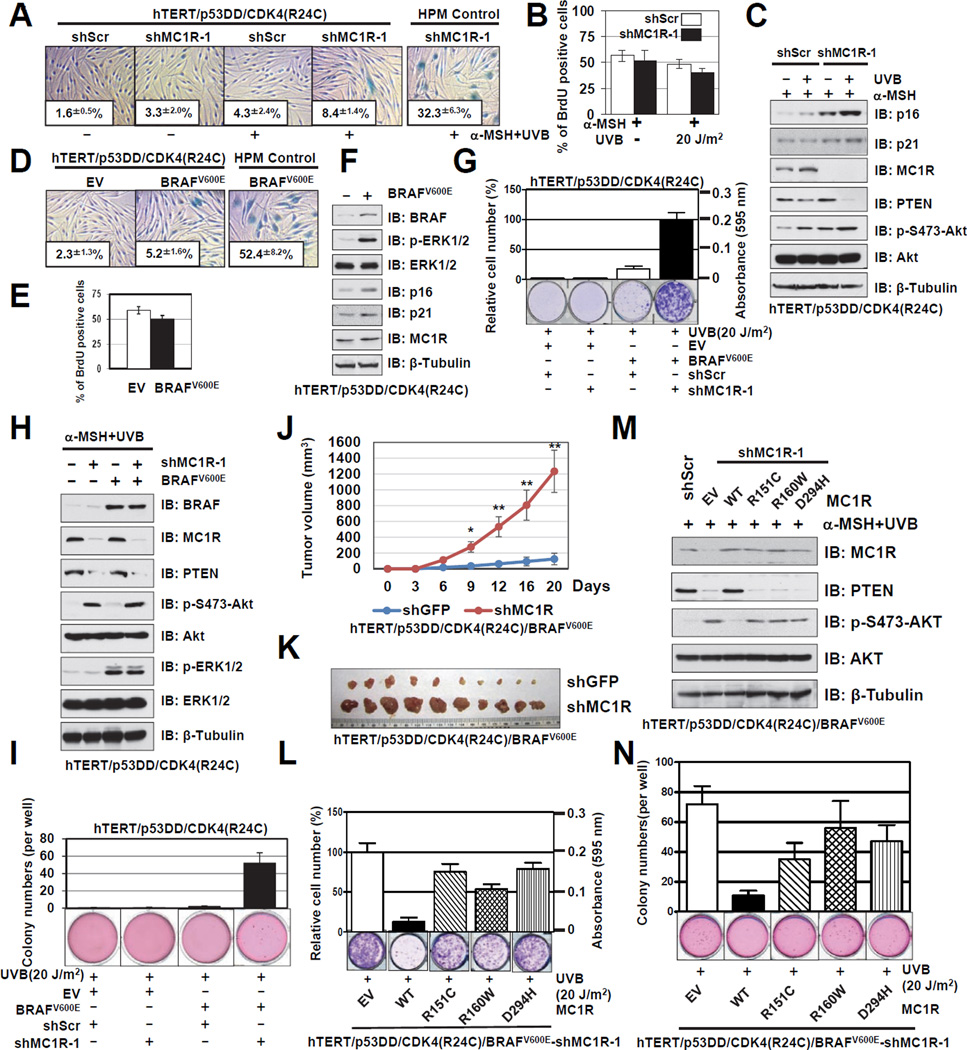
Notably, using genetically engineered human immortal melanocytes (hTERT/p53DD/CDK4(R24C) melanocytes) (Garraway et al., 2005), we demonstrated that depletion of MC1R could not initiate the onset of premature senescence even after UVB exposure (Figures 7A–C). Although bypassing premature senescence is necessary for oncogene-induced transformation, depletion of the MC1R tumor suppressor was not sufficient to confer cellular transformation in hTERT/p53DD/CDK4(R24C) melanocytes (Figures 7G and I). These results indicate that additional genetic alterations are required for cellular transformation of human melanocytes. As oncogenic mutation of BRAFV600E is detected in over 70% of human melanomas and is considered as a driving force to facilitate melanoma development (Vredeveld et al., 2012), we intended to further investigate the potential role of BRAFV600E in MC1R deficiency-induced melanoma.
Figure 7. BRAFV600E Cooperates with MC1R Deficiency to Drive Melanomagenesis.
A. Control or MC1R depleted hTERT/p53DD/CDK4(R24C) human melanocytes were treated with 1 µM α-MSH for 30 min and irradiated with or without UVB (20 J/m2). Cells were then subjected to SA-β-gal staining 10 days after UVR. The quantification of the percentage of SA-β-gal positive cells is indicated within the pictures. The pictures show one representative experiment out of three independent experiments. Data are represented as mean ± SEM, n=3.
B. The cells generated in A were subjected to BrdU incorporation assays. The quantification of the percentage of BrdU positive cells was plotted as mean ± SEM from three independent experiments.
C. The cells generated in A were harvested for IB analysis.
D. Control or BRAFV600E expressing hTERT/p53DD/CDK4(R24C) human melanocytes were subjected to SA-β-gal staining assays 10 days after infection, and quantification of the percentage of SA-β-gal positive cells was indicated within the pictures. The pictures show one representative experiment out of three independent experiments. Data are represented as mean ± SEM, n=3.
E. The cells generated in D were subjected to BrdU incorporation assays, and quantification of the percentage of BrdU positive cells was plotted as mean ± SEM from three independent experiments.
F. The cells generated in D were harvested for IB analysis.
G–H. The BRAFV600E (or EV) expressing cells generated in D were further infected with the shMC1R lentiviral construct (with shScr as a negative control) to deplete endogenous MC1R. The resulting cells were subjected to clonogenic survival assays 15 days after UVR. Crystal violet was used to stain the formed colonies and the colony numbers were counted from three independent experiments. The relative colony numbers were calculated as mean ± SEM, n=3 (G). The resulting cells were also subjected to IB analysis (H).
I. The cells generated in G were seeded (10,000 cells per well) in 0.5% low-melting-point agarose in DMEM with 10% FBS, layered onto 0.8% agarose in DMEM/10% FBS. The plates were cultured for 30 days whereupon the colonies >50 µm were counted under a light microscope. The colony numbers were plotted as mean ± SEM from three independent experiments.
J. Growth curves for the xenograft experiments with the indicated tumor cells that were inoculated subcutaneously. In each flank of ten nude mice, 3×106 cells were injected. The visible tumors were measured at the indicated days. Error bars represent ±SEM, n=10 and * p < 0.05, ** p < 0.01 (Student’s t test).
K. Dissected tumors from the xenograft experiments (J).
L–M. MC1R-depleted hTERT/p53DD/CDK4(R24C)/BRAFV600E melanocytes expressing EV, WT, R151C, R160W or D294H-MC1R were pre-incubated with 1 µM α-MSH for 30min before being irradiated with 20 J/m2 UVB, and were then subjected to clonogenic survival assays 15 days after UVR. Crystal violet was used to stain the formed colonies and the colony numbers were counted from three independent experiments. The relative colony numbers were calculated as mean ± SEM (L), n=3. The resulting cells were also subjected to IB analysis (M).
N. The cells generated in L were seeded (10,000 cells per well) in 0.5% low-melting-point agarose in DMEM with 10% FBS, layered onto 0.8% agarose in DMEM+10% FBS. The plates were cultured for 30 days whereupon the colonies >50 µm were counted under a light microscope. The colony numbers were plotted as mean ± SEM from three independent experiments.
(See also Figure S7 and Supplemental Table 5)
In keeping with previous reports (Vredeveld et al., 2012), ectopic expression of BRAFV600E efficiently triggered the onset of premature senescence (Figure S7A), in part through upregulating both p16INK4a and p21 (Figure S7B). Correspondingly, inhibition of the p53 and Rb pathways by ectopic expression of p53DD and R24C–Cdk4 also bypassed BRAFV600E-induced premature senescence (Figures 7D–F). However, in primary human melanocytes, depletion of either MC1R or PTEN could not rescue the senescent phenotype exhibited by BRAFV600E expression (Figures S7C and S7E), as depletion of either PTEN or MC1R could not suppress BRAFV600E-elevated expression of p16INK4a and p21 (Figures S7D and S7F). Since abrogation of the p53 and Rb pathways allows both MC1R-depleted cells and BRAFV600E-expressing cells to bypass senescence, we further explored whether BRAFV600E cooperates with MC1R deficiency to induce cellular transformation. Notably, we found that unlike ectopic expression of BRAFV600E alone, or singular depletion of MC1R, genetically engineered human melanocytes with simultaneous depletion of MC1R and ectopic expression of BRAFV600E resulted in robust melanocyte media-independent growth in vitro (Figures 7G–H). Mechanistically, only in experimental conditions with depletion of MC1R and overexpression of BRAFV600E, there was a significant co-induction of both p-AKT and p-ERK, both of which could facilitate cellular transformation (Figure 7H). In support of these results, only cells engineered to express BRAFV600E in conjunction with MC1R depletion readily formed anchorage-independent colonies (Figure 7I), or significantly promoted in vivo tumor formation in mouse xenograft (Figures 7J–K and S7G–H).
Lastly, to eliminate possible off-target effects of shRNA treatment, we reintroduced WT-MC1R and various MC1R variants into the BRAFV600E-expressing, MC1R-depleted hTERT/p53DD/CDK4(R24C) melanocytes and further demonstrated that reintroducing WT, but not MC1R-RHC variants that are defective in associating with PTEN, could efficiently block the cellular transformation phenotype (Figures 7L–N). Interestingly, comparing to WT-MC1R, reintroduction of MC1R-RHC variants resulted in elevated sensitivity to AKT inhibitor treatment (Figures S7I–N), presumably due to their deficiency to protect PTEN from WWP2-induced destruction (Figure S7P). These results also indicate that multiple genetic alterations are required for transforming human primary melanocytes. More importantly, our results demonstrated that MC1R variants could synergize with BRAFV600E to facilitate cellular transformation. In keeping with our results, the Fisher group recently demonstrated that melanocyte-targeted expression of BRAFV600E in mice carrying an inactivating MC1R variant that is analogous to red hair-fair skin humans, led to a high incidence of invasive melanomas in vivo (Mitra et al., 2012). These findings altogether provide a possible molecular mechanism for the observation that red-haired individuals harboring MC1R mutations, are more susceptible to UV-induced skin damage than individuals with darker skin, resulting in a 10- to 100-fold higher frequency of non-melanoma and melanoma skin cancers.
PTEN Aberrations are Frequently Detected in Melanomas with Wild-type MC1R
To identify possible correlations between MC1R genetic status and PTEN activity in melanomas, PTEN mutations and homozygous deletions were determined in 79 melanoma cell lines derived from human melanoma samples collected in Brisbane, Australia (Supplemental Table 5). We found that 68 of 79 melanoma cell lines (86%) have wild-type PTEN and of these, only 10 are MC1R wild-type (14.7%). In contrast, of 11 melanoma cell lines carrying PTEN aberrations, 7 (64%) are MC1R wild-type (Supplemental Table 5). Therefore, there seems to exist a significant association between MC1R variants and PTEN activity in melanomas (χ2=8.8, p=0.00301 by Pearson’s Chi-squared test) (Figure S7O). These results suggest that MC1R and PTEN defects are complementary in melanoma development and furthermore, the host genotypes might condition the somatic mutation spectrum in melanoma. Importantly, this phenomenon has previously been postulated for germline MC1R status influencing BRAF mutations in a similar fashion in melanoma (Landi et al., 2006).
Discussion
We show here that the α-MSH/MC1R signaling receptor/ligand pair protects against UV damage by a direct interaction with PTEN, protecting PTEN from proteolysis and consequently acts to suppress the PI3K/AKT signaling. Thus, the MC1R-RHC variants that do not interact with PTEN allow elevated levels of PI3K/AKT signaling following UV irradiation. Strikingly, the consequences of MC1R-RHC variants in failing to suppress the PI3K/AKT signaling are highly context dependent. In primary melanocytes, the hyperactivation of the PI3K/AKT signaling, arising as a consequence of the failure of MC1R-RHC variants to stabilize PTEN following UV irradiation, leading to premature senescence. On the other hand, in the presence of activated BRAF, the same elevated PI3K/AKT signaling drives oncogenic transformation. These data go a long way to explain previous observations that certain MC1R variants promote UV-induced senescence, and delay DNA repair (Dong et al., 2010) and ROS clearance (Henri et al., 2011) following UV exposure.
Importantly, PTEN dysfunctions are frequently detected in melanomas with wild-type MC1R. Our demonstration that MC1R could govern the activity of PTEN under UV exposure provides a mechanistic explanation for the role of MC1R in protecting against UV-induced melanocyte premature senescence, a critical natural barrier for the subsequent cellular transformation induced by additional acquisition of the BRAFV600E mutation, an event detected in nearly 70% of human melanomas. Hence, the identification of the MC1R protective interaction with PTEN will be a starting point for future studies on pigment-independent mechanisms governing UV response and oncogenesis. Furthermore, we found that unlike WT-MC1R, MC1R-RHC variants are defective in associating with PTEN, and do not protect PTEN upon UV exposure, which could adversely affect various cellular functions. Although our studies indicated that UVB-induced secretion of α-MSH might trigger PTEN/MC1R interaction, it remains not fully understood why RHC-type, but not all MC1R sequence variants, are defective in associating with PTEN following UVB exposure. Further crystallization studies are required to identify the crystal structure of the MC1R protein and how this is altered by MC1R-RHC variants to potentially impair its PTEN association.
Notably, similar to loss of other tumor suppressors including TSC2 (Zhang et al., 2003), PTEN (Chen et al., 2005; Nardella et al., 2008) or VHL (Young et al., 2008), we found that loss of the MC1R tumor suppressor in primary human melanocytes induces the onset of premature senescence upon UVB exposure. Importantly, several senescence pathways are usually activated in benign nevi where they work to prevent to further transformation (Michaloglou et al., 2005). Nevi and melanoma are genetically linked (Hayward, 2000). Both of them are more frequent in Caucasians with light-skin than those with a dark-skin phenotype (Halder and Bridgeman-Shah, 1995). Further studies revealed that abrogation of the downstream p53 and Rb tumor suppressor pathways allows primary human melanocytes to bypass MC1R deficiency-induced premature senescence, but are not sufficient to gain a transformation phenotype (Figures 7G–K). These findings further impinge on additional genetic alterations that might be required for melanomagenesis. To this end, we found that additional expression of BRAFV600E efficiently transformed the MC1R-depleted hTERT/p53DD/CDK4(R24C) human melanocytes, but not the control cell lines, and promoted in vivo tumorigenesis in a xenograft model. Consistent with this result, a recent report showed that abrogation of BRAFV600E-induced senescence by PI3K pathway activation (e.g. PTEN deletion) contributes to melanomagenesis (Vredeveld et al., 2012). More importantly, the synergistic effects of gain of BRAFV600E oncogenic function and loss of MC1R tumor suppressor function in facilitating melanoma development in vivo are further illustrated by the Fisher group using elegant engineered mice models (Mitra et al., 2012). These data further indicate the tumor suppressor role of MC1R and the tight connections between MC1R and PTEN in melanocytes.
Experimental Procedures
Cell Lines, Animals and UV Exposure
Mouse strains, cell lines and UV exposure were described previously (Cui et al., 2007; D'Orazio et al., 2006). Primary melanocytes were isolated from normal discarded foreskins as described before (Dunham et al., 1996). Human primary melanocytes were cultured in Medium 254 (Gibco). Immortal human melanocytes (hTERT/p53DD/CDK4(R24C)) (Garraway et al., 2005) were cultured in glutamine containing Ham’s F12 media supplemented with 7% FBS, 0.1 mM IBMX, 50 ng/mL TPA, 1 µM Na3VO4 and 1 µM dbcAMP.
Quantitative Real-time RT-PCR Analysis
Quantitative real-time RT-PCR was utilized to determine relative mRNA expression as described previously (Cui et al., 2007; D'Orazio et al., 2006). The primers used for human MC1R are: Forward, 5’- ACCTGCACTCGCCCATGTATTACT-3’; Reverse, 5’- AATGATGCCCAGGAAGCAGAGACT-3’; Mouse MC1R: Forward, 5’-TGGGCATCATTGCTATAGACCGCT-3’; Reverse, 5’-AACGGCTGTGTGCTTGTAGTAGGT-3’.
BrdU, SA- β-Gal Assays, and Crystal Violet Staining
BrdU labeling was performed at 16 hr after UV irradiation. SA β-Gal and crystal violet staining were performed as described previously (Banito et al., 2009).
Subcellular Fractionations
Saponin subcellular fractionation for MC1R and PTEN were performed as described previously (Odriozola et al., 2007).
Supplementary Material
Highlights.
UVB exposure triggers PTEN interaction with wild-type, but not MC1R RHC variants
WT, but not MC1R RHC variants protect PTEN from WWP2-mediated ubiquitination
MC1R deficiency leads to the onset of premature senescence in primary melanocytes
MC1R deficiency cooperates with BRAFV600E to drive melanomagenesis
Acknowledgments
We thank Drs. Xiaoqi Liu and Jiali Han for helpful comments and suggestions. This work was supported by the National Institutes of Health (RC: 1RO1CA137098 and UL1-TR000157, WW: GM089763), American Cancer Society (RSG-09-022-01-CNE), Harry J. Lloyd Charitable Trust (RC), and the National Health and Medical Research Council of Australia (NHMRC). RC and WW are American Cancer Society Research Scholars and NKH is supported by a senior principal research fellowship from the NHMRC. The authors are grateful to Pamela Pollock, Rick Sturm and Mitchell Stark for generating some of the cell line mutation data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes seven figures, five tables, Supplemental Experimental Procedures and Supplemental References, and can be found with this article online.
References
- Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, Manevich Y, Beeson C, Neumann CA. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D’Orazio J, Fung CY, Schanbacher CF, Granter SR, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- D'Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- Dong L, Wen J, Pier E, Zhang X, Zhang B, Dong F, Ziegler N, Mysz M, Armenta R, Cui R. Melanocyte-stimulating hormone directly enhances UV-Induced DNA repair in keratinocytes by a xeroderma pigmentosum group A-dependent mechanism. Cancer Res. 2010;70:3547–3556. doi: 10.1158/0008-5472.CAN-09-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham WR, Klein SB, Rhodes LM, Marcelo CL. Oleic acid and linoleic acid are the major determinants of changes in keratinocyte plasma membrane viscosity. J Invest Dermatol. 1996;107:332–335. doi: 10.1111/1523-1747.ep12363174. [DOI] [PubMed] [Google Scholar]
- Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003;3:117–130. doi: 10.1016/s1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Borron JC, Sanchez-Laorden BL, Jimenez-Cervantes C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005;18:393–410. doi: 10.1111/j.1600-0749.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Landi MT, Tsang S, Fraser MC, Munroe DJ, Tucker MA. Association of MC1R variants and risk of melanoma in melanoma-prone families with CDKN2A mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:2208–2212. doi: 10.1158/1055-9965.EPI-05-0321A. [DOI] [PubMed] [Google Scholar]
- Ha L, Merlino G, Sviderskaya EV. Melanomagenesis: overcoming the barrier of melanocyte senescence. Cell Cycle. 2008;7:1944–1948. doi: 10.4161/cc.7.13.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667–673. doi: 10.1002/1097-0142(19950115)75:2+<667::aid-cncr2820751409>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hayward N. New developments in melanoma genetics. Curr Oncol Rep. 2000;2:300–306. doi: 10.1007/s11912-000-0022-z. [DOI] [PubMed] [Google Scholar]
- Henri P, Beaumel S, Guezennec A, Poumes C, Stoebner PE, Stasia MJ, Guesnet J, Martinez J, Meunier L. MC1R expression in HaCaT keratinocytes inhibits UVA-induced ROS production via NADPH oxidase- and cAMP-dependent mechanisms. J Cell Physiol. 2011;227:2578–2585. doi: 10.1002/jcp.22996. [DOI] [PubMed] [Google Scholar]
- Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, ter Huurne J, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, Bavinck JN. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117:294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- Kinsler VA, Abu-Amero S, Budd P, Jackson IJ, Ring SM, Northstone K, Atherton DJ, Bulstrode NW, Stanier P, Hennekam RC, et al. Germline melanocortin-1-receptor genotype is associated with severity of cutaneous phenotype in congenital melanocytic nevi: a role for MC1R in human fetal development. J Invest Dermatol. 2012;132:2026–2032. doi: 10.1038/jid.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada T, Lu SZ, Yoshida H, Nishikawa S, Nishikawa S, Mizoguchi M, Hayashi S, Tyrrell L, Williams DA, Wang X, et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998;187:1565–1573. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi MT, Bauer J, Pfeiffer RM, Elder DE, Hulley B, Minghetti P, Calista D, Kanetsky PA, Pinkel D, Bastian BC. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313:521–522. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- Maddika S, Kavela S, Rani N, Palicharla VR, Pokorny JL, Sarkaria JN, Chen J. WWP2 is an E3 ubiquitin ligase for PTEN. Nat Cell Biol. 2011;13:728–733. doi: 10.1038/ncb2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescencelike cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Mihm MC., Jr. Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- Ming M, Feng L, Shea CR, Soltani K, Zhao B, Han W, Smart RC, Trempus CS, He YY. PTEN positively regulates UVB-induced DNA damage repair. Cancer Res. 2010;71:5287–5295. doi: 10.1158/0008-5472.CAN-10-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, Guerrero CR, Lennerz JK, Mihm MC, Wargo JA, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491:449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardella C, Chen Z, Salmena L, Carracedo A, Alimonti A, Egia A, Carver B, Gerald W, Cordon-Cardo C, Pandolfi PP. Aberrant Rheb-mediated mTORC1 activation and Pten haploinsufficiency are cooperative oncogenic events. Genes Dev. 2008;22:2172–2177. doi: 10.1101/gad.1699608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif NT, Lobo GP, Wu X, Henderson CJ, Morrison CD, Eng C, Jalaludin B, Segelov E. PTEN mutations are common in sporadic microsatellite stable colorectal cancer. Oncogene. 2004;23:617–628. doi: 10.1038/sj.onc.1207059. [DOI] [PubMed] [Google Scholar]
- Odriozola L, Singh G, Hoang T, Chan AM. Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J Biol Chem. 2007;282:23306–23315. doi: 10.1074/jbc.M611240200. [DOI] [PubMed] [Google Scholar]
- Peeper DS. Oncogene-induced senescence and melanoma: where do we stand? Pigment Cell Melanoma Res. 2011;24:1107–1111. doi: 10.1111/j.1755-148X.2011.00933.x. [DOI] [PubMed] [Google Scholar]
- Rabinovsky R, Pochanard P, McNear C, Brachmann SM, Duke-Cohan JS, Garraway LA, Sellers WR. p85 Associates with unphosphorylated PTEN and the PTEN-associated complex. Mol Cell Biol. 2009;29:5377–5388. doi: 10.1128/MCB.01649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S, Sera F, Gandini S, Iodice S, Caini S, Maisonneuve P, Fargnoli MC. MC1R variants, melanoma and red hair color phenotype: a meta-analysis. Int J Cancer. 2008;122:2753–2760. doi: 10.1002/ijc.23396. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Phillips SR, Rudzish R, Birch-Machin MA, Wikberg JE, Rees JL. Loss of function mutations of the human melanocortin 1 receptor are common and are associated with red hair. Biochem Biophys Res Commun. 1999;260:488–491. doi: 10.1006/bbrc.1999.0935. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- Stark M, Hayward N. Genome-wide loss of heterozygosity and copy number analysis in melanoma using high-density single-nucleotide polymorphism arrays. Cancer Res. 2007;67:2632–2642. doi: 10.1158/0008-5472.CAN-06-4152. [DOI] [PubMed] [Google Scholar]
- Tap WD, Gong KW, Dering J, Tseng Y, Ginther C, Pauletti G, Glaspy JA, Essner R, Bollag G, Hirth P, et al. Pharmacodynamic characterization of the efficacy signals due to selective BRAF inhibition with PLX4032 in malignant melanoma. Neoplasia. 2010;12:637–649. doi: 10.1593/neo.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NE, Kanetsky PA, Edmiston SN, Alexander A, Begg CB, Groben PA, Hao H, Busam K, Ollila DW, Berwick M, et al. Relationship between germline MC1R variants and BRAF-mutant melanoma in a North Carolina population-based study. J Invest Dermatol. 2010;130:1463–1465. doi: 10.1038/jid.2009.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vredeveld LC, Possik PA, Smit MA, Meissl K, Michaloglou C, Horlings HM, Ajouaou A, Kortman PC, Dankort D, McMahon M, et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev. 2012;26:1055–1069. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Shi Y, Wang J, Huang G, Jiang X. Crucial role of the C-terminus of PTEN in antagonizing NEDD4-1-mediated PTEN ubiquitination and degradation. Biochem J. 2008;414:221–229. doi: 10.1042/BJ20080674. [DOI] [PubMed] [Google Scholar]
- Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–3122. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- Wu Y, Dowbenko D, Spencer S, Laura R, Lee J, Gu Q, Lasky LA. Interaction of the tumor suppressor PTEN/MMAC with a PDZ domain of MAGI3, a novel membrane-associated guanylate kinase. J Biol Chem. 2000;275:21477–21485. doi: 10.1074/jbc.M909741199. [DOI] [PubMed] [Google Scholar]
- Young AP, Schlisio S, Minamishima YA, Zhang Q, Li L, Grisanzio C, Signoretti S, Kaelin WG., Jr. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol. 2008;10:361–369. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest. 2003;112:1223–1233. doi: 10.1172/JCI17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.