Abstract
Acute glucose deprivation rapidly but transiently depolarizes the actin cytoskeleton and inhibits translation initiation in Saccharomyces cerevisiae. Neither rapid actin depolarization nor translation inhibition upon glucose removal occurs in a reg1 disruptant, which is defective in glucose repression, or in the tpk1w mutant, which has weak cAPK activity. In the absence of additional glucose, recovery of either actin polarization or translation initiation relies upon respiration, the Snf1p protein kinase, and the transcription factors Msn2p and Msn4p. The readdition of glucose to glucose-starved cells causes a rapid recovery of actin polarization as well as translation initiation without respiration. These results indicate that the simultaneous regulation of actin polarization and translation initiation is divided into three reactions: 1) rapid shutdown depending on Reg1p and cAPK after glucose removal, 2) slow adaptation depending on Snf1p and Msn2p/4p in the absence of glucose, and 3) rapid recovery upon readdition of glucose. On glucose removal, translation initiation is rapidly inhibited in a rom2 disruptant, which is defective in rapid actin depolarization, whereas rapid actin depolarization occurs in a pop2/caf1 disruptant, which is defective in rapid inhibition of translation initiation. Thus, translation initiation and actin polarization seem to be simultaneously but independently regulated by glucose deprivation.
INTRODUCTION
The actin cytoskeleton provides the structural basis for cell polarity in the yeast Saccharomyces cerevisiae and is organized primarily into two morphologically distinct structures: cortical patches and cables (Botstein et al., 1997). Cortical patches are discrete cytoskeletal bodies at the plasma membrane, whereas actin cables are long bundles of actin filaments that are oriented along the mother-bud axis. Both structures are polarized during the budding phase of the cell cycle. During early G1 phase, cortical patches and cables are randomly distributed. As a bud emerges, cortical patches cluster at the growing tip and cables extend from the mother cell into the bud. Near the end of bud growth, the patches and cables redistribute randomly, disrupting the asymmetric distribution of the actin cytoskeleton. At the end of mitosis, patches accumulate in and cables reorient toward the mother-bud neck in connection with cytokinesis and septum formation (Botstein et al., 1997).
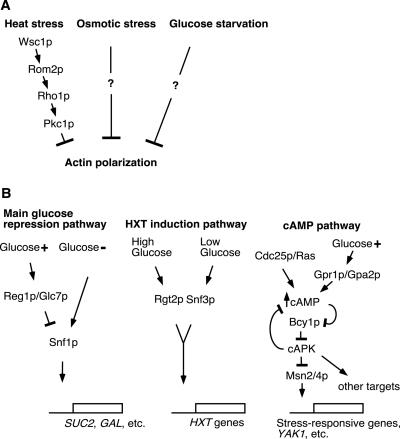
The actin cytoskeleton is also regulated in response to environmental stimuli. Mating pheromone causes polarization of the actin cytoskeleton toward the tip of the mating projection via the Cdc42p GTPase (Read et al., 1992; Leberer et al., 1997). In contrast, stresses such as osmotic shock, heat shock, and glucose starvation depolarize the actin cytoskeleton in budded cells as shown in Figure 1A (Novick et al., 1989; Chowdhury et al., 1992; Delley and Hall, 1999). In particular, the depolarization by heat or osmotic stress is known to be a rapid and transient reaction. A recent report has indicated that the rapid depolarization by heat stress occurs as a consequence of cell wall stress (Delley and Hall, 1999). This stress is sensed by a signal transduction pathway consisting of the Wsc1p plasma membrane protein, the Rom2p guanine nucleotide exchange factor, and the Rho1p GTPase with its effector kinase Pkc1p, a yeast homologue of mammalian protein kinase C (Figure 1A). Although the Pkc1p-activated MAPK cascade is not required for the rapid depolarization of actin, it is involved in the slow repolarization of the actin cytoskeleton after heat stress (Delley and Hall, 1999).
Figure 1.
Summaries of the stress-responsive pathways depolarizing the actin cytoskeleton (A) and the signaling pathways involved in glucose-mediated control (B) in S. cerevisiae. Arrows indicate activating signals, and blunt-ended lines indicate inhibitory signals.
Osmotic stress also rapidly but transiently inhibits translation initiation (Uesono and Toh-e, 2002). This observation suggests that a common mechanism may regulate both the dynamics of the actin cytoskeleton and translation initiation. The Hog1p MAPK cascade has a crucial role in osmotic sensing, and yet it is not required for either rapid actin depolarization or rapid inhibition of translation initiation upon exposure of yeast to osmotic stress. However, this cascade is required for the accurate repositioning and reassembly of the actin cytoskeleton and the recovery of translation initiation after osmotic shock (Brewster and Gustin, 1994; Uesono and Toh-e, 2002; Yuzyuk et al., 2002). Therefore, the precise factors initially controlling both actin depolarization and the inhibition of translation remain unknown.
Glucose, the preferred carbon and energy source for most eukaryotic cells, has significant and varied effects on cell function. There are three major pathways responsible for glucose-regulated gene expression in S. cerevisiae: the main glucose repression pathway, the cAMP-dependent protein kinase (cAPK) pathway, and the hexose transporter gene (HXT) induction pathway (Thevelein, 1994; Colombo et al., 1998; Gancedo, 1998; Carlson, 1999; Rolland et al., 2001; Figure 1B). Glucose deprivation also rapidly inhibits translation initiation (Martinez-Pastor and Estruch, 1996; Ashe et al., 2000). Ashe et al. (2000) reported that several mutants affected in the glucose-sensing signal transduction pathways are resistant to the inhibitory effect of glucose removal on translation initiation. This indicates that these pathways are involved in the regulation of translation initiation as well as the transcriptional control of glucose-regulated genes. Other work has reported that after glucose removal, cells exhibited a somewhat abnormal actin cytoskeleton (Novick et al., 1989). These findings suggest that osmotic stress and glucose deprivation have similar consequences for both the actin cytoskeleton and translation initiation and thus that similar mechanisms might be involved. However, it is not known how glucose regulates the organization of the actin cytoskeleton. Here, we describe the regulation of both actin polarization and translation initiation by glucose and highlight the genes that are involved in these apparently independent phenomena. In addition, we speculate that the regulatory mechanisms that coordinate effects on the actin cytoskeleton with effects on gene expression may be involved in controlling the spatial distribution of gene expression after environmental stress.
MATERIALS AND METHODS
Strains, Plasmids, Media, and Growth Conditions
Standard genetic manipulation of yeast and DNA manipulation were performed as described previously (Guthrie and Fink, 1991). Yeast transformations were performed by the lithium acetate method (Ito et al., 1983; Elble, 1992). The strains used in this study are listed in Table 1. The W303-1A0 (rho°) strain lacking mitochondrial DNA was isolated from W303-1A by treatment with ethidium bromide as described previously (Goldring et al., 1970). Strain YDK069, in which the MSN2 and MSN4 open reading frames (ORFs) are completely deleted, was provided by D. Kaida and Y. Kikuchi (Department of Biological Science, University of Tokyo, Tokyo, Japan). Strain YBC1d was constructed by a cross of BY4741 (bcy1Δ) with BY4742 (bcy1Δ), in which the BCY1 ORFs were fully deleted using the bcy1Δ::URA3 fragment derived from plasmid pKZ8 (a gift from K. Tanaka, Institute for Genetic Medicine, Hokkaido University, Sapporo, Japan). StrainYRS23 was constructed by crosses of Y03836 (BY4741 rgt2Δ::kanMX4) with BY4742 and Y07201 (BY4741 snf3Δ::kanMX4). Strain YRM24 was constructed by deletion of the MSN2 ORF in Y04911 (BY4741 msn4Δ::kanMX4) by using the msn2Δ::HIS3 fragment derived from plasmid YIP-ΔMSN2 (a gift from D. Kaida). StrainYRM24-1C was constructed by a cross of YRG1-1C (BY4742 reg1Δ::kanMX4) with YRM24.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| W303-1A | MATa ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1can1-100 ssd1-d2 | 2 |
| W303-1A0 | W303-1A rho° | 1 |
| YDK069 | W303-1A msn2Δ::HIS3 msn4Δ::URA3 | 3 |
| FY250 | MATα his3-200 leu2-1 trp1-63 ura3-52 | 4 |
| MCY3278 | MATα his3-200 leu2-1 trp1-63 ura3-52 reg1Δ::URA3 | 5 |
| yAS2605 | MATα his3-200 leu2-1 trp1-63 ura3-52 reg1Δ::URA3 snf1Δ::kanMX2 | 6 |
| yAS2605-1 | MATα his3-200 leu2-1 trp1-63 ura3-52 snf1Δ:: :kanMX2 | 1 |
| MCY2616 | MATα his3-200 leu2-1 trp1-63 ura3-52 glc7-T152K | 5 |
| JC821A/pKC886 | MATα his3-11,15 leu2 trp1 ura3 glc7Δ::HIS3 p[GLC7 TRP1 CEN] | 7 |
| JC821A/pKC866-Q48K | MATα his3-11,15 leu2 trp1 ura3 glc7Δ::HIS3 p[GLC7-Q48K TRP1 CEN] | 7 |
| SP1 | MATa ade8 his3 leu2 trp1 ura3 | 8 |
| S18-3B | MATa ade8 his3 leu2 trp1 ura3 tpk1-w1 tpk2Δ::HIS3 tpk3Δ::TRP1 | 8 |
| ASY62 | MATa ade8 his3 leu2 trp1 ura3 tpk1Δ::ADE8 tpk2Δ::HIS3 tpk3Δ::TRP1 msn2Δ::HIS3 msn4Δ::LEU2 | 9 |
| TK161-R2V | MATa ade8 his3 leu2 trp1 ura3 RAS2-val19 | 8 |
| A1961 | MATα leu2 trp1 ura3 met14 | 10 |
| A1954 | MATα leu2 trp1 ura3 his3 aro7 met14 pop2Δ::LEU2 | 10 |
| JK9-3da | MATa his4 leu2-3,112 trp1 ura3-52 rme1 HMLa | 11 |
| PA39-1b | JK9-3da wsc1Δ::kanMX4 | 11 |
| AS138-1b | JK9-3da rom2Δ::URA3 | 11 |
| OHNY1 | MATa ade2 his3 leu2 trp1 ura3 | 12 |
| IOS20a | MATa ade2 his3 leu2 trp1 ura3 rom2Δ::HIS3 | 12 |
| BY4741 | MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 | 13 |
| BY4742 | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | 13 |
| BY4743 | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met15Δ0/MET15 LYS2/lys2Δ0 | 13 |
| Y33967 | BY4743 reg1Δ::kanMX4/reg1Δ::kanMX4 | 13 |
| Y34311 | BY4743 snf1Δ::kanMX4/snf1Δ::kanMX4 | 13 |
| Y33731 | BY4743 gpr1Δ::kanMX4/gpr1Δ::kanMX4 | 13 |
| Y30152 | BY4743 gpa2Δ::kanMX4/gpa2Δ::kanMX4 | 13 |
| Y35280 | BY4743 rom2Δ::kanMX4/rom2Δ::kanMX4 | 13 |
| YBC1d | BY4743 bcy1Δ::URA3/bcy1Δ::URA3 | 1 |
| YRS23 | BY4741 rgt2Δ::kanMX4 snf3Δ::kanMX4 | 1 |
| YRM24 | BY4741 msn2Δ::HIS3 msn4Δ::kanMX4 | 1 |
| YRM24-1C | MATa his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 lys2Δ0 reg1Δ::kanMX4 msn2Δ::HIS3 msn4Δ::kanMX4 | 1 |
Sources of strains listed: this study (1); our strain collection (2); a gift from D. Kaida and Y. Kikuchi (3); Sherwood and Carlson (1997) (4); Tu and Carlson (1995) (5); Ashe et al. (2000) (6); Ramaswamy et al. (1998) (7); Nikawa et al. (1987) (8); Smith et al. (1998) (9); Shimizu-Yoshida et al. (1999) (10); Delley and Hall (1999) (11); Ozaki et al. (1996) (12); and the EUROSCARF collection (13).
Media used in this study were YP (2% polypeptone, 1% yeast extract) and YP containing 2% glucose (YPD), 0.5% glucose (0.5% Glu), 2% sucrose (YPS), 2% galactose (YPG), 2% raffinose (YPR), or 2% glycerol (YPGly). YP, SCD (0.67% yeast nitrogen base, 2% glucose), and STMD (0.17% yeast nitrogen base without ammonium sulfate, 2% glucose) were used for deprivation of glucose (-Glu), amino acids (-aa), or both nitrogen and sulfate (-NS), respectively. YP or YPD containing antimycin A (2 μg/ml; Sigma-Aldrich, St. Louis, MO) was used to inhibit respiration after glucose removal. Cycloheximide (Wako Pure Chemicals, Tokyo, Japan) and rapamycin (Sigma-Aldrich) were added to the culture at final concentrations of 100 μg/ml and 1 μg/ml, respectively. Cells were precultured in a test tube without shaking to minimize adaptation to an aerobic condition. The experimental cultures were then incubated at 25°C in a test tube containing 5 ml of medium with shaking and bubbling by a micropipet. When the OD600 of cultures reached 0.5–1.0, the cells were collected rapidly by centrifugation at 25°C and washed once with the appropriate medium for cytological tests. The washed cells were resuspended in the same medium and cultured further in a test tube with shaking and bubbling or in an Eppendorf tube without shaking (to produce partially anaerobic conditions).
Pulse-labeling Assay
Cells were grown in SCD-Met medium with appropriate supplements (without methionine) at 25°C without shaking. When the OD600 of cultures reached 0.2, the cells were collected by centrifugation and washed once with SC-Met medium lacking glucose. The washed cells were resuspended in SC-Met and cultured with shaking. After cells were collected at the indicated times, the rates of both methionine uptake and protein synthesis were measured by the pulse-labeling method using [35S]methionine (ICN Pharmaceuticals, Costa Mesa, CA) as described previously (Uesono and Toh-e, 2002). The relative uptake rates were calculated as [the cell-associated counts at the indicated times]/[those at time 0]. The protein synthesis rate was calculated as the ratio of trichloroacetic acid (TCA)-precipitated counts to cell-associated counts of the same aliquots.
Ribosome Analysis
Polysome analysis was carried out as described previously (Uesono and Toh-e, 2002) but with a few modifications. Samples containing 5 or 8 A260 units and a sample containing RNaseA (10 μg/ml) were layered onto 11 ml of a continuous 15–50% sucrose gradient, and ultracentrifugation was performed using a p40ST rotor (Himac CP60E; Hitachi, Tokyo, Japan) at 40,000 rpm for 2 h at 4°C. The polysome/monosome ratio was determined as the ratio of the area of polysomes to the area of 80S monosomes using NIH Image (developed and maintained by the National Institutes of Health, Bethesda, MD).
Actin Staining
Actin staining was performed as described previously (Adams and Pringle, 1991) with some modifications. Cells were fixed by adding formaldehyde to a culture at a final concentration of 5%, washed twice with phosphatebuffered saline, and then stained with rhodamine phalloidin (Molecular Probes, Eugene, OR). The percentages of polarization were calculated as the fraction of the cells exhibiting polarized actin cytoskeleton in small- and medium-budded cells (n > 300). Cells with <50% of their actin patches in the bud were considered to have a depolarized actin cytoskeleton, following the convention of Delley and Hall (1999).
ATP Measurement
Extraction of ATP from cells was performed as described by Ashe et al. (2000). Logarithmically growing cells in YPD were exposed to each stress by transfer to YP, shift to 37°C, or adding NaCl to the culture to a final concentration of 0.6 M. Then, 200 μl of each culture was collected rapidly by centrifugation at the indicated times, and the cells were mixed with 20 μl of 5% TCA. Ten microliters of each sample was used for ATP measurement with the Enliten luciferase/luciferin kit (Promega, Madison, WI), according to the manufacturer's instructions. Luminescence was measured at 560 nm by using a spectrofluorometer (F-2000; Hitachi). Another 5 μl of each sample was used to determine the cell density (OD600). ATP concentrations were calculated as units of luminescence per the cell density. The ATP concentrations of the wild-type cells before exposure to each stress were used as the 100% standard in each panel. The relative ATP levels were calculated as [the ATP concentration at the indicated time]/[the 100% standard in each panel].
RESULTS
Glucose Deprivation Elicits a Rapid but Transient Depolarization of the Actin Cytoskeleton
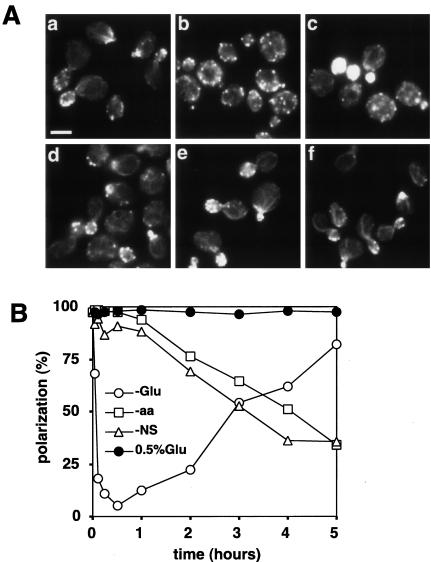
To investigate the effects of nutrient deprivation on the actin cytoskeleton, wild-type cells (W303-1A) grown in YPD were transferred to various media, and the distribution of actin in small- and medium-budded cells was observed at various time points. On transfer to YP (-Glu), the actin cytoskeleton started to depolarize after 3 min, and the polarization reached a minimum level after 30 min (Figure 2A, a and b; and B). Similar results were observed upon transfer to YPG (galactose), YPR (raffinose), or YPS (sucrose) (our unpublished data). In contrast, no depolarization was seen when cells were transferred from YPD to YPD (our unpublished data) or to YP containing 0.5% glucose (Figure 2A, d; and B). Hence, the rapid depolarization is not simply due to the manipulation upon medium replacement or to hypoosmotic stress. Rapid depolarization was also observed upon transfer from YPD to YP medium containing cycloheximide (our unpublished data), indicating that de novo protein synthesis is not required for the rapid depolarization of the actin cytoskeleton. In contrast to the rapid depolarization after glucose deprivation, the actin cytoskeleton was only gradually depolarized over a period of several hours after a shift from YPD to SCD (-aa) or STMD (-NS) (Figure 2A, e and f; and B). A gradual depolarization was also observed after treatment of cells grown in YPD with rapamycin, which inhibits the TOR protein (Barbet et al., 1996), or after a shift from YPG or YPR to YP (our unpublished data). Thus, the rapid depolarization of the actin cytoskeleton is specific to the abrupt deprivation of glucose.
Figure 2.
Glucose deprivation rapidly but transiently depolarizes the actin cytoskeleton. Wild-type (W303-1A) cells were grown in YPD, shifted to YP (-Glu), SCD (-aa), STMD (-NS), or YP containing 0.5% glucose (0.5% Glu), cultured further in test tubes with shaking, and stained for actin after various times. The percentage of budded cells after shifts to YP, SCD, or STMD gradually decreased from 63%, reaching 18, 31, and 8%, respectively, after 5 h. (A) Actin staining of cells growing in YPD (a), 30 min (b), or 5 h (c) after a shift to YP, or 30 min after a shift to YP containing 0.5% glucose (d), SCD (e), or STMD (f). Bar, 5 μm. (B) Percentages of cells exhibiting a polarized actin cytoskeleton at the indicated times. A representative of three experiments is shown.
Interestingly, the actin cytoskeleton gradually repolarized, up to ∼80% of the initial value, after 5 h in the absence of glucose (Figure 2A, c; and B). Thus, the depolarization of the actin cytoskeleton upon glucose removal is transient. It was reported previously that cells showed a somewhat abnormal actin cytoskeleton 2 h after glucose removal (Novick et al., 1989). This is consistent with the transient depolarization observed here, because ∼20% of cells contained repolarized actin 2 h after glucose removal (Figure 2B).
The timing of actin depolarization upon glucose deprivation closely resembled that observed for translation inhibition (Ashe et al., 2000), suggesting that glucose deprivation simultaneously regulates both actin polarization and translation initiation. In addition, the specificity for carbon source on actin depolarization (see above) is similar to that found for the regulation of gene repression/derepression or translation initiation (Thevelein, 1994; Ashe et al., 2000).
Both Actin Repolarization and Translation Initiation after Glucose Removal Require Respiration
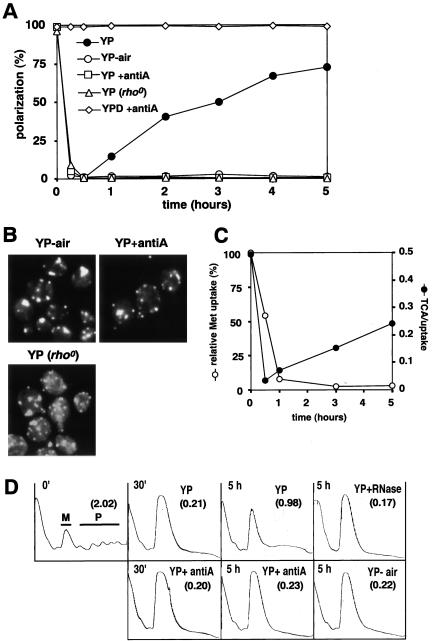
Although the actin cytoskeleton gradually repolarized after glucose removal in an aerated culture (see above), repolarization was not observed in a static nonaerated culture (Figure 3, A and B; YP-air). In addition, when cells were transferred from YPD to YP containing antimycin A, an inhibitor of the electron transfer system in mitochondria, the actin cytoskeleton was not repolarized even with aeration (Figure 3, A and B; YP+antiA). A similar result was observed with a rho° strain defective in mitochondrial function [Figure 3, A and B; YP (rho°)]. In contrast, antimycin A had no effect on the actin cytoskeleton of cells grown continuously in YPD medium (Figure 3A). Together, these results indicate that respiration is required for repolarization of actin in the absence of glucose.
Figure 3.
Repolarization of actin and the resumption of translation require respiration in the absence of glucose. (A and B) Wild-type (W303-1A) cells grown in YPD were transferred to YP with aeration (YP), YP without aeration (YP-air), YP containing antimycin A with aeration (YP+antiA), or YPD containing antimycin A with aeration (YPD+antiA). W303-1A (rho°) cells were transferred to YP with aeration [YP (rho°)]. (A) Percentages of cells exhibiting polarized actin cytoskeletons at the indicated times. A representative of three experiments is shown. (B) Actin cytoskeletons of representative cells after 5 h in the indicated medium. Bar, 5 μm. (C) Time course of the rates of methionine uptake and protein synthesis (TCA/uptake), determined as described in MATERIALS AND METHODS after shifting W303-1A cells from SCD-Met to SC-Met lacking glucose. The relative rates of uptake are plotted as a percentage of the rate in the SCD-Met-grown cells. (D) Polysome profiles (eight A260 units) of W303-1A cells at the indicated times after a shift from YPD to YP, YP+antiA, or YP-air as in A. An aliquot of one sample (YP with aeration, 5 h) was treated with RNaseA (YP+RNase). Gradient fractions were collected from top (left) to the bottom (right) in each panel. M and P indicate 80S monosomes and polyribosomes, respectively. The polysome/monosome (P/M) ratios are indicated in parentheses in each panel.
To know whether translation also recovers in the continued absence of glucose, we performed a pulse-labeling experiment by using [35S]methionine. Interestingly, the rate of methionine uptake decreased after glucose removal and did not recover during the experimental period (Figure 3C). In contrast, the rate of protein synthesis rapidly decreased but then gradually recovered after glucose removal in an aerated culture (Figure 3C). These phenomena seem to resemble the transient inhibition of protein synthesis caused by osmotic stress (Uesono and Toh-e, 2002). We then investigated whether translation initiation resumes after glucose removal, judging this by the polysome/monosome ratio, which has been observed to decrease in several mutants defective in translation initiation (Hartwell and McLaughlin, 1969; Valasek et al., 1998). The polysome/monosome ratio was markedly reduced 30 min after glucose removal (Figure 3D), as reported previously (Ashe et al., 2000). However, after 5 h in an aerated culture, high-molecular-weight materials increased, whereas monosomes decreased (Figure 3D). These high-molecular-weight materials disappeared, whereas 80S monosomes increased, upon RNaseA treatment (Figure 3D), indicating that the high-molecular-weight materials are indeed polysomes despite the absence (for unknown reasons) of discrete peaks in the polysome fraction. The polysome/monosome ratio did not recover after 5 h when the cultures were not aerated or contained antimycin A (Figure 3D). Thus, translation initiation recovers in the continued absence of glucose in a respiration-dependent manner.
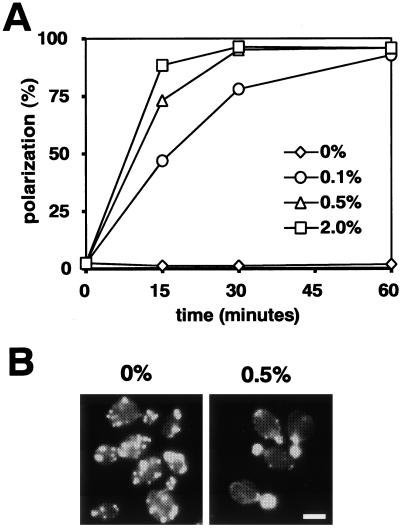
Rapid Repolarization of Actin upon Glucose Readdition
We next examined the effect of glucose addition on cells that had been glucose starved for 30 min. In the presence of antimycin A, repolarization did not occur in the absence of glucose (Figure 4A; and B, left) but was rapid and extensive at each glucose concentration tested (Figure 4A; and B, right). Similar results have been obtained for the regulation of translation initiation after glucose readdition to starved cells (Ashe et al., 2000). Thus, both actin polarization and translation initiation are simultaneously regulated by glucose, and this synchronous regulation can be divided into three individual processes: 1) the rapid shutdown immediately after glucose removal, 2) the slow adaptation in the absence of glucose, and 3) the rapid recovery after readdition of glucose.
Figure 4.
Rapid recovery of actin polarization upon readdition of glucose. Wild-type (W303-1A) cells grown in YPD were transferred to YP containing antimycin A. After 30 min, glucose at a final concentration of 0, 0.1, 0.5, or 2% was added to the starved cells. (A) Percentages of cells with polarized actin cytoskeletons at the indicated times. A representative of three experiments is shown. (B) Actin cytoskeletons of representative cells 30 min after addition of 0 or 0.5% glucose.
Genes Involved in the Loss and Recovery of Both Actin Polarization and Translation Initiation after Glucose Removal
The main glucose-repression pathway, the cAPK pathway, and the HXT induction pathway regulate not only the transcription of many genes (Figure 1B) but also translation initiation (see INTRODUCTION). Therefore, we examined whether these pathways also contribute to the rapid depolarization of the actin cytoskeleton upon glucose removal.
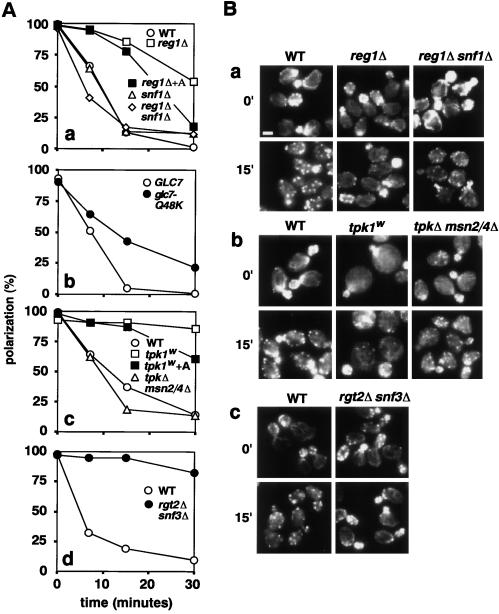
In the main glucose repression pathway, the protein kinase Snf1p is active in the absence of glucose but is negatively regulated in the presence of glucose by a type 1 protein phosphatase (PP1) complex consisting of regulatory (Reg1p) and catalytic (Glc7p) subunits (Gancedo, 1998; Carlson, 1999). In a snf1Δ strain, the actin cytoskeleton was polarized normally during growth on glucose and was depolarized as rapidly as in wild-type after glucose removal (Figure 5A, a). These results indicate that Snf1p plays no direct role in actin cytoskeletal polarization or depolarization and are consistent with the conclusion from the cycloheximide experiment (see above) that new gene expression is not necessary for the rapid depolarization response. In contrast, either a REG1 deletion or the glc7-Q48K allele of the essential gene GLC7 (Ramaswamy et al., 1998; Ashe et al., 2000) caused a slower-than-normal depolarization (Figure 5A, a and b; and B, a). These results suggest that the constitutive derepression of Snf1p-dependent genes that occurs in the reg1 and glc7 mutants might interfere with the rapid depolarization response. In strong support of this hypothesis, a reg1Δ snf1Δ double mutant depolarized as rapidly as wild type (Figure 5, Aa and Ba). These results all parallel those obtained previously with respect to the rapid inhibition of translation initiation upon glucose removal (Ashe et al., 2000).
Figure 5.
Genes required for rapid actin depolarization upon glucose removal. Cells of the indicated strains grown in YPD were transferred to YP at time 0. In two cases (Aa, reg1Δ+A; Ac, tpk1w+A), cells growing in YPD were pretreated with antimycin A for 1 h and then transferred to YP containing antimycin A. (A) Percentages of cells exhibiting a polarized actin cytoskeleton as a function of time. A representative of three experiments is shown. (B) Actin cytoskeletons of cells before (0′) or 15 min after transfer. Strains were as follows: (Aa and Ba) Wild-type, FY250; reg1Δ, MCY3278; snf1Δ, yAS2605-1; snf1Δ reg1Δ, yAS2605; (antibody) wild-type, yAS2506; glc7-Q48K, yAS2507. (Ac and Bb) Wild-type, SP1; tpk1w, S18-3D; tpkΔ msn2/4Δ, ASY62. (Ad and Bc) Wild-type, BY4741; rgt2Δ snf3Δ, YRS23.
In the cAPK pathway, cAPK negatively regulates the stress-responsive transcription factors Msn2p and Msn4p by inhibiting their nuclear import (Ruis and Schuller, 1995; Görner et al., 1998, 2002). A tpk1w mutant (tpk1-1w tpk2Δ tpk3Δ), which has a low level of cAPK activity, was largely resistant to actin depolarization upon glucose removal, whereas a tpk1Δ tpk2Δ tpk3Δ msn2Δ msn4Δ strain depolarized as rapidly as wild type (Figure 5, Ac and Bb). These results suggest that the constitutive activity of Msn2p/4p in the low-cAPK mutant is responsible for the loss of rapid depolarization. Again, these results parallel those obtained in studies of translation initiation (Ashe et al., 2000). To investigate the relationship between the Reg1p/Glc7p/Snf1p and cAPK/Msn2p/4p pathways, we constructed a reg1Δ msn2Δ msn4Δ triple mutant (YRM24-1C). This strain was resistant to rapid depolarization like the reg1Δ single mutant (our unpublished data), indicating that Msn2p and Msn4p are not involved in the reg1Δ effect.
In the HXT induction pathway, glucose is sensed by the high- and low-affinity glucose sensors Rgt2p and Snf3p, which are required for the differential expression of HXT genes (Özcan and Johnston, 1996; Özcan et al., 1998). In an rgt2 snf3 double mutant, translation initiation continues after glucose removal (Ashe et al., 2000), and actin polarization is also maintained (Figure 5, Ad and Bc). Thus, it seems that each of the three pathways for glucose response is required for the normal synchronous regulation of actin depolarization and shutdown of translation initiation upon glucose withdrawal.
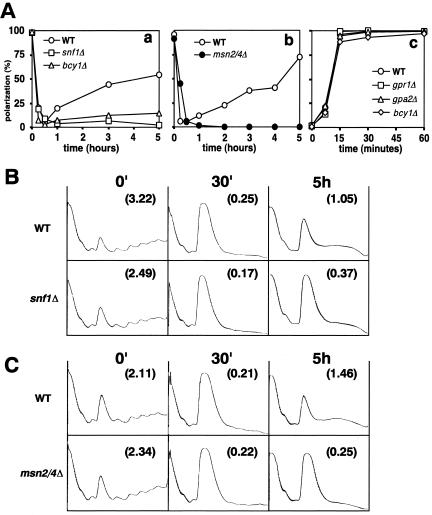
As shown above, respiration is necessary for the gradual recovery of actin polarization and translation initiation in the continued absence of glucose. Snf1p is crucial for the activation of respiration after glucose depletion because it allows the derepression of genes required for utilization of nonfermentable carbon sources and for mitochondrial function (Ulery et al., 1994; Carlson, 1999). Thus, it seemed likely that Snf1p would be necessary for the recovery of actin polarization and translation initiation after glucose removal. Indeed, neither actin polarization nor translation initiation recovered well in a snf1 disruptant within 5 h after glucose removal (Figure 6, Aa and B). In the course of these experiments, we also observed that recovery is also affected by strain background and/or ploidy. Thus, in contrast to W303-1A (Figures 2 and 3) and BY4743 (Figure 6), neither FY250 (the wild-type strain used in Figure 5) nor a haploid of the BY background showed efficient recovery of actin polarization within 5 h after glucose removal (our unpublished data).
Figure 6.
Genes required for both repolarization of the actin cytoskeleton and resumption of translation initiation after glucose removal. (A) Percentages of cells showing a polarized actin cytoskeleton are shown for a representative of three experiments in each case. (a and b) Cells of the indicated strains grown in YPD were transferred to YP. (a) Wild-type, BY4743; snf1Δ, Y34311; bcy1Δ, YBC1d. (b) Wild-type, W303-1A; msn2/4Δ, YDK069. (c) Cells of the indicated strains grown in YPD were transferred to YP containing antimycin A. After 30 min, 2.0% glucose as final concentration was added to the cultures in the continued presence of antimycin A. Wild-type, BY4743; bcy1Δ, YBC1d; gpr1Δ, Y33731; gpa2Δ, Y30152. (B and C) Polysome profiles (8 A260 units) of the indicated strains are shown before (0′) and 30 min and 5 h after transfer from YPD to YP. The P/M ratios are indicated in parentheses in each panel. (B) Wild-type, BY4743; snf1Δ, Y34311. (C) Wild-type, W303-1A; msn2/4Δ, YDK069.
Msn2p and Msn4p are also required for growth on nonfermentable carbon sources (Estruch and Carlson, 1993) and thus presumably would also be necessary for the recovery of actin polarization and translation initiation after glucose removal. Indeed, little or no recovery was observed in an msn2 msn4 disruptant during the experimental period (Figure 6, antibody and C). In addition, a bcy1 disruptant, which has a high level of cAPK activity that negatively regulates Msn2p/4p, also showed little or no recovery (Figure 6A, a; our unpublished data).
It is possible that the constitutive activation of Snf1p and Msn2p/4p in the reg1 and tpk1w mutants, respectively, results in an activation of respiration even in the presence of glucose in the mutant strains. If it is such a preactivation of respiration that prevents rapid actin depolarization and translation inhibition in the mutant strains upon glucose removal (Figure 5), then the effects of the mutations might be prevented by inhibiting respiration before glucose removal. However, both the reg1 and tpk1w mutants were still largely resistant to actin depolarization when the cells were treated with antimycin A for 1 h before glucose removal (Figure 5, Aa and Ac), although in both cases there were small decreases in resistance. These results suggest that it is the expression of Snf1p- and Msn2p/4p-regulated genes other than those involved in respiration that is primarily responsible for the lack of rapid actin depolarization (and perhaps of rapid translation inhibition) upon glucose removal in the reg1 and tpk1w mutant strains.
Recovery of Actin Polarization upon Glucose Readdition Does Not Seem to Require Snf1p, Msn2p/4p, or a Transient Increase in cAMP
The addition of glucose to glucose-starved cells causes a rapid and transient increase of intracellular cAMP level. This transient increase is strongly reduced in a strain with enhanced feedback inhibition of cAMP synthesis by elevated cAPK activity (Mbonyi et al., 1990; Colombo et al., 1998; Figure 1B), such as a bcy1 disruptant, which is also defective in the slow adaptation of the actin cytoskeleton in the absence of glucose (see above). In addition, the transient increase is also partially dependent on Gpr1p, a putative G protein-coupled receptor, and Gpa2p, a G protein subunit (Thevelein, 1984; Kübler et al., 1997; Kraakman et al., 1999). To ask whether this transient increase of cAMP is required for the recovery of actin polarization upon glucose addition, we observed the recovery in bcy1, gpr1, and gpa2 disruptants. In the presence of antimycin A (to avoid the slow adaptation process), the actin cytoskeleton was rapidly polarized after glucose addition in these mutants (Figure 6A, c). Similar results (our unpublished data) were observed in the snf1 and msn2/4 disruptants, which are also defective in the slow adaptation in the absence of glucose (see above). These results suggest that the rapid, glucose-dependent recovery of actin polarization does not require either the transient increase in cAMP or the control of gene expression mediated by Snf1p or Msn2p/4p.
Interactions between the Glucose-Sensing and Stress Pathways during Rapid Actin Depolarization
The actin cytoskeleton is rapidly depolarized via an unidentified pathway upon exposure to osmotic stress, via the Rom2p/Rho1p/Pkc1p pathway upon exposure to heat stress, or via the glucose-sensing pathways upon glucose removal (Brewster and Gustin, 1994; Delley and Hall, 1999; Figures 1 and 5). To investigate possible cross talk between these pathways, we tested whether the various stresses could depolarize actin in the mutants defective in the Rom2p/Rho1p/Pkc1p or glucose-sensing pathways.
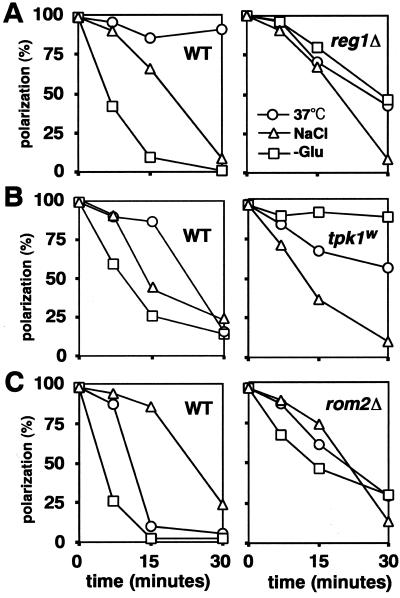
Three different wild-type strains showed similar depolarization of actin after addition of 0.6 M NaCl (Figure 7, A–C, left). This depolarization was typically less rapid than that seen after glucose removal. Addition of 0.6 M NaCl to the congenic reg1, tpk1w, and rom2 mutants led to a similar depolarization (Figure 7, A–C, right). These data suggest that osmotic stress depolarizes the actin cytoskeleton via a pathway different from the glucose-sensing and Rom2p/Rho1p/Pkc1p pathways. The observation on the tpk1w mutant is consistent with studies of the effects of osmotic stress on translation initiation (Uesono and Toh-e, 2002).
Figure 7.
Interrelationship between the stress-responsive pathways for actin depolarization. Cells of the indicated strains growing in YPD were shifted to 37°C, exposed to 0.6 M NaCl, or transferred to YP (-Glu). Percentages of cells exhibiting a polarized actin cytoskeleton are shown for a representative of three experiments in each case. (A) Wild-type, FY250; reg1Δ, MCY3278. (B) Wild-type, SP1; tpk1w, S18-3B. (C) Wild-type, JK9-3da; rom2Δ, AS138-1b.
In two of the wild-type strains, <20% of cells showed a polarized actin distribution at 30 min after a shift to 37°C (Figure 7, B and C, left). However, in the third wild-type strain (FY250), polarization was ∼85% at this time (Figure 7A, left). Thus, the degree of actin depolarization upon heat stress depends upon the strain background. In strain FY250, actin was slightly depolarized at 15 min and then recovered quickly by 30 min, whereas the congenic reg1 disruptant showed more depolarization after 30 min at 37°C (Figure 7A). These data suggest that Reg1p may be involved either in preventing actin depolarization or in allowing a rapid recovery from depolarization upon heat stress in the FY250 strain background. In comparison with the congenic wild-type strain, the tpk1w mutant showed somewhat less actin depolarization upon shift to 37°C (Figure 7B), suggesting that cAPK is involved in heat stress-induced depolarization, at least in this strain background. Thus, there seems to be some common components in the glucose-sensing and heat stress pathways of actin depolarization.
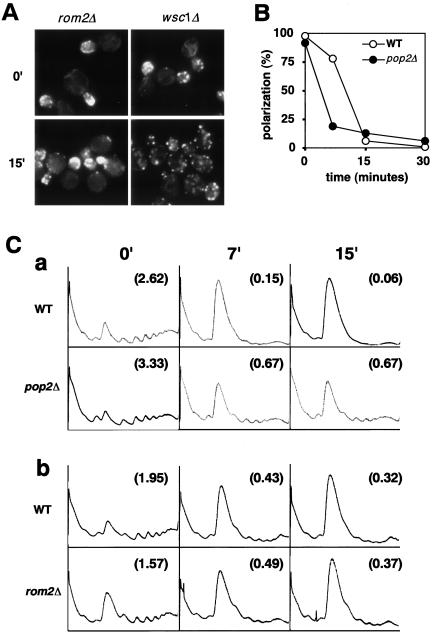
This conclusion was supported by observations on the rom2 disruptant. As expected (Delley and Hall, 1999), this mutant showed less depolarization upon shift to 37°C than did the congenic wild-type strain (Figure 7C, right). It also showed less depolarization in response to glucose removal (Figures 7C and 8A). In contrast, a wsc1 disruptant did not show resistance to depolarization upon glucose removal (Figure 8A). These results suggest that not only heat stress but also glucose deprivation can transmit a signal via the Rom2p. Heat stress is sensed by Wsc1p (Delley and Hall, 1999), whereas glucose deprivation must be sensed by other factors. We also observed that in either the BY or OHNY1 strain background, a rom2 disruptant did not show resistance to actin depolarization upon either a shift to 37°C or glucose removal (our unpublished data). Therefore, Rom2p is not absolutely required for depolarization after either heat stress or glucose removal, but it is clearly involved in the depolarization process in some strain backgrounds.
Figure 8.
Specificity of actin depolarization and inhibition of translation upon glucose removal. (A) The actin cytoskeletons of rom2Δ (AS138-1b) and wsc1Δ(PA39-1b) cells were viewed before (0′) and 15 min after a shift from YPD to YP. (B) Wild-type (A1961) and pop2Δ (A1954) cells grown in YPD were transferred to YP. Percentages of cells exhibiting a polarized actin cytoskeleton are shown at the indicated times for a representative of three experiments. (C) Polysome profiles (five A260 units) are shown for (a) wild-type (A1961) and pop2Δ (A1954) strains or (b) wild-type (JK9-3da) and rom2Δ (AS138-1b) strains before (0′) and at 7 and 15 min after transfer from YPD to YP. The P/M ratios are indicated in parentheses in each panel.
Specific Roles of Rom2p and Pop2p/Caf1p in Actin Depolarization and Translation Inhibition upon Glucose Removal
We have reported that the pop2/caf1 disruptant, which is defective in mRNA deadenylation (Tucker et al., 2001), is resistant to the inhibitory effect of glucose removal on translation initiation (Uesono and Toh-e, 2002). We examined whether the pop2 disruptant is also resistant to actin depolarization upon glucose removal. However, in contrast to mutants that are resistant to both effects, such as reg1 and tpk1w (see above), a pop2 disruptant depolarized actin at least as efficiently as the congenic wild-type strain (Figure 8B), whereas translation initiation was partially maintained (Figure 8C, a). This indicates that Pop2p plays a role in the inhibition of translation initiation but is not involved in the actin depolarization after glucose removal.
In contrast, although actin polarization was partially maintained in a rom2 disruptant upon glucose removal (Figures 7C and 8A), translation initiation was inhibited as in the congenic wild-type strain (Figure 8C, b). In addition, overexpression of activated Pkc1p did not inhibit translation initiation in W303-1A, as judged by polysome profiles (our unpublished data), although it did cause actin depolarization (Delley and Hall, 1999). Thus, at least in these strain backgrounds, the Rom2p/Rho1p/Pkc1p pathway seems to be important for actin depolarization but not for inhibition of translation initiation upon glucose removal. Consistent with a specific role for the Rom2p/Rho1p/Pkc1p pathway in the dynamics of the actin cytoskeleton, these proteins have all been reported to localize to the site of polarized growth (Yamochi et al., 1994; Manning et al., 1997; Andrews and Stark, 2000). Consequently, our findings suggest that Pop2p and the Rom2p/Rho1p/Pkc1p pathways act separately on the translation machinery and the actin cytoskeleton, respectively.
Rapid Actin Depolarization Does Not Seem to Be Due to a Decrease in Intracellular ATP
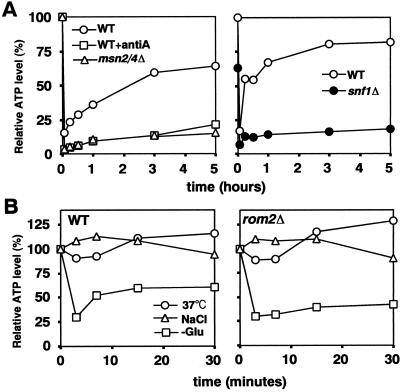
Intracellular ATP decreases transiently after glucose removal (Ashe et al., 2000; Jona et al., 2000). Because polymerization of actin requires ATP, it seemed possible that actin depolarization is linked to this decrease in ATP. Thus, we investigated the relationship between actin polarization and ATP levels in various strains exposed to different stresses. In the wild-type strains W303-1A and BY4743, the ATP levels decreased rapidly after glucose removal, as expected, but then gradually recovered to 70–80% of the initial level after 5 h in an aerated culture (Figure 9A). This recovery was markedly decreased when antimycin A was added (Figure 9A), when the W303-1A rho° strain was examined (our unpublished data), or when a msn2/4 or snf1 disruptant was examined (Figure 9A). In each of these situations, actin polarization also failed to recover (Figures 3, A and B; and 6, Aa and antibody). Moreover, in the reg1Δ and tpk1w mutants, where rapid actin depolarization did not occur (Figure 5, Aa and Ac), there was also no rapid decrease in ATP levels (our unpublished data; Ashe et al., 2000). These data are all consistent with the hypothesis that the transient depolarization of the actin cytoskeleton is due to the transient decrease in ATP levels after glucose removal.
Figure 9.
Relationships between intracellular ATP levels and actin depolarization/repolarization. (A) Wild-type strains W303-1A (left) and BY4743 (right), the msn2/4Δ strain YDK069, and the snf1Δ strain Y34311 were shifted from YPD to YP. W303-1A was also shifted to YP containing antimycin A. Relative ATP levels are plotted as a percentage of the levels in W303-1A (left) or BY4743 (right) at 0 min. (B) Wild-type strain JK9-3da and rom2Δ mutant strain AS138-1b growing in YPD were exposed to 37°C or to 0.6 M NaCl or were transferred to YP. Relative ATP levels are plotted as a percentage of the level in JK9-3da at 0 min. In each case, a representative of three experiments is shown.
However, in contrast, a shift to 37°C or exposure to 0.6 M NaCl did not produce a decrease in ATP levels in the wild-type strain JK9-3da strain (Figure 9B, left), although these conditions caused rapid actin depolarization in this strain (Figure 7C). Moreover, in the related rom2Δ strain, ATP levels decreased to the same degree as in wild-type after glucose removal (Figure 9B, right), although actin was not rapidly depolarized in this mutant (Figure 7C). Thus, in these situations, the occurrence of actin depolarization is not correlated with changes in ATP levels. It is conceivable that Rom2p produces rapid actin depolarization by sensing the decrease in ATP levels upon glucose removal. However, Rom2p is also required for rapid actin depolarization in response to heat stress (Figure 7C; Delley and Hall, 1999), which is not correlated with changes in the ATP level (Figure 9B). Together, the observations suggest that rapid actin depolarization in response to either glucose removal or heat stress is caused by a signal other than a decrease in ATP levels. This signal must act at least partially via Rom2p.
DISCUSSION
We have found that actin polarization and translation initiation are regulated simultaneously and in parallel by glucose: 1) rapid loss of each upon glucose removal depends on Reg1p and cAPK; 2) slow adaptation of each in the continued absence of glucose depends on respiration, Snf1p, and Msn2p/4p; and 3) rapid recovery of each occurs after readdition of glucose and is independent of respiration in this case. Thus, the systems for glucose repression of gene expression are also required for the regulation of both actin polarization and translation initiation.
In relation to both actin polarization and translation initiation, the response to glucose deprivation is divided into two opposite reactions in a way similar to the response to osmotic stress (Uesono and Toh-e, 2002). One is the acute loss of both actin polarization and translation initiation via an unidentified mechanism that has no requirement for changes in gene expression. The other is the slow reactivation of both translation and actin polarization, possibly via gene expression changes brought about by Snf1p and Msn2p/4p, a hypothesis supported by the observation that both the snf1 and msn2/4 disruptants showed incomplete adaptation of actin polarization and translation initiation after glucose removal (Figure 6). These two opposite reactions are likely to start at virtually the same time after glucose removal, because the shutdown is initiated after ∼3 min (Figure 2), whereas Snf1p and Msn2p/4p are rapidly modified by phosphorylation and rapidly imported into the nucleus, respectively (Wilson et al., 1996; Vincent et al., 2001; Görner et al., 2002). As a result of the combination of these opposite reactions, the shutdown may occur only transiently. This relationship closely parallels the transient shutdown of translation initiation caused by osmotic stress, except that the Hog1p pathway instead of Snf1p and Msn2p/4p is involved in the slow reactivation (Uesono and Toh-e, 2002). There seem to be two possibilities for the relationship between the opposite reactions. One is that gene expression via Snf1p and Msn2p/4p overcomes a shutdown pathway that simultaneously regulates both actin polarization and translation initiation after glucose removal. The other is that changes in gene expression directly stimulate an activation pathway that simultaneously regulates both translation and the actin cytoskeleton. We favor the former hypothesis, because the tpk1w mutant, which has activated Msn2p/4p, does not activate bulk protein synthesis in the presence of glucose (Ashe et al., 2000).
Some translation factors are likely to associate physically with cytoskeletal components such as actin or tubulin (Hasek et al., 2000; Palecek et al., 2001). Because the loss of both translation initiation and actin polarization occurs at approximately the same time after glucose removal, it is possible that these shutdown processes are dependent upon each other. However, the polysome/monosome ratio did not decrease rapidly in the presence of glucose in an act1 mutant, which rapidly depolarizes the actin cytoskeleton after a shift to the restrictive temperature (our unpublished data), suggesting that the rapid shutdown of translation initiation does not result from rapid depolarization of the actin cytoskeleton. Conversely, rapid depolarization of the actin cytoskeleton was not observed in the presence of glucose after addition of cycloheximide (100 μg/ml), which rapidly inhibits translation at the elongation step, or after a shift to restrictive temperature of a prt1-1 mutant, which rapidly shuts down translation at the initiation level (our unpublished data). Thus, the rapid depolarization does not seem to result from the loss of translation initiation. [However, the actin cytoskeleton did depolarize slowly after cycloheximide treatment (our unpublished data), suggesting that maintenance of actin polarization may require continuous protein synthesis, as reported previously (Novick et al., 1989)]. In addition, translation initiation was rapidly inhibited in a rom2 disruptant, which is defective in the rapid depolarization of actin upon glucose removal (Figure 8, A and Cb), indicating that shutdown of translation initiation can occur irrespective of actin polarization. Conversely, rapid depolarization occurred in a pop2 disruptant, which is defective in rapid inhibition of translation initiation upon glucose removal (Figure 8, B and Ca), indicating that actin depolarization can occur irrespective of translation initiation. Together, the observations suggest that translation initiation and actin polarization are simultaneously but independently regulated by acute glucose deprivation.
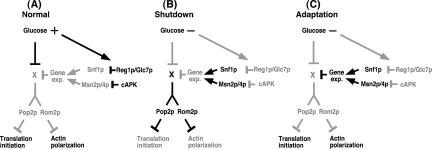
We found that the previously described glucose-sensing pathways regulate actin polarization and translation initiation as well as transcription. These systems may function cooperatively in response to glucose deprivation, as indicated in the possible model shown in Figure 10. When glucose is present, Reg1p/Glc7p and cAPK inactivate Snf1p and Msn2p/4p, which are involved in the transcriptional derepression/activation of glucose-repressed genes (Figure 10A). Immediately after glucose removal, a shutdown pathway (X) that does not involve de novo protein synthesis is activated rapidly and causes actin depolarization via Rom2p and the inhibition of translation initiation via Pop2p before the new gene expression occurs. Simultaneously, however, Snf1p and Msn2p/4p are also activated rapidly via inactivation of Reg1p/Glc7p and cAPK (Figure 10B). Thus, after a few hours in the absence of glucose, the gene products required for cancellation of the shutdown pathway are transcribed by the activated transcription factors and translated (perhaps preferentially). Consequently, the actin repolarizes and bulk translation initiation is reactivated (Figure 10C). Glucose removal cannot induce a rapid shutdown in the reg1 or tpk1w mutant (Figure 5), because preactivation of Snf1p or Msn2p/4p in these mutants prevents the shutdown pathway from functioning by means of the preactivated gene expression. Adaptation does not occur in the snf1 or msn2/4 mutant after glucose removal (Figure 6), because the shutdown pathway is not canceled by activated gene expression in these mutants.
Figure 10.
A possible model for the synchronous regulation by glucose deprivation. The reaction to glucose deprivation is separated into three steps: the normal situation before glucose deprivation, the shutdown phase just after glucose deprivation, and the adaptation phase in the continued absence of glucose. Black and gray lines indicate an active and an inactive state, respectively. X represents the presumed target of glucose that is common to the pathways controlling translation initiation and actin polarization.
What is the biological significance of the transient losses of both actin polarization and translation initiation, which occur synchronously upon exposure to stresses such as glucose deprivation or osmotic stress? It has been reported that many mRNAs and RNA-binding proteins are localized to specific compartments, along with cytoskeletal elements, in a variety of eukaryotic cells (Jansen, 2001; Kloc et al., 2002). In S. cerevisiae, many mRNAs are localized to produce proteins in specific compartments such as the daughter cell or the mitochondria (Corral-Debrinski et al., 2000; Takizawa et al., 2000; Jansen, 2001; Kwon and Schnapp, 2001; Shepard et al., 2003). These observations suggest that individual mRNAs may be consistently transported to specific areas in the yeast cell. One possible scenario for transient shutdown of both actin polarization and translation initiation by stresses is as follows. When cells are exposed to such stresses, the cells immediately suspend both transportation of mRNAs to the specific compartments by loss of actin polarization and production of proteins by shutdown of translation initiation. During the shutdown, the intracellular profile of mRNAs is dramatically changed in response to the stress (Gancedo, 1998; Causton et al., 2001). The shutdown of translation at the initiation but not the elongation level would increase the proportion of mRNAs that are not protected by the ribosome. This would allow the mRNAs that are unnecessary for the stressed condition to be degraded by the mRNA decay pathways. Consequently, the cell can easily change the intracellular pattern of mRNAs, as required for the stressed condition, by changes in transcriptional regulation and/or mRNA stability during the shutdown. Subsequently, some mRNAs are relocated to specific areas, where their translation products are required for the stressed condition, by repolarization of the actin cytoskeleton. Simultaneously, the cell can produce the corresponding proteins by resuming the translation process. Therefore, it seems likely that the dramatic changes in both the actin cytoskeleton and protein synthesis, as described here, form part of a system for the spatial rearrangement of gene expression in response to environmental stress.
Acknowledgments
We thank Alan B. Sachs, Dae-Jin Yun, Marian Carlson, Joseph Heitman, Michael N. Hall, Stephen Garrett, Jose Antonio Prieto, David Botstein, Yasushi Matsui, Akira Sakai, Daisuke Kaida, Yoshiko Kikuchi, and Kenji Irie for strains and plasmids, and K. Tanaka for useful discussion. M.A. is supported by a Wellcome Trust project grant 061867/Z/00/Z. This work was supported by Japan Society for the Promotion of Science grant 13780548.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–12–0877. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-12-0877.
References
- Adams, A.E.M., and Pringle, J.R. (1991). Staining of actin with fluorochromeconjugated phalloidin. Methods Enzymol. 194, 729-731. [DOI] [PubMed] [Google Scholar]
- Andrews, P.D., and Stark, M.J. (2000). Dynamic, Rho1p-dependent localization of Pkc1p to sites of polarized growth. J. Cell Sci. 113, 2685-2693. [DOI] [PubMed] [Google Scholar]
- Ashe, M.P., De Long, S.K., and Sachs, A.B. (2000). Glucose depletion rapidly inhibits translation initiation in yeast. Mol. Biol. Cell 11, 833-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet, N.C., Schneider, U., Helliwell, S.B., Stansfield, I., Tuite, M.F., and Hall, M.N. (1996). TOR controls translation initiation and early G1 progression in yeast. Mol. Biol. Cell 7, 25-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein, D., Amberg, D., Mulholland, J., Huffaker, T., Adams, A., Drubin, D., and Stearns, T. (1997). The yeast cytoskeleton. In: The Molecular and Cellular Biology of the yeast Saccharomyces. Cell Cycle and Cell Biology, ed. J.R. Pringle, J.R. Broach, and E.W. Jones, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1-45.
- Brewster, J.L., and Gustin, M.C. (1994). Positioning of cell growth and division after osmotic stress requires a MAP kinase pathway. Yeast 10, 425-439. [DOI] [PubMed] [Google Scholar]
- Carlson, M. (1999). Glucose repression in yeast. Curr. Opin. Microbiol. 2, 202-207. [DOI] [PubMed] [Google Scholar]
- Causton, H.C., Ren, B., Koh, S.S., Harbison, C.T., Kanin, E., Jennings, E.G., Lee, T.I., True, H.L., Lander, E.S., and Young, R.A. (2001). Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12, 323-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, S., Smith, K.W., and Gustin, M.C. (1992). Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J. Cell Biol. 118, 561-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo, S., et al. (1998). Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 17, 3326-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Debrinski, M., Blugeon, C., and Jacq, C. (2000). In yeast, the 3′ untranslated region or the presequence of ATM1 is required for the exclusive localization of its mRNA to the vicinity of mitochondria. Mol. Cell. Biol. 20, 7881-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delley, P.A., and Hall, M.N. (1999). Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147, 163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble, R. (1992). A simple and efficient procedure for transformation of yeasts. Biotechniques 13, 18-20. [PubMed] [Google Scholar]
- Estruch, F., and Carlson, M. (1993). Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae. Mol. Cell. Biol. 13, 3872-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo, J.M. (1998). Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 62, 334-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring, E.S., Grossman, L.I., Krupnick, D., Cryer, D.R., and Marmur, J. (1970). The petite mutation in yeast: loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J. Mol. Biol. 52, 323-335. [DOI] [PubMed] [Google Scholar]
- Görner, W., Durchschlag, E., Martinez-Pastor, M.T., Estruch, F., Ammerer, G., Hamilton, B., Ruis, H., and Schuller, C. (1998). Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12, 586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görner, W., Durchschlag, E., Wolf, J., Brown, E.L., Ammerer, G., Ruis, H., and Schuller, C. (2002). Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J. 21, 135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G.R. (eds.) (1991). Guide to Yeast Genetics and Molecular Biology: Volume 194: Guide to Yeast Genetics and Molecular Biology, New York: Academic.
- Hartwell, L.H., and McLaughlin. C.S. (1969). A mutant of yeast apparently defective in the initiation of protein synthesis. Proc. Natl. Acad. Sci. USA 62, 468-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasek, J., Kovarik, P., Valasek, L., Malinska, K., Schneider, J., Kohlwein, S.D., and Ruis, H. (2000). Rpg1p, the subunit of the Saccharomyces cerevisiae eIF3 core complex, is a microtubule-interacting protein. Cell Motil. Cytoskeleton 45, 235-246. [DOI] [PubMed] [Google Scholar]
- Ito, H., Fukuda, Y., Murata, K., and Kimura, A. (1983). Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153, 163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, R.P. (2001). mRNA localization: message on the move. Nat. Rev. Mol. Cell. Biol. 2, 247-256. [DOI] [PubMed] [Google Scholar]
- Jona, G., Choder, M., and Gileadi, O. (2000). Glucose starvation induces a drastic reduction in the rates of both transcription and degradation of mRNA in yeast. Biochim. Biophys. Acta 1491, 37-48. [DOI] [PubMed] [Google Scholar]
- Kloc, M., Zearfoss, N.R., and Etkin, L.D. (2002). Mechanisms of subcellular mRNA localization. Cell 108, 533-544. [DOI] [PubMed] [Google Scholar]
- Kraakman, L., Lemaire, K., Ma, P., Teunissen, A.W., Donaton, M.C., Van Dijck, P., Winderickx, J., de Winde, J.H., and Thevelein, J.M. (1999). A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol. Microbiol. 32, 1002-1012. [DOI] [PubMed] [Google Scholar]
- Kübler, E., Mösch, H.U., Rupp, S., and Lisanti, M.P. (1997). Gpa2p, a G-protein alpha-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J. Biol. Chem. 272, 20321-20323. [DOI] [PubMed] [Google Scholar]
- Kwon, S., and Schnapp, B.J. (2001). RNA localization: shedding light on the RNA-motor linkage. Curr. Biol. 11, R166-R168. [DOI] [PubMed] [Google Scholar]
- Leberer, E., Thomas, D.Y., and Whiteway, M. (1997). Pheromone signalling and polarized morphogenesis in yeast. Curr. Opin. Genet. Dev. 7, 59-66. [DOI] [PubMed] [Google Scholar]
- Manning, B.D., Padmanabha, R., and Snyder, M. (1997). The Rho-GEF Rom2p localizes to sites of polarized cell growth and participates in cytoskeletal functions in Saccharomyces cerevisiae. Mol. Biol. Cell 8, 1829-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pastor, M.T., and Estruch, F. (1996). Sudden depletion of carbon source blocks translation, but not transcription, in the yeast Saccharomyces cerevisiae. FEBS Lett. 390, 319-322. [DOI] [PubMed] [Google Scholar]
- Mbonyi, K., van Aelst, L., Arguelles, J.C., Jans, A.W., and Thevelein, J.M. (1990). Glucose-induced hyperaccumulation of cyclic AMP and defective glucose repression in yeast strains with reduced activity of cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 10, 4518-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa, J., Cameron, S., Toda, T., Ferguson, K.M., and Wigler, M. (1987). Rigorous feedback control of cAMP levels in Saccharomyces cerevisiae. Genes Dev. 1, 931-937. [DOI] [PubMed] [Google Scholar]
- Novick, P., Osmond, B.C., and Botstein, D. (1989). Suppressors of yeast actin mutations. Genetics 121, 659-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki, K., Tanaka, K., Imamura, H., Hihara, T., Kameyama, T., Nonaka, H., Hirano, H., Matsuura, Y., and Takai, Y. (1996). Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15, 2196-2207. [PMC free article] [PubMed] [Google Scholar]
- Özcan, S., Dover, J., and Johnston, M. (1998). Glucose sensing and signaling by two glucose receptors in the yeast Saccharomyces cerevisiae. EMBO J. 17, 2566-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan, S., and Johnston, M. (1996). Two different repressors collaborate to restrict expression of the yeast glucose transporter genes HXT2 and HXT4 to low levels of glucose. Mol. Cell. Biol. 16, 5536-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek, J., Hasek, J., and Ruis, H. (2001). Rpg1p/Tif32p, a subunit of translation initiation factor 3, interacts with actin-associated protein Sla2p. Biochem. Biophys. Res. Commun. 282, 1244-1250. [DOI] [PubMed] [Google Scholar]
- Ramaswamy, N.T., Li, L., Khalil, M., and Cannon, J.F. (1998). Regulation of yeast glycogen metabolism and sporulation by Glc7p protein phosphatase. Genetics 149, 57-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, E.B., Okamura, H.H., and Drubin, D.G. (1992). Actin- and tubulin-dependent functions during Saccharomyces cerevisiae mating projection formation. Mol. Biol. Cell 3, 429-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland, F., Winderickx, J., and Thevelein. J.M. (2001). Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem. Sci. 26, 310-317. [DOI] [PubMed] [Google Scholar]
- Ruis, H., and Schuller, C. (1995). Stress signaling in yeast. Bioessays 17, 959-965. [DOI] [PubMed] [Google Scholar]
- Shepard, K.A., Gerber, A.P., Jambhekar, A., Takizawa, P.A., Brown, P.O., Herschlag, D., DeRisi, J.L., and Vale, R.D. (2003). Widespread cytoplasmic mRNA transport in yeast: identification of 22 bud-localized transcripts using DNA microarray analysis. Proc. Natl. Acad. Sci. USA 100, 11429-11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood, P.W., and Carlson, M. (1997). Mutations in GSF1 and GSF2 alter glucose signaling in Saccharomyces cerevisiae. Genetics 147, 557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Yoshida, Y., Sasamoto, M., Yoshida, A., Yoshioka, T., Matsumoto, A., and Sakai, A. (1999). Mouse CAF1, a mouse homologue of the yeast POP2 gene, complements the yeast pop2 null mutation. Yeast 15, 1357-1364. [DOI] [PubMed] [Google Scholar]
- Smith, A., Ward, M.P., and Garrett, S. (1998). Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J. 17, 3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa, P.A., DeRisi, J.L., Wilhelm, J.E., and Vale, R.D. (2000). Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290, 341-344. [DOI] [PubMed] [Google Scholar]
- Thevelein, J.M. (1984). Cyclic-AMP content and trehalase activation in vegetative cells and ascospores of yeast. Arch. Microbiol. 138, 64-67. [DOI] [PubMed] [Google Scholar]
- Thevelein, J.M. (1994). Signal transduction in yeast. Yeast 10, 1753-1790. [DOI] [PubMed] [Google Scholar]
- Tu, J., and Carlson, M. (1995). REG1 binds to protein phosphatase type 1 and regulates glucose repression in Saccharomyces cerevisiae. EMBO J. 14, 5939-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, M., Valencia-Sanchez, M.A., Staples, R.R., Chen, J., Denis, C.L., and Parker, R. (2001). The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell 104, 377-386. [DOI] [PubMed] [Google Scholar]
- Uesono, Y., and Toh-e, A. (2002). Transient inhibition of translation initiation by osmotic stress. J. Biol. Chem. 277, 13848-13855. [DOI] [PubMed] [Google Scholar]
- Ulery, T.L., Jang, S.H., and Jaehning, J.A. (1994). Glucose repression of yeast mitochondrial transcription: kinetics of derepression and role of nuclear genes. Mol. Cell. Biol. 14, 1160-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valasek, L., Trachsel, H., Hasek, J., and Ruis, H. (1998). Rpg1, the Saccharomyces cerevisiae homologue of the largest subunit of mammalian translation initiation factor 3, is required for translational activity. J. Biol. Chem. 273, 21253-21260. [DOI] [PubMed] [Google Scholar]
- Vincent, O., Townley, R., Kuchin, S., and Carlson, M. (2001). Subcellular localization of the Snf1 kinase is regulated by specific beta subunits and a novel glucose signaling mechanism. Genes Dev. 15, 1104-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, W.A., Hawley, S.A., and Hardie, D.G. (1996). Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP: ATP ratio. Curr. Biol. 6, 1426-1434. [DOI] [PubMed] [Google Scholar]
- Yamochi, W., Tanaka, K., Nonaka, H., Maeda, A., Musha, T., and Takai, Y. (1994). Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol. 125, 1077-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzyuk, T., Foehr, M., and Amberg, D.C. (2002). The MEK kinase Ssk2p promotes actin cytoskeleton recovery after osmotic stress. Mol. Biol. Cell 13, 2869-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]