Abstract
Head and neck paragangliomas (HNPGLs) account for approximately 3% of all paragangliomas (PGLs). Most often, HNPGLs are benign, non-secreting, and slowly progressing. The initial physical examination and biochemical diagnosis usually adds very little to the proper diagnosis of these tumors and therefore, radiologists and nuclear medicine physicians play the pivotal role in providing the initial diagnosis, the locoregional staging, and the plan for detecting potential multicentric or metastatic lesions. Based on several current studies, the most accurate use of HNPGL-specific initial and subsequent imaging modalities must be guided by the knowledge of genetics and the specifically measured biochemical profile of these tumors for the proper management of these patients. Thus, this short review article presents the application of the most up-to-date anatomic and functional imaging approaches to HNPGLs tightly linked to the clinical management of these patients. Based on the most recent studies, 18F-FDOPA PET/CT has been shown to be a useful addition to anatomic imaging in the preoperative localization and molecular assessment of HNPGLs. It is estimated that the frequency of metabolically active PGLs on 18F-FDOPA PET/CT in this region is higher than 90%. 18F-FDG PET/CT should be reserved for patients with hereditary PGL syndromes. Imaging of somatostatin receptors using Octreoscan or 68Ga-labeled somatostatin analogs plays an important role for selecting patients for targeted radiation therapy.
This review also concludes that it is expected that in the near future, these patients will indeed benefit from new diagnostic approaches based on the identification of new targets by molecular profiling studies that will result in the development of novel PGL specific radiopharamceuticals.
Keywords: Positron emission tomography, computed tomography, paraganglioma, radiopharmaceuticals, genetics, head and neck
Definition and anatomy of HNPGLs
Head and neck paragangliomas (HNPGLs) are neural crest-derived neuroendocrine neoplasms arising from head and neck paraganglia that are closely aligned along the parasympathetic nervous system, some of which act as chemoreceptors. HNPGLs account for about 0.6% of all neoplasms in the head and neck region and approximately 3% of all PGLs (1). They are widely distributed, with a predilection for the following sites: the jugular foramen (glomus jugulare PGL), cochlear promontory (glomus tympanicum PGL), prestyloid pharyngeal space (vagal PGL), carotid bifurcation (carotid body PGL), or other unusual or exceptional locations (e.g., larynx, thyroid, sinonasal region, nasopharynx, orbit, tongue). Since HNPGLs are usually non-secreting tumors, they are most often discovered on imaging studies carried out due to neck swelling or by symptoms of compression or infiltration of the adjacent head and neck structures (e.g., otologic symptoms, dysphagia, cranial nerve palsies).
Clinical and genetic update on HNPGLs
Although most HNPGLs arise sporadically, PGL susceptibility genes have been identified in approximately one-third of cases with a single, apparently sporadic tumor, and in more than 90% of patients with multifocal tumors (2, 3). Hereditary HNPGLs are most frequently associated with germline mutations in one of the succinate dehydrogenase (SDH) subunits genes (SDHB/D and rarely SDHC/A) or its flavination factor SDHAF2 (4). The spectrum of clinical manifestations in patients with von Hippel-Lindau (VHL) and recently discovered transmembrane protein 127 (TMEM127) mutations has also been extended to rarely include HNPGLs (5, 6). The inheritance pattern of the SDHB and SDHC genes is autosomal dominant, whereas for SDHD and SDHAF2 genes, there is virtually an exclusive autosomal dominant paternal transmission of the disease (7). Major predictors for hereditary HNPGLs are family history of PGL, although less frequently available due to a low penetrance of these tumors, previous history of adrenal or extra-adrenal PGL, and multifocality or characteristic syndromic presentation (2, 8). In addition, the existence of other tumor types (e.g., renal cell carcinoma (9), gastrointestinal stromal tumor and/or pulmonary chondroma in Carney–Stratakis dyad or triad (10), pituitary adenoma (11)) or a dopaminergic biochemical phenotype based on the measurement of a new plasma biomarker, the dopamine metabolite 3-methoxytyramine, can be indicative of SDH-related PGLs (12). Of all the known genetic mutations, mutations in SDHD are currently the leading cause of HNPGLs, followed by SDHB mutations (3, 13). Furthermore, it should be noted that multiple PGLs at a very young age found in several family members are often related to SDAF2 mutations (14). Moreover, PGLs with an underlying SDHB mutations are associated with a higher risk of metastatic and perhaps locally aggressive behavior (15), which may also apply to HNPGLs, although properly designed studies are missing.
Anatomic imaging as the first line imaging in the localization of HNPGLs
At present, anatomic imaging remains the first-line modality in the localization of these tumors, since practically no surgery would be carried out without a precise delineation of these tumors. Multidetector computed tomography (MDCT) and magnetic resonance (MR) are both considered gold standards for HNPGL imaging, although each modality has advantages and disadvantages (Figures 1-7). Current CT technology offers several physical advantages over MR (e.g., better spatial resolution and less motion artifact) and enables better evaluation of the temporal bone extension of glomus jugulare and glomus tympanicum PGLs (Figures 2-4, 6, 7). However, MR provides better soft tissue contrast than CT and thus offers unique diagnostic and prognostic information as well as not using ionizing radiation (Table 1). HNPGLs demonstrate marked enhancement of intra-tumoral vessels following contrast administration on CT and low signal on T1-weighted images and an intermediate to high signal on T2-weighted MRI images; they also often enhance intensely after gadolinium injection on MRI. Flow signal voids in the tumor are typical of PGL, with a “salt and pepper” appearance on spin-echo sequences. MR angiography also demonstrates intra-tumoral arterial vessels (16-20). 3D time-of-flight (11 minutes at 3 Tesla for a 10 cm field of exploration) and 3D gadolinium (58 seconds for a 30 cm field of exploration) sequences were shown to be highly informative, with sensititivies and specificities of 90% and 94% (17) and 100% and 94% (18), respectively. There is a current trend to use time-resolved 4D gadolinium MR angiography (TRICKS, 5 seconds for a 22 cm field of exploration) (20). TRICKS enables evaluation of both intratumoral vessels (early arterial phase) and tumor perfusion, including capillary permeability. Compressive sensing is currently being developed for reconstruction of images and permits acceleration factors approaching 1000 (21). In our experience, fusion images between 3D volumetric interpolated fat-saturated (FATSAT) T1-weighted (VIBE) and 4D-MR angiography are particularly informative (Figures 1-3, 7). Ultrasound is a good non-invasive method, but it is limited to the evaluation of neck PGLs (carotid body and large vagal PGLs) and is mostly performed only when radiation exposure must be limited (e.g., pregnancy, previous excessive exposure to radiation) or due to contrast allergy. Thus, localization of HNPGLs is often easily done by radiological imaging, but these techniques often lack specificity. For example, evaluation of parapharyngeal space tumors involves careful consideration of clinical and imaging information to distinguish vagal PGLs from peripheral nerve sheath tumors (schwannoma, neurofibroma), nodal metastases (nasopharynx/oropharynx, thyroid cancer), other rare primary tumors, and a variety of uncommon miscellaneous lesions. Diffusion-weighted imaging (DWI), which is dependent on tissue cellularity, may be useful in preoperative characterization and prognosis assessment of PGL, but further evaluation is needed (22). Positron emission tomography (PET)/MR using an integrated system with simultaneous acquisition of both techniques also holds promise for HNPGL, most likely for anatomical sites after a previous operation (e.g., recurrence). Angiography is usually not needed for localization of these tumors and when used, is most often performed for pre-operative vascular mapping followed by endovascular embolization in order to minimize blood loss intraoperatively. Furthermore, in some situations it is also required to assess the vascular supply of a HNPGL to plan an appropriate surgical intervention.
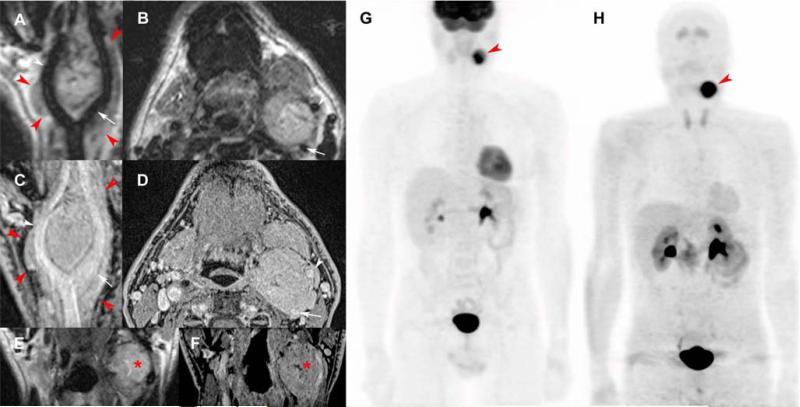
Figure 1. Typical carotid body paraganglioma.
T2-weighted 3D imaging with different flip angle evolutions in curve (A), axial (B), and coronal reconstructions (E); 3D volumetric interpolated fat-saturated (FATSAT) T1-weighted (VIBE) in curve (C), axial (D), and coronal reconstructions (F); 18F-FDG PET/CT (maximal intensity projection (MIP)) (G) and 18F-FDOPA PET/CT (MIP) (H).
MRI shows a “lyre sign” related to a 5-centimeter left carotid body PGL (red arrowheads) arising from the carotid bifurcation, splaying the internal carotid artery (ICE) (A-E, white arrows) and external carotid artery (ECA) (A-D, white arrowheads). Note small central necrosis in the tumor (E, F, red asterisk) and the lack of flow voids due to high temporal resolution of 3D sequences, especially for high-field MRI. 3D reconstructions demonstrate 360° carotid invasion along the common carotid, ICA, and ECA. 18F-FDG PET/CT and 18F-FDOPA PET/CT show a single highly-avid carotid body PGL.
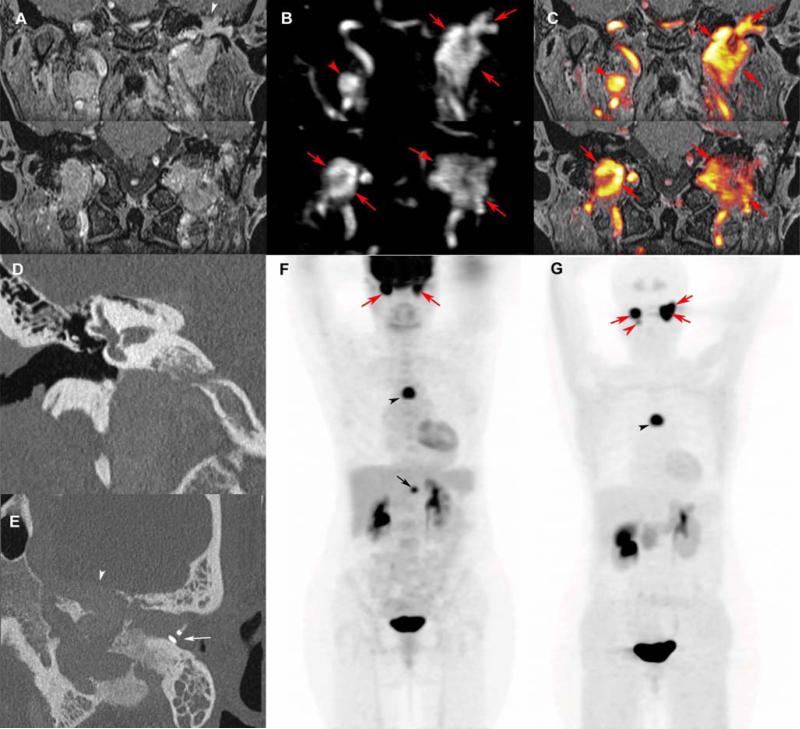
Figure 7. Multifocal and recurrent SDHD-related extra-adrenal PGLs.
Coronal 3D volumetric interpolated fat-saturated (FATSAT) T1-weighted (VIBE) (A), early arterial 4D Dynamic MR Angiography (B), FATSAT T1-weighted/4D-MRA 3D fusion image (C), coronal CT bone reconstruction of the right (D) and axial oblique CT bone reconstruction of the left ear (E), 18F-FDG PET (MIP) (F), and 18F-FDOPA PET (MIP) (G).
MRI with 4D Dynamic MR angiography shows bilateral jugular PGL (red arrows), spreading in the tympanic cavity on the left side, and a right vagal PGL (red arrowheads). CT shows bilateral enlargement of the jugular foramens and sugar bone erosions (D, E). The left tegmen tympani and the anterior wall of the attic are widely eroded by jugulo-tympanic PGL (A, E, white arrowhead). The tumor mass effect is responsible for a complete prosthetic luxation (E, white arrow), explaining the recurrent left conductive hearing loss. 18F-FDG and 18F-FDOPA PE/CT also reveal a mediastinal parasympathetic PGL (F, G, black arrowheads). In comparison to 18F-FDG PET/CT, 18F-FDOPA PET missed an abdominal paraaortic PGL (F, black arrow) and detected one more HNPGL (left vagal PGL, G: red arrowhead). False negative sympathetic PGL on 18F-FDOPA PET is not uncommon in SDHx-related PGL syndromes.
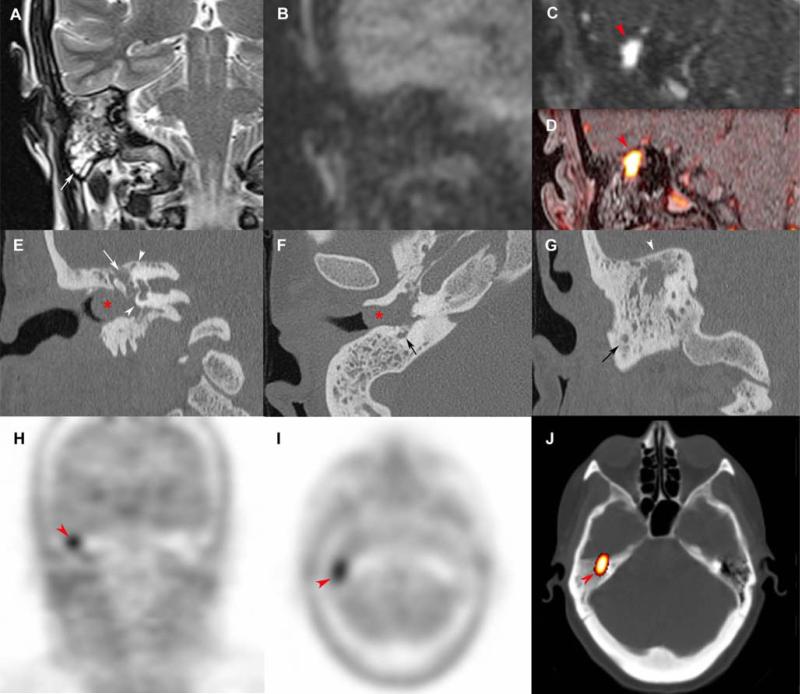
Figure 2. Tympanic paraganglioma extending into the petrous apex.
Axial 3D volumetric interpolated fat-saturated (FATSAT) T1-weighted (VIBE) (A), early arterial 4D Dynamic MR Angiography (MRA) (B), FATSAT T1-weighted/4D-MRA 3D fusion image (C), T2-weighted 3D imaging with different flip angle evolutions (D), CT reconstruction with a bone algorithm in the axial plane (E) and coronal oblique plane (F), axial 18F-FDG PET (G), axial and coronal 18F-FDOPA PET (H,I).
Tympanic PGL (red arrowheads) spreading along Jacobson's nerve canal (A, C-F, white arrows) deeply into the petrous apex along the cochlear aqueduct (white arrowheads, A-C, E). The CT shows integrity of the promontory and round window (F, black arrowheads) before surgical procedure. Note the early enhancement of the superior petrosal sinus suggesting tumor invasion (A-C, asterisk). 18F-FDOPA PET shows a highly avid tympanic PGL with extension in the anteromedial plane. The tumour was barely visible on 18F-FDG PET.
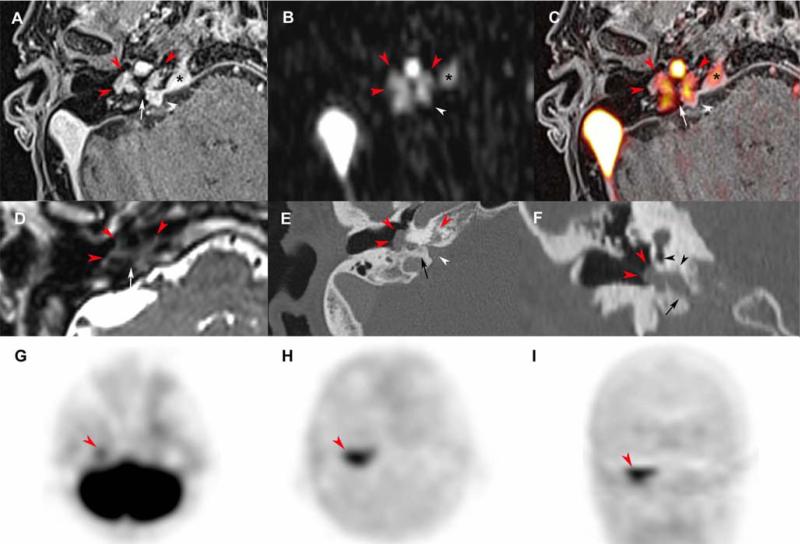
Figure 4. Residual tympanic PGL after surgery.
Axial SE T1-weighted (A), early arterial contrast-enhanced T1-weighted (B), early venous contrast-enhanced T1-weighted (C) and T2- weighted (D), CT scan with a bone algorithm (E), time-intensity curve on dynamic contrast-enhanced MRI (F), axial 18F-FDOPA PET (G), axial 18F-FDOPA PET/CT fusion image (H), coronal 18F-FDOPA PET/CT fusion image (I).
Previous canal wall up tympanomastoidectomy (A-E, asterisk). Residual tympanic PGL (A-E, red arrowheads) located in the petrous apex, posteriorly to the carotid artery (white arrowheads) and lateral to the cochlear aqueduct (arrows). The time-intensity curve shows arterial enhancement of the PGL and enables precise delineation of the lesion prior to sigmoid sinus and nasopharyngeal mucosa enhancements. 18F-FDOPA PET was positive (red arrowheads).
Figure 6. Mutifocal PGL with vagal and jugular locations.
Axial SE T2-weighted (A), T1-weighted (B), FATSAT (3D) T1-weighted (C), CT angiogram in the axial (D) and sagittal planes (E), CT bone reconstruction in the axial plane (F), sagittal 18F-FDG (G) and 18F-FDOPA PET (H), axial 18F-FDOPA PET/CT fusion image.
Vagal PGL (red arrow) arising from the posterior parapharyngeal space, splaying anteriorly the internal carotid artery (white arrow), with typical flow voids and massive enhancement. Imaging reveals an additional jugular PGL (red arrowhead) with typical wet sugar bone erosion of the jugular foramen. Both lesions were positive for 18F-FDG and 18F-FDOPA.
Table 1.
Expression of somatostatin receptor subtypes in PGL and respective affinity profiles of radiolabeled somatostatin agonists.
| SST1 | SST2 | SST3 | SST4 | SST5 | |
|---|---|---|---|---|---|
| % of PGLs with strong overexpression of SSTs | 20% | 80% | − | − | 5% |
| Native SS-28 | +++ | +++ | +++ | +++ | +++ |
| Octreotide (OC) | − | +++ | + | − | ++ |
| [111In]-DTPA-OC (Octreoscan) | − | ++ | + | − | + |
| [68Ga]-DOTA-TOC | − | ++++ | +/− | − | ++ |
| [68Ga]-DOTA-TATE | − | ++++ | − | + | +/− |
| [111In]-DOTA-NOC | − | ++++ | ++ | + | +++ |
SS-28: Native somatostatin (SRIF28)
Affinity (IC50, nM): <5++++, 5 − <40 = +++, 40 − <100 = ++, 100 − <300 = +, 300−<1000 = +/−, ≥1000 = −.
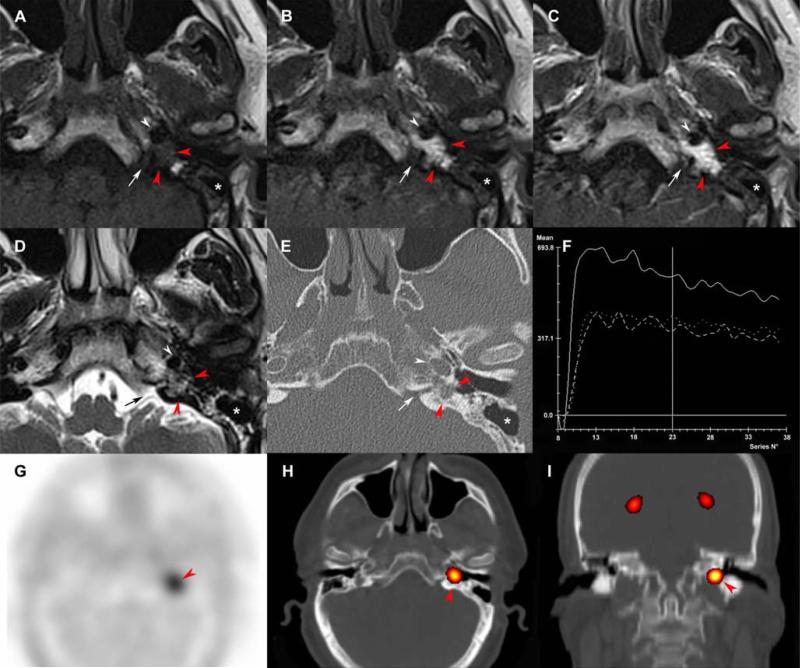
Figure 3. Tympanic paraganglioma in a rare attical location.
Coronal SE T2-weighted (A), non-EPI-b1000-DWI (B), early arterial 4D Dynamic MR Angiography (MRA) (C), fat-saturated (FATSAT) T1-weighted/4D-MRA fusion image (D), CT reconstructions with a bone algorithm (E, F, G), coronal 18F-FDOPA PET (H), axial 18F-FDOPA PET (I), and axial 18F-FDOPA PET/CT fusion image (J).
Chronic otitis media with full field tympanic cavity (A, white arrow) associated with an 8 mm inflammatory polyp in the external auditory canal (E, F, red asterix). Absence of cholestatoma on non-EPI-b1000-DWI (B). MRI shows a 7 mm attic paraganglioma, located in the superior and medial side of the uncudo-mallear joint, with a typical hyper-vascularization pattern (C, D, red arrowhead). CT reconstructions were useful in the preoperative evaluation of the tegmen tympani's integrity (G, white arrowhead), the facial nerve canal (E: white arrows, F: black arrow), and the otic capsule (E, arrow heads). The tumor was highly avid for 18F-FDOPA.
Current functional imaging in the localization of HNPGLs
Radionuclide imaging techniques now play a crucial and unavoidable role in the evaluation of HNPGLs. This is due to the increasing availability of radiopharmaceuticals that have been developed to target receptors or metabolic pathways specific to these tumors, essentially and uniquely performing so-called in vivo histology. Traditionally, the most successful strategy has been to target the cell membrane norepinephrine transporter (NET) and intracellular vesicular monoamine transporter (VMAT) systems, used for cellular entry and catecholamine storage, with 123I/131I-metaiodobenzylguanidine (123I/131I-MIBG). 123I-MIBG scintigraphy is highly specific for chromaffin-derived tumors. Its sensitivity ranges from 90-100% in sporadic PHEOs, but significantly decreases in SDHx-related tumors(23), especially in HNPGLs (24).
More recently, somatostatin receptor scintigraphy using 111In-DTPA-pentetreotide (also called Octreoscan) has been used to localize PGLs, since they overexpress somatostatin receptors, especially subtype 2 (SST2). Several excellent studies have demonstrated the superiority of Octreoscan for HNPGLs compared to 123I/131I-MIBG, with sensitivities of 89-100% and 18-50%, respectively (25-30). However, the sensitivity of this imaging modality needs to be revised downwards because some lesions can be only a few millimeters in size and therefore are not detectable by even the best available cameras (sensitivity 75% with SPECT) (Table 1) (31). The recently introduced hybrid SPECT/CT cameras have increased diagnostic confidence in image interpretation and enhanced sensitivity, but practical constraints such as long imaging times remain important limitations. Based on these studies, 123I-MIBG has been practically abandoned in the evalution of these tumors, except in situations where targeted radiotherapy can be planned using 131I-MIBG. In contrast, the presence of SST receptors has lead to the further evaluation of somatostatin peptide analogs in the localization and subsequent treatment of these tumors. More recently, PET/CT imaging has been growing rapidly in the imaging of these tumors, paralleled by great efforts towards the development of PGL-specific radiopharmaceuticals. PET imaging with 68Ga-labeled somatostatin analogs ([68Ga]SSTa) has been introduced as an alternative to Octreoscan (32-35). Currently, three DOTA-coupled peptides are available: DOTATOC (Tyr3-octreotide), DOTATATE (Tyr3-octreotate), and DOTANOC (Nal3-octreotide); all have an excellent affinity mainly for SST2 receptors (IC50: 2.5 nM; 0.2 nM; and 1.9 nM, respectively) (36-38). DOTANOC also binds specifically to SST3, SST4, and SST5 receptors, but the expression of SST3 and SST4 is absent in PGLs and SST5 is strongly expressed in only 5% of cases (Table 2).
Table 2.
Comparison of different imaging techniques.
| Sensitivity | Specificity | Locoregional staging | Mean estimated effective dose equivalent | |
|---|---|---|---|---|
| MDCT (pre-contrast and angio-CT) | 80-90% | 90% | +++ (>MRI for bony structures) | 3 mSv for unenhanced CT and 5 mSv for CT angiogram |
| MRI | 80-90% | 90% | +++ (>CT for soft tissue) | None |
| Octreoscan | 65-75% | 90-95% | + | 0.06 mSv/MBq for Octreoscan and 1 mSv for low-dose CT |
| PET/CT - 18F-FDOPA | 90-100% | >95% | +/++ | 0.025 mSv/MBq for 18F-FDOPA and 3 mSv for low-dose CT |
| 123I-MIBG | 30-40% | >95% | + | 0.013 mSv/MBq for 123I-MIBG and 1 mSv for low-dose CT |
| PET/CT - 18F-FDG | 80% | 80-90% | + | 0.019 mSv/Mbq for 18F-FDG and 3 mSv for low-dose CT |
| PET/CT - 18F-FDA | 45-55% | >95% | + | 0.0068 mSv/Mbq for 18F-FDA and 3 mSv for low-dose CT |
Two other radiopharmaceuticals have been developped to investigate the catecholaminergic metabolism system: 11C-hydroxyephedrine (11C-HED) and 18F-fluorodopamine (18F-FDA). They were first developed to investigate the sympathetic innervation of the myocardium. 11C-HED has shown excellent results in the setting of PHEOs (39) but has limited use because of the very short half-life of 11C (20 min). Because of this major drawback, 18F-FDA was first developed and initially evaluated in PGLs of sympathetic origin with excellent results. However, subsequent studies found this radiopharmaceutical to be suboptimal for HNPGLs (46%) (24), and its use in the localization of these tumors should be limited and perhaps abandoned.
Because of initial disappointing results with 18F-fluorodeoxyglucose (18F-FDG) PET/CT (40), its evaluation in PGL has been delayed until several recent excellent and large studies evaluating various sympathetic PGLs (overall sensitivity 80%) (41-43). The latest SDHx discoveries in the pathogenesis of these tumors have suggested a role for the specific use of 18F-FDG PET in certain tumors, since it has been shown that PGL-specific imaging phenotypes might be different across different genotypes. 18F-FDG PET has been found to be highly sensitive for sympathetic SDHB-related PGLs (42, 43). Less data is available for patients with HNPGLs (24), which are parasympthetic and do not follow the rule of 18F-FDG PET/CT superiority over other imaging modalities in the evaluation of SDHB-related PGLs. Therefore, 18F-FDG PET is not indicated in HNPGLs as the first-line imaging modality (Figures 1, 2, 6, 7).
18F-fluorodopa (18F-FDOPA), which was initially developed to investigate dopaminergic neurotransmission, is taken up through a neutral amino acid transporter (LAT1/4F2hc complex) and decarboxylated into 18F-FDA by aromatic L-amino acid decarboxylase (AADC). Hoegerle et al. were the first to uniquely demonstrate the utility of 18F-FDOPA PET to localize PGLs (44). Hoegerle et al. found 100% sensitivity in a small series of 10 patients (15 tumors) and demonstrated that the high signal-to-noise uptake ratio enabled the detection of sub-centimetric lesions (45). Subsequent studies compared 18F-FDOPA to other imaging modalites and radiopharmaceuticals across different PGL locations and genotypes (46). 18F-FDOPA PET/CT was found to be a sensitive (80-90% for abdominal PGLs and >95% for HNPGLs) and specific (95-100%) imaging modality for the detection and staging of non-metastatic PGLs (24, 46-49) (Figures 1-7). 18F-FDOPA PET was also found to be superior to Octreoscan (24, 47) in the detection of HNPGLs and to be excellent for both SDHx (24) and non-SDHx (48) PGLs. In the largest series, 116 PHEO/PGL patients from two academic endocrine tumour centers were investigated with 18F-FDOPA PET or PET/CT imaging. The detection rate was higher for parasympathetic PGLs (98.2%) than for sympathetic PGLs and not affected by the genetic status of patients. This study and the previous ones (comprising a total sample size of about 200 HNPGLs from 6 studies) suggest without any reservation that 18F-FDOPA PET/CT is currently the first-line imaging modality in the localization of HNPGLs, regardless of genetic background (24, 42, 47, 48, 50-53).
The optimal timing for acquisition remains to be evaluated, as well as the use of oral premedication with carbidopa, an inhibitor of AADC (54, 55).
However, 18F-FDOPA is not routinely available at most imaging centers worldwide. Furthermore, the current leading role of 18F-FDOPA PET/CT in the evaluation of HNPGL will need to be compared to newly introduced and promising agents such as 68Ga-SST receptor analogs. In the absence of 18F-FDOPA or 68Ga-SST receptor analogs, SRS with 111In-pentetreotide SPECT (/CT) acquisition may be used as the first-line evaluation. In patients with multifocal disease, elevated metanephrines, or SDHx mutations, additional investigations are required to fully evaluate the extent of the disease.
Proposed imaging algorithm in the localization of HNPGLs and their management
Based on the currently available imaging techniques in the diagnosis and staging of PGLs, we propose the following approach to a patient with HNPGL:
The first step is to confirm the diagnosis of HNPGL and to determine whether solitary or multifocal tumors are present. For this purpose the initial information provided by MRI is superior to CT.
The next step is to further characterize these tumors, first to confirm that these tumors are indeed PGLs and second to determine whether additional tumors are present, since MRI is inferior to functional imaging using 18F-FDOPA PET/CT. Based on the most recent studies, both SDHx and non-SDHx PGLs are well detected by 18F-FDOPA PET/CT.
The third step is to fully evaluate the temporal bone extension of tympanic and jugular HNPGLs for surgical decision-making based on MR imaging.
The last step is to determine patients who are not candidates for surgery, which in our experience is most sucessfully done by 18F-FDOPA PET/CT, which has the highest sensitivity, and thus select patients who would benefit from radiotherapy. For external beam radiation therapy, there is no need to perform additional imaging techniques. For targeted radiation therapy, the use of Octreoscan or PET imaging using 68Ga-labeled somatostatin analogs should be used for selecting potential candidates for 177Lu-DOTA-Octreotate (Lutathera®). Since 123/131I-MIBG is much less sensitive for HNPGLs than radiolabeled somatostatin peptide analogs, we suggest that the use of 131I-MIBG should be limited and perhaps abandoned.
Accurately localizing HNPGLs plays a critical role in the management of patients with these tumors, especially in determining the feasibility of surgery.
Surgical removal of HNPGLs remains the primary treatment of choice but can be associated with some morbidity. The current aim of surgery is two-fold: oncologic and functional. The functional risk of radical resection needs to be balanced with the usual slow growth of most of these tumors. Therefore, some advocate that tumors less than 2 cm in size sould be followed rather than risk surgical removal. On the other hand, curative surgery after early detection of a HNPGL is often expected and even demanded by patients in order to avoid subsequent complications related to mass effect and its related neurological complications, and thus must be considered. In these situations, surgery is usually performed if complete tumor excision is medically and surgically feasible, particularly in younger patients, in the case of large tumors that soon may or already present with cranial nerve deficits, and in rare catecholamine-secreting HNPGL to prevent cardiovascular consequences. By classifying tumor extension according to different classifications (i.e., Fisch and Mattox's, Glasscock and Jackson's, Netterville's, Shamblin's), radiological imaging can help predict surgical outcome (56-59). For example, for Shamblin II (partial surrounding of internal and external carotid arteries) and III (complete surrounding of the carotid vessels) carotid body PGLs, complete resection is challenging and may require temporary interruption of the cerebral circulation and major vessel reconstruction (Figure 1).
The detection of multifocality as well as knowledge of the genetic status of these tumors also has an important impact on the decision-making process, especially whether surgery should be an option or not (Figure 6 and 7). Current recommendations are to treat bilateral cervical PGLs sequentially due to the morbidity risk (60). The choice of which side to operate on first is based on the location, tumor size, and preoperative functional status of the facial and lower cranial nerves.
In high-risk situations where surgical options are limited or suboptimal, alternatives include watchful waiting or radiotherapy. New fractionated radiotherapy modalities (i.e, conformal radiotherapy, 3D conformal intensity-modulated radiotherapy, conventional stereotactic radiosurgery) allow escalated radiation dose delivery to tumors and better preservation of surrounding normal tissues at risk. Single and hypofractionated stereotactic radiotherapy using gamma-knife radiosurgery and Cyberknife®, respectively, also appear very promising, with excellent tumor control and a low morbidity rate (61-64). Cyberknife® also offers the opportunity to treat large tumors with extracranial sites, including moving tumor targets by using real-time tracking and automatic correction. Long-term results are not yet available to definitively evaluate the outcomes (i.e., the therapeutic efficiency or toxicity) of these different approaches. Intensity-modulated proton therapy also enables excellent target coverage, homogeneity within the planned target volume, and sparing of the organs at risk. However, its clinical relevance in the setting of HNPGLs has not yet been evaluated. Radiotherapy planning can also benefit from 18F-FDOPA PET/CT and other imaging, which might be useful to help delineate the biologically active tumor volume and to distinguish between viable tumor and non-specific changes due to previous treatments.
Another approach is to target isolated tumor cells directly with beta emitters such as 131I-MIBG or 177Lu and/or 90Y radiolabeled somatostatin analogs. The role of 123I-MIBG should be limited to evaluting patients in whom tumors cannot be surgically removed to see if they are potential candidates for 131I-MIBG therapy.
In summary, successful management of patients requires an interdisciplinary team approach. Precise identification of the clinical context and genetic status of patients enables a personalized use of functional imaging modalities.
Future directions
Future studies are also needed to evaluate the performance of 18F-FDOPA PET/CT and other radiopharmaceuticals in the detection and characterization of VHL/SDHAF2/TMEM-127-related HNPGLs, detection of recurrent HNPGLs, the ability of imaging studies to predict metastatic behavior based on specific cellular characteristics, and the role of quantification in the use of various radiopharmaceuticals in the evaluation of treatment response.
The calculation of more reliable quantitative parameters besides the standardized uptake value (SUV) could be of particular value in the in vivo assessment of these tumors at the molecular level.
Excellent results from molecular profiling studies now provide important and promising information in the identification of new therapeutic and imaging targets, particularly for various types of PGLs (65, 66). New radiopharmaceuticals that are targeted to angiogenesis (radiolabeled RGD peptides), apoptosis, hypoxia, and glycolysis are of particular interest in the characterization of these tumors (67). SDH-related PGLs are known to result in the dysfunction and destabilization of the SDH complex (mitochondrial complex II) and the preferential activation of the HIF-2α signaling pathway. Currently, there is no radiopharmaceutical to assess the degree of mutation-specific mitochondrial dysfunction or the degree of HIF-α stabilization. It is expected that these radiopharmceuticals will be introduced in the future, after specific HIF-2α inhibitors or mitochondrial-specific targeted compounds are introduced (68).
Nanomedicine applications have recently gathered great attention in the field of imaging. It is expected that synthetized multifunctional and multivalency agents may increase tracer delivery, enable co-delivery of therapeutic or diagnostic agents, and enable the targeting of intracellular proteins (69).
The diagnosis of malignancy, which remains challenging, may also benefit from these new imaging approaches. Malignancy, which may occur in about 5% of HNPGLs, is often discovered several years after the resection of the primary tumor when locoregional and/or distant metastases are discovered. Many radiopharmaceuticals are expected to have the potential to serve as predictive biomarker classifiers.
As for other cancers, functional imaging including PET must and will rapidly shift towards tumor specific characterization tightly linked to appropriate therapy selections and individualized medicine, resulting in more optimal treatment with cost-effective outcomes.
Figure 5. Post-therapeutic evaluation of a jugular PGL.
Axial reconstructions at the level of the petrous apex (A-F) and basilar process of the occipital bone (G-I), T2-weighted (A), inverted b1000 DWI MRI (B), T2-weighted/MRI-inverted b1000 DWI MRI fusion images (C), Dixon FATSAT (3D) T1-weighted 3D (D), axial 18F-FDOPA PET (E, H), axial 18F-FDOPA PET/MRI (T2-weighted) fusion images (F, I).
Post-operative status with canal wall down tympano-mastoidectomy (A, D, white arrows). Post-operative cholesteatoma in the tympanic cavity with a typical hypersignal on T2-weighted and b1000 DWI MRI (A, B, C, arrowheads). Double recurrent 18F-FDOPA-positive PGL: the first involving the edge of the right petrous apex (D-F, red arrowheads) and the second involving the basilar process of the occipital bone, retro-pharyngeal, and para-pharyngeal spaces (G-I, red arrows). (Image kindly provided by Prof. Minerva Becker and Prof. Osman Ratib, University Hospital of Geneva, Switzerland).
Acknowledgements
The authors would like to thank Victoria Martucci for her excellent technical assistance in the preparation of this manuscript. This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare no conflict of interest.
References
- 1.Sykes JM, Ossoff RH. Paragangliomas of the head and neck. Otolaryngol Clin North Am. 1986;19:755–767. [PubMed] [Google Scholar]
- 2.Jafri M, Whitworth J, Rattenberry E, et al. Evaluation of SDHB, SDHD and VHL gene susceptibility testing in the assessment of individuals with non-syndromic phaeochromocytoma, paraganglioma and head and neck paraganglioma. Clin Endocrinol (Oxf) 2013;78:898–906. doi: 10.1111/cen.12074. [DOI] [PubMed] [Google Scholar]
- 3.Piccini V, Rapizzi E, Bacca A, et al. Head and neck paragangliomas: genetic spectrum and clinical variability in 79 consecutive patients. Endocr Relat Cancer. 2012;19:149–155. doi: 10.1530/ERC-11-0369. [DOI] [PubMed] [Google Scholar]
- 4.Hao HX, Khalimonchuk O, Schraders M, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann HP, Sullivan M, Winter A, et al. Germline mutations of the TMEM127 gene in patients with paraganglioma of head and neck and extraadrenal abdominal sites. J Clin Endocrinol Metab. 2011;96:E1279–1282. doi: 10.1210/jc.2011-0114. [DOI] [PubMed] [Google Scholar]
- 6.Gaal J, van Nederveen FH, Erlic Z, et al. Parasympathetic paragangliomas are part of the Von Hippel-Lindau syndrome. J Clin Endocrinol Metab. 2009;94:4367–4371. doi: 10.1210/jc.2009-1479. [DOI] [PubMed] [Google Scholar]
- 7.Baysal BE. Mitochondrial complex II and genomic imprinting in inheritance of paraganglioma tumors. Biochim Biophys Acta. 2013;1827:573–577. doi: 10.1016/j.bbabio.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Neumann HP, Erlic Z, Boedeker CC, et al. Clinical predictors for germline mutations in head and neck paraganglioma patients: cost reduction strategy in genetic diagnostic process as fall-out. Cancer Res. 2009;69:3650–3656. doi: 10.1158/0008-5472.CAN-08-4057. [DOI] [PubMed] [Google Scholar]
- 9.Ricketts C, Woodward ER, Killick P, et al. Germline SDHB mutations and familial renal cell carcinoma. Journal of the National Cancer Institute. 2008;100:1260–1262. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 10.Pasini B, McWhinney SR, Bei T, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. 2008;16:79–88. doi: 10.1038/sj.ejhg.5201904. [DOI] [PubMed] [Google Scholar]
- 11.Xekouki P, Pacak K, Almeida M, et al. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: a new association for SDH? J Clin Endocrinol Metab. 2012;97:E357–366. doi: 10.1210/jc.2011-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48:1739–1749. doi: 10.1016/j.ejca.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baysal BE, Willett-Brozick JE, Lawrence EC, et al. Prevalence of SDHB, SDHC, and SDHD germline mutations in clinic patients with head and neck paragangliomas. J Med Genet. 2002;39:178–183. doi: 10.1136/jmg.39.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunst HP, Rutten MH, de Monnink JP, et al. SDHAF2 (PGL2-SDH5) and hereditary head and neck paraganglioma. Clin Cancer Res. 2011;17:247–254. doi: 10.1158/1078-0432.CCR-10-0420. [DOI] [PubMed] [Google Scholar]
- 15.Timmers HJ, Gimenez-Roqueplo AP, Mannelli M, Pacak K. Clinical aspects of SDHx-related pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2009;16:391–400. doi: 10.1677/ERC-08-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MH. Head and neck vascular anatomy. Neuroimaging Clin N Am. 1998;8:119–141. [PubMed] [Google Scholar]
- 17.van den Berg R, Schepers A, de Bruine FT, et al. The value of MR angiography techniques in the detection of head and neck paragangliomas. Eur J Radiol. 2004;52:240–245. doi: 10.1016/j.ejrad.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Neves F, Huwart L, Jourdan G, et al. Head and neck paragangliomas: value of contrast-enhanced 3D MR angiography. AJNR Am J Neuroradiol. 2008;29:883–889. doi: 10.3174/ajnr.A0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg R. Imaging and management of head and neck paragangliomas. Eur Radiol. 2005;15:1310–1318. doi: 10.1007/s00330-005-2743-8. [DOI] [PubMed] [Google Scholar]
- 20.Arnold SM, Strecker R, Scheffler K, et al. Dynamic contrast enhancement of paragangliomas of the head and neck: evaluation with time-resolved 2D MR projection angiography. Eur Radiol. 2003;13:1608–1611. doi: 10.1007/s00330-002-1717-3. [DOI] [PubMed] [Google Scholar]
- 21.Grist TM, Mistretta CA, Strother CM, Turski PA. Time-resolved angiography: Past, present, and future. J Magn Reson Imaging. 2012;36:1273–1286. doi: 10.1002/jmri.23646. [DOI] [PubMed] [Google Scholar]
- 22.Dong Y, Liu Q. Differentiation of malignant from benign pheochromocytomas with diffusion-weighted and dynamic contrast-enhanced magnetic resonance at 3.0 T. J Comput Assist Tomogr. 2012;36:361–366. doi: 10.1097/RCT.0b013e31825975f8. [DOI] [PubMed] [Google Scholar]
- 23.Fonte JS, Robles JF, Chen CC, et al. False-negative (1)(2)(3)I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocr Relat Cancer. 2012;19:83–93. doi: 10.1530/ERC-11-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King KS, Chen CC, Alexopoulos DK, et al. Functional imaging of SDHx-related head and neck paragangliomas: comparison of 18F-fluorodihydroxyphenylalanine, 18F-fluorodopamine, 18F-fluoro-2-deoxy-D-glucose PET, 123I-metaiodobenzylguanidine scintigraphy, and 111In-pentetreotide scintigraphy. J Clin Endocrinol Metab. 2011;96:2779–2785. doi: 10.1210/jc.2011-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bustillo A, Telischi F, Weed D, et al. Octreotide scintigraphy in the head and neck. Laryngoscope. 2004;114:434–440. doi: 10.1097/00005537-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Duet M, Sauvaget E, Petelle B, et al. Clinical impact of somatostatin receptor scintigraphy in the management of paragangliomas of the head and neck. J Nucl Med. 2003;44:1767–1774. [PubMed] [Google Scholar]
- 27.Koopmans KP, Jager PL, Kema IP, Kerstens MN, Albers F, Dullaart RP. 111In-octreotide is superior to 123I-metaiodobenzylguanidine for scintigraphic detection of head and neck paragangliomas. Journal of Nuclear Medicine. 2008;49:1232–1237. doi: 10.2967/jnumed.107.047738. [DOI] [PubMed] [Google Scholar]
- 28.Muros MA, Llamas-Elvira JM, Rodriguez A, et al. 111In-pentetreotide scintigraphy is superior to 123I-MIBG scintigraphy in the diagnosis and location of chemodectoma. Nucl Med Commun. 1998;19:735–742. doi: 10.1097/00006231-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M, Fischer E, Dietlein M, et al. Clinical value of somatostatin receptor imaging in patients with suspected head and neck paragangliomas. Eur J Nucl Med Mol Imaging. 2002;29:1571–1580. doi: 10.1007/s00259-002-0939-6. [DOI] [PubMed] [Google Scholar]
- 30.Telischi FF, Bustillo A, Whiteman ML, et al. Octreotide scintigraphy for the detection of paragangliomas. Otolaryngol Head Neck Surg. 2000;122:358–362. doi: 10.1016/S0194-5998(00)70048-9. [DOI] [PubMed] [Google Scholar]
- 31.Gimenez-Roqueplo AP, Caumont-Prim A, Houzard C, et al. Imaging work-up for screening of paraganglioma and pheochromocytoma in SDHx mutation carriers: a multicenter prospective study from the PGL.EVA Investigators. J Clin Endocrinol Metab. 2013;98:E162–173. doi: 10.1210/jc.2012-2975. [DOI] [PubMed] [Google Scholar]
- 32.Naji M, Zhao C, Welsh SJ, et al. 68Ga-DOTA-TATE PET vs. 123I-MIBG in identifying malignant neural crest tumours. Mol Imaging Biol. 2011;13:769–775. doi: 10.1007/s11307-010-0396-8. [DOI] [PubMed] [Google Scholar]
- 33.Naji M, Al-Nahhas A. (68)Ga-labelled peptides in the management of neuroectodermal tumours. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):61–67. doi: 10.1007/s00259-011-1990-y. [DOI] [PubMed] [Google Scholar]
- 34.Maurice JB, Troke R, Win Z, et al. A comparison of the performance of (6)(8)Ga-DOTATATE PET/CT and (1)(2)(3)I-MIBG SPECT in the diagnosis and follow-up of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39:1266–1270. doi: 10.1007/s00259-012-2119-7. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P, Thakar A, Suman Kc S, et al. 68Ga-DOTANOC PET/CT for Baseline Evaluation of Patients with Head and Neck Paraganglioma. J Nucl Med. doi: 10.2967/jnumed.112.115485. in press. [DOI] [PubMed] [Google Scholar]
- 36.Wild D, Macke HR, Waser B, et al. 68Ga-DOTANOC: a first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur J Nucl Med Mol Imaging. 2005;32:724. doi: 10.1007/s00259-004-1697-4. [DOI] [PubMed] [Google Scholar]
- 37.Wild D, Schmitt JS, Ginj M, et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging. 2003;30:1338–1347. doi: 10.1007/s00259-003-1255-5. [DOI] [PubMed] [Google Scholar]
- 38.Reubi JC, Schar JC, Waser B, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto S, Hellman P, Wassberg C, Sundin A. 11C-hydroxyephedrine positron emission tomography imaging of pheochromocytoma: a single center experience over 11 years. J Clin Endocrinol Metab. 2012;97:2423–2432. doi: 10.1210/jc.2011-3342. [DOI] [PubMed] [Google Scholar]
- 40.Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC. Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-D-glucose PET. Radiology. 1999;212:35–41. doi: 10.1148/radiology.212.1.r99jl3035. [DOI] [PubMed] [Google Scholar]
- 41.Timmers HJ, Kozupa A, Chen CC, et al. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. Journal of Clinical Oncology. 2007;25:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 42.Taieb D, Sebag F, Barlier A, et al. 18F-FDG avidity of pheochromocytomas and paragangliomas: a new molecular imaging signature? Journal of Nuclear Medicine. 2009;50:711–717. doi: 10.2967/jnumed.108.060731. [DOI] [PubMed] [Google Scholar]
- 43.Timmers HJ, Chen CC, Carrasquillo JA, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. Journal of the National Cancer Institute. 2012;104:700–708. doi: 10.1093/jnci/djs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoegerle S, Nitzsche E, Altehoefer C, et al. Pheochromocytomas: detection with 18F DOPA whole body PET--initial results. Radiology. 2002;222:507–512. doi: 10.1148/radiol.2222010622. [DOI] [PubMed] [Google Scholar]
- 45.Hoegerle S, Ghanem N, Altehoefer C, et al. 18F-DOPA positron emission tomography for the detection of glomus tumours. Eur J Nucl Med Mol Imaging. 2003;30:689–694. doi: 10.1007/s00259-003-1115-3. [DOI] [PubMed] [Google Scholar]
- 46.Timmers HJ, Chen CC, Carrasquillo JA, et al. Comparison of 18F-Fluoro-L-DOPA, 18F-Fluoro-Deoxyglucose, and 18F-Fluorodopamine PET and 123I-MIBG Scintigraphy in the Localization of Pheochromocytoma and Paraganglioma. Journal of Clinical Endocrinology and Metabolism. 2009;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charrier N, Deveze A, Fakhry N, et al. Comparison of [(1)(1)(1)In]pentetreotide-SPECT and [(1)F]FDOPA-PET in the localization of extra-adrenal paragangliomas: the case for a patient-tailored use of nuclear imaging modalities. Clin Endocrinol (Oxf) 2011;74:21–29. doi: 10.1111/j.1365-2265.2010.03893.x. [DOI] [PubMed] [Google Scholar]
- 48.Gabriel S, Blanchet EM, Sebag F, et al. Functional characterization of non-metastatic paraganglioma and pheochromocytoma by (18) F-FDOPA PET: focus on missed lesions. Clin Endocrinol (Oxf) doi: 10.1111/cen.12126. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miederer M, Fottner C, Rossmann H, et al. High incidence of extraadrenal paraganglioma in families with SDHx syndromes detected by functional imaging with [(18)F]fluorodihydroxyphenylalanine PET. Eur J Nucl Med Mol Imaging. doi: 10.1007/s00259-013-2346-6. in press. [DOI] [PubMed] [Google Scholar]
- 50.Rischke HC, Benz MR, Wild D, et al. Correlation of the Genotype of Paragangliomas and Pheochromocytomas with Their Metabolic Phenotype on 3,4-Dihydroxy-6-18F-Fluoro-L-Phenylalanin PET. J Nucl Med. 2012;53:1352–1358. doi: 10.2967/jnumed.111.101303. [DOI] [PubMed] [Google Scholar]
- 51.Taieb D, Neumann H, Rubello D, Al-Nahhas A, Guillet B, Hindie E. Modern nuclear imaging for paragangliomas: beyond SPECT. J Nucl Med. 2012;53:264–274. doi: 10.2967/jnumed.111.098152. [DOI] [PubMed] [Google Scholar]
- 52.Timmers HJ, Taieb D, Pacak K. Current and future anatomical and functional imaging approaches to pheochromocytoma and paraganglioma. Horm Metab Res. 2012;44:367–372. doi: 10.1055/s-0031-1299712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Treglia G, Cocciolillo F, de Waure C, et al. Diagnostic performance of 18F-dihydroxyphenylalanine positron emission tomography in patients with paraganglioma: a meta-analysis. Eur J Nucl Med Mol Imaging. 2012;39:1144–1153. doi: 10.1007/s00259-012-2087-y. [DOI] [PubMed] [Google Scholar]
- 54.Hentschel M, Rottenburger C, Boedeker CC, Neumann HP, Brink I. Is there an optimal scan time for 6-[F-18]fluoro-L-DOPA PET in pheochromocytomas and paragangliomas? Clinical nuclear medicine. 2012;37:e24–29. doi: 10.1097/RLU.0b013e318238f550. [DOI] [PubMed] [Google Scholar]
- 55.Timmers HJ, Hadi M, Carrasquillo JA, et al. The effects of carbidopa on uptake of 6-18F-Fluoro-L-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma. J Nucl Med. 2007;48:1599–1606. doi: 10.2967/jnumed.107.042721. [DOI] [PubMed] [Google Scholar]
- 56.Fisch U, Mattox D. Microsurgery of the Skull Base. Georg Thieme Verlag; Stuttgart/New York: 1988. pp. 148–281. [Google Scholar]
- 57.Fisch U. Infratemporal fossa approach to tumours of the temporal bone and base of the skull. J Laryngol Otol. 1978;92:949–967. doi: 10.1017/s0022215100086382. [DOI] [PubMed] [Google Scholar]
- 58.Netterville JL, Jackson CG, Miller FR, Wanamaker JR, Glasscock ME. Vagal paraganglioma: a review of 46 patients treated during a 20-year period. Arch Otolaryngol Head Neck Surg. 1998;124:1133–1140. doi: 10.1001/archotol.124.10.1133. [DOI] [PubMed] [Google Scholar]
- 59.Shamblin WR, ReMine WH, Sheps SG, Harrison EG., Jr Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;122:732–739. doi: 10.1016/0002-9610(71)90436-3. [DOI] [PubMed] [Google Scholar]
- 60.Netterville JL, Reilly KM, Robertson D, Reiber ME, Armstrong WB, Childs P. Carotid body tumors: a review of 30 patients with 46 tumors. Laryngoscope. 1995;105:115–126. doi: 10.1288/00005537-199502000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Bianchi LC, Marchetti M, Brait L, et al. Paragangliomas of head and neck: a treatment option with CyberKnife radiosurgery. Neurol Sci. 2009;30:479–485. doi: 10.1007/s10072-009-0138-3. [DOI] [PubMed] [Google Scholar]
- 62.Chen PG, Nguyen JH, Payne SC, Sheehan JP, Hashisaki GT. Treatment of glomus jugulare tumors with gamma knife radiosurgery. Laryngoscope. 2010;120:1856–1862. doi: 10.1002/lary.21073. [DOI] [PubMed] [Google Scholar]
- 63.Navarro Martin A, Maitz A, Grills IS, et al. Successful treatment of glomus jugulare tumours with gamma knife radiosurgery: clinical and physical aspects of management and review of the literature. Clin Transl Oncol. 2010;12:55–62. doi: 10.1007/s12094-010-0467-y. [DOI] [PubMed] [Google Scholar]
- 64.Guss ZD, Batra S, Limb CJ, et al. Radiosurgery of glomus jugulare tumors: a meta-analysis. Int J Radiat Oncol Biol Phys. 2011;81:e497–502. doi: 10.1016/j.ijrobp.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cascon A, Tennant DA. From transcriptional profiling to tumor biology in pheochromocytoma and paraganglioma. Endocr Pathol. 2012;23:15–20. doi: 10.1007/s12022-012-9195-x. [DOI] [PubMed] [Google Scholar]
- 66.Shankavaram U, Fliedner SM, Elkahloun AG, et al. Genotype and tumor locus determine expression profile of pseudohypoxic pheochromocytomas and paragangliomas. Neoplasia. 2013;15:435–447. doi: 10.1593/neo.122132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Favier J, Plouin PF, Corvol P, Gasc JM. Angiogenesis and vascular architecture in pheochromocytomas: distinctive traits in malignant tumors. Am J Pathol. 2002;161:1235–1246. doi: 10.1016/S0002-9440(10)64400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogers JL, Bayeh L, Scheuermann TH, et al. Development of Inhibitors of the PAS-B Domain of the HIF-2alpha Transcription Factor. J Med Chem. doi: 10.1021/jm301847z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lo ST, Kumar A, Hsieh JT, Sun X. Dendrimer nanoscaffolds for potential theranostics of prostate cancer with a focus on radiochemistry. Mol Pharm. 2013;10:793–812. doi: 10.1021/mp3005325. [DOI] [PMC free article] [PubMed] [Google Scholar]