Abstract
Second-generation antipsychotics can greatly improve symptoms of psychosis-spectrum disorders. Unfortunately, these drugs are associated with weight gain, which increases a patient’s risk for developing chronic diseases including Type 2 diabetes, cardiovascular diseases or other obesity-related complications. There are interindividual differences in weight gain resulting from antipsychotic drug use that may be explained by pharmacodynamic characteristics of these agents as well as clinical factors. In addition, genetic variations in pathways associated with satiety are increasingly recognized as potential contributors to antipsychotic-associated weight gain. Polymorphisms in the leptin gene, as well as the leptin receptor gene, are potential pharmacogenetic markers associated with these outcomes. This article summarizes evidence for the associations of the leptin gene and the leptin receptor gene polymorphisms with antipsychotic-induced weight gain, potential mechanisms underlying these relationships, and discusses areas for future pharmacogenetic investigation.
Keywords: antipsychotic, leptin, leptin receptor, obesity, polymorphism, weight gain
Antipsychotic-associated weight gain and related metabolic complications are major side effects of many second-generation antipsychotics (SGA) [1]. This phenomenon is especially concerning in patients with psychosis-spectrum disorders who are nearly twice as likely as the general population to be obese [2]. Although weight gain is a relatively common side effect from many SGAs, the amount of weight gained by patients is highly variable [1,3]. Considering that the pathways responsible for antipsychotic-associated weight gain are not yet fully understood, investigating relationships between this outcome and genetic variants may help to account for some of the variability observed and explain the mechanisms by which it occurs. Over the past decade, a number of pharmacogenetic investigations have studied polymorphisms in genes believed to be involved in antipsychotic-associated weight gain [4]. Thus far, candidates such as HTR2C have yielded promising results [5,6]. Building upon this foundation, an increasing number of studies also suggest a relationship between drug-associated weight gain and genes related to neuropeptides known to influence appetite and satiety such as leptin. This article summarizes the available literature investigating the relationship between LEP and LEPR polymorphisms and antipsychotic-associated weight gain, with a focus on psychosis- spectrum disorders such as schizophrenia.
Obesity is a growing epidemic & patients with schizophrenia are at an increased risk
Obesity is increasing in the general population and places those affected at an increased risk for serious diseases. Data from the 2007–2008 National Health and Nutrition Examination Survey (NHANES) illustrate that 68% of the US population has an overweight BMI (≥25 kg/m2), and 33.8% are considered obese (BMI ≥30 kg/m2) [7]. There are numerous consequences of obesity which include an increased risk of developing chronic diseases including diabetes, hypertension, stroke and coronary heart disease [8]. Metabolic syndrome is a related outcome that is characterized by abdominal obesity, dyslipidemia, hypertension and impaired fasting glucose [8]. People with metabolic syndrome are at a twofold higher risk of cardiovascular disease, stroke and cardiovascular-related mortality [9].
Psychiatric patient populations such as those with schizophrenia are more likely to develop obesity, metabolic dysregulation and cardiovascular disease than the general population [10–12]. This is due in part to the effects of antipsychotic treatment, but evidence also suggests an increased risk independent of drug therapy. Some clinical characteristics associated with risk for obesity in schizophrenia include negative symptoms and poor self-care, which often results in poor diet choices (e.g., high in fat, low in fiber) and inadequate exercise [13]. Moreover, patients with schizophrenia often have less access to and are less likely to seek medical attention [13], making it even more difficult to intervene and prevent complications owing to metabolic disturbances and weight gain.
Weight gain & SGAs
The likelihood of significant weight gain associated with antipsychotic medications varies and appears to reflect differences in the pharmacodynamic properties of these drugs. The degree of weight gained from SGAs is typically greatest early in treatment as meta-analyses have determined mean weight increases from 1.4 to 11 lb (0.6–5 kg) over the initial 4–12 weeks of therapy [3]. Antipsychotic-associated weight gain is believed to reach a plateau by year 1 of treatment [14,15], although there is still controversy regarding the timing of this plateau for each SGA [16]. Antipsychotics with the highest degree of weight gain observed after 10 weeks of exposure in clinical trials include clozapine, olanzapine and risperidone, while ziprasidone is less likely to cause weight gain [1]. While SGAs such as olanzapine and clozapine have the greatest relative risks for weight gain, they are also two of the most effective and efficacious agents [17–19]. This dilemma often forces prescribers to balance the risks of weight gain-associated sequelae versus the potential for improved symptom control and function.
Clinical & genetic factors related to weight gain during antipsychotic treatment
In addition to drug-specific factors, clinical factors – psychiatric and nonpsychiatric – may also influence the likelihood of an individual being overweight. Most studies suggest that a low or normal baseline BMI is predictive of greater weight gain in the context of antipsychotic treatment [16]. Family history may also influence BMI status as illustrated through positive correlations between the BMIs of the parents of study participants and weight gain observed over the course of treatment [20]. Sex [21,22] and age [23–25] may also be contributing factors since females and younger patients (particularly children and adolescents) appear to be more vulnerable to antipsychotic-induced weight gain.
The relationship between psychiatric history or diagnosis and the magnitude of weight gained is still under investigation. In studies of olanzapine and risperidone, weight gain was associated with a diagnosis of undifferentiated schizophrenia [26] and presence of negative symptoms [25]. In addition, some findings suggest that clinical outcomes are positively associated with weight gain over 6 weeks of treatment [22,27], although other literature has not confirmed this relationship in cases of long-term treatment [16].
Genetic factors have also emerged as potential predictors of antipsychotic-associated weight gain. A series of twin and sibling studies suggest that there may be 60–80% heritability of weight gain from clozapine, olanzapine or risperidone [20,28,29]. Several genetic association studies have also linked SGA-associated weight gain to variants in many genes, including BDNF [30], HTR2A [31], HTR2C [32], DRD2 [33] and ADRA2A [34,35]. Therefore it appears likely that some combination of genetic variations may be the key to predicting and understanding antipsychotic-associated weight gain.
Pharmacology of antipsychotic-associated weight gain: the usual suspects
Despite some correlations with clinical factors, it is clear that antipsychotic medication utilization is related to the extent of weight gained from these drugs, although the mechanism(s) by which this occurs is still under exploration. Several studies suggest that selectivity for known pharmacodynamic targets of SGAs such as the serotonin 2C (5-HT2C), histamine 1 (H1) and dopamine 2 (D2) receptors are at least partially responsible for weight gain-associated side effects [36,37].
Transgenic research in rodents has helped to clarify the role of the 5-HT2C receptor as a vital component in the regulation of food intake, adiposity and levels of blood-based bio-markers of metabolic function (e.g., glucose, insulin and leptin) [38,39]. 5-HT2C receptors are believed to influence metabolic function by regulating transmission of melanocortin and its precursor pro-opiomelanocortin (POMC), which are two neuropeptides with anorexigenic effects (i.e., decrease feeding and increase energy expenditure) [36,37]. Olanzapine and clozapine both have strong affinity for, and antagonize the 5-HT2C receptors, which may explain their associations with greater weight gain. Ziprasidone, which is less likely to induce weight gain, also has moderate affinity for 5-HT2C receptors [36]. This suggests that the involvement of additional pharmacodynamic factors is important.
Antipsychotic drugs also block D2 receptors, which are part of another pharmacologic pathway that may regulate appetite and satiety. All first- and second-generation antipsychotics have some degree of activity at D2 receptors [40,41]. Thus far, D2 receptors have been implicated in addictive and impulsive behaviors like pathological eating, obesity and gambling [42,43]. Consequently, dopamine signaling, particularly in the mesocorticolimbic system, is believed to be involved in hyperphagia and reward-seeking behaviors [44,45].
Finally, there is strong evidence of an association between H1 receptor antagonism by antipsychotics and weight gain [36,37]. Animal studies have established histamine as an anorexigenic factor [46,47], while antagonism of H1 receptors has been demonstrated to cause increased food intake [48]. Chronic olanzapine exposure can downregulate H1 receptor expression in the arcuate nucleus and ventromedial hypothalamus [49], which can subsequently impair appetite suppression.
Leptin physiology & signaling
Recently, the search for a comprehensive mechanistic explanation for antipsychotic-associated weight gain has expanded to include the roles of other hormones involved in appetite and satiety. One such hormone is leptin, an anorexigenic factor that was discovered in the mid-1990s when knockout mice for the ob gene encoding leptin were created [50]. These ob/ob mice became hyperphagic, extremely obese, diabetic and completely leptin deficient.
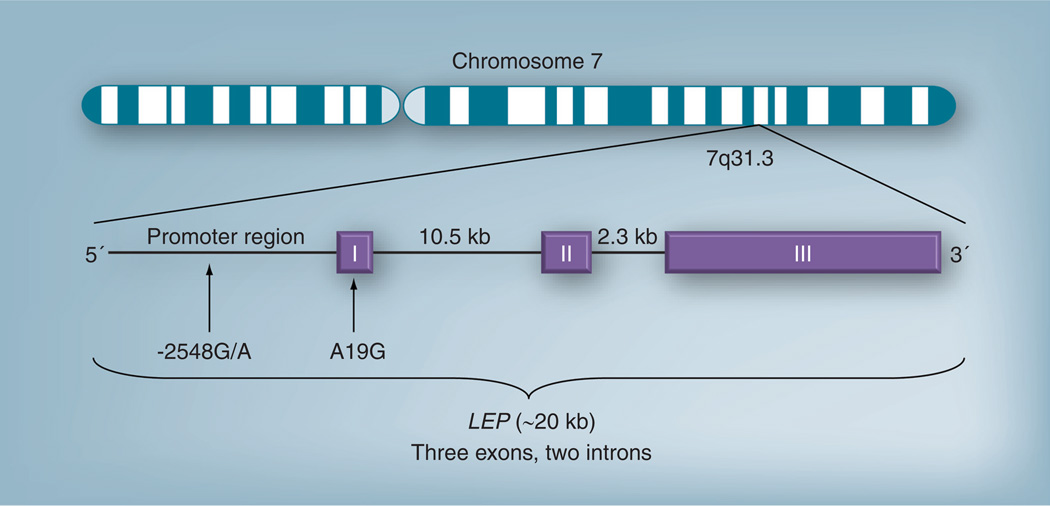
The leptin neuropeptide in humans is encoded by LEP, which maps to chromosome 7q31.3 and consists of three exons separated by two introns [51] (see Figure 1). An N-terminus signaling sequence is cleaved from the 167 amino acid-long product to form the leptin peptide [50]. In humans, leptin circulates in two forms: as an unbound peptide or; bound to a soluble leptin receptor (sOB-R) [52]. Leptin is primarily secreted by white adipose tissue in a pulsatile fashion [53], such that leptin levels are elevated in the evening and early morning [54].
Figure 1. Proposed structure and organization of the leptin gene.
Exon 1 is thought to be an untranslated 29 bp sequence. The 5´ flanking region in intron 1 contains a TATA box-like sequence at −87 to −81; and three GC boxes at −79 to −74, −155 to −150, and −160 to −155. Exon 2 is 172 bp long. Exon 3 is 1039 bp long.
LEP: Leptin.
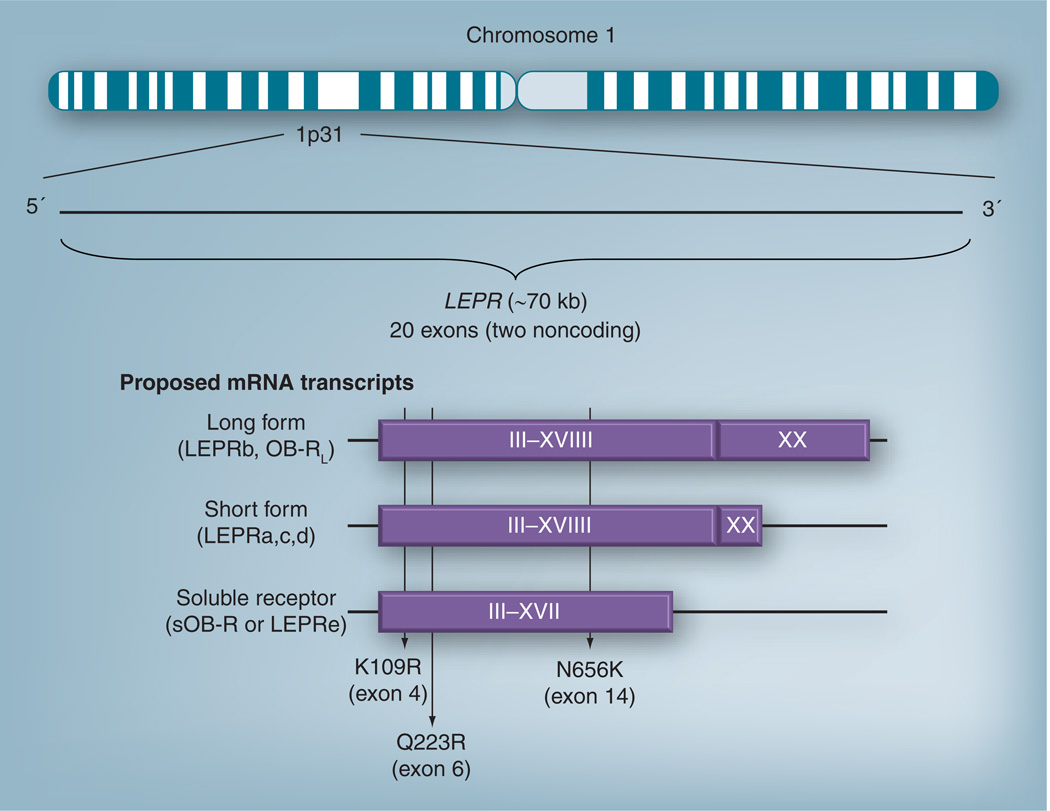
Leptin signaling is mediated through the leptin receptor, which belongs to the class I family of cytokine receptors [55]. In humans, LEPR maps to chromosome 1p31 and contains 20 exons, two of which are noncoding (see Figure 2) [56]. The LEPR transcript is alternatively spliced to several different isoforms that all share the same extracellular and transmembrane domains [57]. The only known long isoform (also known as LEPRb or OB-RL) is primarily involved in hypothalamic signaling, whereas the short isoforms (OB-Rs also known as LEPRa, LEPRc and LEPRd) are thought to mediate leptin transport [58]. The soluble leptin receptor (sOB-R or LEPRe) only has the extracellular domain [59] and circulates throughout the periphery [60].
Figure 2. Proposed structure and organization of the leptin receptor gene.
Exons 1 and 2 are noncoding, but have the potential to form secondary structures [56]. Exon 3 contains the initiation codon [56]. Exons 3–17 code for the extracellular domain that all isoforms possess [58]; this extracellular domain is approximately 816 amino acids long [110]. Exon 18 codes for the transmembrane domain, which comprises approximately 23 amino acids [110]. Exons 19 and 20 code for the intracellular domain. Exon 20 codes for the last 274 amino acid residues of LEPRb [56]. The proposed splice site is located between exon 19 and intron 19 [56]. The location of SNPs K109R, Q223R and N656K are indicated. The silent P1019 polymorphism is at nucleotide position 3037 [56]. Structure and function of leptin receptor isofroms: LEPRa: no intracellular domain; facilitates leptin transport across the blood–brain barrier [58]. LEPRb: full intracellular domain necessary for cell signaling via JAK2/STAT3 and phosphoinositide-3 kinase pathways; contains 203 [59] or 303 [110] intracellular amino acid residues. LEPRb is primarily found in the hypothalamus, but is also present in adipocytes, pancreatic islet cells, skeletal muscle, heart, kidneys, adrenals, immune cells and liver [59]. LEPRe is the soluble form of leptin thought to inhibit transporter action of LEPRa [58] and circulate throughout the body [59]. The isoforms are formed either by splicing or post-translational editing [59].
LEPR: Leptin receptor.
Leptin exerts its action via its receptors throughout the human body. In particular, LEPRb is highly expressed in the hypothalamus and, unlike short LEPR isoforms, possesses docking sites responsible for intracellular signal transduction [61]. After the leptin peptide binds to this receptor, signal transduction at LEPRb occurs by autophosphorylation of a tyrosine kinase, Janus-kinase2 (Jak2), leading to the phosphorylation of several tyrosine residues that activate signal transducers and activators of transcription isoforms (STAT3 and STAT5) [62,63]. Other downstream signals of LEPRb that have yet to be fully characterized include phosphoinositide 3-kinase, mammalian target of rapamycin and AMP-activated protein kinase [64].
Much of what is understood about the effects of leptin on energy regulation comes from understanding its actions at the arcuate nucleus. There, leptin acts on receptors in the POMC/melanocortin neuropathway, which suppresses appetite and increases energy expenditure. Furthermore, leptin inhibits production of two orexigenic (appetite stimulating) peptides: neuropeptide Y and agouti-related peptides [65]. In addition to hypothalamic targets, leptin is also believed to act alongside gut peptides (e.g., glucagon-like peptide 1 and cholecystokinin) at the nucleus of the solitary tract to signal for satiety [64,65].
Role of leptin in obesity & antipsychotic-associated weight dysregulation
Leptin is produced by adipose tissue, and circulating leptin levels are positively correlated with BMI [52]. Thus, increased adipose tissue is generally associated with greater leptin levels [66]. The relationship between leptin levels and adipose tissue may explain why in cross-sectional studies, women (who have a larger percentage of adipose mass than men) tend to have higher leptin levels than men [67,68]. Another hypothesis is that the discrepancy in leptin levels is owing to the effect of sex steroids on leptin production. Estrogen, progesterone and androgen receptors are found in adipose tissue [69–71], and there is evidence that estrogen stimulates leptin expression in women, whereas in men, testosterone levels are negatively correlated with leptin levels [72,73].
Some studies indicate that obese individuals have higher serum leptin levels than nonobese populations [52,74]. Therefore, many investigators hypothesize that obese people may actually be resistant to the effects of leptin, which include inducing the sensation of satiety and increasing energy expenditure. The hypotheses that antipsychotics may somehow contribute to leptin dysregulation or that a genetic predisposition to leptin resistance may influence the degree of weight gain experienced by a patient have recently been tested.
Several studies have attempted to determine what effect(s) antipsychotics have on leptin. It is generally understood that the use of antipsychotics (especially SGAs), and not the diagnosis of a psychosis-spectrum disorder, is associated with both an increase in weight and an increase in serum leptin levels [14,75]. However, after controlling for BMI, there does not appear to be a strong correlation between antipsychotic use and changes in leptin levels [14]. In addition, the effect of treatment on leptin levels is evident regardless of the dose of SGA used [75]. These observations suggest that increases in serum leptin are most likely owing to weight gained during treatment rather than a direct effect of antipsychotics on leptin production. The increase in leptin serves as the body’s attempt to curb the increased food intake. Still, as previously mentioned, there is a great deal of interin-dividual variance in the amount of weight gain experienced while taking antipsychotic drugs. One interesting hypothesis is that genetic variation in leptin system genes influences the body’s ability to effectively modify appetite and energy expenditure in the context of increased food consumption and resulting weight gain.
Function of LEP & LEPR genetic polymorphisms
There is evidently a relationship between antipsychotic use, weight gain or metabolic disturbances and leptin; however, what exactly links these variables is still under investigation. Studies of genetic variability in leptin system genes might provide answers and help us account for some of the interindividual differences observed in patients who develop metabolic disturbances while on antipsychotics.
Many SNPs have been discovered in LEP and LEPR. In LEP, −2548G/A (rs7799039) and A19G (rs2167270) are candidate SNPs studied for associations with obesity in addition to cancer [76] and hypertension [77]. In humans, the LEP −2548G/A polymorphism appears to influence leptin transcription, expression and secretion such that AA genotype carriers have twice the rate of leptin secretion and 60% more leptin mRNA transcription than G allele carriers [78]. In an obese population, those homozygous for G alleles of the LEP A19G polymorphism had lower leptin concentrations [79]. The functional consequences of the A19G SNP have not been described, but its close chromosomal proximity to the −2548 promoter variant indicates that linkage disequilibrium between the markers could mean that they are markers for the same causal variation. Other genetic variations in the 5´- and 3´-UTRs have also been linked with obesity and BMI status [80]; however their biological functions have not yet been elucidated.
A number of variants within LEPR have been studied for relationships with obesity or weight status [81]. These include Gln223Arg or 668G/A (rs1137101), Lys656Asn (rs8179183), Ser343Ser (rs1805134) and Lys109Arg (rs1137100). At this point, the biological functions of these polymorphisms have not been well studied in humans. In vitro experiments suggest that the LEPR Q223R polymorphism has no effect on leptin signaling [82]. Nonetheless, candidate gene association studies have found that the Q223R polymorphism is a marker for fat mass, obesity and increased BMI [83–85]. The functional characterization of other LEPR polymorphisms is still limited, although the LEPR Ser343Ser polymorphism was also associated with overweight status in a female French population [86]. Despite these positive association reports, meta-analyses of studies on the LEPR Q223R SNP [87] and other commonly studied LEPR SNPs (e.g., LEPR Q223R, K109R and N656K [88]) have not found significant evidence for associations with obesity. Interestingly, a recent genome-wide association study of sOB-R in women of European ancestry identified a number of polymorphisms associated with the sOB-R serum level phenotype – all of which were located in LEPR [89]. It is plausible that the LEPR variants selected for candidate gene association studies do not represent the causal variants, but rather are linked to the causal variant(s). Alternatively, known differences in allele frequencies across racial and ethnic groups, or the use of less specific phenotypes (e.g., weight or BMI instead of sOB-R levels) may have contributed to the inconsistent associations and mechanistic information regarding these variants.
Pharmacogenetics of LEP & LEPR
Many studies have examined the relationships between LEP and LEPR polymorphisms and metabolic dysfunction. However, only 14 have been conducted in the context of antipsychotic use. Six of the reviewed studies were cross-sectional (see Table 1) and seven were prospective (see Tables 2 & 3). One study is only available in Chinese and is not reviewed extensively herein [90]. The available studies all represent candidate gene association investigations, where variants were selected based on a priori knowledge of associations with being overweight, obesity outcomes or by virtue of being located in known coding regions of LEP or LEPR. Many focused on the LEP −2548G/A (rs7799039) polymorphism, while others also examined polymorphisms within LEP (rs4731426 C/G) and several in the leptin receptor: LEPR Q223R (rs1137101), LEPR N656K (rs8179183), rs7602 and rs1171276.
Table 1.
Summary of cross-sectional studies examining the association of leptin polymorphisms and metabolic disturbances in antipsychotic use.
| Author (year) | n (% male) | Population/ diagnosis |
Medications | Dose | Treatment duration |
SNP(s) studied | Summary of results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ruano et al (2007) | 168(66) | Caucasian (84%) African–American (14%) Diagnoses not reported† | Olanzapine Risperidone | Not available | Patients already on AP; duration unknown | LEPR rs7602 rs1171276 N656K‡ rs8179183 | Significant association between LEPR N656K (rsS179183) and weight in risperidone-treated patients only; G allele carriers were protected against higher weight | [91] |
| Yevtushenko et al. (2008) | 134(65) | Schizophrenia and schizoaffective disorder | Olanzapine Clozapine Risperidone | Not available | Unknown duration of AP exposure | LEP −2548G/A (rs7799039) HTR2C −759C/T (rs3813929) | Among subjects with the HTR2C −759C/CC genotype, those with LEP −2548 G allele versus AA homozygotes had higher BMI, larger waist circumference and presence of metabolic syndrome (BMI 29.89 ± 6.66 kg/m2 vs 26.09 ± 5.53 kg/m2; p = 0.021 waist circumference: 103.86 ±15.61 cm vs 93.78 ± 16.00 cm; p = 0.012: metabolic syndrome 33 patients vs two patients; p = 0.003) | [92] |
| Gregoor et al (2009)§ | 200 (67) | Caucasian Schizophrenia, schizoaffective and other psychotic disorders | Clozapine Olanzapine Risperidone Other unspecified | Not available | At least 3 months | LEP −2548G/A (rs7799039) | No significant association with obesity | [93] |
| LEPR Q223R‡ (rs1137101) | Female QQ homozygotes had higher rate of obesity (defined as BMI >30 kg/m2) versus R carriers (70.6% obese in QQ vs 38.5% in QR; p = 0.007: vs 40% in RR; p = 0.018) | |||||||
| Gregoor et al (2010)§ | 200 (67) | Caucasian Schizophrenia, schizoaffective and other psychotic disorders | Clozapine Olanzapine Risperidone Other unspecified | Not available | At least 3 months | LEP −2548G/A (rs7799039) HTR2C −759C/T (rs3813929) | Combined absence of HTR2C T allele and presence of LEP-2548G allele associated with obesity in persons without an HTR2C T allele (33.9% of subjects vs 22.2% in other genotype groups; OR: 2.88; 95% CI: 1.05–7.95) | [94] |
| LEPR Q223R (rs1137101) HTR2C −759C/T (rs3813929) | No significant association with obesity | |||||||
| Opgen-Rhein et al. (2010)¶ | 128(62.5) | European (92%) Turkish (8%) Schizophrenia and schizoaffective disorder | Olanzapine Clozapine Risperidone Amisulpride Quetiapine Clozapine Some FGAs | Not available | At least 6 weeks | LEP −2548G/A (rs7799039) HTR2C −759C/T (rs3813929) | No significant association with clinically significant weight gain (>7% initial bodyweight) after 6 weeks | [95] |
| Fernandez et al (2010) | 56(78.6) | Schizophrenia | Clozapine | Not available | At least 3 months | LEP −2S48G/A (rs7799039) LEPR Q223R† (rs1137101) | No association between LEP and LEPR variants and frequency of metabolic syndrome or obesity when comparing homozygotes for LEP −2548 GG versus A allele carriers and LEPR 223 QQ vs R allele carriers in all subjects. LEPR 223 QQ genotype was associated with lower triglyceride levels in males after controlling for dose | [96] |
| Moons et al (2011) | 261 (68.6) | Schizophrenia and schizoaffective disorder | Risperidone Olanzapine Clozapine Quetiapine Amisulpride Aripiprazole | Not available | At least 3 months | LEPR N656K‡ (rs8179183) | No association found with LEPR N656K genotype and weight, BMI, waist circumference, hip circumference or waist:hip ratio | [97] |
Participants recruited from hospitals treating severe, persistent mental illness.
LEPR Q223R (rs1137101) polymorphism: G allele codes for R (arginine), A allele codes for Q (glutamine); LEPR N656K (rs8179183) polymorphism: C allele codes for N (asparagine), G allele codes for K (lysine).
Both studies by Gregoor et al. utilized the same subject population.
Case-control, retrospective study.
AP: Antipsychotic; FGA: First-generation antipsychotic; OR: Odds ratio.
Table 2.
Summary of short-term (≤6 weeks exposure) prospective studies examining the association of leptin polymorphisms and metabolic disturbances in antipsychotic use.
| Author (year) | n (% male) | Population/ diagnosis |
Medication | Dose | Treatment duration |
SNPs studied | Summary of results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ellingrod et al (2007) | 37(81) | Caucasian, schizophrenia | Olanzapine | 12.43 ± 3.04 mg/day | 6 weeks (min. 4 weeks) | LEP −2548G/A (rs7799039) LEPR Q223R† (rs1137101) | Significant interaction between the G allele of both LEPR and LEP variants and greater weight gain at higher serum concentrations (3.82 ± 3.31 kg/m2 vs 1.27 ± 1.74 kg/m2; p = 0.049) | [98] |
| Srivastava et al (2008) | 130(41) | North Indian, schizophrenia and schizoaffective disorder | Olanzapine | 16.5 ± 3.0 mg/day | 6 weeks (3-day washout if prior AP exposure) | LEP (rs4731426) C/G | G allele was associated with above average (>2.17 kg) weight gain from baseline after 6 weeks (OR: 11.43; 95% CI: 1.49–87.55; p = 0.019) | [99] |
| Leptin haplotype A-C-A-A-G‡ | This haplotype associated with above average weight gain (>2.17 kg) | |||||||
For LEPR Q223R polymorphism, G allele codes for R (arginine), A allele codes for Q (glutamine).
Haplotype A-C-A-A-G: rs10487506 A/G–rs4731426 C/G–rs2278815 A/G–rs3828942 A/G–rs17151922 GIT.
AP: Antipsychotic; min.: Minimum; OR: Odds ratio.
Table 3.
Summary of longer-term (≥6 weeks exposure) prospective studies examining the association of leptin polymorphisms and metabolic disturbances in antipsychotic use.
| Author (year) | n (% male) |
Population/ diagnosis |
Medications | Dose | Treatment duration |
SNPs studied | Summary of results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Templeman et al (2005) | 73 (75) | Spanish Caucasian, first episode psychosis | Risperidone Olanzapine Quetiapine Haloperidol Ziprasidone Amisulpride | Not available | 6-week trial, then re-evaluation | LEP −2S48G/A (rs7799039) HTR2C-759C/T (rs3813929) | 9 months post-treatment initiation, LEP −2548 GG homozygotes had greater increase in BMI versus A allele carriers (5.81 ± 0.85 kg/m2 vs 3.58 ± 0.47 kg/m2; p = 0.033) | [100] |
| Zhang et al (2007) | 102(66) | Chinese, schizophrenia | Clozapine Other APs previously used† | 225.4 ± 105.1 mg/day | At least 2 years on stable dose (mean 6.7 ±2.8 years) | LEP −2S48G/A (rs7799039) | G allele carriers had a greater change in BMI than AA homozygotes (4.4 ± 3.5 kg/m2 vs 2.6 ± 3.5 kg/m2; p = 0.014) | [101] |
| Kang et al. (2008) | 74 (68) | Korean, schizophrenia | Olanzapine | 14.0 ± 5.1 mg/day | At least 3 months (453 ± 289 days) | LEP −2S48G/A (rs7799039) | Subjects with AG genotype had more weight gain than AA genotype (8.6 ± 5.3 kg vs 4.4 ± 6.4 kg; p = 0.029) (no subjects had GG genotype) | [102] |
| Calarge et al (2009)‡ | 74(91) | Caucasian and African–American children and adolescents (7–17 years old, ADHD (84% irritability and aggression) | Risperidone | 0.03 mg/kg/day (median) | At least 6 months (median 2.6 years) | LEP −2S48G/A (rs7799039) | A allele carriers had a higher rate of weight gain than GG after 1 year of risperidone treatment (0.56 mean z-score increase vs 0.36) and 2 years (0.78 vs 0.56) A allele also associated with higher BMI z-scores vs GG genotype after risperidone treatment | [104] |
| Perez-lglesias et al. (2010) | 205(57.5) | Caucasian (first episode, drug-naive), schizophrenia and schizophreniform disorder | Haloperidol Olanzapine Risperidone Ziprasidone Aripiprazole Quetiapine | Not available | 12 months | LEP −2S48G/A (rs7799039) LEPR Q223R+ (rs1137101) | No association between either LEP or LEPR variants and change in BMI or weight gain | [103] |
For LEPR Q223R polymorphism, G allele codes for R (arginine), A allele codes for Q (glutamine).
Some patients included in this study were also taking SSRIs or α2-agonists, which can independently cause weight gain. Further analysis demonstrated that this drug did not affect the results (when controlling for use of SSRIs/α2-agonists). Psychostimulants were also concomitantly taken, but did not appear to affect weight z-score changes over time.
ADHD: Attention deficit hyperactivity disorder; AP: Antipsychotic; SSRI: Selective serotonin-reuptake inhibitor.
Leptin system genes & antipsychotic-associated weight gain: cross-sectional studies
Four of the seven available cross-sectional studies reported at least one significant association between a LEP or LEPR polymorphism and BMI or weight with details summarized in Table 1. Highlights of these investigations are reviewed here in chronological order. The first study reviewed was an investigation of 168 predominantly Caucasian subjects who had a severe and persisting mental illness requiring treatment with either risperidone or olanzapine [91]. No data on drug dose or duration of exposure was reported. The primary goal of this investigation was to perform a ‘physiogenomic’ study comparing weight profiles between patients on risperidone and olanzapine and to determine whether selected candidate SNPs were associated with any of the study end points. Upon relating polymorphisms in LEPR to patient weight, there was a significant association between participants carrying the minor allele (G) coding for K at LEPR N656K and ‘protection’ against higher weight while on risperidone; in fact, the K allele frequency was 0% in weights over 100 kg.
A second cross-sectional study published in 2008 included 134 participants with a Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) diagnosis of schizophrenia or schizoaffective disorder [92]. The primary goal of this study was to characterize associations between LEP −2548G/A and HTR2C −759C/T genotypes and several metabolic parameters (e.g., weight, BMI and waist circumference) in these individuals. The participants were prescribed olanzapine, clozapine or risperidone for any duration prior to evaluation. Associations between LEP or HTR2C genotype groups and BMI did not reach statistical significance; however, within subjects of the HTR2C −759 C/CC genotype group, there was a significant association between LEP −2548 G allele carriers and BMI. Those participants with the −759 C/CC and −2548G combination had an approximately 2 kg/m2 higher BMI, approximately a 10 cm greater waist circumference and were significantly more likely to have metabolic syndrome than those without this genotype combination. This effect remained statistically significant when the results were stratified by medication prescribed (e.g., either olanzapine/clozapine use vs risperidone).
Gregoor et al. conducted an investigation of the LEP −2548G/A SNP in conjunction with the LEPR Q223R SNP in 200 subjects with schizophrenia or schizoaffective disorder [93]. These participants were primarily taking clozapine, olanzapine or risperidone and had been treated with one of these agents for at least 3 months. In this investigation there was no evidence for association between genotype and obesity (BMI >30 kg/m2). However, the authors did report evidence of a potential sex difference in associations with LEPR variants. Women with the LEPR 223QQ genotype (i.e., homozygous for AA) were approximately twice as likely to be obese than R allele carriers (i.e., G allele carriers).
In a follow-up study using the same subject population, Gregoor et al. further investigated interactions between LEP, LEPR and HTR2C polymorphisms and weight-related outcomes [94]. The primary objective of this study was to characterize associations between obesity and two HTR2C SNPs (rs3813929/759C/T and rs1414334), and two leptin polymorphisms (LEP rs7799039/-2548G/A and LEPR rs1137101/Q223R). In this analysis, the authors identified a significant association between obesity and the absence of the HTR2C −759 T allele and the presence of the LEP −2548 G allele. This association is consistent with results from the study by Yevtushenko et al. [92]. No other significant associations were identified, although the authors reported a trend towards significance among subjects with the HTR2C rs1414334 C allele in combination with either LEP −2548 G allele or the LEPR 223 R allele.
A case–control retrospective study of subjects diagnosed with schizophrenia or schizoaffective disorder also examined weight gain in the context of LEP and HTR2C SNPs, as well as insulin-induced gene 2 (INSIG2) variants [95]. The study population (n = 128) was predominantly male and of European or Turkish descent. A total of 54% of participants were prescribed monotherapy with an SGA (primarily clozapine or olanzapine), and 46% were on two or more antipsychotics. A total of 43% of participants were also taking other psychotropics associated with weight gain (e.g., mood stabilizers or antidepressants). The primary end point was to characterize the relationship between clinically significant weight gain after being treated with an antipsychotic for at least 6 weeks and LEP −2548G/A, INSIG2 and HTR2C variants (including the −759C/T variant). No association was found between clinically significant weight gain and the LEP −2548G/A polymorphism.
A two-part candidate gene association study in Venezuela examined LEP −2548G/A and LEPR Q223R variants in conjunction with baseline physical characteristics and metabolic response to metformin during chronic clozapine use [96]. Participants with schizophrenia were included if they had been prescribed clozapine for at least 3 months. The primary end point of the first phase of this study was to characterize the relationships between LEP −2548G/A and LEPR Q223R variants and differences in the frequency of metabolic syndrome, obesity and other metabolic variables (e.g., waist circumference, blood glucose, hemoglobin A1c, lipids, leptin and cortisol). Among the 56 participants, there was no significant association between either LEP or LEPR SNP and the frequency of metabolic syndrome or obesity. However, there was an association between observable LEPR 223QQ and lower baseline triglyceride levels, only in males after controlling for clozapine dose.
The LEPR N665K polymorphism was examined in a Belgium-based study that also investigated ‘clock genes’ (e.g., MC3R, PER2, SDC3 and NR3C1) and their relationships with weight and other anthropometric parameters [97]. This was a candidate gene association study of 261 subjects with schizophrenia who were on a stable dose of antipsychotic (risperidone, olanzapine, clozapine, quetiapine, amisulpride, aripiprazole or a first-generation antipsychotic) for at least 3 months. Subjects were allowed to take concomitant medications (including benzodiazepines, anticholinergic agents, mood stabilizers and antidepressants). No significant associations were observed between the LEPR N665K polymorphism and weight, BMI, waist circumference, hip circumference or waist:hip circumference ratio.
To date, cross-sectional studies have identified some associations between LEP and LEPR SNPs and weight gain, particularly when LEP −2548G/A variants were examined in the context of HTR2C variants [92,94] or when LEPR Q223R variants were studied for sex differences [93]. However, some of these studies did not find leptin genotypes to be significant on their own. This could be owing in part to the nature of cross-sectional studies and the recruitment of participants with varying histories of antipsychotic use. While these associations were not consistent across studies, there is enough evidence to suggest a pharmacogenetic relationship between LEP polymorphisms and obesity-associated outcomes in patients on chronic antipsychotic therapy for 3 months or longer.
Leptin system genes & antipsychotic-associated weight gain: short-term prospective studies
Ellingrod et al. conducted a small candidate gene association study of 37 Caucasian schizophrenia subjects taking olanzapine for 6 weeks which investigated the relationships between LEP and LEPR genotypes with weight gain early on in treatment [98]. In this study, subjects underwent a 4–7 day washout prior to olanzapine treatment whereby participants were titrated to a fixed dose for the duration of the study. Subjects who were carriers of the G allele for both the LEP −2548G/A and LEPR Q223R SNPs and had high olanzapine serum levels (defined as plasma olanzapine >20.6 ng/ml) had a greater increase in BMI after 6 weeks versus AA homozygotes. Interestingly, the high proportion of males (81%) in this population combined with the association of G allele presence in LEPR Q223R and a greater change in BMI mimics a similar trend, albeit not statistically significant, found by Gregoor et al. [93] which associated the G allele with obesity in Caucasian males.
Srivastava et al. recruited 130 North Indian subjects with schizophrenia or schizoaffective disorder taking olanzapine for 6 weeks with the goal of studying polymorphisms in genes involved in appetite regulation – LPL, TAG, CL and LEP – and olanzapine-associated weight gain [99]. Participants already taking an antipsychotic prior to the study underwent a 3 day washout period. Subjects with the G allele for the LEP rs4731426 C/G polymorphism and one LEP haplotype (see Table 2) were associated with greater than average weight gain relative to the rest of the sample population. There was no evidence for association when a commonly used definition of ‘clinically significant’ weight gain (>7% from baseline) was used to define this outcome.
Leptin system genes & antipsychotic-associated weight gain: longer-term prospective studies
Additional prospective studies have also examined relationships between LEP or LEPR genotypes and weight gain during chronic treatment, defined here as investigations with greater than 6 weeks of antipsychotic exposure. Templeman et al. studied 73 drug-naive Caucasian subjects experiencing their first significant episode of psychosis [100]. Study participants were enrolled in this 9-month prospective study of flexibly-dosed SGA agents (including risperidone, olanzapine, quetiapine, ziprasidone and amisulpride). The investigators selected one candidate SNP (LEP −2548G/A) for analysis with weight gain after 6 weeks, 3 months and 9 months of treatment. There was no evidence for an association between the LEP −2548G/A genotype early in treatment, but at 9 months, LEP −2548GG homozygous subjects had approximately 2 kg/m2 greater change in BMI in comparison to A allele carriers.
Zhang et al. investigated Han Chinese inpatients diagnosed with chronic schizophrenia who were prescribed a stable dose of clozapine for at least 2 years [101]. The primary goal of this investigation was to examine the relationship between the LEP −2548G/A genotype and clozapine-induced weight gain over this time period. Similar to the aforementioned findings in Caucasian populations by Templeman et al. [100], subjects carrying the G allele for LEP −2548G/A had an approximately 2 kg/m2 greater increase in BMI than AA homozygotes. When stratifying the results by sex, the G allele effect on BMI was observed in men but not in women.
In a similar study, Korean inpatients with schizophrenia were recruited with the primary goal of characterizing the relationship between the LEP −2548G/A SNP and weight gain during olanzapine treatment. Participants had no prior use of olanzapine or clozapine and were administered olanzapine for a minimum of 3 months [102]. Consistent with previously reviewed studies, subjects carrying the G allele for LEP −2548 had greater weight gain than AA homozygous patients after 3 months of treatment. None of the 74 patients carried the GG genotype. Though not statistically significant, the authors noted a trend towards the AG genotype being associated with clinically significant weight gain (77% with AG genotype vs 48% with AA genotype; p = 0.054).
The most recent prospective study investigated the LEP −2548G/A and LEPR Q223R SNPs as well as FTO and SH2B1 variants in association with BMI and weight before and after 12 months of exposure to antipsychotics [103]. This study included predominantly Caucasian subjects experiencing their first episode of schizophrenia or schizophreniform disorder. The study population was predominantly drug naive, and participants were prescribed a variety of first- and second-generation antipsychotics over the course of the study. In this study, 71% of subjects experienced clinically significant weight gain but no significant associations with either LEP or LEPR variants were observed.
All of the aforementioned investigations studied antipsychotic use, leptin genotypes and weight implications in adult populations. To the best of our knowledge, only one published study to date has examined this phenomenon in a pediatric population. This was a candidate gene association study of children aged 7–17 years old with the primary objective of investigating associations between risperidone-induced weight gain, plasma leptin concentration and LEP −2548G/A genotype [104]. Interestingly, the findings of this pediatric study are in contrast to what many of the other studies in this article have found. A total of 74 participants, who were primarily male Caucasians were prescribed risperidone for at least 6 months. The authors observed that LEP −2548 A carriers had greater increases in weight and BMI z-score than GG homozygotes after 1 year and 2 years of treatment. The same relationship held true even after controlling for concomitant use of selective serotonin-reuptake inhibitors and α2-agonists, which have also been associated with weight gain in this population.
These prospective studies provide clearer support for a significant relationship between the LEP −2548G/A variant and weight gain or increased BMI. Of note is that two studies [100,103] utilized antipsychotic-naive first-episode subjects, which are ideal for studies of weight gain and treatment response. Only one [98] of two studies found an association between the LEPR Q223R variant and weight gain; however, this was in the context of high drug concentration and a LEP −2548G/A genotype interaction.
Discussion
Studies investigating the relationship of LEP and LEPR polymorphisms and metabolic outcomes in psychiatric populations prescribed antipsychotic drugs have yielded somewhat conflicting results. However, a careful look at these investigations reveals a few notable trends. A total of six of the 13 reviewed studies identified associations between the LEP −2548 G allele and weight gain or increased BMI [92,98,100–102,105]. Subjects in these investigations were predominantly prescribed olanzapine or clozapine, which are the SGAs most associated with treatment-associated weight gain [2] and have high 5-HT2C affinity [36]. Conversely, the observation by Calarge et al. [104] that the LEP −2548 A allele is associated with increased BMI, is in agreement with findings from an earlier study conducted in a Han Chinese population that also found the LEP −2548 AA genotype to be associated with weight gain after 10 weeks [90]. Participants in these two studies were primarily treated with risperidone or antipsychotics other than olanzapine or clozapine. These findings suggest that the relationship between LEP −2548G/A genotype and weight gain may be dependent upon the type of antipsychotic a patient is currently or has previously taken (particularly clozapine and olanzapine vs others). If LEP genotype associations indeed differ across medication groups, this is consistent with a unique or additional mechanism by which clozapine and olanzapine influence weight or obesity outcomes relative to other antipsychotics studied. While this is not a new concept, the growing body of literature supporting the role of leptin gene variants in weight gain from clozapine or olanzapine use is a step forward in clarifying the mechanisms by which this occurs. Some studies have proposed that this unique mechanism may be in part related to the immunological effects of olanzapine and clozapine. Specifically, alterations in the levels of cytokines (including leptin, TNF-α and sTNFR-1 and 2) have been linked to weight gain by these two SGAs [106]. In addition, variations in H1 and muscarinic 3 (M3) receptors, for which olanzapine and clozapine have a stronger affinity than other SGAs, may also provide an explanation for these high effects on weight [107], although M3 affinity has only been linked with a risk of developing Type 2 diabetes thus far [37].
The LEPR polymorphism at codon 223 (rs1137101) was also associated with weight gain or increased BMI, but only in the context of analyses stratified by sex [93] or interactions with LEP variants [98]. Two studies did not observe LEPR Q223R associations with weight gain [96,103]. However, neither of these two studies specifically tested for sex differences, and the use of several different SGAs with varying weight gain liabilities may have limited the ability of the investigators to detect potential associations. Findings regarding the LEPR N656K (rs8179183) polymorphism are less conclusive [91,97], although it appears that this variant may be associated with weight gain in the context of risperidone use [91]. These data should be interpreted with caution owing to the limitations of the cross-sectional design employed by both studies as well as the use of concomitant medications [97]. Additional studies of the N656K polymorphism as well as another reported as significant by Srivastava et al. [99] (LEP rs4731426) are needed before any conclusions can be drawn. Other genetic markers within LEP and LEPR, such as the polymorphisms identified in a genome-wide association study on plasma sOB-R levels [89], have yet to be further studied in the context of antipsychotic-associated weight gain and may prove to be causal variants.
Only two studies published to date have examined serum leptin levels during antipsychotic exposure [100,104]. Templeman et al. observed a trend towards an association between LEP −2548 A allele carriers and higher baseline plasma leptin levels in comparison to GG homozygotes, and further noted that plasma leptin levels increased for almost all patients during treatment [100]. Calarge et al. also determined that LEP −2548 A allele carriers (who had lower BMI z-scores) had significantly higher leptin concentrations than did GG participants (p < 0.04) [104]. These findings are consistent with in vitro data identifying greater leptin secretion and leptin mRNA transcription in LEP −2548 AA homozygotes compared with G allele carriers [78]. This suggests that individuals with the LEP −2548 A allele may have higher leptin levels during antipsychotic treatment which are consistent with normal, anorexigenic signaling. However, it is unclear why associations between the LEP-2548 A allele and increased weight gain were observed in two studies [90,104]. As previously mentioned, these studies did not involve treatment with clozapine or olanzapine and therefore suggest that alternative mechanisms of weight gain need to be explored in treatments with other medications like risperidone. In addition, the effects observed in younger patients [104] underscore the importance of examining these phenomena early on in treatment.
Some limitations of the reviewed studies include the aforementioned use of data from patients on a variety of different antipsychotic medications [93,95,97,100,103,105]. In studies with heterogeneous drug treatment, pooling data across many SGA treatment groups may have limited the ability of investigators to detect significant associations between genotype(s) and weight gain or obesity if results from weight-neutral SGAs counterbalanced the effects of weight gain from olanzapine or clozapine within a given genotype or allele group. Although stratification of data by antipsychotic used may have lessoned the significance of this issue, the subsequent sample sizes were likely to be too small to detect any true associations of moderate or small effect sizes. Nonetheless, these study samples represent patient populations observed in clinical practice and illustrate that the findings observed may prove to be clinically important and applicable.
Studies of chronic schizophrenia patients [101,102] are generally complicated by an extensive and variable history of antipsychotic use. A history of prior antipsychotic treatment may minimize the magnitude of metabolic effects observed during subsequent treatments. Investigations of weight gain in patients with minimal to no prior antipsychotic use are ideal to avoid this confounder. Opgen-Rhein et al. [95] recruited subjects who had not been on antipsychotics 2 months prior to administering study medications, and both Templeman et al. [100] and Perez-Iglesias et al. [103] studied first-episode populations with limited to no prior antipsychotic history. Larger sample sizes, which could allow for data stratification by drug, may be needed to detect additional associations by specific drugs or groups of drugs.
Trends based on sex [93,101] or those conducted in study samples heavily weighted towards males [96–98,100,104], provide mechanistic insights as well as important information regarding differences between males and females. Of note, previous studies of antipsychotic-associated weight gain and the X-linked HTR2C have identified some trends suggestive of sex effects, although these studies were also limited by treatment heterogeneity and smaller sample sizes [5]. In the context of studies reviewed here, two have identified significant interactions or differential associations when HTR2C and LEP/ LEPR variants were analyzed together [92,105]. It is plausible that the satiety-regulating activities of 5-HT2C and leptin signaling work in concert or in parallel. Furthermore, differences in weight-related outcomes may exist in males and females in the context of exposure to agents with differential 5-HT2C affinity owing to inherent differences in gene expression and leptin signaling. Superimposing LEP/LEPR variability on this complex association is likely to be a source for some of the inconsistencies observed across studies of leptin and antipsychotic-associated weight gain. In the context of high 5-HT2C affinity (e.g., with clozapine or olanzapine treatment), increased appetite resulting from reduced 5-HT2C expression conferred by the −759C polymorphism could result in a pharmacologically and genetically influenced state of hyperphagia in which the body’s ability to compensate for increased food intake and adipose accumulation may be further impaired in those with lower baseline leptin production owing to the LEP −2548G allele.
While it has been established that leptin levels may increase as a result of antipsychotic use, surprisingly, no studies have objectively examined whether these leptin levels or leptin polymorphisms affect feeding behavior or appetite while on antipsychotics. Such an investigation may provide additional information characterizing whether antipsychotic-associated increases in leptin are indeed a reflection of increased feeding and development of adipose tissue or if leptin production or leptin receptor sensitivity is compromised during drug exposure.
Additional value may be derived from further characterization of the relationships between leptin polymorphisms and abnormalities in other metabolic markers such as insulin, blood glucose, cholesterol, low-density lipoproteins and triglycerides. These may serve as more specific phenotypes that may help researchers focus on multiple mechanistic pathways that converge to result in obesity outcomes following antipsychotic treatment. Of the studies reviewed here, two revealed an association between leptin polymorphisms, antipsychotic use and metabolic markers. Yevtushenko et al. [92] determined that in the context of the HTR2C −759C/CC genotype, the LEP −2548 G allele was associated with metabolic syndrome. Fernandez et al. [96] reported that after controlling for clozapine dose, LEPR 223QQ males had significantly lower triglyceride levels. Identifying other genetic markers relating to specific metabolic components associated with obesity might further clarify why patients on olanzapine and clozapine are at a higher risk of developing rapid-onset diabetes compared with people on risperidone or quetiapine [108].
Future perspective
Weight gain and obesity-related sequelae are serious complications of SGAs that affect some patients more than others. Although lifestyle and clinical factors may partly contribute, the studies reviewed here suggest that some individuals taking antipsychotics may be genetically predisposed to gain more weight than other antipsychotic users and that variability in LEP and LEPR are important in this regard. Differential relationships observed in studies of olanzapine and clozapine when compared with other drugs suggest that genetic influences are likely to differ for all antipsychotics. More specifically, the influence of specific polymorphisms may differ depending on the drug(s) being studied. Complicating the picture more is a hypothesis that these relationships may be different in males and females. The studies reviewed in this article suggest the involvement of more than one genetic factor as some determined significant associations in change in BMI when linking LEP −2548G/A genotype with LEPR Q223R [98] or HTR2C −759C/T genotype [92]. The idea that obesity is a multifactorial process should come as no surprise since there are a multitude of biological factors involved in energy intake, thermogenesis and energy metabolism.
Accordingly, future investigations should continue to examine the interaction between LEP and LEPR polymorphisms along with other genes involved in metabolic regulation in the context of antipsychotic-associated weight gain. Examining these relationships in medications other than clozapine and olanzapine will be particularly informative. It is generally accepted that agents such as aripiprazole, ziprasidone and iloperidone are less likely to induce significant weight gain than olanzapine or clozapine. However, weight gain is still observed in some individuals taking these drugs [109]. Thus, identifying genetic signatures that better characterize risk for weight- and obesity-related outcomes across all available antipsychotic drugs will be clinically important.
The candidate genes discussed herein focused on leptin signaling as well as 5-HT2C. This is obviously one small piece of a multifactorial network of pathways which influence metabolic outcomes in antipsychotic-treated patients. Other metabolic pathway genes may also be associated with antipsychotic-associated weight gain (e.g., MC3R [97], INSIG2 [95], FTO and SH2B1 [103]). A comprehensive, multifactorial approach is now needed to move the field forward. Further research in this area will hopefully generate consensus data that are mechanistically and therapeutically informative. If genetic markers can reliably predict a patient’s risk for significant weight gain from these drugs, metabolic-related morbidity and mortality may be reduced and patient adherence and treatment outcomes will likely improve.
Executive summary.
Antipsychotic-associated weight gain
-
▪
Second-generation antipsychotics (SGA) are effective in the treatment of psychosis-spectrum disorders. However, certain SGAs (especially clozapine and olanzapine) are known to cause weight gain and related sequelae (e.g., diabetes, dyslipidemia and cardiovascular disease).
-
▪
Interindividual variations in SGA-associated weight gain may be explained by variations in genes that regulate appetite and metabolism.
Impact of LEP/LEPR variants on weight gain in the context of antipsychotic use
-
▪
Many studies identified associations between the LEP −2548 G allele and weight gain, particularly in studies of olanzapine or clozapine. Associations between LEPR Q223R variants and weight gain are unclear; however, there is preliminary evidence that suggests that sex and LEP genotype may influence these relationships.
Future areas for investigation
-
▪
Additional studies are needed to clarify the effects of sex and HTR2C variants in combination with leptin variants in SGA-associated weight gain.
-
▪
Prospective studies with larger, drug-naive populations are needed. Controlling for specific SGAs used (particularly for those with greater weight-gain liability) may help to differentiate drug-specific mechanisms of weight gain.
-
▪
Future pharmacogenetic studies should also examine SGA-associated alterations in metabolic biomarkers (e.g., blood glucose, insulin and cholesterol), with the goal of better predicting risk of diabetes or heart disease.
Acknowledgments
Jeffrey Bishop has received research grant support from Ortho-McNeil Janssen and an honoraria from Eli Lilly and Ortho-McNeil Janssen for CME presentations. These activities were unrelated to the information presented in this manuscript. NIH funding was also received by Jeffrey Bishop: K08MH083888.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am. J. Psychiatry. 1999;156(11):1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 3.Parsons B, Allison DB, Loebel A, et al. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr. Res. 2009;110(1–3):103–110. doi: 10.1016/j.schres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Arranz MJ, De Leon J. Pharmacogenetics and pharmacogenomics of schizophrenia: a review of last decade of research. Mol. Psychiatry. 2007;12(8):707–747. doi: 10.1038/sj.mp.4002009. [DOI] [PubMed] [Google Scholar]
- 5.de Luca V, Mueller DJ, de Bartolomeis A, Kennedy JL. Association of the HTR2C gene and antipsychotic induced weight gain: a meta-analysis. Int. J. Neuropsychopharmacol. 2007;10(5):697–704. doi: 10.1017/S1461145707007547. [DOI] [PubMed] [Google Scholar]
- 6.Sicard MN, Zai CC, Tiwari AK, et al. Polymorphisms of the HTR2C gene and antipsychotic-induced weight gain: an update and meta-analysis. Pharmacogenomics. 2010;11(11):1561–1571. doi: 10.2217/pgs.10.123. [DOI] [PubMed] [Google Scholar]
- 7.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 8.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 9.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Citrome L, Blonde L, Damatarca C. Metabolic issues in patients with severe mental illness. South Med. J. 2005;98(7):714–720. doi: 10.1097/01.smj.0000167621.49292.11. [DOI] [PubMed] [Google Scholar]
- 11.Holt RI, Peveler RC. Obesity, serious mental illness and antipsychotic drugs. Diabetes. Obes. Metab. 2009;11(7):665–679. doi: 10.1111/j.1463-1326.2009.01038.x. [DOI] [PubMed] [Google Scholar]
- 12.Newcomer JW. Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J. Clin. Psychiatry. 2007;68(Suppl. 1):20–27. [PubMed] [Google Scholar]
- 13.Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol. Med. 1999;29(3):697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- 14.Jin H, Meyer JM, Mudaliar S, Jeste DV. Impact of atypical antipsychotic therapy on leptin, ghrelin, and adiponectin. Schizophr. Res. 2008;100(1–3):70–85. doi: 10.1016/j.schres.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinon BJ, Basson BR, Gilmore JA, Tollefson GD. Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. J. Clin. Psychiatry. 2001;62(2):92–100. [PubMed] [Google Scholar]
- 16.Gentile S. Contributing factors to weight gain during long-term treatment with second-generation antipsychotics. A systematic appraisal and clinical implications. Obes. Rev. 2009;10(5):527–542. doi: 10.1111/j.1467-789X.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 17.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373(9657):31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 18.Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am. J. Psychiatry. 2009;166(2):152–163. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman JA, Stroup TS, Mcevoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 20.Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, et al. Antipsychotic-induced body weight gain: predictors and a systematic categorization of the long-term weight course. J. Psychiatr. Res. 2009;43(6):620–626. doi: 10.1016/j.jpsychires.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Aichhorn W, Whitworth AB, Weiss EM, Marksteiner J. Second-generation antipsychotics: is there evidence for sex differences in pharmacokinetic and adverse effect profiles? Drug Saf. 2006;29(7):587–598. doi: 10.2165/00002018-200629070-00004. [DOI] [PubMed] [Google Scholar]
- 22.Basson BR, Kinon BJ, Taylor CC, Szymanski KA, Gilmore JA, Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. J. Clin. Psychiatry. 2001;62(4):231–238. doi: 10.4088/jcp.v62n0404. [DOI] [PubMed] [Google Scholar]
- 23.Kinon BJ, Kaiser CJ, Ahmed S, Rotelli MD, Kollack-Walker S. Association between early and rapid weight gain and change in weight over one year of olanzapine therapy in patients with schizophrenia and related disorders. J. Clin. Psychopharmacol. 2005;25(3):255–258. doi: 10.1097/01.jcp.0000161501.65890.22. [DOI] [PubMed] [Google Scholar]
- 24.Safer DJ. A comparison of risperidone-induced weight gain across the age span. J. Clin. Psychopharmacol. 2004;24(4):429–436. doi: 10.1097/01.jcp.0000130558.86125.5b. [DOI] [PubMed] [Google Scholar]
- 25.Strassnig M, Miewald J, Keshavan M, Ganguli R. Weight gain in newly diagnosed first-episode psychosis patients and healthy comparisons: one-year analysis. Schizophr. Res. 2007;93(1–3):90–98. doi: 10.1016/j.schres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saddichha S, Ameen S, Akhtar S. Predictors of antipsychotic-induced weight gain in first-episode psychosis: conclusions from a randomized, double-blind, controlled prospective study of olanzapine, risperidone, and haloperidol. J. Clin. Psychopharmacol. 2008;28(1):27–31. doi: 10.1097/jcp.0b013e3181602fe6. [DOI] [PubMed] [Google Scholar]
- 27.Ascher-Svanum H, Stensland MD, Kinon BJ, Tollefson GD. Weight gain as a prognostic indicator of therapeutic improvement during acute treatment of schizophrenia with placebo or active antipsychotic. J. Psychopharmacol. 2005;19(Suppl. 6):110–117. doi: 10.1177/0269881105058978. [DOI] [PubMed] [Google Scholar]
- 28.Theisen FM, Gebhardt S, Haberhausen M, et al. Clozapine-induced weight gain: a study in monozygotic twins and same-sex sib pairs. Psychiatr. Genet. 2005;15(4):285–289. doi: 10.1097/00041444-200512000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Wehmeier PM, Gebhardt S, Schmidtke J, Remschmidt H, Hebebrand J, Theisen FM. Clozapine: weight gain in a pair of monozygotic twins concordant for schizophrenia and mild mental retardation. Psychiatry Res. 2005;133(2–3):273–276. doi: 10.1016/j.psychres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XY, Zhou DF, Wu GY, et al. BDNF levels and genotype are associated with antipsychotic-induced weight gain in patients with chronic schizophrenia. Neuropsychopharmacology. 2008;33(9):2200–2205. doi: 10.1038/sj.npp.1301619. [DOI] [PubMed] [Google Scholar]
- 31.Lane HY, Liu YC, Huang CL, et al. Risperidone-related weight gain: genetic and nongenetic predictors. J. Clin. Psychopharmacol. 2006;26(2):128–134. doi: 10.1097/01.jcp.0000203196.65710.2b. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds GP, Zhang ZJ, Zhang XB. Association of antipsychotic drug-induced weight gain with a 5-HT2C receptor gene polymorphism. Lancet. 2002;359(9323):2086–2087. doi: 10.1016/S0140-6736(02)08913-4. [DOI] [PubMed] [Google Scholar]
- 33.Lencz T, Robinson DG, Napolitano B, et al. DRD2 promoter region variation predicts antipsychotic-induced weight gain in first episode schizophrenia. Pharmacogenet. Genomics. 2010;20(9):569–572. doi: 10.1097/FPC.0b013e32833ca24b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sickert L, Muller DJ, Tiwari AK, et al. Association of the a 2A adrenergic receptor −1291C/G polymorphism and antipsychotic-induced weight gain in European–Americans. Pharmacogenomics. 2009;10(7):1169–1176. doi: 10.2217/pgs.09.43. [DOI] [PubMed] [Google Scholar]
- 35.Wang YC, Bai YM, Chen JY, Lin CC, Lai IC, Liou YJ. Polymorphism of the adrenergic receptor α 2A −1291C>G genetic variation and clozapine-induced weight gain. J. Neural Transm. 2005;112(11):1463–1468. doi: 10.1007/s00702-005-0291-7. [DOI] [PubMed] [Google Scholar]
- 36.Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28(3):519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 37.Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol. Psychiatry. 2008;13(1):27–35. doi: 10.1038/sj.mp.4002066. [DOI] [PubMed] [Google Scholar]
- 38.Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat. Med. 1998;4(10):1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 39.Tecott LH, Sun LM, Akana SF, et al. Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature. 1995;374(6522):542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 40.Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J. Pharmacol. Exp. Ther. 1989;251(1):238–246. [PubMed] [Google Scholar]
- 41.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 2004;3(4):353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 42.Blum K, Braverman ER, Holder JM, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoactive Drugs. 2000;32(Suppl. i–iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 43.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 44.Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31(10):2091–2120. doi: 10.1038/sj.npp.1301051. [DOI] [PubMed] [Google Scholar]
- 45.Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav. Pharmacol. 2009;20(1):1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- 46.Jorgensen EA, Knigge U, Warberg J, Kjaer A. Histamine and the regulation of body weight. Neuroendocrinology. 2007;86(3):210–214. doi: 10.1159/000108341. [DOI] [PubMed] [Google Scholar]
- 47.Ookuma K, Sakata T, Fukagawa K, et al. Neuronal histamine in the hypothalamus suppresses food intake in rats. Brain Res. 1993;628(1–2):235–242. doi: 10.1016/0006-8993(93)90960-u. [DOI] [PubMed] [Google Scholar]
- 48.Lecklin A, Tuomisto L. The blockade of H1 receptors attenuates the suppression of feeding and diuresis induced by inhibition of histamine catabolism. Pharmacol. Biochem. Behav. 1998;59(3):753–758. doi: 10.1016/s0091-3057(97)00465-6. [DOI] [PubMed] [Google Scholar]
- 49.Han M, Deng C, Burne TH, Newell KA, Huang XF. Short- and long-term effects of antipsychotic drug treatment on weight gain and H1 receptor expression. Psychoneuroendocrinology. 2008;33(5):569–580. doi: 10.1016/j.psyneuen.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 51.Isse N, Ogawa Y, Tamura N, et al. Structural organization and chromosomal assignment of the human obese gene. J. Biol. Chem. 1995;270(46):27728–27733. doi: 10.1074/jbc.270.46.27728. [DOI] [PubMed] [Google Scholar]
- 52.Sinha MK, Opentanova I, Ohannesian JP, et al. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J. Clin. Invest. 1996;98(6):1277–1282. doi: 10.1172/JCI118913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Licinio J, Mantzoros C, Negrao Ab, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat. Med. 1997;3(5):575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 54.Sinha MK, Ohannesian JP, Heiman ML, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J. Clin. Invest. 1996;97(5):1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baumann H, Morella KK, White DW, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl Acad. Sci. USA. 1996;93(16):8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson DB, Ravussin E, Bennett PH, Bogardus C. Structure and sequence variation at the human leptin receptor gene in lean and obese Pima Indians. Hum. Mol. Genet. 1997;6(5):675–679. doi: 10.1093/hmg/6.5.675. [DOI] [PubMed] [Google Scholar]
- 57.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 58. Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am. J. Physiol. Endocrinol. Metab. 2009;297(6):E1247–E1259. doi: 10.1152/ajpendo.00274.2009. ▪ Comprehensive review of leptin molecular signaling as well as proposed mechanism of leptin resistance as it relates to weight gain/appetite regulation
- 59.Zhang F, Chen Y, Heiman M, Dimarchi R. Leptin: structure, function and biology. Vitam. Horm. 2005;71:345–372. doi: 10.1016/S0083-6729(05)71012-8. [DOI] [PubMed] [Google Scholar]
- 60.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem. Biophys. Res. Commun. 2001;283(4):982–988. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 61.Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG., Jr Regulation of Jak kinases by intracellular leptin receptor sequences. J. Biol. Chem. 2002;277(44):41547–41555. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 62.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Munzberg H, Myers MG., Jr The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J. Biol. Chem. 2007;282(42):31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 63.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat. Genet. 1996;14(1):95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 64.Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiol. Behav. 2008;94(5):637–642. doi: 10.1016/j.physbeh.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 66.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int. J. Obes. Relat. Metab. Disord. 2002;26(11):1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 67.Sentissi O, Grouselle D, Viala A, et al. Ghrelin and leptin levels in schizophrenic patients treated with antipsychotic monotherapy. J. Clin. Psychopharmacol. 2009;29(3):304–306. doi: 10.1097/JCP.0b013e3181a390ed. [DOI] [PubMed] [Google Scholar]
- 68.Wang HC, Yang YK, Chen PS, Lee IH, Yeh TL, Lu RB. Increased plasma leptin in antipsychotic-naive females with schizophrenia, but not in males. Neuropsychobiology. 2007;56(4):213–215. doi: 10.1159/000122267. [DOI] [PubMed] [Google Scholar]
- 69.Dieudonne MN, Pecquery R, Boumediene A, Leneveu MC, Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am. J. Physiol. 1998;274(6 Pt 1):C1645–C1652. doi: 10.1152/ajpcell.1998.274.6.C1645. [DOI] [PubMed] [Google Scholar]
- 70.Mizutani T, Nishikawa Y, Adachi H, et al. Identification of estrogen receptor in human adipose tissue and adipocytes. J. Clin. Endocrinol. Metab. 1994;78(4):950–954. doi: 10.1210/jcem.78.4.8157726. [DOI] [PubMed] [Google Scholar]
- 71.O’Brien SN, Welter BH, Mantzke KA, Price TM. Identification of progesterone receptor in human subcutaneous adipose tissue. J. Clin. Endocrinol. Metab. 1998;83(2):509–513. doi: 10.1210/jcem.83.2.4561. [DOI] [PubMed] [Google Scholar]
- 72.Elbers JM, Asscheman H, Seidell JC, Frolich M, Meinders AE, Gooren LJ. Reversal of the sex difference in serum leptin levels upon cross-sex hormone administration in transsexuals. J. Clin. Endocrinol. Metab. 1997;82(10):3267–3270. doi: 10.1210/jcem.82.10.4284. [DOI] [PubMed] [Google Scholar]
- 73.Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140(4):1567–1574. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- 74.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 75.Sentissi O, Epelbaum J, Olie JP, Poirier MF. Leptin and ghrelin levels in patients with schizophrenia during different antipsychotics treatment: a review. Schizophr. Bull. 2008;34(6):1189–1199. doi: 10.1093/schbul/sbm141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ribeiro R, Vasconcelos A, Costa S, et al. Overexpressing leptin genetic polymorphism (−2548 G/A) is associated with susceptibility to prostate cancer and risk of advanced disease. Prostate. 2004;59(3):268–274. doi: 10.1002/pros.20004. [DOI] [PubMed] [Google Scholar]
- 77.Ma D, Feitosa MF, Wilk JB, et al. Leptin is associated with blood pressure and hypertension in women from the national heart, lung, and blood institute family heart study. Hypertension. 2009;53(3):473–479. doi: 10.1161/HYPERTENSIONAHA.108.118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoffstedt J, Eriksson P, Mottagui-Tabar S, Arner P. A polymorphism in the leptin promoter region (−2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm. Metab. Res. 2002;34(7):355–359. doi: 10.1055/s-2002-33466. [DOI] [PubMed] [Google Scholar]
- 79.Hager J, Clement K, Francke S, et al. A polymorphism in the 5´ untranslated region of the human ob gene is associated with low leptin levels. Int. J. Obes. Relat. Metab. Disord. 1998;22(3):200–205. doi: 10.1038/sj.ijo.0800567. [DOI] [PubMed] [Google Scholar]
- 80.Dahlman I, Arner P. Obesity and polymorphisms in genes regulating human adipose tissue. Int. J. Obes. (Lond.) 2007;31(11):1629–1641. doi: 10.1038/sj.ijo.0803657. [DOI] [PubMed] [Google Scholar]
- 81.Rosmond R. Association studies of genetic polymorphisms in central obesity: a critical review. Int. J. Obes. Relat. Metab. Disord. 2003;27(10):1141–1151. doi: 10.1038/sj.ijo.0802397. [DOI] [PubMed] [Google Scholar]
- 82.Stratigopoulos G, Leduc CA, Matsuoka N, et al. Functional consequences of the human leptin receptor (LEPR) Q223R transversion. Obesity (Silver Spring) 2009;17(1):126–135. doi: 10.1038/oby.2008.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Furusawa T, Naka I, Yamauchi T, et al. The Q223R polymorphism in LEPR is associated with obesity in Pacific Islanders. Hum. Genet. 2010;127(3):287–294. doi: 10.1007/s00439-009-0768-9. [DOI] [PubMed] [Google Scholar]
- 84.Quinton ND, Lee AJ, Ross RJ, Eastell R, Blakemore AI. A single nucleotide polymorphism (SNP) in the leptin receptor is associated with BMI, fat mass and leptin levels in postmenopausal Caucasian women. Hum. Genet. 2001;108(3):233–236. doi: 10.1007/s004390100468. [DOI] [PubMed] [Google Scholar]
- 85.Yiannakouris N, Yannakoulia M, Melistas L, Chan JL, Klimis-Zacas D, Mantzoros CS. The Q223R polymorphism of the leptin receptor gene is significantly associated with obesity and predicts a small percentage of body weight and body composition variability. J. Clin. Endocrinol. Metab. 2001;86(9):4434–4439. doi: 10.1210/jcem.86.9.7842. [DOI] [PubMed] [Google Scholar]
- 86.Mammes O, Aubert R, Betoulle D, et al. LEPR gene polymorphisms: associations with overweight, fat mass and response to diet in women. Eur. J. Clin. Invest. 2001;31(5):398–404. doi: 10.1046/j.1365-2362.2001.00843.x. [DOI] [PubMed] [Google Scholar]
- 87.Heo M, Leibel RL, Fontaine KR, et al. A meta-analytic investigation of linkage and association of common leptin receptor (LEPR) polymorphisms with body mass index and waist circumference. Int. J. Obes. Relat. Metab. Disord. 2002;26(5):640–646. doi: 10.1038/sj.ijo.0801990. [DOI] [PubMed] [Google Scholar]
- 88.Paracchini V, Pedotti P, Taioli E. Genetics of leptin and obesity: a HuGE review. Am. J. Epidemiol. 2005;162(2):101–114. doi: 10.1093/aje/kwi174. [DOI] [PubMed] [Google Scholar]
- 89.Sun Q, Cornelis MC, Kraft P, et al. Genome-wide association study identifies polymorphisms in LEPR as determinants of plasma soluble leptin receptor levels. Hum. Mol. Genet. 2010;19(9):1846–1855. doi: 10.1093/hmg/ddq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang ZJ, Yao ZJ, Mou XD, et al. Association of −2548G/A functional polymorphism in the promoter region of leptin gene with antipsychotic agent-induced weight gain. Zhonghua Yi Xue Za Zhi. 2003;83(24):2119–2123. [PubMed] [Google Scholar]
- 91.Ruano G, Goethe JW, Caley C, et al. Physiogenomic comparison of weight profiles of olanzapine- and risperidone-treated patients. Mol. Psychiatry. 2007;12(5):474–482. doi: 10.1038/sj.mp.4001944. [DOI] [PubMed] [Google Scholar]
- 92.Yevtushenko OO, Cooper SJ, O'Neill R, Doherty JK, Woodside JV, Reynolds GP. Influence of 5-HT2C receptor and leptin gene polymorphisms, smoking and drug treatment on metabolic disturbances in patients with schizophrenia. Br. J. Psychiatry. 2008;192(6):424–428. doi: 10.1192/bjp.bp.107.041723. [DOI] [PubMed] [Google Scholar]
- 93.Gregoor JG, Van Der Weide J, Mulder H, et al. Polymorphisms of the LEP- and LEPR gene and obesity in patients using antipsychotic medication. J. Clin. Psychopharmacol. 2009;29(1):21–25. doi: 10.1097/JCP.0b013e31819359be. [DOI] [PubMed] [Google Scholar]
- 94.Gregoor JG, Mulder H, Cohen D, et al. Combined HTR2C-LEP genotype as a determinant of obesity in patients using antipsychotic medication. J. Clin. Psychopharmacol. 2010;30(6):702–705. doi: 10.1097/jcp.0b013e3181fa05a2. [DOI] [PubMed] [Google Scholar]
- 95.Opgen-Rhein C, Brandl EJ, Muller DJ, et al. Association of HTR2C, but not LEP or INSIG2, genes with antipsychotic-induced weight gain in a German sample. Pharmacogenomics. 2010;11(6):773–780. doi: 10.2217/pgs.10.50. [DOI] [PubMed] [Google Scholar]
- 96.Fernandez E, Carrizo E, Fernandez V, et al. Polymorphisms of the LEP- and LEPR genes, metabolic profile after prolonged clozapine administration and response to the antidiabetic metformin. Schizophr. Res. 2010;121(1–3):213–217. doi: 10.1016/j.schres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 97.Moons T, Claes S, Martens GJ, et al. Clock genes and body composition in patients with schizophrenia under treatment with antipsychotic drugs. Schizophr. Res. 2011;125(2–3):187–193. doi: 10.1016/j.schres.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 98.Ellingrod Vl, Bishop JR, Moline J, Lin YC, Miller DD. Leptin and leptin receptor gene polymorphisms and increases in body mass index (BMI) from olanzapine treatment in persons with schizophrenia. Psychopharmacol. Bull. 2007;40(1):57–62. [PubMed] [Google Scholar]
- 99.Srivastava V, Deshpande SN, Nimgaonkar VL, Lerer B, Thelma B. Genetic correlates of olanzapine-induced weight gain in schizophrenia subjects from north India: role of metabolic pathway genes. Pharmacogenomics. 2008;9(8):1055–1068. doi: 10.2217/14622416.9.8.1055. [DOI] [PubMed] [Google Scholar]
- 100. Templeman LA, Reynolds GP, Arranz B, San L. Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet. Genomics. 2005;15(4):195–200. doi: 10.1097/01213011-200504000-00002. ▪ First prospective pharmacogenetic study of weight gain and leptin polymorphisms in a primarily drug-naive population.
- 101.Zhang XY, Tan YL, Zhou DF, et al. Association of clozapine-induced weight gain with a polymorphism in the leptin promoter region in patients with chronic schizophrenia in a Chinese population. J. Clin. Psychopharmacol. 2007;27(3):246–251. doi: 10.1097/jcp.0b013e3180582412. [DOI] [PubMed] [Google Scholar]
- 102.Kang SG, Lee HJ, Park YM, et al. Possible association between the −2548A/G polymorphism of the leptin gene and olanzapine-induced weight gain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(1):160–163. doi: 10.1016/j.pnpbp.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Perez-Iglesias R, Mata I, Amado JA, et al. Effect of FTO, SH2B1, LEP, and LEPR polymorphisms on weight gain associated with antipsychotic treatment. J. Clin. Psychopharmacol. 2010;30(6):661–666. doi: 10.1097/jcp.0b013e3181fae248. [DOI] [PubMed] [Google Scholar]
- 104. Calarge CA, Ellingrod VL, Zimmerman B, Acion L, Sivitz WI, Schlechte JA. Leptin gene −2548G/A variants predict risperidone-associated weight gain in children and adolescents. Psychiatr. Genet. 2009;19(6):320–327. doi: 10.1097/ypg.0b013e3283328e06. ▪ The first study of leptin polymorphisms and antipsychotic weight gain in a pediatric population
- 105.Gregoor JG, van der Weide J, Loovers HM, van Megen HJ, Egberts TC, Heerdink ER. Association between LEP and LEPR gene polymorphisms and dyslipidemia in patients using atypical antipsychotic medication. Psychiatr. Genet. 2010;20(6):311–316. doi: 10.1097/YPG.0b013e32833b6378. [DOI] [PubMed] [Google Scholar]
- 106. Kluge M, Schuld A, Schacht A, et al. Effects of clozapine and olanzapine on cytokine systems are closely linked to weight gain and drug-induced fever. Psychoneuroendocrinology. 2009;34(1):118–128. doi: 10.1016/j.psyneuen.2008.08.016. ▪ First randomized study comparing immunomodulatory effects of olanzapine and clozapine with discussion of how cytokines may influence weight gain.
- 107.Vehof J, Risselada AJ, Al Hadithy AF, et al. Association of genetic variants of the histamine H1 and muscarinic M3 receptors with BMI and HbA1c values in patients on antipsychotic medication. Psychopharmacology (Berl.) 2011;216(2):257–265. doi: 10.1007/s00213-011-2211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(Suppl. 1):1–93. doi: 10.2165/00023210-200519001-00001. ▪▪ Comprehensive review of relationship of second-generation antipsychotics and metabolic complications.
- 109.Thompson A, Lavedan C, Volpi S. Absence of weight gain association with the HTR2C −759C/T polymorphism in patients with schizophrenia treated with iloperidone. Psychiatry Res. 2010;175(3):271–273. doi: 10.1016/j.psychres.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 110.Tartaglia LA. The leptin receptor. J. Biol. Chem. 1997;272(10):6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]