Abstract
Complex signal-transduction cascades are known to be involved in regulating cardiomyocyte function, death and survival during acute cardiac ischemia–reperfusion process, but detailed survival signalling pathways are not clear. This review presents and discusses the recent findings bearing upon the evidence on the cardioprotective effect of sphingosine-1-phosphate (S1P) and bradykinin in acute cardiac ischemia–reperfusion and underlying signalling mechanisms, particularly, through activation of P21 activated kinase.
Keywords: Pak1, S1P, Cardiac ischemia
1. Introduction
Ischemic heart disease (IHD) remains the leading cause of human mortality with some 7.6 million deaths worldwide. (Mathers and Loncar, 2006), despite tremendous advances in the knowledge of the causes that lead to IHD, it still remains the leading Ischemia/reperfusion (I/R) injury is a major contributory factor to cardiac dysfunction and infarct size, which determines patient prognosis after acute myocardial infarction in IHD. However, such injury can be minimized by a cardiac self-protective mechanism called ischemic pre-conditioning, in which a brief period of myocardium ischemia/reperfusion significantly reduces injury resulting from subsequent long-term I/R. Since the appearance of the first publication on ischemic pre-conditioning in 1986 (Murry et al., 1986), our knowledge of this phenomenon has increased immensely. It has been demonstrated that cardioprotective pathways can also be induced effectively by ischemic pre- and post-conditioning or pharmaceutical post-conditioning treatment during the reperfusion period. The application of increasingly more refined experimental strategies has provided insights into the complex signal transduction cascades involved in regulating cardiomyocyte death and survival in ischemia–reperfusion. Although precise mechanisms are far from clear, it is now known that multiple signalling pathways regulate the critical balance between cell death and cell survival in ischemia–reperfusion.
New evidence indicates a definitive role of sphingolipid metabolites, particularly, sphingosine-1-phosphate (S1P) as an important member of the ischemic pre-conditioning mediated intracellular signalling process (Karliner, 2009). Experimental studies have also demonstrated protection against cardiac I/R injury achieved by pre-treatment with exogenous S1P (Karliner, 2009). More recently, Vessey and colleagues also demonstrated that S1P is an important endogenous cardioprotectant released by ischemic pre- and post-conditioning in experimental animal models (Vessey et al., 2009).We and other groups have further identified the downstream signalling pathways of S1P cardiac protective effect signalling (Egom et al., 2009; Hofmann et al., 2009). This review highlights the recent progress in understanding the cardiac of S1P protective effect signalling in I/R injury and raises the question of whether modulating the sphingolipid pathway may lead to potential therapeutic benefit both before and during an I/R injury in ischemic heart disease (IHD).
2. Expression of S1P and S1P receptors in cardiac tissues
The lysophospholipid, sphingosine-1-phosphate (S1P), is a circulating bioactive lipid metabolite that has been known for many years to induce cellular responses, including proliferation, migration, contraction, and intracellular calcium mobilization (Jin et al., 2007; Lecour et al., 2002; Vessey et al., 2008; Zhang et al., 2007). S1P is present in human plasma and serum in high nanomolar concentrations, associated with some lipoproteins, especially the high-density lipoprotein (HDL) (Okajima, 2002). One of the main sources for S1P in plasma and serum are platelets. High concentrations of S1P are stored in platelets as these do not have the enzyme S1P lyase, an enzyme that catalyses the degradation of S1P to hexadecanal and ethanolamine-phosphate (Yatomi et al., 1997). The stored S1P is released upon activation of the platelets. S1P has unique properties as it can act both as an intracellular messenger and also as an intercellular/extracellular messenger (Payne et al., 2002; Pyne and Pyne, 2000; Spiegel and Milstien, 2003).
The extracellular effects of S1P are mediated via its interaction with G-protein-coupled receptors (GPCR). To date, a family of five structurally related receptors with high affinity for S1P has been identified. The first member of the Endothelial Differentiation Gene (EDG) family of GPCR to be cloned, S1P1/EDG-1, was originally identified as an inducible transcript of endothelial cell differentiation in vitro and therefore, it was named EDG-1 (Hla and Maciag, 1990). At the time, the ligand for the receptor was unknown, but subsequent investigation revealed S1P to be a high affinity ligand for EDG-1 (Kon et al., 1999; Lee et al., 1998; Sato et al., 2000). The other GPCRs in this subfamily bind either S1P or a structurally related lysophospholipid (lysophosphatidic acid, LPA) as high affinity ligands. Thus, this family of receptors has been grouped into two subfamilies; one whose receptors bind S1P as their high affinity ligand (S1P1/EDG-1, S1P2/EDG-5, S1P3/EDG-3, S1P4/EDG-6 and S1P5/EDG-8) and a second whose members bind LPA as their high affinity ligand (LPA1/EDG-2, LPA2/EDG-4 and LPA3/EDG-7).
Tissue expression of the S1P1/EDG-1, S1P3/EDG-3, and S1P2/EDG-5 receptors in mice indicates that heart and lung have the highest overall expression of these genes (Ishii et al., 2001; Zhang et al., 1999), whereas S1P4/EDG-6 and S1P5/EDG-8 receptors are mainly expressed in lymphoid and brain tissues, respectively (Graler et al., 1998; Im et al., 2000).
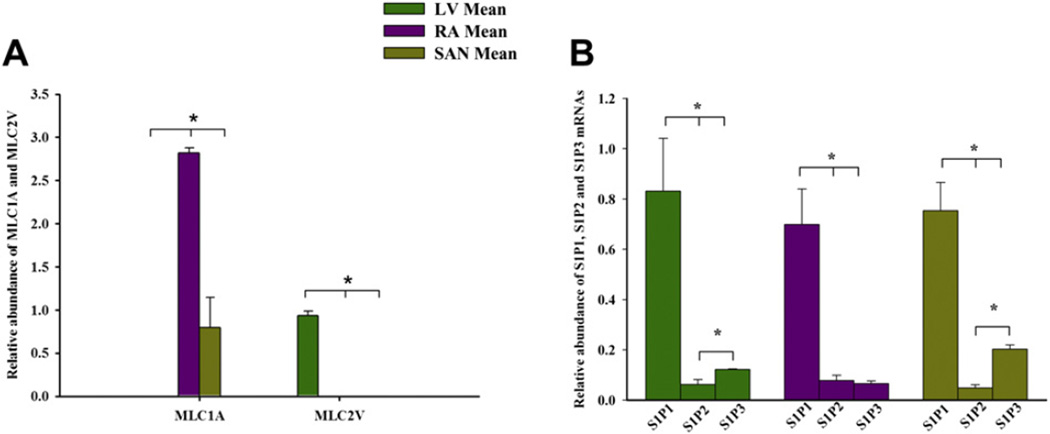
We employed real-time (RT)-PCR to determine the expression of S1P1, S1P2 and S1P3 transcript pools in rat cardiac tissues. The transcripts were detected and analysed by in tissues dissected from sinoatrial node (SAN), right atrium (RA) and left ventricle (LV). The S1P1 receptor isoform expression level was higher than S1P2 and S1P3 in these tissue types (Fig. 1), which indicates that the S1P1 receptor is the dominant isoform in rat heart tissues. Our Western blot and immunocytochemistry analysis further confirmed the expression profile of S1P1–3 receptors in rat heart tissues and myocytes.
Fig. 1.
Expression profiles of S1P receptor mRNAs in rat heart tissue. A: Expression of regionally distributed MLCA1 and MLC2V in the atria, SAN and ventricle tissues. B: Expression profile of S1P receptors mRNAs in sinoatrial node tissue, in atrial tissue and in ventricular tissue. Means ± SEM (n = 7) shown. *p < 0.05; One way ANOVA. LV, left ventricle; RA, right atrium; SAN, sinoatrial node; MLC1A, atrial myosin light chain gene; MLC2V, ventricular myosin light chain gene.
The differential G-protein-coupling of the individual S1P receptor subtypes and associated signalling pathways have been extensively studied and the major pathways have been proposed (for review see, Brinkmann, 2007). Whereas the S1P1 receptor couples exclusively to Gi proteins, the S1P2 and S1P3 receptors are more promiscuous, coupling to the Gi, Gq, and G12/13 families of heterotrimeric G-proteins. Coupling of S1P2 and S1P3 receptors to these G-proteins has been confirmed by GTPγS binding assays. Analysis of the signalling pathways downstream of these receptors, which includes activation of phospholipase C and Rho also implicates Gi, Gq, and G12/13 in mediating the effects of the S1P2 and S1P3 receptors (Karliner, 2008).
3. Cardioprotection by S1P and its analogue, FTY720, in myocardial I/R injury
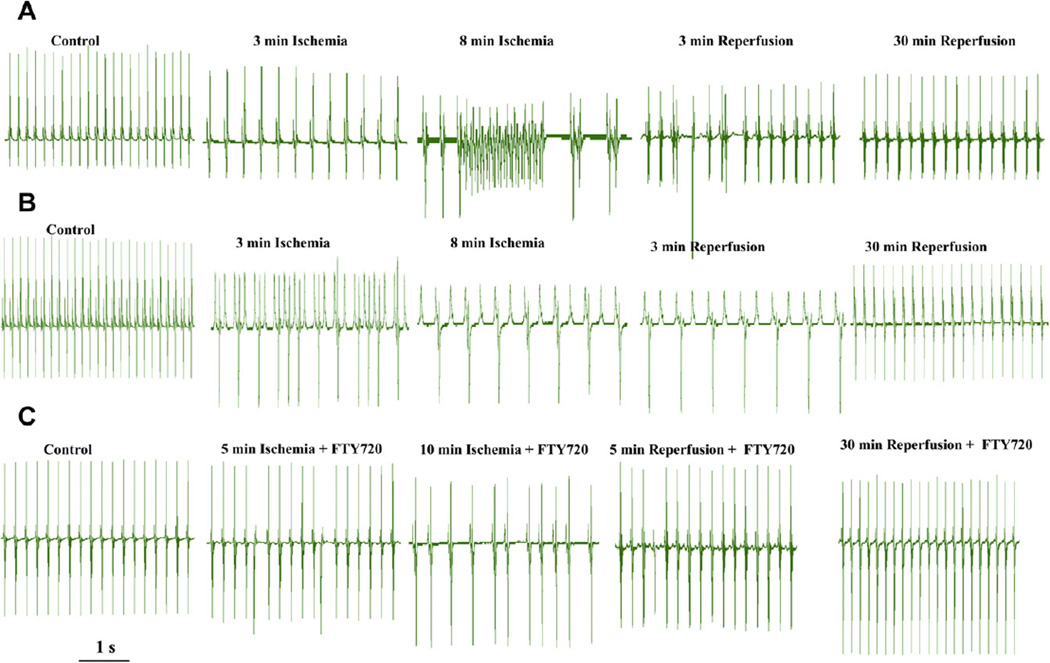
Several recent studies (Jin et al., 2007; Vessey et al., 2008; Karliner, 2008; Peters and Alewijnse, 2007) have provided evidence for a role of S1P signalling in protection against I/R injury, in particular its role in pre-conditioning and post-conditioning mechanisms of rescue of hearts from I/R injury (Karliner, 2008; Peters and Alewijnse, 2007; Karliner et al., 2001). The addition of S1P to neonatal rat ventricular myocytes has been demonstrated to confer cardioprotection against hypoxia (Karliner et al., 2001), and S1P also protects against global I/R damage in isolated mouse hearts (Jin et al., 2002). Hofmann et al. (2009) have recently shown that S1P and its agonists, FTY720 and SEW2871, can limit cell death even when applied during reperfusion and can induce cardioprotective effect. Moreover, Vessey et al. (2008) also reported showed that both sphingosine and S1P were able to protect the ex vivo rat heart from ischemia reperfusion injury when added to the perfusion medium at the time of reperfusion after a 40 min ischemia (post-conditioning). Activation of sphingosine kinase (SK), the upstream kinase responsible for producing S1P, has more recently been suggested to protect the isolated perfused heart from I/R damage (Jin et al., 2004). Moreover, an S1P agonist FTY720 has been shown to attenuate small-for-size liver graft injury by activation of cell-survival Akt signalling and down regulation of the MAPK pathway (Zhao et al., 2004). We also have evidence that S1P analogue FTY720 effectively antagonized both brady- and tachy-arrhythmias induced by I/R and protected against hypoxic and ischemic insults (Egom et al., 2009). Fig. 2 shows representative ECG recordings from hearts treated with 25 nM FTY720 in ischemic and reperfusion conditions. In 15 hearts examined in our experiments, FTY720 significantly prevented I/R injury induced arrhythmic events. Treatment of hearts with 25 nM FTY720 greatly reduced the occurrence of premature ventricular beats, ventricular tachycardia (VT) and sinus bradycardia as well as atrio-ventricular (A–V) conduction block caused by I/R injury. Ectopic beats occurred in 1 of 15 hearts in ischemia and in 3 of 15 hearts in reperfusion conditions; non-sustained and sustained VT was not observed in any of the ischemia and reperfusion conditions. With FTY720 treatment, severe sinus bradycardia or A–V conduction block occurred in 3 of 15 hearts in ischemia and in 2 of 15 hearts in reperfusion conditions. Thus, FTY720 significantly prevented both brady- and tachy- cardiac arrhythmic events induced by I/R injury in the Langendorff ex vivo heart model.
Fig. 2.
The effect of FTY720 on rhythm disturbance induced by ischemia/reperfusion in rat heart preparations. ECG recordings from Langendorff perfused heart in the presence and absence of FTY720. A_B: in the absence of FTY720, RR interval, P–R, QRS and QT intervals were significantly increased during ischemia and reperfusion, but in the presence of FTY720, RR interval, P–R, QRS and QT intervals were not significantly different than those during the control condition or during ischemia and reperfusion. Both tachycardia-related (ectopic beats, non-sustained and sustained episodes of ventricular tachycardia (VT) and ventricular fibrillation (VF)) and bradycardia-related (sinus bradycardia, sinus pause, sinus arrest, and A–V conduction block) arrhythmic events were frequently observed after 5–10 min in the presence of ischemic conditions. C: FTY720 greatly reduced the occurrence of premature ventricular beats, VT and sinus bradycardia as well as A-V conduction block caused by I/R injury.
4. Pak1/Akt signalling as potential intracellular pathways underlying FTY720 cardiac protection effect
The underlying key mechanism(s) and signalling pathway(s) for S1P cardioprotection remain largely unknown until recently (Karliner, 2009). Hofmann and colleagues first showed the activation of Akt, a major molecule underlies the protective effects of S1P receptor agonist treatment after myocardial ischemia–reperfusion. These findings open the door for understanding key mechanism(s) and signalling pathway(s) for S1P cardioprotection (Hofmann et al., 2009). Another significant clue as to the mechanism came from experiments in a mammalian cell line, which demonstrated that p21 activated kinase (Pak1), a Ser/Thr kinase downstream of small G-proteins, is activated by sphingosine and several related long chain sphingoid bases in a time- and dose-dependent activation manner (Bokoch et al., 1998). There is a large body of evidence that Pak1 activity is a key regulator of a number of cellular functions, including cytoskeletal dynamics, cell motility, growth and proliferation, cardiac ion channel activity, and contractility (Ke et al., 2007, 2004). Pak1 also facilitates Akt stimulation and aids recruitment of Akt to the membrane. This reveals an important scaffolding function of Pak1 in the Akt pathway (Higuchi et al., 2008). However, the signal-transduction pathways mediating these effects have not been established. Neither has an in vivo metabolism for endogenously released S1P been demonstrated during acute cardiac ischemic conditions. On the basis of these data, we speculated that Pak1 participates in the cardiac effect of S1P signalling.
We investigated the effect of S1P analogue, FTY720, on I/R injury induced cardiac arrhythmias in an ex vivo rat heart models. Our results demonstrate a cardioprotection by FTY720 signalling through the S1P cascade to Pak1. To determine Pak1 activation we employed Western blotting with an anti-phosphoThr 423 Ab and Akt activation with an anti-phospho-Thr 308 Ab (Higuchi et al., 2008) and we probed the same hearts used for arrhythmias studies (in the presence or absence of FTY720) for phosphorylation of Pak1/Akt. Compared to baseline levels under the control condition, there was a significant depression in the levels of phospho-Pak1 and phospho-Akt decreased by 62% and 64% in ischemic conditions and by 73% and 63.5% in reperfusion conditions. However, in the presence of FTY720, phospho-Pak1 and Akt levels decreased by only 22% and 24%, in ischemic conditions and by 30% and 29%, respectively in reperfusion conditions. In the presence of S1P, phospho-Pak1 and Akt levels increased by 10% and decreased only by 26%, respectively in ischemic and decreased by only 13% and 28.8%, respectively in reperfusion conditions, compared to baseline level under the control condition (Egom et al., 2009).
We then addressed the important questions of whether FTY720-mediated Pak1 and Akt activation was through Gi by treating neonatal rat cardiac myocytes with 100 ng/ml PTX overnight and then stimulating with 25 nM FTY720 for 5 min. FTY720 induced a 1.6-fold increase in Pak1 phosphorylation, and a 1.45-fold increase in Akt phosphorylation relative to vehicle. After PTX treatment, FTY720-mediated activation of Pak1 was reduced by 87%, and activation of Akt was reduced by 53%. These data demonstrate that a significant component of FTY720-mediated Akt and Pak1 activation in cardiomyocytes occurs through a Gi-coupled S1P receptor (Egom et al., 2009).
5. FTY720 stimulates nitric oxide (NO) production via a PTX-sensitive PI3K/Akt/eNOS cascade
In our recent study, we further demonstrated that FTY720 is able to stimulate Pak1 and Akt autophosphorylation and to activate and trigger NO release through eNOS (Egomet al., 2009, unpublished observation). We found that NO release via S1P receptors is mediated by PI3K and Akt activation. NO release induced by FTY720, which is an agonist at S1P1 as well as S1P3 receptors, was sensitive to Gi inhibition.
We examined NO release in cultured neonatal cardiac myocytes by the NO sensitive dye DAF-FM staining. Myocytes exposed to 25nM FTY720 and 25 nM SEW2871 (a specific S1P1 receptor agonist) displayed NO vesicles that are localized in specific areas of the cell membrane. This FTY720 induced appearance of NO vesicles was still observed, when cells were pre-treated for 60 min with 10 µM l-NIL, a potent and selective inhibitor of iNOS and 1 µM SMLT, a potent inhibitor of nNOS. However, NO vesicles were not visible when cells were pre-incubated for 1 h with 1 mM l-NAME (potent inhibitor of eNOS, iNOS, nNOS, or with 1 µM l-NIO (potent inhibitor of eNOS)) or with 50 µM of LY294002 (PI3K inhibitor) or for 30 min with 1 µMof A6730 (Akt1/2 kinase inhibitor), respectively. These data indicate that the effect of FTY720 is mainly through PI3K/Akt/eNOS signalling pathway (Egom et al., 2009, unpublished observation).
We also determined whether FTY720-mediated Pak1 and Akt activation and NO release was through Gi by treating myocytes with 100 ng/ml PTX overnight and then stimulating with 25 nM FTY720 for 5 min. FTY720 induced a 1.6-fold increase in Pak1 phosphorylation, and a 1.45-fold increase in Akt phosphorylation relative to vehicle. After PTX treatment, FTY720-mediated activation of Pak1 was reduced by 94%, and activation of Akt was reduced by 55%. FTY720 induced NO release was abolished. Our data demonstrate that a significant component of FTY720-mediated PI3K/Akt and Pak1 activation and NO release in cardiomyocytes occurs through a Gi-coupled S1P receptor via activation of eNOS (Egom et al., 2009, unpublished observation). Thus, signalling through the activation of S1P receptors by the S1P and its analogue FTY720, to Pak1/Akt/eNOS might serve as a mechanism underlying the S1P/FTY720 cardioprotective effect.
6. Bradykinin signalling
Substantial evidence indicates a critical cardioprotective role for bradykinin during pre-conditioning and ischemia/reperfusion (Baxter and Ebrahim, 2002; Downey et al., 2007). Bradykinin is derived from protein degradation consisting of a group of peptides with 5–9 amino acids, which interact with cells of the heart in a paracrine or autocrine manner (Su, 2006). As with S1P receptors, receptors, bradykinin receptors are coupled to G-proteins, primarily Gi (Liebmann et al., 1990). The signalling pathways downstream of bradykinin receptors beyond large G-proteins are poorly understood. Some evidence indicates that PKC may be the protein kinase effector (Wolfrum et al., 2002). In the heart, bradykinin induces dephosphorylation of major cardiac regulatory proteins, such as troponin I (cTnI) and phospholamban (Ke et al., in revision). Thus signalling to these important regulatory proteins is likely to an important functional readout of bradykinin cascades.
We have determined the effect of bradykinin on cardiac ventricular myocyte protein phosphorylation by following 32P incorporation. Phosphorylation of both phospholamban and cTnI were significantly reduced when the myocytes were treated with bradykinin at 1 and 10 µM. In the presence of bradykinin, Ca-uptake by vesicles of the sarcoplasmic reticulum was inhibited. This suggested that phosphatase activities may be involved in the bradykinin signalling pathways. Our previous studies have demonstrated that Pak1 executes its intracellular function through activation of phosphatase PP2A in ventricle myocytes and in sinoatrial nodal cells as well. In addition, in fibroblasts, Cdc42 and bradykinin produce the same cytoskeletal changes (Kozma et al.,1995). These findings prompted us to identify a functional link between bradykinin and Pak1. Indeed, treatment of the ventricular myocytes with bradykinin induced translocation of endogenous Pak1, which is an indication of Pak1 activation. More direct evidence comes from biochemical analysis demonstrating that autophosphorylation of Pak1 at threonine 423 was significantly increased when cardiac cells are treated with bradykinin at different concentrations (Ke et al., in revision). These observations have provided novel evidence suggesting Pak1 and PP2A as downstream signalling molecules for bradykinin in cardiac cells.
7. Conclusion and future directions
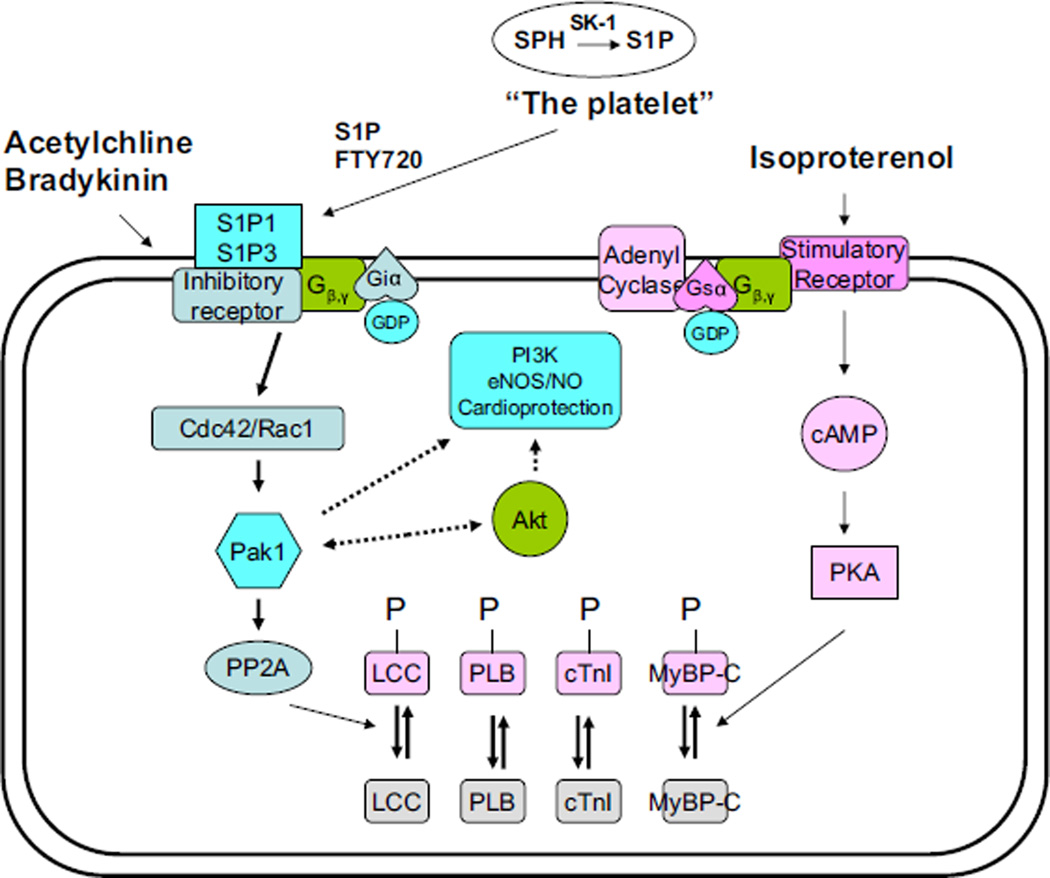
Our data are the first to demonstrate a down regulation of phospho-Pak1 during I/R model. Moreover, we found a strong correlation of the activity of Pak1 and Akt with the incidence of I/R induced arrhythmias with and without FTY720. The detailed mechanisms underlying the cardioprotective effect of Pak1/Akt activation on I/R induced arrhythmias is likely to be complex, and may involve primary effects on ion channels/transporters and secondary effects to protect cardiac myocytes from hypoxia-induced stress and cell death. Earlier studies also indicated that sphingosine and several related long chain sphingoid bases can directly activate Pak1 in vitro at a higher concentration than the FTY720 we used in this study (Bokoch et al., 1998; Ke and Solaro, 2008). Whether FTY720, which is structurally similar to S1P, can directly activate Pak1 in cardiac cells without prior conversion to FTY720 phosphate remains unclear. Recent studies also indicate that FTY720 activates PP2A and induces dephosphorylation of Erk-1/2 in immuno-cells, which also provides amechanistic insight into understanding the cardiac effects of FTY720 demonstrated in this study (Liu et al., 2008). Fig. 3 illustrates our proposed possible mechanisms underlying cardiac protective effect of S1P. It is unclear about the exact relationship among many different cardioprotective factors. It has recently demonstrated that bradykinin signalling is also involved in generation of ROS and modulation of nitric oxide (NO) (Cohen et al., 2001; Ebrahim et al., 2001).
Fig. 3.
Previous studies of ours have indicated that Pak1 executes its anti-adrenergic function in the heart through activation of phosphatase PP2A. Both Pak1 and Akt may play a significant role in S1P1 and S1P3 mediated cardioprotective effects during cardiac ischemia and reperfusion. Pak1 is not only involved in regulation of myofilament activity, but also regulates activities of ion channels which are directly related to ischemia/reperfusion induced arrhythmia. It remains unclear if Pak1 and Akt are related to other cardioprotective signals, such as PI3 kinase, eNOS etc. The inhibitory G protein is a key upstream signal for Pak1 in cardiomyocytes. Abbreviations: LCC–L-type Ca channel; PLB–phospholamban; cTnI–cardiac troponin I; MyBP-C–myosin binding protein C; SK-1–sphingosine-1 kinase, S1P–sphingosine-1-phosphate.
Further work needs to be done to illuminate the molecular mechanisms underling all the cardioprotective signals. Although the precise mechanisms underlying the activation of Pak1/Akt signalling by S1P in preventing cardiac I/R injury require further investigation, recent results reported by us and the others suggest that activation of Pak1 and Akt signalling pathways play a role in the FTY720/S1Pmediated cardioprotection mechanism in the heart. Our evidence that FTY720 prevents arrhythmias induced by I/R injury and can be a potentially important and novel agent protecting against I/R injury and its associated arrhythmias. The detailed mechanisms for the production of sphingolipids as a consequence of cardiac ischemia or hypoxia require further investigations. Such investigations should raise the question of whether modulating the sphingolipid pathway may lead to potential therapeutic benefit both before and during an ischemic coronary event.
Acknowledgement
The work was supported by The Wellcome Trust (ML), The British Heart Foundation (ML) and National Institute of Health grants RO1 HL 64035 and PO1 HL 62426 (Project 1) (RJS).
References
- Baxter GF, Ebrahim Z. Role of bradykinin in preconditioning and protection of the ischaemic myocardium. Br. J. Pharmacol. 2002;135:843–854. doi: 10.1038/sj.bjp.0704548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM, Reilly AM, Daniels RH, King CC, Olivera A, Spiegel S, Knaus UG. AGTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J. Biol. Chem. 1998;273:8137–8144. doi: 10.1074/jbc.273.14.8137. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Cohen MV, Yang XM, Liu GS, Heusch G, Downey JM. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial K(ATP) channels. Circ. Res. 2001;89:273–278. doi: 10.1161/hh1501.094266. [DOI] [PubMed] [Google Scholar]
- Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail. Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- Ebrahim Z, Yellon DM, Baxter GF. Bradykinin elicits “second window” myocardial protection in rat heart through an NO-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1458–H1464. doi: 10.1152/ajpheart.2001.281.3.H1458. [DOI] [PubMed] [Google Scholar]
- Egom EA, Ke Y, Musa H, Mohamed T, Wang T, Cartwright E, Solaro RJ, Lei M. FTY720 prevents ischemia/reperfusion injury associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signalling. JMCC. 2009 doi: 10.1016/j.yjmcc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graler MH, Bernhardt G, Lipp M. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics. 1998;53:164–169. doi: 10.1006/geno.1998.5491. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat. Cell Biol. 2008;10:1356–1364. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J. Biol. Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- Hofmann U, Burkard N, Vogt C, Thoma A, Frantz S, Ertl G, Ritter O, Bonz A. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemiaereperfusion. Cardiovasc. Res. 2009;83:285–293. doi: 10.1093/cvr/cvp137. [DOI] [PubMed] [Google Scholar]
- Im DS, Heise CE, Ancellin N, O'Dowd BF, Shei GJ, Heavens RP, Rigby MR, Hla T, Mandala S, McAllister G, George SR, Lynch KR. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J. Biol. Chem. 2000;275:14281–14286. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, Kingsbury MA, Zhang G, Brown JH, Chun J. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J. Biol. Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–1989. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- Jin ZQ, Zhang J, Huang Y, Hoover HE, Vessey DA, Karliner JS. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc. Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1970–H1977. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- Karliner JS. Sphingosine kinase regulation and cardioprotection. Cardiovasc. Res. 2008 doi: 10.1093/cvr/cvn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karliner JS. Sphingosine kinase regulation and cardioprotection. Cardiovasc. Res. 2009;82:184–192. doi: 10.1093/cvr/cvn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiacmyocytes. J. Mol. Cell. Cardiol. 2001;33:1713–1717. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- Ke Y, Lei M, Collins TP, Rakovic S, Mattick PA, Yamasaki M, Brodie MS, Terrar DA, Solaro RJ. Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21-activated kinase-1 in guinea pig sinoatrial node pacemaker cells. Circ. Res. 2007;100:1317–1327. doi: 10.1161/01.RES.0000266742.51389.a4. [DOI] [PubMed] [Google Scholar]
- Ke Y, Sheehan C, Egom Emmanuel Eroume A, Lei M, Solaro RJ. Novel bradykinin signaling in adult rat cardiac myocytes through activation of p21 activated kinase. Am. J. Physiol. Heart Circ. Physiol. doi: 10.1152/ajpheart.01070.2009. (in revision). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Solaro RJ. Use of a decoy peptide to purify p21 activated kinase -1 in cardiac muscle and identification of ceramide related activation. Biologics Target Ther. 2008;2:903–909. doi: 10.2147/btt.s3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ. Res. 2004;94:194–200. doi: 10.1161/01.RES.0000111522.02730.56. [DOI] [PubMed] [Google Scholar]
- Kon J, Sato K, Watanabe T, Tomura H, Kuwabara A, Kimura T, Tamama K, Ishizuka T, Murata N, Kanda T, Kobayashi I, Ohta H, Ui M, Okajima F. Comparison of intrinsic activities of the putative sphingosine 1-phosphate receptor subtypes to regulate several signaling pathways in their cDNA-transfected Chinese hamster ovary cells. J. Biol. Chem. 1999;274:23940–23947. doi: 10.1074/jbc.274.34.23940. [DOI] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J. Mol. Cell. Cardiol. 2002;34:509–518. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Van B, Jr, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Liebmann C, Offermanns S, Spicher K, Hinsch KD, Schnittler M, Morgat JL, Reissmann S, Schultz G, Rosenthal W. A high-affinity bradykinin receptor in membranes from rat myometrium is coupled to pertussis toxin-sensitive G-proteins of the Gi family. Biochem. Biophys. Res. Commun. 1990;167:910–917. doi: 10.1016/0006-291x(90)90610-y. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–284. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers Loncar. Projections of global mortality and burden of disease from 2002–2006 to. PLoS Med. 2030;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim. Biophys. Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- Payne SG, Milstien S, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 2002;531:54–57. doi: 10.1016/s0014-5793(02)03480-4. [DOI] [PubMed] [Google Scholar]
- Peters SL, Alewijnse AE. Sphingosine-1-phosphate signaling in the cardiovascular system. Curr. Opin. Pharmacol. 2007;7:186–192. doi: 10.1016/j.coph.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem. J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Ui M, Okajima F. Differential roles of Edg-1 and Edg-5, sphingosine 1-phosphate receptors, in the signaling pathways in C6 glioma cells. Brain Res. Mol. Brain Res. 2000;85:151–160. doi: 10.1016/s0169-328x(00)00262-x. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem. Soc. Trans. 2003;31:1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- Su JB. Kinins and cardiovascular diseases. Curr. Pharm. Des. 2006;12:3423–3435. doi: 10.2174/138161206778194051. [DOI] [PubMed] [Google Scholar]
- Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and post-conditioning. Am. J. Physiol. Heart Circ. Physiol. 2009 doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey DA, Li L, Kelley M, Karliner JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem. Biophys. Res. Commun. 2008;375:425–429. doi: 10.1016/j.bbrc.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum S, Schneider K, Heidbreder M, Nienstedt J, Dominiak P, Dendorfer A. Remote preconditioning protects the heart by activating myocardial PKC epsilon-isoform. Cardiovasc. Res. 2002;55:583–589. doi: 10.1016/s0008-6363(02)00408-x. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- Zhang G, Contos JJ, Weiner JA, Fukushima N, Chun J. Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene. 1999;227:89–99. doi: 10.1016/s0378-1119(98)00589-7. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Man K, Lo CM, Ng KT, Li XL, Sun CK, Lee TK, Dai XW, Fan ST. Attenuation of small-for-size liver graft injury by FTY720: significance of cell-survival Akt signaling pathway. Am. J. Transplant. 2004;4:1399–1407. doi: 10.1111/j.1600-6143.2004.00527.x. [DOI] [PubMed] [Google Scholar]