Abstract
The concept of neuroinflammation has evolved over the past two decades from an initially controversial viewpoint to its present status as a generally accepted idea whose mechanisms and consequences are still actively under research and debate, particularly with regard to Alzheimer’s disease (AD). This review summarizes the current status of neuroinflammation research as it specifically relates to AD. Neuroinflammation is discussed mechanistically with emphasis on the role of redox signal transduction linked to the activation of central nervous system-relevant innate immune pathways. Redox signaling is presented both as a causal factor and a consequence of sustained neuroinflammation. Functional relationships are discussed that connect distinct neuroinflammatory components such as cytokines, eicosanoids, classic AD pathology (amyloid plaques and neurofibrillary tangles), and the recently emergent notion of “damage-associated molecular patterns”. The interaction of these paracrine factors likely can produce positive as well as negative effects on the AD brain, ranging from plaque clearance by microglia in the short term to glial dysfunction and neuronal compromise if the neuroinflammation is chronically sustained and unmitigated. Recent disappointments in AD clinical trials of anti-inflammatory drugs are discussed with reference to possible explanations and potential avenues for future pharmacological approaches to the disease.
Keywords: Alzheimer’s disease, cytokines, damage-associated molecular patterns (DAMPs), eicosanoids, neuroinflammation, redox signaling
INTRODUCTION: A BRIEF HISTORY OF NEUROINFLAMMATION IN ALZHEIMER’S DISEASE RESEARCH
Prior to the early 1990s, prevailing scientific dog-ma held that the brain was immunologically privileged by virtue of the blood-brain barrier (BBB) impeding passage of immune cells and humoral factors; and by an inherent inability of brain cells to mount an innate immunological response. Over the past two decades, this dogma has been completely overturned in a major paradigm shift. The ascendency of neuroinflammation occurred through initial epidemiological observations, combined with direct observational evidence from postmortem brain, and more recently has been substantiated by experiments that show brain tissue has a complex and inherently flexible ability to alter its innate paracrine systems using autonomously-produced and regulated inflammatory molecules.
Pioneering epidemiology by McGeer, Rogers, and colleagues first noted that arthritis patients who chronically medicated their condition with non-steroidal anti-inflammatory drugs (NSAIDs) had approximately half the risk for developing Alzheimer’s disease (AD) than did the broader population [1–4]. Subsequent prospective population-based studies suggest that NSAID use may diminish AD risk by 80% if used for more than two years, amongst subjects > 55 years of age who were not demented prior to the observation period [5]. Currently there are more than twenty published epidemiological studies that suggest anti-inflammatory drugs, and NSAIDs specifically, may protect against AD [4]. Unfortunately, several clinical trials designed to slow progression of AD through medication of already-afflicted AD patients with either prednisone or cyclooxygenase-inhibiting NSAIDs have thus far failed to produce a clear benefit [6–12]. Most notably a moderately powered very recent study (127 subjects) of ibuprofen for mild-moderate AD found that the drug had no overall effect on slowing cognitive decline in general, in contrast to earlier epidemiological suggestions referenced above [1–4], though the possibility of effect was observed in 27 apolipoprotein E4 (ApoE4) carriers [12]. Thus the early epidemiological findings regarding NSAIDs are only now being considered in the context of clinical intervention, and results to date strongly suggest that more refinement of treatment strategy are needed with special attention to patient selection and intervention stage timing.
Concurrently during this early historical period of neuroinflammation research, basic science investigations were identifying microglia (brain-resident macrophage-typic cells) as proliferative brain cells recruited to AD-associated amyloid-β (Aβ) plaques [13, 14]. Histological and biochemical analyses have, over the years, convincingly demonstrated a myriad of classic immune molecules associated with AD brain parenchyma especially in and around AD-defining histological lesions, the senile plaques and neurofibrillary tangles (NFTs). The long and growing list of these molecules include complement components [4,15]; inflammatory cytokines, especially interleukin 1 [13,16, 17] and interleukin 6 [17–21]; macrophage colony-stimulating factor (M-CSF) [22]; transforming growth factor-α [23]; C-reactive protein (CRP) and S100β [13, 16,21]. In some studies, cytokine message has been found increased in AD brain, implying a local source for the cytokine [20,21].
Lipid paracrine signaling from arachidonate metabolites also may be relevant to AD neuroinflammation. Arachidonic acid is metabolized through either the cyclooxygenase (COX) pathway or the lipoxygenase pathway to yield paracrine substances called eicosanoids, which can be either prostaglandins (COX and downstream products) or leukotrienes (products of 5- or 12/15-lipoxygenase, LOX enzymes). The inflammatory eicosanoid prostaglandin E2, the production of which NSAIDs are designed to inhibit, was found 5-fold elevated in cerebrospinal fluid (CSF) from patients with clinical AD [24]. Although it has been less well-studied than the prostaglandin pathway, arachidonic acid lipoxygenation products can also be pro-inflammatory and exacerbate cytokine signaling (discussed below). The pro-inflammatory arachidonate 5-lipoxygenase (5LOX) generally increases with age in mammalian brain and has been found increased at the protein level in AD brain [25,26]. Inflammatory markers are inexorably associated with the production of potentially neurotoxic substances such as reactive oxygen and nitrogen species, whose elevated presence in AD brain have been amply evidenced [27–30].
Arguably, the inflammatory markers found in late-stage AD brain might represent peripheral immune incursions secondary to severe AD pathology and BBB disruption; however, current knowledge of glial cell biology and two decades of observational study of AD brain belay the need to invoke strong peripheral immune origins for inflammatory markers that are so readily observed in the AD brain. Aside from the fact that microglia proliferate endogenously in the brain, and are profligate sources of reactive oxygen species (ROS), cytokines, and eicosanoids, it is now clear that astrocytes and neurons are quite capable of locally synthesizing inflammatory cytokines and paracrine molecules. Astrocytes in particular synthesize high levels of PGE2, leukotrienes, cytokines, and chemokines which challenged with ROS, cytokine receptor ligands, or certain toxins [31–33]. Thus the brain is quite capable of initiating an innate and localized immunological response, such as seems to occur during the course of AD. At the same time that glia actively produce inflammatory factors, stressed astroglia suffer loss-of-functions of oxidative-sensitive enzyme activities and homeostatic behaviors, thus diminishing their ability to protect and nourish neurons, and to scavenge reactive paracrine factors [34].
Despite the general current consensus that neuroinflammation is a prominent feature of AD, important questions remain about its primacy and consequences. That is to say, is neuroinflammation a process that initiates or accelerates AD? Alternatively, might some aspects of neuroinflammation represent a defensive response whose biological purpose is to protect the damaged brain? Or is much of neuroinflammation an epiphenomenon with little role in determining AD onset or progression rate? These questions likely will not have simple declarative answers but are crucial to address in order to improve pharmacotherapy in AD. Emerging genetic and clinical research suggests that neuroinflammation is an early and continuous feature of AD with discrete components contributory to AD pathology that likely will prove amenable to pharmacological exploitation.
The purpose of this review, therefore, is to discuss the relationship between causes and consequences of neuroinflammation in the context of the AD brain, so as to challenge the linear notion that innate brain immune responses must either precede and cause damage or, alternatively, must result from prior, separate pathology. An argument is made that the aging mammalian brain exists in a meta-stable state that is predisposed to enter a pro-inflammatory “spiral” that propagates forward through cycles of cellular damage, neuroinflammation, and further cellular damage. The cyclical, positive-feedback nature of the neuroinflammatory process, it follows, would be more amenable to mitigation earlier in the disease (or prophylactically) than during its fulminant phase, thus suggesting that research efforts might best concentrate on ways to predict, diagnose, and medicate AD early in the process.
MECHANISMS DRIVING NEUROINFLAMMATION IN THE AGING BRAIN
Oxidative stress as a driver of neuroinflammation: redox signaling themes
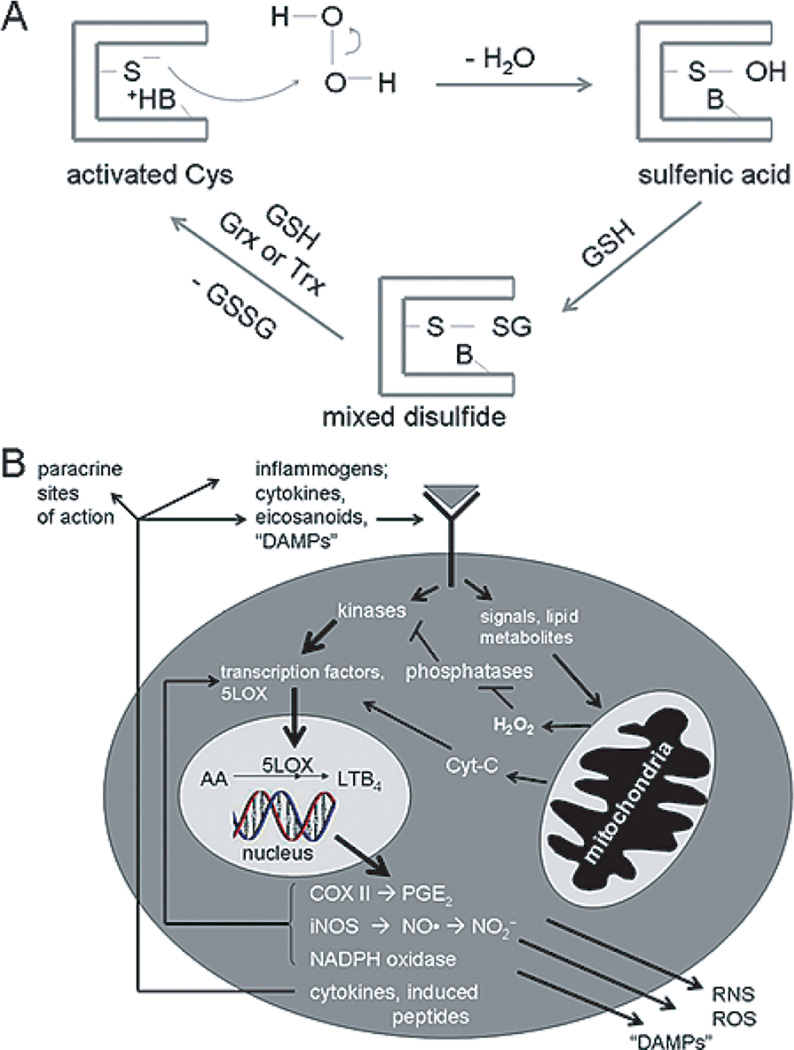
Oxidative stress arguably can be viewed both as a cause, and as a consequence, of neuroinflammation (Fig. 1). To consider oxidative stress as a causal or mechanistic factor in the onset and progression of a neuroinflammatory cycle, one need consider the role of ROS as signal transduction mediators or second messengers. This topic has been treated extensively in many reviews [33,35,36]. Briefly, diffusible oxidants including, notably, hydrogen peroxide (H2O2) and nitric oxide (NO) can be generated either intracellularly through receptor-triggered signal transduction processes or can diffuse into the cell from the outside. An example of the former, intracellular ROS generation is the tumor necrosis factor α (TNFα)-mediated production of intracellular ROS, which might occur through ceramide-triggered mitochondrial ROS leakage [37]. Cytokine-triggered ROS production through NADH oxidases (NOX) is another, ubiquitous mechanism often implicated for inflammogen-triggered intracellular ROS generation. In the case of NOX, ROS may be generated on the outside of the plasma membrane through a “phagocytic burst” type response, especially in cases of inflammogens-activated microglia. The NADPH oxidase catalyzes oxygen reduction to superoxide anion superoxide anion (), released either at the cell surface or within internal compartments where signaling components are localized (reviewed in [38]). Disproportionation of the superoxide yields H2O2 that diffuses across nearby plasma membranes. Nitric oxide can be similarly generated by astrocytes and microglia through the high-yield inducible nitric oxide synthase (iNOS) and in smaller transient bursts through neuronal nNOS isoforms, and then acts on targets either inside the NO-generating cell or upon other, nearby cells (reviewed in [39]).
Fig. 1.
A Representation of the glutathionylation cycle of protein regulation through glutathione conjugation, a principal means of redox signal transduction control at the level of post-translational modification. The protein modified by glutathionylation is typically (but not always) a phosphatase containing an especially nucleophilic cysteine. B) A broader view of the relationships amongst the phenomena of redox signal transduction, oxidative stress, and neuroinflammation. The hypothetical cell in this schematic is intended to be a microglial or astrocyte, which would be involved in reciprocal autocrine and paracrine signaling with ambient neurons and other glial cell types. The glutathionylation cycle depicted in panel A would be implicit in panel B, along with other types of reversible and irreversible protein post-translational modifications that mediate redox signal transduction mechanisms. In the context of AD specifically, perturbations in the intracellular signaling pathways would lead to aberrant phosphorylation, protein aggregation, and processing that could contribute ultimately to both NFTs and amyloid plaque deposition. These phenomena would superimpose upon and interact with the neuroinflammatory forces depicted explicitly in panel B.
ROS/reactive nitrogen species (RNS) act predominantly upon cellular thiols, and also in the case of NO, upon heme-containing redox sensors. Thiols react to form sulfenic acids (RSOH) or S-nitrosothiols (RSNO). Both these moieties are transient and undergo exchange with ambient cellular glutathione (GSH) to form mixed protein-SSG species [33,35] (Fig. 1A). Typically the reactive thiol is a catalytic or regulatory cysteine of a protein, such as a phosphatase, that co-regulates a signaling pathway. When oxidized or glutathionylated, the regulator is “off” thus allowing cognate kinase cascades to operate at much higher gain than otherwise would occur. The protein-SSG is recycled to its active state by reaction of a second GSH equivalent catalyzed by glutaredoxin (Grx) or in some cases thioredoxin (Trx) [33] (Fig. 1A). The above described “glutathionylation cycle” is one common theme in redox signaling but other mechanisms of redox signaling have been documented, including ROS/RNS reaction with heme cofactors [40] and directly with specific transcription factors [35].
The net result of ROS/RNS-mediated redox signaling in the neuroinflammatory context is typically an enhancement of downstream expression and/or activation for transcription factors controlling the expression of cytokines, chemokines, paracrine molecule metabolizing-enzymes [e.g., cyclooxygenase-2 (COX-II) and arachidonic acid 5-lipoxygenase (LOX)], and other ROS/RNS-generating enzymes (e.g., iNOS) (Fig. 1B) The function of ROS/RNS in cytokine elaboration has been amply documented. In cultured astrocytes, in fact, exogenously-supplied H2O2 directly leads to TNFα, IL1, and IL6 mRNA transcription virtually identical to the transcription elicited through application of authentic cytokine receptor ligands [33]. Mitochondrial poisons that increase intracellular ROS “leakage” create a very similar cytokine message up-regulation [33].
Furthermore, genetic alterations that predispose to neurodegenerative pathology have been shown to exacerbate astrocyte and microglia sensitivity. For example, mutations in cytosolic Cu, Zn-superoxide dismutase (SOD1) cause heritable forms of the motor neuron disease amyotrophic lateral sclerosis. Mutant SOD1-bearing astrocytes generate demonstrably more iNOS, NO, and cytokines in response to inflammogen challenge than do non-transgenic astrocytes [32] and similar findings have been reported for mutant SOD 1-bearing microglia [41]. It remains to be determined whether AD-associated genetic risk factors may render glia similarly hypersensitive to activation of redox-regulated, pro-inflammatory signal transduction.
The intimate and perhaps mutually causal relationship between neuroinflammatory signaling and oxidative stress might suggest that appropriate antioxidant strategies could also antagonize negative CNS immune reactions. This is a topic of much research and discussion, details of which are outside the scope of the present review, but has been treated in other recent reviews (e.g., [42]).
Autocrine and paracrine signaling networks: cytokines, eicosanoids and their interactions
Immune responses, by their very nature, need to “ramp up” rapidly in order to defend effectively against foreign incursions or rogue neoplastic events. Of course, after a (hopefully) successful defense, the immunologic response needs to abate. Evolution has designed the immune system with necessary checks and balances, but on balance the need for an aggressive positive immune response probably outweighs the potential evolutionary costs of a sluggish immune down-regulation. In terms of neuroinflammation, this general immune sentiment is reflected by the tendency of endogenous cytokine expression to beget further cytokine expression in a type of autocrine, feed-forward spiral. Such events are easily modeled in cell culture where treatment of astrocytes or microglia with small boluses of TNFα, IL1β, or IL6 produce a robust and accelerating transcription and translation of the same cytokine series [31–33]. Moreover small increments of the same cytokines interact synergistically (multiplicatively) to produce a disproportionate glial response especially in terms of ROS/RNS production [31,32].
While cytokines and inflammatory eicosanoids are often discussed in the context of glial cell biology, the relationship between the two paracrine systems is not so often investigated. It is likely that arachidonate metabolites likewise synergize with cytokines in propagating innate brain immune reactions. Cytokine treatment of rodent astrocytes or microglia can increase synthesis of both the cyclooxygenase product PGE2 and the leukotriene LTB4 [32], while direct application of either PGE2 or LTB4 sensitizes mouse astrocytes to TNFα-stimulated iNOS expression and NO production (Table 1). Such synergies could represent evolutionarily-favored mechanisms of accelerating local immune responses that may be poorly balanced by anti-inflammatory compensation mechanisms in the aging or diseased brain.
Table 1.
Interaction of prostaglandin E2 (PGE2) or leukotriene B4 (LTB4) with tumor necrosis factor α (TNFα) to increase nitrite production in the medium of primary mouse cortical astrocytes. Astrocytes isolated from brain cortices of mouse pups [31,32] were treated with the inflammogens and the medium assayed for by the Griess reaction 24 h later.
| Treatment | at 24 h |
|---|---|
| control | 2.66 ± 1.23 µM |
| 5 ng/mL TNFα | 6.13 ± 2.28 µM |
| 10 µM LTB4 | 0.14 ± 0.22 µM |
| 10 µM PGE2 | 1.54 ± 0.15 µM |
| TNFα + LTB4 | 11.12 ± 3.56 µM* |
| TNFα + PGE2 | 13.72 ± 0.61 µM* |
p < 0.05 relative to TNFα alone, by two-tailed T-test. Data indicate mean ± SD for a typical experiment with n = 4 culture wells/treatment group
Emerging genetic data suggest that polymorphisms in cytokine genes or genes controlling cytokine expression, may predispose for risk of AD, which would point to an early mechanism of incipient neuroinflammation in certain subsets of AD. Several studies have reported that homozygosity of polymorphisms in the ILIA gene (coding for ILlα), in a negative regulatory element upstream from the transcription start site, create a 2–3 fold increased for AD [43] which can be further exacerbated by polymorphisms in exon 5 of the ILIB gene (coding for IL1β) [44,45]. Similar AD risk-increasing polymorphisms have been reported associated with genes for TNFα [46,47], TGFβ [48], IL-6 [49], and MCP-1 amongst Italians [50]. Not all these associations have been rigorously confirmed and widely accepted, however, as some studies have failed to find AD associations with IL-1β, TNFα, and IL6 [51–54]. It is worth noting that AD is not uniquely associated with genetic variation in cytokine-encoding genes, as other neurode-generative diseases including Lewy body disease and Parkinson’s disease overlap clinically with AD [18], which complicates the elucidation of neuroinflammatory etiologies that are specific to AD.
More recently, genome-wide associations studies using very large populations have searched for single nucleotide polymorphisms (SNPs) associated with neurological diseases including AD. These approaches represent unbiased searches for statistically important variations in the human genome that may create relatively small, but highly prevalent, changes in risk for a particular affliction. In the largest AD-oriented GWAS study to date, using over 16,000 individuals a suggestive association of the gene for complement-receptor-1 (CR1) while an independent GWAS of late-onset AD reported a statistically significant association with CR1 [55,56]. These two studies also reported highly significant associations with genes whose products either bind amyloid peptide or regulate lipid and protein trafficking through cellular vesicles and synaptic exocytosis [55,56]. The GWAS approach is noteworthy in its implication of relatively few and specific biochemical pathways and may suggest a need to investigate neuroinflammatory mechanisms relating specifically to cytoskeletal restructuring and vesicle trafficking.
In summary, there have been numerous independent research projects using very different strategies to document inflammatory factors that are perturbed in AD or even altered inherent, at the genetic level, which would imply a more causal involvement with AD. To date this work suggests that neurinflammation in AD is likely to be genetically multifactorial, very dependent upon specific individual’s genetic lineage and lifetime history, and upon co-morbid factors including the degree of AD overlap with other neurological or vascular conditions. The common theme that different neuroinflammatory factors tend to synergize to drive the glial elaboration of other, different factors might suggest a situation where “all roads lead to neuroinflammation” once the process is initiated. This would complicate the genetic elucidation of common pathways to AD, as well as complicating the pharmacologic approach to AD, as discussed further below.
Alzheimer’s disease plaques and neurofibrillary tangles as drivers of neuroinflammation
AD is defined by clinical dementia, neuron and synapse loss, and the appearance of classic histopathology comprised of dense amyloid plaques and NFTs. Volumes have been composed discussing the possible toxic properties of plaque and tangle components, particularly plaque-associated Aβ peptide, so that these topics will not be treated in the present review. It is essential to note in this review that both plaques and tangles may represent nodes of origination for neuroinflammatory stress.
First, Aβ plaques are frequently associated with both reactive astrocytes and activated microglia [4,57] plus neuroinflammatory markers such as interleukin [58], TGFα [23], 5LOX [26], CRP [59,60] and complement [61]. Mitogen-activated protein kinase cascades such as the p38 module that largely regulate inflammatory gene expression are clearly activated in microglia and neurons in and around senile plaques [62]. The p38 MAP kinase module may be particularly interesting in eicosanoid metabolism because p38 directly phosphorylates 5LOX, prompting nuclear translocation and leukotriene production (reviewed in [63]). Amyloid plaques may serve as neuroinflammatory foci both passively, by “capture” and slow release of lipophilic or amphipathic paracrine molecules, and by more active means wherein Aβ activates ambient glial cells. Aβ has been well-documented to promote microglial activation through action on scavenger receptors, chemokine receptors, receptors for advanced glycation end products (RAGE) and possibly by other means [64—66]. Aβ-activated glia can produce ROS/RNS and Aβ itself possesses interesting redox properties through its capacity to promote metal-catalyzed redox cycling reactions and ROS production in certain circumstances (reviewed in [42,67]). ROS/RNS production, as discussed above, can be viewed both as a consequence and as a driving force in neuroinflammatory cycles. Thus, the Aβ peptide itself, or perhaps small oligomeric forms, is inherently neuro-inflammatory.
NFTs, composed of abnormally phosphorylated and polymerized cytoskeletal proteins, represent a prominent inclusion body in AD brain whose presence is retained in a histologically-recognized form after death of the encompassing cells [57]. As in the case of plaques, NFTs have been physically associated with inflammatory markers including CRP [60] and TGFβ [23]. Similar to the case for ROS/RNS, NFTs may be viewed as a toxic consequence of neuroinflammation as well as a driving force for the process. For instance, Neumann’s group has shown that tau accumulates in neurites of cultured neurons upon treatment with TNFα or co-culture with activated microglia, a process that seems to involve cytokine-stimulated ROS [68]. Recently, the MIF receptor CD74 has also been immunohistologically co-localized with NFTs [69], suggesting that tangle-bearing neurons might attract and/or capture activated microglia. Dysfunctional cytoskeletal tangles would be expected to compromise multiple aspects of neuron function to a degree possibly neurotoxic. Debris released during cell death and residual NFT “ghosts” could plausible trigger further immune activation, thus exacerbating neuroinflammatory cycles.
“Damage Associated Molecular Patterns” (DAMPs) in neuroinflammation
This last point is worth elaboration when considering the self-propagating nature of neuroinflammation. The immune system must constantly monitor the organism for signs of foreign incursion as well as rogue cancer cells. Glial cells appear to have particularly dangerous oncogenic potential, as evidenced by the extremely malignant nature of glioblastomas. In recent years it has become apparent that one of the strategies used by the mammalian immune system to sense a potential extrinsic or intrinsic threat is the recognition of “Damage Associated Molecular Patterns” or DAMPs (reviewed in [70,71]). DAMPs are molecules or systems of molecules released from dying pathogens, or from necrotic endogenous tissue (but not from apoptotic cells) that are recognized by the immune system as a signal for rapid response. Thus, DAMPs are a type of endogenous immune adjuvant substance [72]. The release and response to DAMPs occurs early in an adaptive response prior to, or during the classic antibody evolutionary phase [70], but represent a separate process by which immune cells prepare to handle the tissue challenge. DAMPs are classified as either pathogen-associated molecular patterns (PAMPs) or endogenous, paracrine-acting factors produced by the necrotic host cells (alarmins) [70]. Alarmins can be proteinacious or not, and probably include small metabolites such as urate and cellular breakdown products originating from membranes and nuclear material [70,72], but thus far have been defined to generally exclude more classical molecules like cytokines that are purposefully built by the cell for the dedicated purpose of intercellular signaling. Thus molecules such as amyloid peptides that have not been considered often as paracrine-acting immune molecules, gain such recognition under DAMP theory [71].
DAMP recognition probably occurs through multiple receptors that have been implicated in neuroinflammation such as toll-like receptors (TLRs), IL-1R, NOD-like receptors, formyl peptide and scavenger receptors, pentraxins, and RAGE [70–72]. It may prove to be the case that DAMP recognition is a type of pattern recognition that requires combinatoric activation of multiple receptor pathways so as to maximize organismal sensitivity while minimizing the risk of accidental activation. The DAMP concept is relatively new, having been formally defined around 2006 by several European groups during the EMBO Workshop on Innate Danger Signals and HMGB1 [70]. Thus the concept is still in the proving stages and nomenclature is being developed and integrated into the context of other immunological parlance, so it is subject to evolution with new discoveries and insights.
With respect to AD, the key point about DAMPs or alarmins is that the system may react inappropriately to the presence of persistent alarmin-type substances including cytokines, eicosanoid metabolites, and cellular detritus including amyloid peptides and NFTs [71]. What is intended as a host-defense and repair-coordinated response may be misdirected to diminish function and viability of ambient, healthy cells. Thus, the DAMP concept may prove somewhat synonymous with neuroinflammation but would more clearly place neuroinflammation within the totality of the mammalian immune system, i.e., the neuroinflammation may come to be viewed as a principal alarmin-driven branch of the innate immune system that is shared with extra-CNS tissue, but which lacks strong involvement of peripheral leukocytes, autoimmunity and classic humoral features especially in the early stages.
As one speculates about DAMPs, alarmins and the like, it naturally occurs to ask whether there are inherent molecular patterns that antagonize DAMPs, perhaps to signal a healthful tissue condition or else to turn off inflammatory reactions after resolution of a tissue injury. To our knowledge this concept, which might be called “anti-DAMPs” or perhaps SAMPs (Stasis-Associated Molecular Patterns), has not been explicitly described in the literature but has been hinted at, perhaps, by certain recent discoveries. Small ubiquitous molecules or molecules previously thought to serve specific neuro-transmitter functions now are being recognized for their unexpected immune-suppressive actions. For example, acetylcholine or nicotinic receptor antagonists reportedly diminish inflammogen-stimulated cytokine elaboration and iNOS expression and suppress microglial TNFα synthesis in cell culture, in vivo in LPS-induced models of neuroinflammation and in the experimental autoimmune encephalitis (EAE) model of multiple sclerosis [73–77]. This action apparently occurs through stimulation of α7-nicotinic acetylcholine receptors on microglia or T-cells [73,76,77]. The selective loss of cholinergic neurons in AD thus could reflect both a consequence of pathology but also an early causal contributor, if incremental decrease in acetylcholine neurotransmission removes a natural brake on neuroinflammation. In a similar vein, one might consider the general tendency of antioxidant molecules to decrease in conditions of inflammation and AD brain specifically (e.g. [30]) by asking whether the antioxidant/oxidant balance might represent another, conditional immune sensor ala “SAMPs” in the CNS. Such questions might be fruitful avenues for future research inquiry.
NEUROINFLAMMATION IS NOT ALL BAD: A CONTRARIAN VIEWPOINT, WITH CAVEATS FOR PHARMACEUTICAL DEVELOPMENT AND CLINICAL TRIALS
There is an easy bias to view neuroinflammation as an unqualified negative process that undermines tissue homeostasis and damages neurons, but such a bias is logically unfounded. The bias stems from the obvious ability of inflammatory reactions to kill cells. Destroying invading pathogens or rogue cells is the principal function of the immune system. Nonetheless, one must remember than most normal inflammatory reactions are designed to save the organism from an imminent danger and therefore mechanisms exist whereby inflammation is down-regulated and in fact, engenders a tissue repair response. In the context of AD, therefore, an objective consideration must take into account possible beneficial aspects of the neuroinflammation that is reported to occur in the aging or AD-challenged brain.
In fact, significant literature suggests that particular subsets of activated microglia may serve protective functions partially through the clearance of nascent amyloid plaques (reviewed in [78]). Emerging evidence suggests that microglia specifically bearing toll-like receptors (TLRs) efficiently phagocytose Aβ, whereas microglia negative for TLRs do not [79–81]. There is some debate as whether the TLR+ microglia are actually brain-derived or represent peripheral-derived macrophages [81,82]. If bone marrow-derived macrophages do infiltrate the aging human brain in significant relative numbers to native brain microglia that originate from embryonic mesoderm to populate the brain decades before AD onset, then the neuroinflammatory concept becomes yet more complex and will have to be reconsidered appropriately. The possible importance of TLRs in amyloid clearance has been underscored by findings that TLR induction via cytosine-guanosine-containing oligonucleotides can reduce cortical amyloid burden by more than 60% in amyloid plaque-bearing Tg2576 mice with corresponding improvement in cognitive performance [83].
A second point of logic argues for a microglial role in plaque clearance. Amyloid-β protein precursor (AβPP) transgenic mice engineered to produce Aβ plaques have proven to be valuable models in the field of AD vaccinology. Immunization of such mice with anti-amyloid antibodies induces plaque clearance [78, 80,81]. Intracranial injection of anti-Aβ appears to induce two phases of plaque clearance, an immediate phase and a latter phase which is dependent upon microglial activation [78]. Immunization of so-called “triple transgenic mice” bearing AβPP, presenilin, and tau mutations that create both plaques and tangles suggests that an immune reaction reduces both plaque and tangle burden in these animals, with associated improvement in cognitive performance [84,85]
Further insights from AβPPswe/PS1dE9 transgenic mice are offered by O’Banion and colleagues who engineered mice to produce sustained levels of IL1β in response to activation by Cre recombinase [86]. These mice were crossed with AβPP/PS1 mice to magnify the IL1-driven microglial activation and neuroinflammation. Interestingly, the IL1β overexpression resulted in decreased Aβ plaque burden one month after IL1β induction, in seven month-old mice [86]. The decreased plaque content was associated with increased association of phagocytic microglia in contact with amyloid deposits. This study was especially relevant because IL1β was induced rather than expressed constitutively from birth, ruling out developmental compensation artifacts; however, the study was of relatively short duration and did not assess the global neuropathological consequences of IL1β overexpression during the latter months of mouse lifetime during which cognitive deterioration would be most pronounced.
Similarly, very recent work by Chakrabarty and colleagues found that AβPP transgenic mice engineered to overexpress murine IL-6 (mIL6) exhibited suppressed net amyloid deposition concurrent with extensive gliosis and apparent amyloid phagocytosis by activated microglia [87]. Chakrabarty et al. did not sample the entire lifespan of transgenic mice but studied animals induced to express mIL-6 up to 5 months of age and from 4–6 months [87] leaving open questions about the consequences of IL6 expression during the latter half of the disease process. Also, neither the IL1β nor the IL6-driven experiment directly addressed aspects of possible collateral damage to ambient tissue consequent to the activation of phagocytic microglia. Nonetheless both studies suggest that short-term specific inflammatory cytokine elevation can beneficially affect amyloid deposition and clearance dynamics, early in disease.
In contrast to research pointing to either a positive or negative role for microglia in amyloid deposition, additional very recent data suggests that microglia may play a minimal role in amyloid dynamics, at least in the absence of additional inflammatory activation [88]. Heppner, Jucker, and colleagues crossed AβPP transgenic mouse strains with CD11b-HSVTK mice containing a herpes simplex thimidine kinase “suicide gene” in myeloid cells that can be induced to die upon ganciclovir (GCV) treatment, and found that neither amyloid plaque deposition nor clearance seemed to be affected by near complete microglial ablation, at least for a short window of several weeks. Neuronal dystrophy was observed even in microglial absence [88]. The Heppner and Jucker study could not assess consequences of HSVTK treatment beyond four weeks due to complications from thalamic microhemorrhages and did not consider consequences in aged animals specifically, however, the implication of this thorough and well-controlled study would be that microglia play little role in the initial murine amyloidopathy in the absence of microglia activating stimuli.
Of course, mice are not humans and there may be fundamental limitations to insights one can gain into human disease based solely on transgenic murine experiments. In recent years, however, actual human trials have been conducted with active immunization of AD-afflicted patients in the hope of generating a protective antibody response, and these studies may begin to corroborate murine observations that neuroinflammation counteracts amyloid plaque deposition partly through microglial phagocytosis. In a safety study initiated in 2000 involving 80 patients with mild to moderate AD, patients were immunized with full-length Aβ1−42 peptide plus adjuvant. This study found a robust anti-Aβ antibody response and no adverse reactions [89]. Unfortunately, a subsequent larger-scale efficacy study employing 372 subjects was halted when 6% of the patients developed menengioencephalitis [90,91]. Postmortem examination did reveal evidence that the active immunization induced some Aβ plaque clearance concomitant with electron microscopic evidence for microglial phagocytosis of Aβ [91–94]. The cause of encephalitis in this trial is uncertain, however, the presence of T lymphocytes in the leptomeninges of these patients near sites of cerebrovascular amyloid angiopathy (CAA) suggests a possible origin of the clinically undesirable events [78] and raises a caveat about extrapolating from animal models of amyloidopathy that generally do not display CAA to the extent that is found in natural human AD.
Activated microglia are not the only component of the neuroinflammatory phenomenon to display positive, as well as negative, activities towards neural tissue. For example TNFα is well-known for its neurotrophic as well as neurotoxic potential. TNF receptors include both a p75 neurotrophic receptor that promotes neuron survival when expressed alone, and a p55 receptor capable of initiating an extrinsic apoptotic program when co-expressed with p75 (reviewed in [95]). To complicate matters further, the dose of ambient cytokine and presence of other interacting paracrine factors may influence a spectrum from neurotrophic to neurotoxic potential. For instance, Li and colleagues find that NSC-34 motor neuron-like cells treated with low concentrations of activated BV1 microglia-conditioned medium display improved viability and neuritic extensivity, whereas higher concentrations of the same microglia-conditioned medium reduce neuron viability [96]. Thus caution is warranted in assuming that any indicator of neuroinflammation in the aging and diseased human brain is a causal factor in neurodegeneration, or is a potential target for safe and effective therapeutic inhibition, and microglial complexity in particular must be appreciated when considering conceptually novel pharmacological approaches.
CONCLUSIONS
Neuroinflammation as a concept is now substantiated by a broad and deep body of corroborating scientific evidence, but is still in its infancy as a scientific theory. As is often the case with scientific ideas, initial enthusiasm that early discoveries will lead quickly to transformative applications has proven overly optimistic. Neuroinflammation is coming to be recognized as a complex process that has both beneficial, salubrious aspects in maintenance of brain homeostasis and injury resolution; but also can be detrimental if sustained chronically, over years and decades, in which case the activated brain immune pathways can cause debilitation or death to otherwise healthy tissue collateral to sites of injury or histological insult (e.g., amyloid plaques and NFTs in the case of AD).
Despite early epidemiological evidence that chronic use of nonsteroidal anti-inflammatory drugs diminished the risk for AD, clinical intervention trials have thus far failed to slow disease progression or provide convincing evidence of symptomatic improvements in patients with early or moderate AD. Reasons for the clinical failure could be many, including considerations that apply to any clinical trial: There may be a therapeutic window early in the disease process where the intervention could benefit, but trials are initiated too late or for too short a duration; the drugs may not reach therapeutic concentrations at the correct sites of action within the bounds of safe and tolerable dosage regimens; there could be confounding off-target effects; etc. Additionally, one must consider the complex nature of neuroinflammation in AD, with special attention to findings that recognize some aspects of neuroinflammation as beneficial (for example, plaque clearance by activated microglia). Some anti-inflammatory approaches might benefit the brain through classic actions on prostaglandin synthesis, for instance, but produce a confounding effect by diminishing beneficial neuroinflammation.
It may prove the case that current anti-inflammatory drugs are too blunt, inadequate tools to address AD-specific neuroinflammation. Most NSAIDs were originally designed to block prostaglandin synthesis more or less through inhibition of cyclooxygenase, and modern selective COX inhibitors can block inducible COX-II dependent prostaglandin production. Brain cells and particularly astrocytes, however, produce considerable PGE2 in the basal (presumptively healthy) state and eicosanoid synthesis in neuroinflammation may be subtly moderated in a spatial manner through compartmentalization and/or local changes in enzyme activity. Additionally, the contribution of arachidonate 5LOX also may be significant but clinically underappreciated in AD, a feature that would be unaffected or possibly even agonized by COX inhibitors that might shunt arachidonate towards lipoxygenation pathways. Finally, the disappointments of recent clinical trials for NSAIDs in AD suggest that care should be taken to better identify subsets of patients who might respond more favorably to the intervention strategy. Towards this end, it may be necessary to develop better diagnostics relying upon peripheral inflammatory biomarkers to help guide patient selection, in addition to focusing on treatment of incipient rather than established neurodisease.
In conclusion, there remains compelling logic to pursue neuroinflammation as a pathway toward better understanding and ultimately interceding in the AD pathological process. More basic science research is needed in this area in order to identify key molecular targets that might be selectively and safely exploited for therapeutic benefit. Ultimately, clinical strategies might require combination therapies (cocktails) using agents that selectively modulate different, involved molecular pathways. Additionally, it may very well prove that AD can be slowed only if the disease is treated early or perhaps even prophylactically. These types of trials are difficult and expensive to conduct due to the need for recruiting large numbers of participants and following their clinical progress for years, in order to measure rates of AD incidence. For this reason, and the expense of the drugs themselves, research into dietary and lifestyle factors that influence AD risk might prove economically most advantageous to society as a whole.
Important key questions remain to be answered, that might move forward the concept of neuroinflammation pharmacotherapy in quantum fashion. Chief amongst these is the origin of the complex process in AD: What is proximal and causal, rather than consequential? This review has attempted to emphasize the complex topography of the neuroinflammatory landscape, which defies understanding through linear thinking. Focus of research upon the earliest events in AD, including particularly aspects of impaired redox signal transduction, presynaptic synapse function, or cholinergic neurotransmission, may prove particularly fruitful in understanding how minor genetic or environmentally-mediated physiological impairment could propagate into an AD phenotype through the process of detrimental neuroinflammation. Certainly, neuroinflammation remains a young discipline whose pursuit will yield substantial scientific knowledge and probably significant clinical advances in the coming years and decades.
ACKNOWLEDGMENTS
This work was supported in part by the Oklahoma Center for Advancement of Science and Technology; the National Institutes of Health (NS044154, AG023519-01); the Hereditary Disease Foundation; and the Jean and Judith Pape Adams Charitable Foundation.
Footnotes
The author’s disclosure is available online (http://www.j-alz.com/disclosures/view.php?id=280).
REFERENCES
- 1.McGeer PL, Rogers J, McGeer EG, Sibley J. Anti-inflammatory drugs and Alzheimer’s disease? Lancet. 1990;335:1037. doi: 10.1016/0140-6736(90)91101-f. [DOI] [PubMed] [Google Scholar]
- 2.Rogers J. The inflammatory response in Alzheimer’s disease. J Periodontol. 2008;79:1535–1543. doi: 10.1902/jop.2008.080171. [DOI] [PubMed] [Google Scholar]
- 3.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: Epidemiological, animal model and clinical studies. Neumbiol Aging. 2007;88:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 4.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: A review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 5.In’t Veld BA, Ruitenberg A, Hofman A, Launer LJ, vn Duijn CM, Stijnen T, Breteler MM, Strieker BH. Nonsteroidal anti-inflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 6.Rogers J, Kirby LC, Hempelman SR, Berry DL, McGeer PL, Kasniak AW, Zalinski J, Cofleld M, Mansukhani L, Willson P, et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology. 1993;43:1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- 7.Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease. Neurology. 1999;53:197–201. doi: 10.1212/wnl.53.1.197. [DOI] [PubMed] [Google Scholar]
- 8.Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, Weiner MF, Farlow MR, Sano M, Grundman M, Thal LJ. A randomized controlled trial of prednisone in Alzheimer’s disease. Neurology. 2000;54:588–593. doi: 10.1212/wnl.54.3.588. [DOI] [PubMed] [Google Scholar]
- 9.Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide in Alzheimer’s disease. Neurology. 2002;58:1050–1054. doi: 10.1212/wnl.58.7.1050. [DOI] [PubMed] [Google Scholar]
- 10.Aisen PS, Schafer A, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs. placebo on Alzheimer disease progression: A randomized controlled trial. JAMA. 2003;289:2819–2816. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 11.Wilcock GK, Black SE, Hendrix SB, Zavitz KH, Swabb EA, Laughlin MA. Tarenflurbil Phase II Study Investigators, Efficacy and safety of tarenflurbil in mild to moderate Alzheimer’s disease: A randomized phase II trial. Lancet Neurol. 2008;7:483–493. doi: 10.1016/S1474-4422(08)70090-5. [DOI] [PubMed] [Google Scholar]
- 12.Pasqualetti P, Bonomin C, Dal Forno G, Paulon L, Sinforiani E, Mara C, Zanetti O, Rossini PM. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s diease. Aging Clin Exp Res. 2009;21:101–110. doi: 10.1007/BF03325217. [DOI] [PubMed] [Google Scholar]
- 13.Griffin WST, Stanley LC, Ling C, MacLeod V, Perrot LJ, White CL, III, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: Relationship to the pathology of Alzheimer’s disease. Neumbiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 15.Rogers J, Coopers NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Ward P, Liebberburgh I. Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mrak RE, Sheng JG, Griffin WST. Glial cytokines in Alzheimer’s disease: Review and pathogenic implications. Human Pathol. 1995;26:816–823. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mrak E, Griffin WST. Potential inflammatory biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2005;8:369–375. doi: 10.3233/jad-2005-8406. [DOI] [PubMed] [Google Scholar]
- 18.Mrak RE, Griffin WST. Griffin, Common inflammatory mechanisms in Lewy Body Disease and Alzheimer Disease. J Neuropathol Exp Neural. 2007;66:683–686. doi: 10.1097/nen.0b013e31812503e1. [DOI] [PubMed] [Google Scholar]
- 19.Bauer J, Strauss S, Schreiter-Gasser U, Ganter U, Schlegel P, Witt I, Yolk B, Berger M. Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 1987;285:525–526. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- 20.Luterman JD, Haroutunian V, Yemul S, Ho L, Purohit D, Aisen PS, Mohs R, Pasinetti GM. Cytokine gene expression as a function of clinical progression of Alzheimer disease dementia. Arch Neurol. 2000;57:1153–1160. doi: 10.1001/archneur.57.8.1153. [DOI] [PubMed] [Google Scholar]
- 21.Wood JA, Wood PL, Ryan R, Graff-Radford NR, Pilapil C, Robitaille Y, Quiron R. Cytokine indices in Alzheimer’s temporal cortex: No changes in mature IL-1 beta or IL-1RA but increases in the associated acute phase proteins IL-6, alpha 2-macroglobulin and C-reactive protein. Brain Res. 1993;629:245–252. doi: 10.1016/0006-8993(93)91327-o. [DOI] [PubMed] [Google Scholar]
- 22.Du Yan S, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashisth T, Chen X, Godman GC, Stern D, Schmidt AM. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: A proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:5296–5301. doi: 10.1073/pnas.94.10.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Wai EA, Gomez-Pinilla F, Cotman CW. Transforming growth factor-beta 1 is in plaques in Alzheimer and Down pathologies. Neuroreport. 1993;4:69–72. doi: 10.1097/00001756-199301000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Montine TJ, Sidell KR, Crews BC, Markesbery WR, Marnett LJ, Roberts LJ, 2nd, Morrow JD. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53:1495–1498. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
- 25.Uz T, Pesold C, Longone P, Manev H. Aging-associated up-regulation of neuronal 5-lipoxygenase expression: Putative role in neuronal vulnerability. FASEB J. 1998;12:439–449. doi: 10.1096/fasebj.12.6.439. [DOI] [PubMed] [Google Scholar]
- 26.Ikonomovi MD, Abrahamson EE, Uz T, manev H, Dekosky ST. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer’s disease. J Histochem Cytochem. 2008;56:1065–1073. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reich EE, Markesbery WR, Roberts LJ, 2nd, Swift LL, Morrow JD, Montine TJ. Brain regional quantification of F-ring and D/E-ring isoprostanes and neuroprostanes in Alzheimer’s disease. Am J Pathol. 2001;158:293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aksenov MY, Aksenova MV, Butterfield DA, Geddes JR, Markesbery WR. Protein oxidation in the brain in Alzheimer’s diease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 29.Williamson KS, Gabbita SP, Mou S, West MS, Pye QN, Markesbery WR, Cooney RV, Grammas P, Reimann-Philipp U, Floyd RA, Hensley K. The nitration product 5-nitro-gamma tocopherol is increased in the Alzheimer brain. Nitric Oxide. 2002;6:221–227. doi: 10.1006/niox.2001.0399. [DOI] [PubMed] [Google Scholar]
- 30.Hensley K, Maidt ML, Yu ZQ, Sang H, Markesbery WR, Floyd RA. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J Neurosci. 1998;18:8126–8132. doi: 10.1523/JNEUROSCI.18-20-08126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hensley K, Fedynyshyn J, Ferre S, Floyd RA, Bordon B, Grammas P, Hamdheydari L, Mhatre MC, Mou S, Pye QN, Stewart CA, West MS, West S, Williamson KS. Message and protein level elevation of tumor necrosis factor (TNFα) and TNFα-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14:74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 32.Hensley K, Abd El-Moaty H, Hunter J, Mhatre MC, Mou S, Nguyen K, Potapova T, Pye QN, Qi M, rice H, Stewart CA, Stroukoff K, West MS. Primary glia expressing the G93A-SOD1 mutation present a neuroinfiammatory phenotype and provide a cellular system for studies of glial inflammation. J Neuminflam. 2006;3:2–10. doi: 10.1186/1742-2094-3-2. PMCID: PMC 1360663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabbita SP, Robinson KA, Stewart CA, Floyd RA, Hensley K. Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys. 2000;376:1–13. doi: 10.1006/abbi.1999.1685. [DOI] [PubMed] [Google Scholar]
- 34.Fuller S, Steele M, Imholz P, Munch G. Activated astroglia during chronic inflammation in Alzheimer’s disease-Do they neglect their neurosupportive roles? Mutat Res. 2010;10 doi: 10.1016/j.mrfmmm.2009.08.016. in press. [DOI] [PubMed] [Google Scholar]
- 35.Brandes N, Schmitt S, Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal. 2009;11:997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mieyall JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chern. 1997;272:11369–11388. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 38.Brown DI, Griendling KK. NOX proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calabrese V, Bates TE, Stella AM. NO synthase and NO-dependent signaling pathways in brain aging and neurode-generative disorders: The role of oxidant/antioxidant balance. Neumchem Res. 2000;25:1315–1341. doi: 10.1023/a:1007604414773. [DOI] [PubMed] [Google Scholar]
- 40.Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A. Nitric oxide-sensitive guanylyl cyclase: Structure and regulation. Neumchem Int. 2004;45:813–819. doi: 10.1016/j.neuint.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Weydt P, Yuen EC, Ransom BR, Möller T. Increased cytotoxic potential of microglia from ALS-transgenic mice. Glia. 2004;48:179–182. doi: 10.1002/glia.20062. [DOI] [PubMed] [Google Scholar]
- 42.Kamat CD, Gadal S, Mhatra M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: Preclinical promise and translational strategies. J Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du Y, Dodel RC, Eastwood BJ, Bales KR, Gao F, Lohmuller F, Muller U, Kurz A, Zimmer R, Evans RM, Hake A, Gasser T, Oertel WH, Griffin WS, Paul SM, Farlow MR. Association of an interleukin la polymorphism with Alzheimer’s disease. Neurology. 2000;55:480–483. doi: 10.1212/wnl.55.4.480. [DOI] [PubMed] [Google Scholar]
- 44.Grimaldi LM, Casadei VM, Ferri C, Veglia F, Licastro F, Annoni G, Biunno I, De Bellis G, Sorbi S, Mariani C, Canal N, Griffin WS, Franceschi M. Association of early-onset Alzheimer’s disease with an interleukin-1β gene polymorphism. Ann Neurol. 2000;47:361–365. [PubMed] [Google Scholar]
- 45.Nicoll JA, Mrak RE, graham DI, Stewart J, Wilcock G, Mac-Gowan S, Esiri MM, Murray LS, Dewar D, Love S, Moss T, Griffin WST. Association of interleukin-1 gene polymorphisms with Alzhemer’s disease. Ann Neurol. 2000;47:365–368. [PMC free article] [PubMed] [Google Scholar]
- 46.Collins JS, Perry RT, Watson B, Jr, Harrell LE, Acton RT, Blacker D, Albert MS, Tanzi RE. Association of a haplotype for tumor necrosis factor in siblings with late-onset Alzheimer’s disease: The NIMH Alzheimer Disease Genetics Initiative. Am J Med Genet. 2000;96:823–830. doi: 10.1002/1096-8628(20001204)96:6<823::aid-ajmg26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 47.Culpan D, MacGowan SH, Ford JM, Nicoll JA, Griffin WS, Dewar D, Cairns NJ, Hughs A, Kehoe PG, Wilcock GK. Tumor necrosis factor-alpha gene polyhnorphisms and Alzheimer’s disease. Neurosci Lett. 2003;350:61–65. doi: 10.1016/s0304-3940(03)00854-1. [DOI] [PubMed] [Google Scholar]
- 48.Luedecking EK, DeKosky ST, Mehdi H, Ganguli M, Kamboh MI. Analysis of genetic polymorphisms in the transforming growth factor-beta 1 gene and the risk of Alzheimer’s disease. Hum Genet. 2000;106:565–569. doi: 10.1007/s004390000313. [DOI] [PubMed] [Google Scholar]
- 49.Papassotiropoulos A, Bagli M, Jessen F, Bayer TA, Maier W, Rao ML, Heun R. A genetic variation of the inflammatory cytokine interleukin-6 delays the initial onset and reduces the risk for sporadic Alzheimer’s disease. Ann Neurol. 2000;47:365–368. doi: 10.1002/1531-8249(199905)45:5<666::aid-ana18>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 50.Pola R, Flex A, Gaetani E, Praia AS, Papaleo P, Giogio AD, Straface G, Pekorini G, Serricchio M, Pola P. Monocyte chemoattractant protein-1 (MCP-1) gene polymorphism and risk of Alzheimer’s disease in Italians. Exp Gerontol. 2004;39:1293–1298. doi: 10.1016/j.exger.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Bertram L, Blacker D, Crystal A, Muffin K, Keeney D, Jones J, Basu S, Yhu S, Guenette M, Mclnnis R, Go R, Tanzi R. Candidate genes showing no evidence for association or linkage with Alzheimer’s disease using family-based methodologies. Exp Gerontol. 2000;35:2353–2361. doi: 10.1016/s0531-5565(00)00193-5. [DOI] [PubMed] [Google Scholar]
- 52.Bhojak TJ, DeKosky M, Ganguli M, Kamboh MI. Genetic polymorphisms in the cathepsis D and interleukin-6 genes and the risk of Alzheimer’s disease. Neurosci Lett. 2000;288:21–24. doi: 10.1016/s0304-3940(00)01185-x. [DOI] [PubMed] [Google Scholar]
- 53.Li XG, Zhang JW, Zhang ZX, Chen D, Qu QM. Interleukin-1 gene cluster polymorphisms and risk of Alzheimer’s disease in Chinese Han population. J Neural Transm. 2004;111:1183–1190. doi: 10.1007/s00702-004-0148-5. [DOI] [PubMed] [Google Scholar]
- 54.Mocali A, Cedrola C, Delia Malva N, Bontempelli M, Mitidieri VA, Bavazzano A, Comolli R, Paoletti F, La Porta CA. Increased plasma levels of soluble CD40, together with the decrease of TGF beta 1, as possible differential markers of Alzheimer’s disease. Exp Gerontol. 2004;39:1555–1561. doi: 10.1016/j.exger.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nature Gen. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Frank A, Helisalmi S, Porcellini E, Harmon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez A, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanch H, Darrigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P The The European Alzheimer’s Disease Initiative Investigators. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 57.Markesbery WR. The neuropathology of dementing disorders. London: Edward Arnold (Publishers) Limited; 1998. [Google Scholar]
- 58.Sheng JG, Griffin WS, Royston MC, Mrak RE. Distribution of interleukin-1-immunoreactive microglia in cerebral cortical layers: Implications for neuritic plaque formation in Alzheimer’s disease. Neuropathol Appl Neurobiol. 1998;24:278–283. doi: 10.1046/j.1365-2990.1998.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwamoto N, Nishiyama E, Ohwada J, Arai H. Demonstration of CRP immunoreactivity in brains of Alzheimer’s disease: Immunohistochemical study using formic acid pre-treatment of tissue sections. Neurosci Lett. 1994;177:23–26. doi: 10.1016/0304-3940(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 60.Duong T, Nikolaeva M, Acton PJ. C-reactive protein-like immunoreactivity in the neurofibrillary tangles of Alzheimer’s disease. Brain Res. 1997;749:152–156. doi: 10.1016/s0006-8993(96)01359-5. [DOI] [PubMed] [Google Scholar]
- 61.Veerhuis R, Van Breemen MJ, Hoozemans JM, Morbin M, Ouladhadj J, Tagliavini F, Eikelenboom P. Amyloid beta plaque-associated proteins Clq and SAP enhance the Aβ (1–42) peptide-induced cytokme secretion by adult human microglia in vitro. Acta Neuropathol. 2003;105:135–144. doi: 10.1007/s00401-002-0624-7. [DOI] [PubMed] [Google Scholar]
- 62.Hensley K, Floyd RA, Zheng NY, Nael R, Robinson KA, Nguyen X, Pye QN, Stewart CA, Geddes J, Markesbery WR, Patel E, Johnson GV, Bing G. p38 kinase is activated in the Alzheimer’s disease brain. J Neurochem. 1999;72:2053–2058. doi: 10.1046/j.1471-4159.1999.0722053.x. [DOI] [PubMed] [Google Scholar]
- 63.Werz O. 5-lipoxygenase: Cellular biology and molecular pharmacology. Curr Drug Targets Inftamm Allerg. 2002:23–44. doi: 10.2174/1568010023344959. [DOI] [PubMed] [Google Scholar]
- 64.Lorton D, Schaller J, Lala A, De Nardin E. Chemotactic-like receptors and Aβ peptide induce responses in Alzheimer’s disease. Neurobiol Aging. 2000;21:463–473. doi: 10.1016/s0197-4580(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 65.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stem D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 66.El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adlard PA, bush AI. Metals and Alzheimer’s disease. J Alzheimers Dis. 2006;10:145–163. doi: 10.3233/jad-2006-102-303. [DOI] [PubMed] [Google Scholar]
- 68.Gorlovoy P, Larionov S, Pham TT, Neumann H. Accumulation of tau induced in neurites by microglial proinflammatory mediators. FASEB J. 2009;23:2502–2513. doi: 10.1096/fj.08-123877. [DOI] [PubMed] [Google Scholar]
- 69.Bryan KJ, Zhu Z, Harris PL, Perry G, Castellani RJ, Smith MA, Casadesus G. Expression of CD74 is increased in neurofibrillary tangles in Alzheimer’s disease. Mol Neurodegener. 2008;3:13. doi: 10.1186/1750-1326-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bianchi ME. DAMPs, PAMPs and alarmins: All we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 71.Salminen A, Ojaja J, Kauppinen A, Kaarniranta K, Suronen T. Inflammation in Alzheimer’s disease: Amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87:181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Andrews NA. Membrane repair and immunological danger. EMBO Reports. 2005;6:826–830. doi: 10.1038/sj.embor.7400505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brenner T, ANizri E, Irony-tur-Sinai M, Hamra-Amitay Y, Wirguin I. Acetylcholinesterase inhibitors and cholinergic modulation in Myasthenia Gravis and neuroinflammation. J Neuroimmunol. 2008;201–202:121–127. doi: 10.1016/j.jneuroim.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki T, Hide I, Matsubara A, Hama C, Harada K, Miyano K, Andra M, Matsubayashi H, Sakai N, Kohsaka S, Inouc K, Nakata Y. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res. 2006;83:1461–1470. doi: 10.1002/jnr.20850. [DOI] [PubMed] [Google Scholar]
- 75.Nizri E, Irony-Tur-Sinai M, Faranesh N, Lavon I, Lavi E, Weinstock M, Brenner T. Suppression of neuroinflammation and immunomodulation by the acetylcholinesterase inhibitor rivastigmine. J Neuroimmunol. 2008;203:12–22. doi: 10.1016/j.jneuroim.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 76.Tyagi E, Agrawal R, Nath C, Shukla R. Cholinergic protection via alpha 7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS induced neuroinflammation. Neurochem Int. 2010;56:135–142. doi: 10.1016/j.neuint.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by a7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 78.Boche D, Nicoll JAR. The role of the immune system in clearance of A? from the brain. Brain Pathol. 2008;18:267–278. doi: 10.1111/j.1750-3639.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signaling in Aβ uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clark K, Ugen KE, Gordon MN, Morgan D. Intracranially administered anti-Aβ antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. J Neurosci. 2003;23:3745–3751. doi: 10.1523/JNEUROSCI.23-09-03745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akiyama H, Mcgeer PL. Specificity of mechanisms for plaque removal after Aβ immunotherapthy for Alzheimer disease. Nat Med. 2004;10:117–118. doi: 10.1038/nm0204-117. [DOI] [PubMed] [Google Scholar]
- 82.Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- 83.Schultzova H, Kascsak RJ, Bates KA, Boutajangout A, Kerr DJ, Meeker HC, Mehta PD, Spinner DS, Wisniewski T. Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer’s disease-related pathology. J Neurosci. 2009;29:1846–1854. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble A? and tau but not soluble Aβ alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;381:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 86.Shaftel SS, Kyrkanides S, Olshowka JA, Miller J-NH, Johnson RE, O’Banion MK. Sustained hippocampal IL-1β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P. Massive gliosis induced by interleukin-6 suppresses Aβ deposition in vivo: Evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24:548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grathwohl SA, Kalin RE, Bolmon T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, Aguzzi A, Staufenbiel M, Mathews PM, Wolburg H, Heppner FL, Jucker M. Formation and maintenance of Alzheimer’s disease β-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12:1358–1260. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Evaluation of the safety and immunogenicity of synthetic Aβ42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 90.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jounny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningioencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 91.Ferrer I, Boada-Rovira M, Sanchez-guerra ML, Rey MJ, Costa-Jusa F. Neuropathology and pathogenesis of encephalitis following amyloid beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Aβ vaccination effects on plaque pathology in the absence of encephaliltis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 93.Nicoll JA, Wilkinson D, Holmes C, Steart P, markham H, Weller RO. Neuropathology of human Alzheimer’s disease after immunization with amyloid-beta peptide: A case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 94.Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinon D, Bayer A, Games D, Seubert P, Schenk D, Holmes C. Aβ species removal after Aβ42 immunization. J Neuropathol Exp Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 95.Kraft AD, McPherson CA, Harry GJ. Heterogeneity of microglia and TNF signaling as determinants for neuronal death or survival. Neurotox. 2009;30:785–793. doi: 10.1016/j.neuro.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li L, Lu J, Tay SS, Moochhala SM, He BP. The function of microglila, either neuroprotection or neurotoxicity, is determined by the equilibrium among factors released from activated microglia in vitro. Br Res. 2007;1159:8–17. doi: 10.1016/j.brainres.2007.04.066. [DOI] [PubMed] [Google Scholar]