Abstract
Auroras (A and B) are oncogenic serine/threonine kinases that play key roles in the mitotic phase of the eukaryotic cell cycle. Analysis of the Leukemia Lymphoma Molecular Profiling Project (LLMPP) database indicates Aurora over-expression correlates with poor prognosis. A tissue microarray (TMA) composed of 20 paired mantle cell lymphoma (MCL) patients demonstrated >75% of patients had high levels Aurora expression. Aurora A and B were also found elevated in 13 aggressive B-NHL cell lines. MLN8237, an Aurora inhibitor induced G2/M arrest with polyploidy and abrogated Aurora A and histone-H3 phosphorylation. MLN8237 inhibited aggressive B-NHL cell proliferation at an IC50 of 10-50 nM and induced apoptosis in a dose- and time-dependent manner. Low dose combinations of MLN8237 + docetaxel enhanced apoptosis by ∼3-4-fold in cell culture compared to single agents respectively. A mouse xenograft model of MCL demonstrated that MLN8237 (10 or 30 mg/kg) or docetaxel (10 mg/kg) alone had modest anti-tumor activity. However, MLN8237 plus docetaxel demonstrated a statistically significant tumor growth inhibition and enhanced survival compared to single agent therapy. Together, our results suggest that MLN8237 plus docetaxel may represent a novel therapeutic strategy that could be evaluated in early phase trials in relapsed/refractory aggressive B-cell NHL.
Keywords: non-Hodgkin lymphoma (NHL), Mantle cell lymphoma (MCL), Aurora A and B, Aurora Inhibitor, MLN8237, Docetaxel
1. Introduction
Aurora kinases are a family of mitotic serine/threonine (S/T) protein kinases that play fundamental roles in the eukaryotic cell division cycle [1, 2]. In Homo sapiens 3 Aurora homologs have been identified (A, B and C), all related to the prototypic molecule increase-inploidy 1 (Ipl1) of the yeast Saccharomyces cerevisiae [2]. They all share a highly conserved catalytic domain located in the carboxyl terminus of ∼300 amino acid residues with a small C-terminal extension, but their N-terminal extensions are of variable lengths with no sequence similarity. Aurora A localizes to centrosomes, functions in centrosome maturation and separation and proper mitotic spindle formation [3-6]. Disruption of Aurora A results in defects in centrosome maturation and separation, mitotic spindle formation and chromosome alignment [7-11] leading to aneuplody. Knockdown or pharmacologic inhibition of Aurora A in tumor cells delays mitotic entry and progression, resulting in G2/M cell cycle arrest [12-15]. Aurora B is a ‘chromosomal passenger protein’ which localizes to the centromere regions of chromosomes in the early stages of mitosis and guides chromosomal alignment on the bipolar spindle. Later in mitosis it re-localizes from the centromeres to the microtubules at the spindle equator and promotes the completion of cytokinesis [3, 16]. Aurora B is essential for chromosomal segregation. Inhibition of Aurora B prevents proper alignment of chromosomes on the spindle plate and also inhibits cytokinesis [17-20] and results in polyploidy. Aurora C similar to Aurora B is a chromosome passenger protein, appears to complement Aurora B functions and is expressed exclusively in reproductive organs [21].
All 3 Aurora homologs are strongly associated with human malignancy due to frequent over-expression and map to distinct regions of chromosomal loci known to be tumor associated-amplicons. This implies that Auroras play important roles in tumor initiation and/or progression in an appropriate genetic context. Both Aurora A and B are able to transform rodent cells that are p53 defective by overriding the p53-dependent G1 checkpoint leading to tumor formation in xenograft mice and attendant metastasis for Aurora B transformed cells [22-24]. In humans Aurora A and B are over-expressed simultaneously in numerous solid (breast, colorectal, pancreas, ovary, gastric, prostate) and hematological malignancies (acute myeloid leukemia) implicating a collaborative-cooperative mechanism for tumor progression [25]. Several groups have shown Auroras to be over-expressed by gene expression profiling (GEP) in aggressive B-and T-cell non-Hodgkin's lymphomas (NHL) such as diffuse large B-cell lymphoma (DLBCL) [26], MCL [27], peripheral T-cell lymphoma (not otherwise specified) (PTCL [NOS]) [28] and are thought to be a key component of the ‘proliferative’ gene signature in NHL.
Given the oncogenic amplification and transforming potential of Auroras, inhibiting their enzyme activity with small molecular inhibitors (SMI) to the catalytic domain ATP-binding site is regarded as feasible for targeted cancer therapy. Currently 12 Aurora SMIs (A-specific, B-specific, pan-Aurora with or without multi-targeting) are in early phase clinical trials [29, 30]. Solid tumor trials have shown Aurora inhibitors alone to have modest activity while in leukemia trials partial responses have been observed. Since treatment options are limited for a substantial number of patients with certain aggressive B-cell NHL subtypes (MCL, DLBCL, Burkitt's lymphoma [BL], transformed follicular lymphoma [TFL]) novel therapeutic evaluations are warranted. We demonstrate that Aurora A and B are highly over-expressed in MCL patients utilizing a tissue microarray (TMA) that are also markers of poor prognosis. Hence, we investigated MLN8237 (Millennium/Takeda Pharmacueticals), an orally active Aurora A SMI that is in early clinical development in aggressive B-cell NHL focusing on MCL. MLN8237 significantly inhibits cell proliferation and induces G2/M arrest with a polyploid phenotype. It also inhibits Aurora A auto-phosphorylation (Thr288) and histone-H3 (Ser10) phosphorylation and promotes apoptosis in aggressive B-NHL cell lines in a dose- and time-dependent manner. Dual targeting of mitosis with docetaxel (microtubule targeting agent, MTA) and MLN8237 to overcome the spindle assembly checkpoint (SAC) induces more apoptosis in cell culture and is an active combination in a MCL xenograft mouse model. The data provide compelling evidence that warrants early phase clinical trial evaluation of this combination in MCL.
2. Materials and Methods
2.1 Cells and reagents
DLBCL cell lines: TOLEDO, DB and RL; MCL cell lines: JEKO, REC-1, Granta-519, Granta-4 and Granta-22; transformed FL cell lines: SUDHL-4, SUDHL-6 and SUDHL-16; Burkitt's lymphoma cell lines: Raji, Rj2.2.5 used in this study were from Drs. S. Grant (Virginia Commonwealth University, VA) and C. Jordan (University of Rochester, NY) and maintained in RPMI 1640 medium (Mediatech, VA) supplemented with 10% fetal bovine serum, 2mM sodium pyruvate and 100units/ml penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. MLN8237 was kindly provided by Millennium Pharmaceuticals Inc (Cambridge, MA) and docetaxel as a kind donation by the Arizona Cancer Center Clinic. The compounds were dissolved at 5 mM in distilled water or DMSO as a stock solution, and then further diluted to desired concentrations for in vitro experiments. Nocodazole was purchased from Calbiochem (LaJolla, CA). Anti-Aurora A (ab1287) and anti-Aurora B (ab2254) antibodies were purchased from abcam (Cambridge, MA). Anti-phospho-Aurora A (Thr288) (C39D8), anti-phospho-Histone H3 (Ser10) (6G3), anti-Histone H3 and anti-GAPDH (14C10) antibodies were from Cell Signaling Technology (Danvers, MA). Anti-PARP (H-250) was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin antibody was from Sigma (St Louis, MO).
2.2 Normal B-cell isolation
Tonsils were received fresh from the operating room under sterile conditions (consented at surgery), and a mononuclear cell suspension was prepared. The sample was placed in a sterile petri dish with RPMI, adequate to fill approximately ¼ of the dish. The sample was disrupted with sterile blades to give a cell suspension with a slightly cloudy appearance to the RPMI. This RPMI-cell suspension was then placed in a conical centrifuge tube and centrifuged at 1800 rpm for 10 minutes. It was washed twice with normal saline under similar conditions and the supernatant resuspended in sterile RPMI to a volume of 2 ml. B-cells were purified from this suspension using human B cell enrichment kit (Catlog # 19054, StemCell Technologies Inc., Vancouver, Canada) according to the manufacture's procedure. Isolated B-cells were cultured for two days and then collected for Aurora A and B expression analysis.
2.3 Cell proliferation assay
Lymphoma cells were seeded at 8,000 per well in 96-well culture plates and allowed to grow for 24 hr followed by the desired treatment with increasing concentrations of the indicated agents for 4 days. Viable cell densities were determined using a CellTiter 96 Cell Proliferation Assay (Promega, Madison, WI). The studies were performed in triplicates × 4 and IC50 values were estimated by Calcusyn software (Biosoft, UK).
2.4 Apoptosis assay
Using Annexin V staining to detect apoptosis, treated cells were harvested and rinsed with cold PBS once. After centrifugation for 5 min, cells were resuspended in 500 μl of 1× Annexin V binding buffer (BioVision, Mountain View, CA, Annexin V-FITC Reagent Kit, Cat.#1001-1000) and then added 1μl of Annexin V-FITC and 1μl of Propidium Iodide (BioVision, Annexin V-FITC Reagent Kit). After incubation for 5 min at room temperature in the dark, the samples were analyzed by flow cytometry. All studies were performed in triplicate.
2.5 Cell cycle analysis
Cells were treated with 2 μM of MLN8237 for 72 hr and then the cells were centrifuged at 1,500 × g for 5 min at 4°C and resuspended in PBS, fixed by drop-wise addition of ice-cold ethanol (100%) to a final concentration of 70%, and incubated for 30 min on ice. Fixed cells were pelleted and treated with 100μl of RNase A (0.2 mg/ml in PBS) for 5 min at room temperature, then suspended in 1 ml ddH2O. After staining with 4 μg/ml propidium iodide, the DNA content was determined using a Becton Dickson flow cytometer and the cell cycle profile was analyzed by ModFit software. Cell aggregates were gated out of the analysis, based on the width of the propidium iodide fluorescence signal. Each profile was compiled from 10,000 gated events. All studies were performed in triplicate.
2.6 Immunoblotting
The cells were lysed in NP-40 lysis buffer containing 50 mM Tris.Cl (pH 7.4), 0.15 M NaCl, 0.5% NP-40, 1 mM DTT, 50 mM Sodium Fluoride, and 2 μl/ml Protease inhibitor cocktail (Sigma, St. Louis, MO). Protein concentrations were determined using the BioRad protein assay kit (Hercules, CA) and 50 μg of protein was resolved by electrophoresis on a 10% SDS-PAGE gel. The proteins were then transferred onto a nitrocellulose membrane and nonspecific binding was blocked by incubating with 5% nonfat milk in TBST buffer (0.01 M Tris-Cl, 0.15 M NaCl, 0.5% Tween-20, pH 8.0) at room temperature for 1 hr. The membrane was subjected to the indicated antibodies and the proteins were detected by a LI-COR Odyssey Infrared Imaging System.
2.7 Gene Expression Profiling Analysis
The Aurora Kinase A (gene AURKA) and B (gene AURKB) expression and patient survival data were taken from the Rosenwald et al data files [31] provided in the Leukemia/Lymphoma Molecular Profiling Project [LLMPP] database (http://llmpp.nih.gov/MCL). Diagnostic specimens were collected from 92 mantle cell lymphoma (MCL) patients [31]. Patients subsequently received multi-agent chemotherapy and were followed to assess treatment outcome. There were three probes for Aurora Kinase A and five probes for Aurora Kinase B. One of the Aurora kinase A probes was incorrectly annotated and was not used. The means of the log values for each probe were divided into 4 quartiles using the R (http://www.r-project.org) quartile function with default settings. Individuals with expression data missing were not used. The quartiles were used to produce Kaplan-Meier survival plots by means of the R survival package, using the survfit and survdiff functions with default settings.
2.8 Tissue microarray
Tissue microarrays (TMA) of 20 MCL (IRB approved protocol) were created by punching 1 mm cores (in duplicate) from representative areas of paraffin embedded blocks as identified by pathologist review of the corresponding hematoxylin and eosin stained sections. The cores were then embedded into a single recipient block and cut at 5 μ thickness for immunohistochemical stains. Normal LN and Tonsil were used as the controls. The staining was scored blindly by a pathologist and rated as 1+, 2+ and 3+.
2.9 Immunohistochemical analysis
Immunohistochemistry (IHC) was performed using Aurora A rabbit polyclonal antibody (Calibiochem, Gibbstown, NJ, cat#PC742) diluted 1:40, and Aurora B rabbit polyclonal antibody (Abcam, Cambridge, MA, cat# ab2254) diluted 1:40. Tissue sections were stained on a Discovery XT Automated Immunostainer (Ventana Medical Systems, Inc, Tucson, Arizona). All steps were performed on this instrument using VMSI validated reagents, including deparaffinization, cell conditioning (antigen retrieval with a borate-EDTA buffer), primary antibody staining, detection and amplification using a biotinylated-streptavidin-HRP and DAB (Diaminobenzidine) system and hematoxylin counterstaining. Aurora A and Aurora B were detected separately using a goat anti-Rabbit secondary antibody. Following staining on the instrument, slides were dehydrated through graded alcohols to xylene and cover slipped with mounting medium (Richard Allan, Logan, UT, Cat #4112).
2.10 Aurora A knockdown by small interference or hairpin RNA
STK6 (Aurora A) siRNA (AM51323) and control siRNA (AM4611) were purchased from Ambion (Austin, TX). ARK-1 (Aurora A) shRNA (h) lentiviral particles (sc-29731-V) and control shRNA lentiviral particles (sc-108080) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Transfection reagent (DharmaFECT3) was from Dharmacon, Inc. (Lafayette, CO). Cells were grown to 70% confluence and transfected by siRNA or infected by shRNA according to the manufacture's instruction. 96 hr later the cells were harvested for protein and cell cycle analysis.
2.11 Interactive docking studies
Crystal structures of Aurora A (pdb: 1OL5) [32] bound to ADP and Aurora B (pdb: 2VGO) [33] bound to a SMI were downloaded from PubMed (NIH) and viewed in Sybyl (Version 8.0, Tripos, St. Louis, MO). A crystal structure of Aurora A bound to a MLN8237 analog (MLN8054) (pdb: 2WTV) was also downloaded. MLN8237 generated utilizing chemdraw (Ultra 12.0), energy minimized and superimposed on the MLN8237 analog and interactively docked into the active sites of the crystal structures of Aurora A and B and compared to MLN8237 analog bound to Aurora A. Interactive docking scores are based on hydrogen bonding, van der Waals and hydrophobic interactions provided by Sybyl software package SurfDock which were calculated and compared.
2.12 MCL SCID mouse xenograft model
Animal care and treatment were performed at Arizona Cancer Center's experimental mouse shared services (EMSS) core facility. SCID mice were injected with 1×107 Granta519 MCL cells subcutaneously into the right hind flank. When tumors reached a volume of ∼100 mm3, animals were divided randomly (pair-matched) into 6 test groups with 12 mice per cohort: control group (Saline), MLN8237 (10 mg/kg oral [PO] QD × 3 weeks) group, MLN8237 (30 mg/kg PO QD × 3 weeks) group, docetaxel (10 mg/kg intra-peritoneal [IP] Q1W × 4) group, MLN8237 (10 mg/kg PO QD × 3 weeks) + docetaxel (10 mg/kg IP Q1W × 4) group and MLN8237 (30 mg/kg PO QD × 3 weeks) + docetaxel (10 mg/kg IP Q1W × 4) group. The length (L) and width (W) of the subcutaneous tumors were measured by calipers and the tumor volume (TV) was calculated as: TV = (L×W2)/2. Mice were sacrificed at the end of treatment (3 mice per cohort), end of study or if they reached >2500 mm3 at any time during the study. Excised tumors (end of treatment or study) were either fixed in paraffin or snap frozen for IHC analysis.
2.13 Statistical Analysis
Statistical analysis of the mouse xenograft model data was performed by estimating the tumor growth for each mouse by fitting the least squares regression line of the tumor volume by day. The cube root of the observed tumor volumes was used to induce linearity in the raw values. The slope of the regression line measures the tumor growth rate. Analysis of variance was used to test for the overall treatment effects on tumor growth inhibition. Tukey's studentized range test was used to assess the significance of pair-wise differences between the groups adjusted for multiple comparisons. Survival of the mice was measured from the date of pair matching to sacrifice (event) or end of study (censored). The Kaplan-Meier method was used to estimate survival. The log rank test was used to compare survival between the respective treatment groups. No statistical adjustments were made for multiple comparisons. Analysis was performed using Prism (Graphpad, La Jolla, CA). All p-values ≤0.05 were considered statistically significant.
3. Results
3.1 Correlation of increased Aurora A and B with poor prognosis in MCL
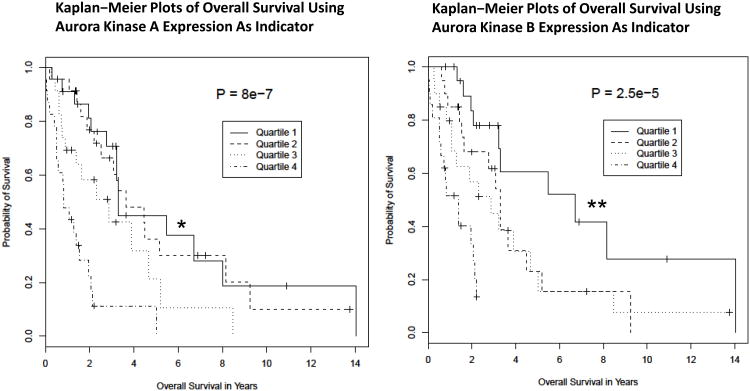
Previously, Rosenwald et al. 2003 [31], determined that an increased level of expression of a set of 22 cell proliferation genes was a predictor of reduced survival of a sample of 92 patients diagnosed with mantle cell lymphoma (MCL). This same data set was analyzed for the effect of expression of the AURKA (aurora kinase A) and AURKB (aurora kinase B) genes on patient survival (Figures 1A). The Aurora (A and B) genes were not included in the original proliferation analysis. There was enough variation in expression for each gene (12-fold for AURKA, and 11-fold for AURKB), that the patient values could be divided into 4 distinct quartiles of approximately equal patient numbers for analysis, with quartile 1 representing patients with the lowest expression values and quartile 4 representing the highest expression values for each gene. The resulting Kaplan-Meier survival curves revealed that, for both AURKA and AURKB, the patients with the highest expression levels (quartile 4) had a much reduced survival than those in quartile 1 with the lowest expression levels, with P-values of 2.5e-5 and 8.0e-7, respectively. The results indicate a significant and greatly increased risk associated with higher levels of expression of Aurora A and B kinases.
Figure 1. Aurora A and B are over-expressed poor prognosis markers in MCL.
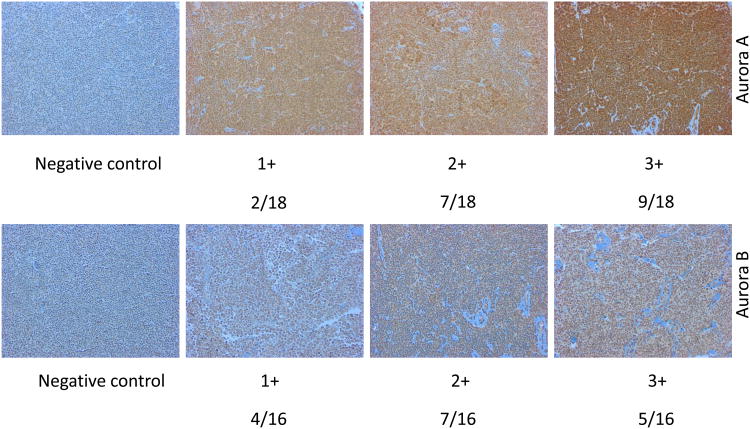
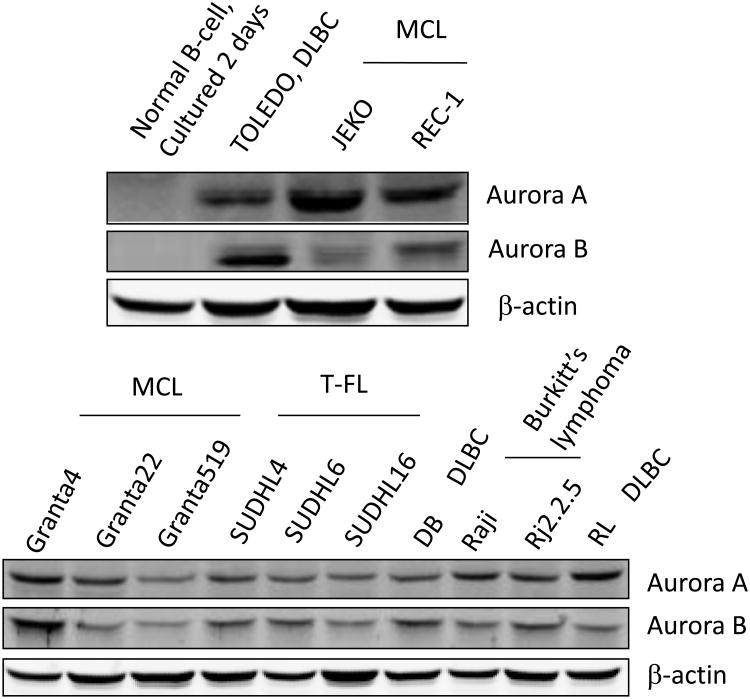
(A) Kaplan-Meier survival curves for patients with Mantle Cell Lymphoma based on the gene expression profile obtained from the LLMPP for Aurora kinase over-expressers (quartile 4) versus the lowest expressers (quartile 1) showing high expressers have a worse survival (months) for Aurora A (* p=8.0e-7) and B (** p=2.5e-5). (B) A representative TMA IHC staining demonstrating negative control, 1+, 2+ and 3+ staining for Aurora A and B (magnification 200× for all panels) by IHC. Positive Aurora (A or B) staining/total population evaluated is shown beneath each IHC panel. (C) Western blotting analysis demonstrated that Aurora A and B are over-expressed in a panel of aggressive B-cell NHL cell lines compared to the normal B-cells.
(Figure 1A). Therefore, over-expression of Auroras portends a marker of poor prognosis. A tissue microarray (TMA) of 20 paired MCL patient samples showed 2+ to 3+ staining for Aurora A in 89% of the patients and for Aurora B in 75% of the patients (Figure 1B) compared to normal/reactive lymph nodes. Several patients also showed 1+ staining of both Aurora A and B. Together LLMPP and TMA demonstrates over-expression Aurora A and B in MCL. The differential protein expression among 13 aggressive human B-cell NHL cell lines for Aurora A and B expression was established. Both Aurora A and B are over-expressed in a panel of B-NHL cell lines including DLBCL (n=3), MCL (n=5), Burkitt's lymphoma (n=2) and TFL (n=3) compared to normal tonsil B-cells in culture (Figure 1C). Therefore, over-expression of Aurora A and B may play a role in B-NHL proliferation by dysregulation of the cell cycle.
3.2 MLN8237 inhibits Aurora A and B kinase activity and promotes polyploidy
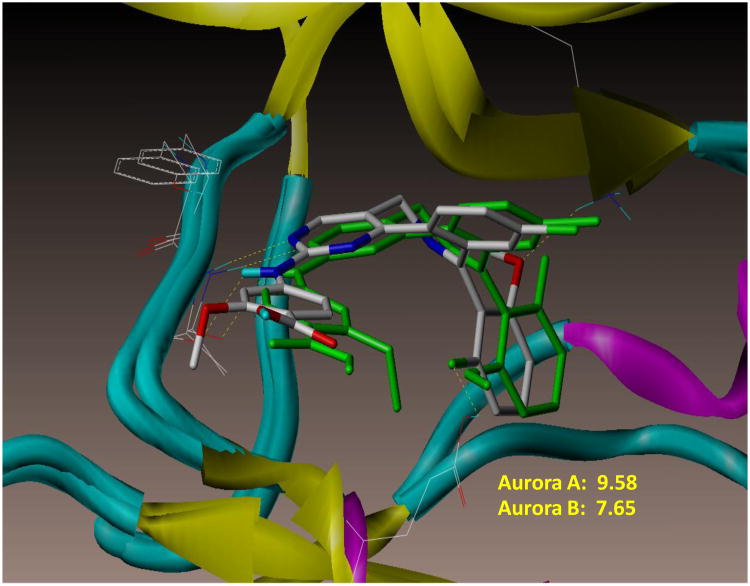
Several Aurora ATP-site SMIs of distinct chemotypes have been discovered (fragment based X-ray crystallography or high throughput screening) implicating the versatility of the ATP-binding site. Some are pan-Aurora inhibitors while others are Aurora A or B specific[34]. MLN8237 is more Aurora A than B specific by in vitro enzyme assays. In support of this conclusion, interactive docking of MLN8237 into the ATP-binding site of the crystal structures of Aurora A and B indicates a higher docking score (binding affinity) for Aurora A (9.58) than B (7.65), corroborating the in vitro enzyme activity data (Figure 2A). The mode of docking of MLN8237 into Aurora A and B although not identical is very similar such that at 0.5-1.0 μM concentrations achievable in mice and humans would occupy both active sites leading to inhibition of both enzymes.
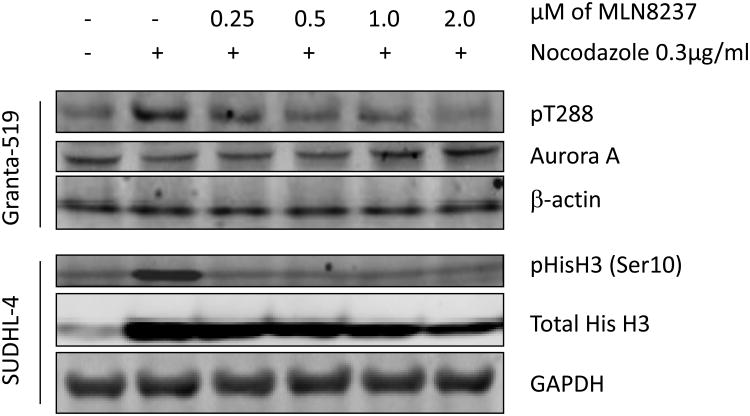
Figure 2. MLN8237 inhibits Aurora A and B kinase activity and promotes polyploidy.
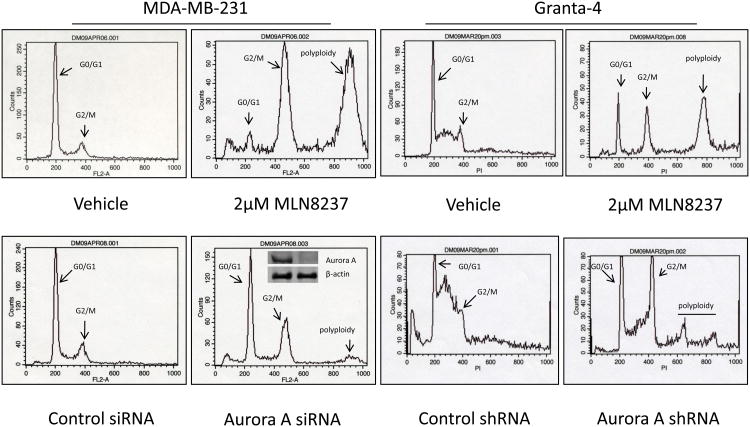
(A) Interactive docking studies of MLN8237 with the crystal structures of the active forms of Aurora A (color by atom type) and B (green) show binding mode and score that predicts for MLN8237 to interact with both enzyme active sites with Aurora A > B. (B) Granta-519 and SUDHL-4 cells were synchronized with nocodazole without or with various concentrations of MLN8237 for 16 h. pThr288, Aurora A, pHisH3 (Ser10) and HisH3 were analyzed by Western blotting. GAPDH and β-actin were used as loading controls. (C) MLN8237 treatment results in cellular phenotypes consistent with Aurora A deficiency. Top, DNA profiles of MDA-MB-231 and Granta-4 cells treated with vehicle or MLN8237 at 2 μM for 72 h and evaluated by flow cytometry. Bottom, MDA-MB-231 and Granta-4 cells were transfected or infected by Aurora A siRNA or shRNA lentiviral particles, respectively and DNA contents were analyzed by flow cytometry at 96 h. Inset: The levels of Aurora A expression was determined for control and Aurora A knock-down cells by Western blotting analysis.
Based on the interactive docking studies it was predicted that MLN8237 would inhibit both Aurora A and B activity. Aurora A kinase activity depends on auto-phosphorylation of Thr288 within the activation loop. Granta-519 MCL cells synchronized with nocodazole lead to increased Aurora A auto-phosphorylation on Thr-288 (pT288). Treatment of these cells with MLN8237 for 16 h at 0.25, 0.5, 1.0 and 2.0 μM leads to potent inhibition of Aurora A auto-phosphorylation on Thr288. Total Aurora A protein level was unchanged upon MLN8237 treatment, indicating that the decreased pT288 was due to inhibition of phosphorylation and not to Aurora A degradation or down-regulation (Figure 2B). Similar results were also demonstrated in RL and Granta-4 cell lines (data not shown). The structurally related Aurora B kinase activity was also evaluated in SUDHL-4 cells for detection of phospho-Histone H3 (pHisH3) on Ser10, an Aurora B-specific substrate [3]. As predicted, MLN8237 also inhibited HisH3 phosphorylation without affecting Aurora B protein levels (Figure 2B). Therefore, MLN8237 at 0.25 μM to 2 μM shows inhibition of both Aurora A (pThr288) and B (pHisH3 Ser10) activity and this observation corroborates well with the docking studies (Figure 2A).
Pharmacologic inhibition of Auroras (A and B) with ATP-site SMIs or siRNA knockdown leads to G2/M arrest and induction of a polyploid phenotype has been reported for solid malignancies [12]. The effect of MLN8237 on the cell cycle was examined by evaluating DNA content using flow cytometry (Figure 2C). Treatment of the human breast cancer cell line MDA-MB-231 which over-expresses Aurora A as a positive control and Granta-4 MCL cell line with 2 μM MLN8237 for 72 h dramatically increased 4N and 8N cells relative to untreated cells. Knockdown of Aurora A by siRNA or shRNA in both cell lines also resulted in an increased 4N and 8N cell population compared to control siRNA or shRNA (Figure 2C). Similar results were also obtained with Granta-519, RL and SUDHL-4 B-NHL cell lines (data not shown). This implicates that a lack of enzyme activity either by pharmacologic inhibition or lack of protein leads to G2/M arrest and a polyploid phenotype. Therefore shRNA knockdown of Aurora A or treatment with MLN8237 in Granta 4 cells leads to G2/M arrest, endo-reduplication and results in tetraploid and polyploid states.
3.3 MLN8237 inhibits aggressive B-NHL cell viability and induces apoptosis
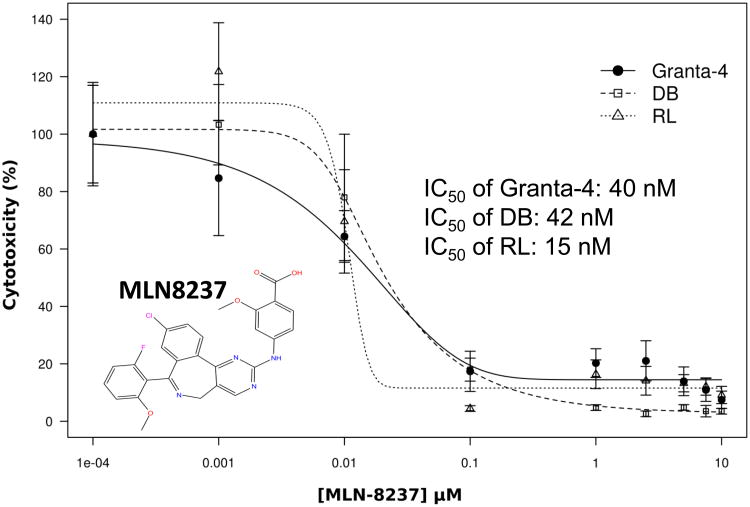
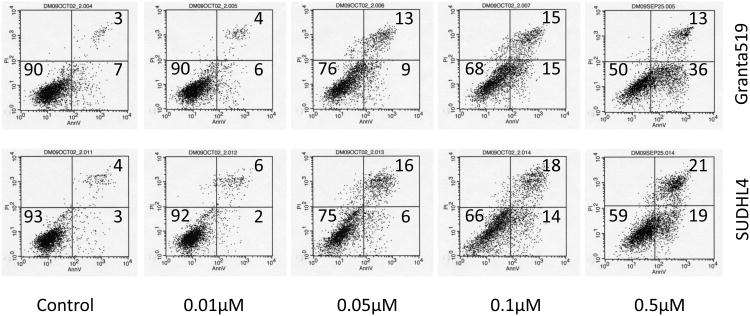
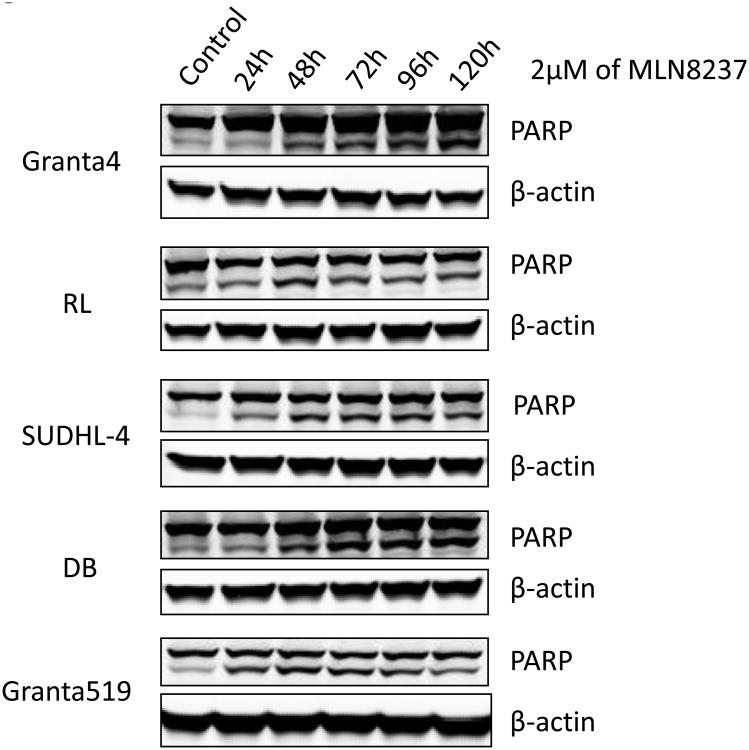
MTS cell viability assays with 3 different aggressive B-NHL cell lines (Granta-4, DB, RL) indicates IC50 of ∼10-50 nM for MLN8237 (Figure 3A) consistent with in vitro enzyme assays [35]. It has been previously shown that inhibition of Auroras leads to apoptosis in cell culture models [36]. Flow cytometry assays following Annexin V and PI staining were utilized to examine apoptosis in different aggressive B-NHL cell lines (Granta-519, SUDHL-4, Granta-4, DB, RL) treated with MLN8237. As expected, MLN8237 induced apoptosis in a dose-dependent manner (Figure 3B, shows 2 representative B-NHL cell lines). These results were confirmed by increased cleaved PARP in treated cells in a time-dependent (24, 48, 72, 96, 120 hr) manner with 2 μM MLN8237 (Fihure 3C). Hence, together the data demonstrate that Aurora inhibition with MLN8237 leads to anti-proliferation, polyploidy by endo-reduplication and subsequent initiation and progression of apoptosis.
Figure 3. MLN8237 inhibits aggressive B-NHL cell viability and induces apoptosis.
(A) Granta-4, DB and RL cells were exposed to varying concentrations of MLN8237 for 7 days. Cell viability was assessed by MTS analysis. Points are the means of triplicate determinations ± SD. Inset: IC50 values of MNL8237 in Granta-4, DB and RL cells. (B) Granta-519 and SUDHL-4 cells were treated with vehicle (control) or MLN8237 at various concentrations for 72h. Apoptosis was detected by flow cytometric analysis based on propidium iodide (Y-axis) and annexin V staining (X-axis). Percentages of survival (the lower left quadrant) and apoptotic cells (the lower and upper right quadrants) are indicated. (C) Granta-4, Granta-519, SUDHL-4, RL and DB cells were treated with MLN8237 at 2 μM for 24 h, 48 h, 72 h, 96 h and 120 h. Apoptosis was evaluated with PARP cleavage utilizing Western blotting analysis.
3.4 MLN8237 plus docetaxel abrogates mitotic delay and enhances apoptosis
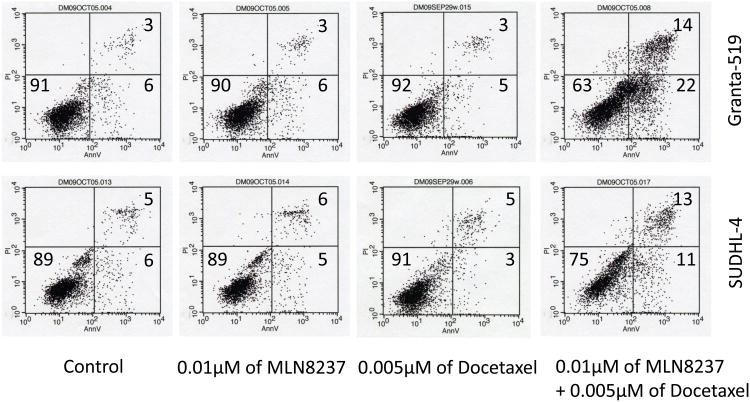
Studies have shown that Aurora A amplification overrides the spindle assembly checkpoint (SAC) which induces paclitaxel resistance [37]. Further, inhibition of Aurora A abrogates the mitotic delay induced by paclitaxel [38]. Aurora kinase inhibitors in combination with paclitaxel or docetaxel show synergy in vitro cell culture models of apoptosis and in vivo anti-tumor activity[39]. Here we treated Granta-519 and SUDHL-4 cells with MLN8237 (10 nM) plus docetaxel (5 nM) and individual single agent at same dose. The apoptotic fraction was enhanced by ∼3-4 fold with the combination compared to single agent treatment (Figure 4). The data indicate that MLN8237 could potentiate anti-tumor growth of docetaxol in aggressive B-cell NHL subtypes.
Figure 4. MLN8237 plus docetaxel abrogates mitotic delay and enhances apoptosis.
Granta-519 and SUDHL-4 cells were treated with a low dose of MLN8237 (10 nM) or docetaxel (5 nM) alone or in combination for 72h. Apoptosis was analyzed by flow cytometry after annexin V and PI staining which showed a ∼3-4-fold higher apoptotic fraction with combination therapy compared to single agent therapy.
3.5 MLN8237 plus docetaxol inhibits tumor growth and increases survival in a MCL xenograft mouse model
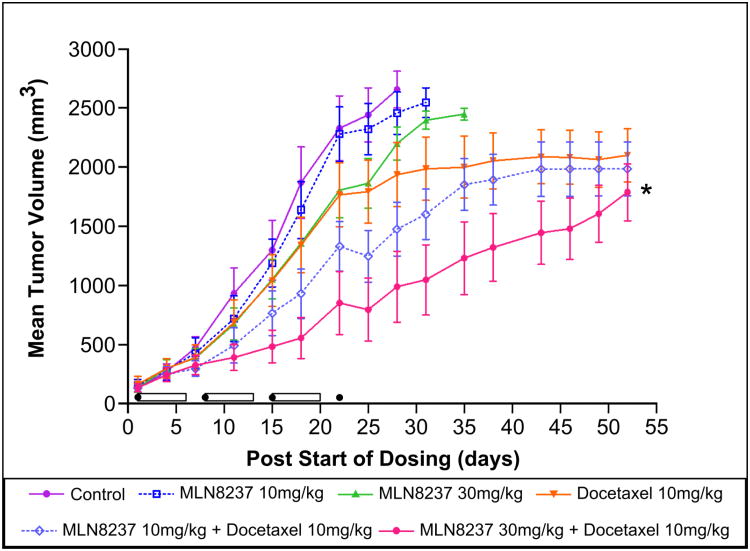
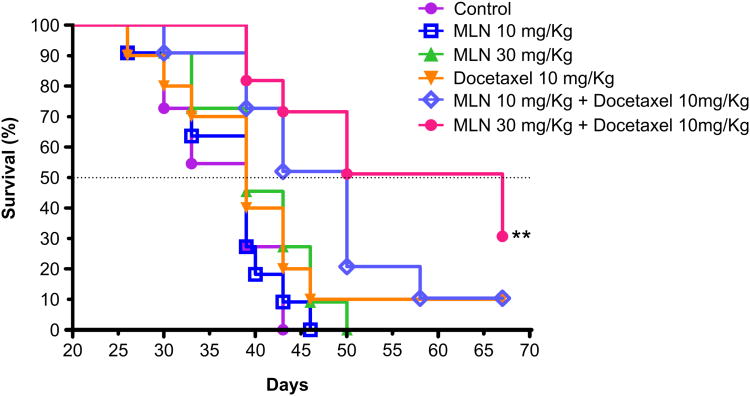
Based on our in vitro data that targeting Aurora A in combination with a microtubule targeting agent (docetaxel) was more effective in inducing apoptosis, we evaluated this combination in a SCID mouse xenograft model of MCL (Granta-519). There were 6 cohorts of 12 mice: vehicle control, MLN8237 at 10 mg/kg and 30 mg/kg PO once a day for 3 weeks, docetaxol at 10 mg/kg IP once/week × 4, MLN8237 at 10 mg/kg or 30 mg/kg for 3 weeks + docetaxel 10 mg/kg once/week × 4. MLN8237 doses were chosen based on prior dose finding studies provided by Millennium Pharmaceuticals, while docetaxel dose was based on a clinically relevant dose in mouse xenograft tumor model. Individual treatments by MLN8237 or Docetaxel had no significant anti-tumor activity. However, MLN8237 (10 mg/kg) + docetaxel showed significant tumor growth inhibition (TGI) compared to control (p < 0.05), and MLN8237 (30 mg/kg) + docetaxel demonstrated significant TGI compared to control (p < 0.001), MLN8237 (10 mg/kg, p < 0.001; 30 mg/kg, p < 0.01) and Docetaxel (p < 0.05) (Figure 5A). The body weights of all mice in all cohorts did not change significantly (within 10%) during the study and mice appeared to tolerate treatment(s) well. Kaplan-Meier analysis of overall survival showed that mice treated with MLN8237 30 mg/kg + docetaxel 10 mg/kg survived the longest (median 28 days) followed by those treated with MLN8237 10 mg/kg + docetaxel 10 mg/kg survived (median 11 days) over vehicle control (p=0.0003 and p=0.0043, respectively) (Figure 5B). However, mice in the single doses of MLN8237 and docetaxel had no superior survival over vehicle (p>0.05). Additionally, both combination treatments with docetaxel significantly increased survival over single agent treatments using MLN8237 (10 mg/kg and 30 mg/kg), and high MLN8237 + docetaxel combination treatment increased survival over single agent treatment with docetaxel (median 28 days more, p=0.0192). Survival of >20 days with treatment(s) in mouse xenograft models generally predict for higher responses and survival in human clinical trials [40].
Figure 5. MLN8237 plus docetaxol inhibits tumor growth in a MCL xenograft mouse model and increases survival.
(A). A MCL Granta-519 xenograft mouse model (n=12 per cohort) shows significant TGI in mice treated with MLN8237 plus docetaxel in a dose-dependent manner in comparison to control (* p < 0.001), MLN8237 or docetaxel alone. The cylinders represent duration of therapy with MLN8237 while the black sphere represents treatment with docetaxel. (B). A MCL Granta-519 xenograft mouse model showed significant overall survival difference with MLN8237 plus docetaxel in comparison to control (** p=0.0003), MLN8237 or docetaxel alone.
4. Discussion
Novel therapies and combinations based on mechanism of action studies provide a rationale for designing effective therapies for subtypes of aggressive B-cell non-Hodgkin's lymphomas that are not curable with current therapies. Here we present data that provide a rationale for targeting Auroras (mitotic S/T kinases) in aggressive B-cell NHL and focus on MCL a challenging subtype that is in need of novel effective therapeutic options. MCL is a B-cell neoplasm composed of monomorphic small-to-medium sized lymphocytic cells with irregular nuclear contours diagnosed by flow cytometry (CD5+/CD23-) and cyclin D1 translocation detected by FISH [t(11;14) ] and IHC for over-expression of cyclin D1. Gene expression profiling of MCL by the LLMPP showed a proliferation gene expression signature (22 genes) that implies dysregulation of the cell cycle [31] as a major defect driving tumorigenesis and suggests that cell cycle inhibitors may change the natural history of the disease.
Since Aurora A and B are strongly associated with mitosis and cell proliferation, the association of increased expression of these genes with reduced survival may be due to their role in more rapid tumor cell growth in MCL and correlates well with decreased survival in MCL (Figure 1A).
A tissue microarray of 20 MCL patients showed both Auroras to be over-expressed in a majority of patients (Figure 1B) compared to normal or reactive lymph nodes. Given that both Aurora A and B are transforming genes within an abnormal genetic background data support the conclusion that both Auroras are factors of poor prognosis and are potential targets for aggressive B-NHL therapy. All of the 13 aggressive B-cell NHL cell lines evaluated showed increased Aurora A and B expression compared to normal B-cells isolated from tonsil (Figure 1C) implicating an oncogenic role for Auroras in lymphomas.
The absence (knockdown) or over-expression of Aurora A or B leads to tetraploid phenotypes with different cellular consequences [41]. Absence or lack of Aurora A is not well tolerated by cells, while a lack of Aurora B is better tolerated. However, over-expression of Aurora A leads to transformation (overrides the SAC), while over-expression of Aurora B (SAC activation compromised) leads to metastasis [41]. Small hairpin RNA knockdown of Aurora A elicited a smaller population of cells with 8N DNA content (Figure 2C). However, treatment with MLN8237 at 2μM resulted in a substantially larger population of 8N cells (Figure 2C). The 8N phenotype is observed with Aurora B inhibition. The data do suggest that MLN8237 inhibits both Auroras, as demonstrated by interactive docking (Figure 2A), pThr288 (Aurora A) and pHisH3 Ser10 inhibition (Aurora B) (Figure 2B). It is likely that at nM concentrations MLN8237 is Aurora A selective but at low μM doses achieved in mouse models and patients are likely to inhibit both Auroras.
Aurora A over-expression has been demonstrated to override the SAC and induce resistance to MTA- (e.g. paclitaxel) induced apoptosis. This raised the possibility that Aurora A over-expression could contribute to drug resistance in the setting of cancer chemotherapy [38]. Inhibition of Aurora A either with SMIs or siRNA synergizes with paclitaxel or docetaxel to induce apoptosis in colon, ovarian and head/neck squamous cell carcinoma cells in vitro [38, 39]. Moreover, combining Aurora A SMI SNS-314 with docetaxel at doses without significant inhibition of HCT116 tumor growth as single agents produced significant TGI in HCT116 xenografts [42]. In B-NHL cell lines our results corroborated these findings as shown in cell culture modeling where a low dose of MLN8237 plus docetaxel has ∼3-4-fold greater apoptosis than individual agents (Figure 4). It has been shown that activation of the SAC followed by its bypass or slippage can trigger a massive apoptotic response in cancer cells [37]. A recent study demonstrated that inhibition of Aurora A in paclitaxel- or nocodazole-treated cells induces mitotic slippage and massive apoptosis [38]. Therefore, combination therapy of MLN8237 and MTA (docetexol) in B-NHL was evaluated in a MCL mouse xenograft model.
We chose and conducted mouse xenograft studies with Granta-519 cells derived from a patient with blastoid MCL with several cell cycle abnormalities [43]. MLN8237 at 10 mg/kg and 30 mg/kg given orally daily for 3 weeks had a dose-response that was modest (Figure 5A) in comparison to a DLBCL mouse xenograft model. However, when MLN8237 was combined with once/week IP docetaxel there was a significant anti-lymphoma dose-dependent response that lead to a ∼28 day median overall survival benefit compared to control and single doses of MLN8237 and docetaxel (Figure 5B). These results predict higher responses for the combination in human clinical studies. Paclitaxel has been evaluated in relapsed/refractory B-NHL as continuous intravenous infusion over 3 h [200 mg/m2] [44], 24 h [175 mg/m2] [45], 96 h [140 mg/m2] [46] and 3 h [250 mg/m2] [47] with response rates of 17% to 50% that were deemed modest. Lower dose infusions of 100 mg/m2 Q3W [48], 80 mg/m2 Q1W [49] and 90 mg/m2 Q1W [50] produced response rates of 23% to 42%. Together the data support the interpretation that taxol, as a single agent is not effective in B-NHL and therefore has not been incorporated into combination therapy [51]. Resistance to paclitaxel in B-NHL therapy is unlikely due to increased MDR1/P-gp (ABC-cassette transporters) expression but most likely due to ineffective targeting of the cell cycle spindle check point as it leads to mitotic delay and escape from apoptosis. However, inhibition of Auroras abrogates taxol-induced mitotic delay and increased mitotic bypass or slippage leading to massive apoptosis.
The molecular and cellular mechanisms underpinning this approach have pharmacologic implications (e.g. duration of treatment, order of drugs) and are likely to play an important role in achieving therapeutic benefits for lymphoma patients.
Acknowledgments
We wish to thank the Lymphoma SPORE (1 P5O CA B080501A1) for funding this project. We also thank Dr. R. I. Fisher (PI, Lymphoma SPORE), E. Chase and A. Stejskal for encouragement and support and Dr. M. Berman for critical review of the manuscript. We also thank Drs. C. Spier, S.T. Wilkinson and D. Mount (Director, Bioinformatics, AZCC) for providing critical analysis of the histopathology and gene expression profiling of the LLMPP respectively. The mouse xenograft study was conducted by the AZCC experimental mouse shared services (EMSS), statistical analysis by Dr. S. R. Brown (AZCC, Biometry) and TMA/IHC was conducted by the tissue acquisition and cellular/molecular analysis (AZCC, TACMASS, Dr. R. Nagle).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 2.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 3.Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, et al. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002;22:874–85. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giet R, Prigent C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J Cell Sci. 1999;112(Pt 21):3591–601. doi: 10.1242/jcs.112.21.3591. [DOI] [PubMed] [Google Scholar]
- 5.Kufer TA, Nigg EA, Sillje HH. Regulation of Aurora-A kinase on the mitotic spindle. Chromosoma. 2003;112:159–63. doi: 10.1007/s00412-003-0265-1. [DOI] [PubMed] [Google Scholar]
- 6.Terada Y, Uetake Y, Kuriyama R. Interaction of Aurora-A and centrosomin at the microtubule-nucleating site in Drosophila and mammalian cells. J Cell Biol. 2003;162:757–63. doi: 10.1083/jcb.200305048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 8.Giet R, McLean D, Descamps S, Lee MJ, Raff JW, Prigent C, et al. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J Cell Biol. 2002;156:437–51. doi: 10.1083/jcb.200108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roghi C, Giet R, Uzbekov R, Morin N, Chartrain I, Le Guellec R, et al. The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J Cell Sci. 1998;111(Pt 5):557–72. doi: 10.1242/jcs.111.5.557. [DOI] [PubMed] [Google Scholar]
- 10.Hannak E, Kirkham M, Hyman AA, Oegema K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J Cell Biol. 2001;155:1109–16. doi: 10.1083/jcb.200108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, et al. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786–95. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- 12.Hata T, Furukawa T, Sunamura M, Egawa S, Motoi F, Ohmura N, et al. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res. 2005;65:2899–905. doi: 10.1158/0008-5472.CAN-04-3981. [DOI] [PubMed] [Google Scholar]
- 13.Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, et al. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–98. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- 14.Marumoto T, Hirota T, Morisaki T, Kunitoku N, Zhang D, Ichikawa Y, et al. Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells. 2002;7:1173–82. doi: 10.1046/j.1365-2443.2002.00592.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Ruderman JV. Aurora A, mitotic entry, and spindle bipolarity. Proc Natl Acad Sci U S A. 2006;103:5811–6. doi: 10.1073/pnas.0601425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–54. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 17.Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–94. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–41. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murata-Hori M, Wang YL. The kinase activity of aurora B is required for kinetochore-microtubule interactions during mitosis. Curr Biol. 2002;12:894–9. doi: 10.1016/s0960-9822(02)00848-5. [DOI] [PubMed] [Google Scholar]
- 20.Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–80. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasai K, Katayama H, Stenoien DL, Fujii S, Honda R, Kimura M, et al. Aurora-C kinase is a novel chromosomal passenger protein that can complement Aurora-B kinase function in mitotic cells. Cell Motil Cytoskeleton. 2004;59:249–63. doi: 10.1002/cm.20039. [DOI] [PubMed] [Google Scholar]
- 22.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–50. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–93. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–65. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortlock AA, Keen NJ, Jung FH, Heron NM, Foote KM, Wilkinson RW, et al. Progress in the development of selective inhibitors of aurora kinases. Curr Top Med Chem. 2005;5:807–21. doi: 10.2174/1568026054637719. [DOI] [PubMed] [Google Scholar]
- 26.Hamada M, Yakushijin Y, Ohtsuka M, Kakimoto M, Yasukawa M, Fujita S. Aurora2/BTAK/STK15 is involved in cell cycle checkpoint and cell survival of aggressive non-Hodgkin's lymphoma. Br J Haematol. 2003;121:439–47. doi: 10.1046/j.1365-2141.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 27.Camacho E, Bea S, Salaverria I, Lopez-Guillermo A, Puig X, Benavente Y, et al. Analysis of Aurora-A and hMPS1 mitotic kinases in mantle cell lymphoma. Int J Cancer. 2006;118:357–63. doi: 10.1002/ijc.21370. [DOI] [PubMed] [Google Scholar]
- 28.Mahadevan D, Spier C, Della Croce K, Miller S, George B, Riley C, et al. Transcript profiling in peripheral T-cell lymphoma, not otherwise specified, and diffuse large B-cell lymphoma identifies distinct tumor profile signatures. Mol Cancer Ther. 2005;4:1867–79. doi: 10.1158/1535-7163.MCT-05-0146. [DOI] [PubMed] [Google Scholar]
- 29.Pinel S, Barbault-Foucher S, Lott-Desroches MC, Astier A. Inhibitors of aurora kinases. Ann Pharm Fr. 2009;67:69–77. doi: 10.1016/j.pharma.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–66. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 31.Rosenwald A, Wright G, Wiestner A, Chan WC, Connors JM, Campo E, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–97. doi: 10.1016/s1535-6108(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 32.Bayliss R, Sardon T, Vernos I, Conti E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol Cell. 2003;12:851–62. doi: 10.1016/s1097-2765(03)00392-7. [DOI] [PubMed] [Google Scholar]
- 33.D'Alise AM, Amabile G, Iovino M, Di Giorgio FP, Bartiromo M, Sessa F, et al. Reversine, a novel Aurora kinases inhibitor, inhibits colony formation of human acute myeloid leukemia cells. Mol Cancer Ther. 2008;7:1140–9. doi: 10.1158/1535-7163.MCT-07-2051. [DOI] [PubMed] [Google Scholar]
- 34.Mahadevan D, Bearss DJ, Vankayalapati H. Structure-based design of novel anti-cancer agents targeting aurora kinases. Curr Med Chem Anticancer Agents. 2003;3:25–34. doi: 10.2174/1568011033353524. [DOI] [PubMed] [Google Scholar]
- 35.Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, et al. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci U S A. 2007;104:4106–11. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–7. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 37.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 38.Wysong DR, Chakravarty A, Hoar K, Ecsedy JA. The inhibition of Aurora A abrogates the mitotic delay induced by microtubule perturbing agents. Cell Cycle. 2009;8:876–88. doi: 10.4161/cc.8.6.7897. [DOI] [PubMed] [Google Scholar]
- 39.Shimomura T, Hasako S, Nakatsuru Y, Mita T, Ichikawa K, Kodera T, et al. MK-5108, a highly selective Aurora-A kinase inhibitor, shows antitumor activity alone and in combination with docetaxel. Mol Cancer Ther. 9:157–66. doi: 10.1158/1535-7163.MCT-09-0609. [DOI] [PubMed] [Google Scholar]
- 40.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meraldi P, Honda R, Nigg EA. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev. 2004;14:29–36. doi: 10.1016/j.gde.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 42.VanderPorten EC, Taverna P, Hogan JN, Ballinger MD, Flanagan WM, Fucini RV. The Aurora kinase inhibitor SNS-314 shows broad therapeutic potential with chemotherapeutics and synergy with microtubule-targeted agents in a colon carcinoma model. Mol Cancer Ther. 2009;8:930–9. doi: 10.1158/1535-7163.MCT-08-0754. [DOI] [PubMed] [Google Scholar]
- 43.Jadayel DM, Lukas J, Nacheva E, Bartkova J, Stranks G, De Schouwer PJ, et al. Potential role for concurrent abnormalities of the cyclin D1, p16CDKN2 and p15CDKN2B genes in certain B cell non-Hodgkin's lymphomas. Functional studies in a cell line (Granta 519) Leukemia. 1997;11:64–72. doi: 10.1038/sj.leu.2400555. [DOI] [PubMed] [Google Scholar]
- 44.Younes A, Sarris A, Melnyk A, Romaguera J, McLaughlin P, Swan F, et al. Three-hour paclitaxel infusion in patients with refractory and relapsed non-Hodgkin's lymphoma. J Clin Oncol. 1995;13:583–7. doi: 10.1200/JCO.1995.13.3.583. [DOI] [PubMed] [Google Scholar]
- 45.Press OW, LeBlanc M, O'Rourke TJ, Gagnet S, Chapman RA, Balcerzak SP, et al. Phase II trial of paclitaxel by 24-hour continuous infusion for relapsed non-Hodgkin's lymphomas: Southwest Oncology Group trial 9246. J Clin Oncol. 1998;16:574–8. doi: 10.1200/JCO.1998.16.2.574. [DOI] [PubMed] [Google Scholar]
- 46.Wilson WH, Chabner BA, Bryant G, Bates S, Fojo A, Regis J, et al. Phase II study of paclitaxel in relapsed non-Hodgkin's lymphomas. J Clin Oncol. 1995;13:381–6. doi: 10.1200/JCO.1995.13.2.381. [DOI] [PubMed] [Google Scholar]
- 47.Casasnovas RO, Haioun C, Dumontet C, Gabarre J, Richard B, Lederlin P, et al. Phase II study of 3-hour infusion of high dose paclitaxel in refractory and relapsed aggressive non-Hodgkin's lymphomas. Groupe d'Etude des Lymphomes de l'Adulte Haematologica. 2000;85:502–7. [PubMed] [Google Scholar]
- 48.Zekri JM, Hough RE, Davies JM, Molife R, Hancock BW, Lorigan PC. Phase II study of docetaxel in patients with relapsed or refractory malignant lymphoma. Br J Cancer. 2003;88:1335–8. doi: 10.1038/sj.bjc.6600914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rizzieri DA, Sand GJ, McGaughey D, Moore JO, DeCastro C, Chao NJ, et al. Low-dose weekly paclitaxel for recurrent or refractory aggressive non-Hodgkin lymphoma. Cancer. 2004;100:2408–14. doi: 10.1002/cncr.20245. [DOI] [PubMed] [Google Scholar]
- 50.Kahl BS, Bailey HH, Smith EP, Turman N, Smith J, Werndli J, et al. Phase II study of weekly low-dose paclitaxel for relapsed and refractory non-Hodgkin's lymphoma: a Wisconsin Oncology Network Study. Cancer Invest. 2005;23:13–8. [PubMed] [Google Scholar]
- 51.Younes A. Paclitaxel-based treatment of lymphoma. Semin Oncol. 1999;26:123–8. [PubMed] [Google Scholar]