Summary
Background/purpose
The present study explores whether photodynamic therapy (PDT)-induced apoptosis can increase the number of tolerogenic regulatory T cells (Treg) and limit collateral tissue damage.
Methods
BALB/c mice were vaccinated subcutaneously three times with PDT-induced apoptotic or thaw-frozen, necrotic non-infected autologous macrophages (MΦ). Two weeks after the last vaccination, mice were infected intradermally with 106 promastigotes of Leishmania major.
Results
Mice that received PDT-induced apoptotic MΦ had fewer parasites and higher numbers of Treg than mice vaccinated with thaw-frozen necrotic MΦ or phosphate-buffered saline (PBS). Interleukin (IL)-4 and IL-6 were significantly suppressed, while IL-10 was increased in mice that received the PDT-induced apoptotic MΦ. The role of Treg in this process was confirmed through Treg transfer from vaccinated to naïve mice. Mice receiving CD4+CD25+ cells from mice vaccinated with PDT-induced apoptotic MΦ showed smaller lesions 3 weeks after infection and lower parasitic burdens than mice that received Tregs from mice of thaw-frozen necrotic MΦ or PBS groups. These changes were mediated by the depletion of CD3+CD8+ and NKT cells and increased levels of IL-12p70 and interferon-γ, IL-10, and TGF-β in the cutaneous leishmaniasis lesions.
Conclusion
Vaccination with apoptotic MΦ-induced tolerogenic Treg cells that limited collateral tissue damage and diminished parasitic burden.
Keywords: Leishmania, macrophage, tolerance, T regulatory cells, vaccination
The mode of cell death and uptake of dead cell by antigen-presenting cells influences a finely tuned balance between immune tolerance and a pathogenic auto-inflammatory response (1). Photodynamic therapy (PDT) induces apoptosis via permeabilization of the mitochondrial inner membrane by reactive oxygen species, which leads to mitochondrial swelling, cytochrome C release into the cytosol, and a caspase cascade (2–4). The uptake of apoptotic cells by immature dendritic cells has been shown to promote immune tolerance, whereas cell debris can elicit an inflammatory immune response (1). In the present study, we intend to test whether PDT-mediated apoptosis is tolerogenic and can suppress collateral tissue damage during infectious processes.
We chose cutaneous leishmaniasis (CL) in susceptible BALB/c mice as our experimental model as in this model, an immune response is not only incapable of eliminating the etiologic agent but also results in collateral tissue damage (5, 6). Progressive involvement of the new tissues may lead to the complete destruction and self-amputation of the affected parts of the host (7). The understanding of the mechanisms underlying the counter-regulation of this inflammatory reaction may have therapeutic implications.
There is some evidence that tolerance following presentation of apoptotic cells is mediated by CD4+CD25+Foxp3+ T regulatory (Treg) cells (8). Treg have been shown to control the severity of an inflammatory response, preserving surrounding tissues from unnecessary damage in many infectious diseases (9–11), including the early stage of CL (12, 13).
We found that vaccination with the apoptotic, but not necrotic non-infected macrophages (MZ), was beneficial for the restriction of parasitic replication during an early stage of Leishmania infection and was mediated by Treg activation. The adoptive transfer of spleen-derived Treg from mice vaccinated with PDT-treated apoptotic, but not necrotic MZ restrained disease progression. These changes were mediated by the depletion of CD3+CD8+ and NKT cells and increased levels of IL-12p70 and interferon (IFN)-γ, IL-10, and TGF-β in the CL lesions.
Methods
Parasites
Leishmania major NIH Friedlin V1 strain (MHOM/IL/80/FN), isolated from a patient with localized CL in Israel, was used in this study. All Leishmania parasites were cultured at 24 °C without CO2, in medium 199 (M199) supplemented with 20% heat-inactivated fetal calf serum, 60 ng/ml penicillin G sodium salt, 100 ng/ml kanamycin sulfate, 50 ng/ml flucytosine, 10 ng/ml chloramphenicol, 2 mM L-glutamine, 40 mM HEPES, 0.1 mM adenine (in 50 mM HEPES), 5 mg/ml hemin (in 50% triethanolamine), and 1 mg/ml 6-biotin (medium 199 complete; M199-C). Infective-stage metacyclic promastigotes were isolated from stationary cultures (4 days old) using a uniform procedure based on a modification of a recently described method of density gradient purification (14).
Bone-marrow-derived macrophage (BMDM) generation
Bone marrow cells were flushed from femurs and tibias of 4-week-old mice using a standard technique (15). A monoclonal antibody cocktail was used to deplete CD8+, CD45R+, I-A+, CD4+, and Ly6C/Ly6G+ cells. The progenitor cells were incubated at 37 °C with 5% CO2 in RPMI supplemented with 500 U/ml M-CSF, 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. On the seventh day of culture, the adherent cells (denoted MΦ, for BMDMs) were harvested.
Animals
Six- to 8-week-old female BALB/c mice were obtained from Charles River Laboratories (Wilmington, MA, USA) for this study. All animal procedures were performed according to protocols approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Infections
For in vitro infections, MΦ were co-incubated with metacyclic parasites at a ratio of 1 : 20 for 24 h (the infection rate was 98%, with 5–7 amastigotes/cell). For in vivo infection, 1×106 metacyclic parasites in 20 µl of phosphate-buffered saline (PBS) were inoculated intradermally into the ear of 6–8-week-old BALB/c female mice using a 27.5 G needle. The evolution of the lesion was monitored for 5 weeks. The following formula was used to estimate the volume of the lesion:
where Dmax is the maximal diameter of the lesion; Dmin was the minimal diameter of the lesion; and T was the thickness of the lesion. The diameters and thickness of the lesions were measured using a dual gauge Vernier Caliper.
Preparation of the apoptotic or necrotic MΦ
0.25 × 10 MΦ were incubated for 1 h in the dark with 50 nM of 5-ethylamino-9-diethylaminobenzo[a]phenol-selenazinium chloride (EtNBSe) in RPMI supplemented with 10% FCS at 37 °C. After centrifugation of the cell suspension at 400 g for 15 min at 4 °C, the medium containing EtNBSe was removed; the MΦ were then washed with RPMI, and a fresh medium was added. MΦ were transferred to 35 mm Petri dishes and then exposed to a light source (HPD diode laser source model 7401, High Power Devices Inc., North Brunswick, NJ, USA) with wavelength 635 nm and a fluence of 10J/cm2. The light intensity was measured using a Coherent Lasermate power meter (Coherent Inc., Santa Clara, CA, USA) and was 100 mW/cm2. Eighteen hours after PDT, the cellular debris was collected at a concentration of 1 × 106 cell equivalent/100 µl of PBS. As the lysate of apoptotic MΦ could not be stored, it was prepared freshly for each injection. The lysate of necrotic MΦ was prepared by three consecutively repeated freeze/thaw cycles of 1×106 cells/100 µl of PBS.
Injections of the lysates
Each mouse received three subcutaneous injections of 100 µl of lysate containing 1 × 106 cell equivalents within a 2-week interval. The control group of mice received three injections of PBS. For in vivo infections, 3 weeks after the last injection, 1×106 metacyclic parasites in 20 µl of PBS were inoculated intradermally into the ear of 6–8-week-old BALB/c female mice using a 27.5 G needle. The evolution of the lesion was monitored for 4–6 weeks.
Cytospin preparations
Cytospins were prepared using a Shandon Cytospin® 4 cytocentrifuge (Shandon Lipshaw, Pittsburgh, PA, USA) set at 60 g for 10 min. After Quick-Dip staining (Mercedes Medical, Sarasota, FL, USA), light microscopic analysis of the cells was performed on an Axiophot (Göttingen, Zeiss, Germany).
Quantification of parasite loads
Parasite loads in the ears were determined as described previously (16). The number of viable parasites in each tissue sample was determined from the highest dilution at which promastigotes could be grown after 7 days of incubation at 24 °C.
Histology
The excised ears or regional lymph nodes were fixed in 10% formalin and embedded in paraffin. After de-waxing and rehydration, 5 µm thick paraffin sections were stained with hematoxylin and eosin.
Adoptive cell transfer
Mice (10 per group) were immunized with the apoptotic or necrotic MΦ according to the protocol described above. Two weeks after the last immunization, the spleens were harvested. CD4+ T cells were pre-enriched by negative selection using magnetic beads, and CD4+ CD25+ and CD4+CD25− were purified by MACs® separation using the CD4+ CD25+ Regulatory T cell isolation Kit (Miltenyi Biotec Inc., Auburn, CA, USA). The cells were routinely analyzed by flow cytometry, and the purity of the CD4+ CD25+ and CD4+ CD25− T cell populations was > 90%. Ninety-five percent of CD4+ CD25+ Treg were Foxp3+ in all the groups as confirmed by flow cytometry. 1×106 cells/ mouse were infused into a non-infected non-immunized recipient. The day after adoptive transfer, all mice were infected with 1×106 L. major. After 3 weeks, mice were sacrificed and the number of parasites was established in a serial dilution assay.
CD25 depletion experiment
BALB/c mice were infected with 1×106 L. major intradermally in the ears. Three weeks after infection, mice received three intravenous injections every 4 days of 400 mg/mouse anti-CD25 mAb derived from hybridoma PC 61 5.3 (ATCC, Manassas, VA, USA) purified using T- and Melon Gel Kits (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions. Control groups for this experiment were (a) infected mice that received PBS injections, (b) infected mice that received serum-derived irrelevant IgG, and (c) infected mice without any treatment. Scarification of mice had been carried out 12 days after the first injection of mAb to prevent an immune response to foreign proteins.
Flow cytometry
The ventral and dorsal dermal sheets of the ears were separated and incubated for 1 h dermal side down in RPMI 1640 with penicillin/ streptomycin containing 125 U/ml collagenase A (Sigma-Aldrich, St. Louis, MO, USA). The dermal sheets from five animals were pooled, homogenized by tissue grinders (PowerGen 125, Fisher Emergo, Pittsburgh, PA, USA and Kontes Duall Tissue Grinder, Fischer Scientific, Pittsburgh, PA, USA), and filtered through a 70 µm nylon cell strainer, and washed twice in RPMI 1640 with 10% FCS. The cells were washed, fixed in 2% paraformaldehyde, and stained with antibodies directly conjugated to fluorescent dye. The following antibodies were used: anti-CD11c-PE (N418) for dendritic cell recognition from eBioscience (San Diego, CA, USA), anti-F4/80-Pe-Cy5 (BM8) for MΦ recognition from Caltag Laboratories, anti-CD3e-Alexa Fluor 647 (17A2) for T-lymphocyte recognition from eBioscience, anti-CD4-FITC (H129.19) for T helper/inducer lymphocyte recognition from BD Pharmingen (San Diego, CA, USA), anti-CD8a-FITC (53–6.7) for T cytotoxic/suppressor lymphocytes recognition from BD Pharmingen, anti-CD25-PE (PC61.5), anti-CD94-PE (18d3) for NK and NKT cells recognition from eBioscience, and anti-Foxp3-Pe-Cy5 (7979) for Treg recognition from eBioscience. The samples were also incubated with rat IgG2a-FITC and rat IgG2a-PE-Cy5, which served as isotype controls. The cells were analyzed using a FACSCalibur flow cytometer equipped with CellQuest Pro software (Becton Dickinson, Heidelberg, Germany). FlowJo software (Tree Star Inc., Ashland, OR, USA) was used for the analysis of data obtained during flow cytometry.
Evaluation of necrosis and apoptosis
To detect DNA fragmentation, terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-FITC nick end-labeling (TUNEL) was performed according to the manufacturer’s instruction (In Situ Cell Death Detection Kit, Fluorescein, Roche Diagnostics GmbH, Mannheim, Germany). The ability of annexin V to bind to phosphatidylserin on the external surface of the cells and DNA-intercalation by propidium iodide were also evaluated using the Annexin-V-FITC Kit (Beckman Coulter, Fullerton, CA, USA).
Cytometric bead array (CBA)
The level of cytokines (IL-6, IL-10, IFN-γ, TNF-α and IL-12p70) was measured in the CL lesion and draining lymph nodes using a BD™ Mouse Inflammation CBA kit (Becton Dickinson, San Diego, CA, USA). The assays were performed according to the manufacturer’s instructions.
ELISA
The amounts of TGF-β1, IL-4 and granzyme B were evaluated in the CL lesions by Qunatikine® according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Statistical analyses
Statistical analysis was based on the calculation of the arithmetic mean and standard deviation. One-way ANOVA was used for group comparisons. Bonferroni’s multiple comparison test was chosen when certain pairs had to be analyzed. The relationship between two or more variables was measured by correlation analysis (Pearson’s coefficient). A P-value of < 0.05 was considered statistically significant.
Results
MΦ treated with PDT underwent apoptosis
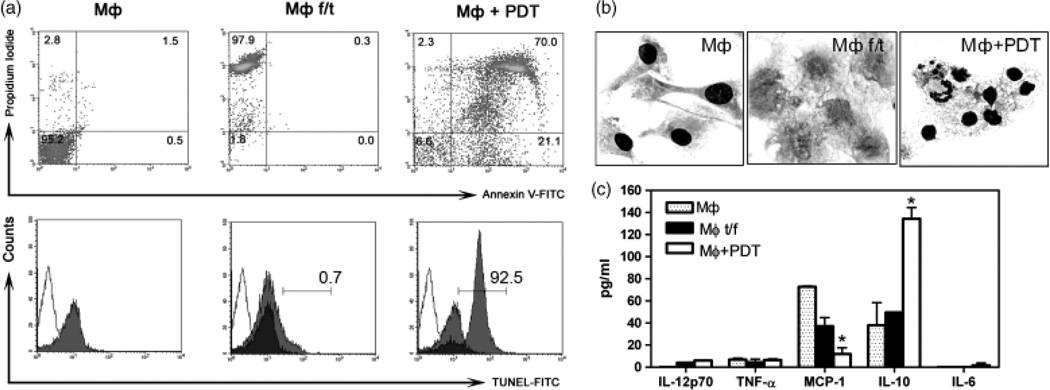
PDT induces apoptosis efficiently in non-infected MF (Fig. 1a and b). PDT yields a pure cellular lysate without any residual toxins. The PS induces apoptosis only during light irradiation, and is not harmful for cells under dark conditions. In contrast, trace amounts of apoptosis-inducing chemicals (e.g., camptothecin) in the vaccination lysate can cause leukopenia (17, 18) and may interfere with host immune response. For comparison, three freeze/thaw cycles were used. We acquired necrotic cells of 97.9% purity, without any features of apoptosis, while 3 h after PDT treatment, 91.1% of the cells were apoptotic as evident with annexin V and TUNEL-positive staining (Fig. 1a). Morphologically, necrotic cells lost their cellular integrity, while apoptotic cells exhibited typical features of chromatin condensation (Fig. 1b). CBA of the supernatant revealed a significantly higher amount of IL-10 and a lower level of MCP-1 in the PDT-treated, apoptotic MΦ in comparison with the necrotic MΦ (Fig. 1c), indicating that PDT induces apoptosis and stimulates the release of IL-10.
Fig. 1.
Photodynamic therapy (PDT) induces apoptosis in MΦ. (a) FACS analysis of the MΦ prepared by freeze/thaw cycles or by PDT. First row: Double staining with Annexin V and propidium iodide; Second row: FACS analysis of TUNEL-positive cells. □, control for autofluorescence of cells, without incubation with label solution; ■, negative control, incubated with label solution; ░, test sample, incubated with TUNEL reaction mixture. (b) Cytologic changes in MΦ f/t or MΦ+PDT. Non-treated MΦ are showed as control (MΦ). Cytospin preparation. Diff-Quick staining, × 1000. (c) Cytokine profiles of the supernatant of the MΦ f/t or the MΦ+PDT lysates. Non-treated MΦ provided as a control group (MΦ) (BD CBA). Data presented are representative of three experiments. *p < 0.05.
Vaccination with PDT-induced apoptotic MΦ diminished the load of L. major in CL lesions
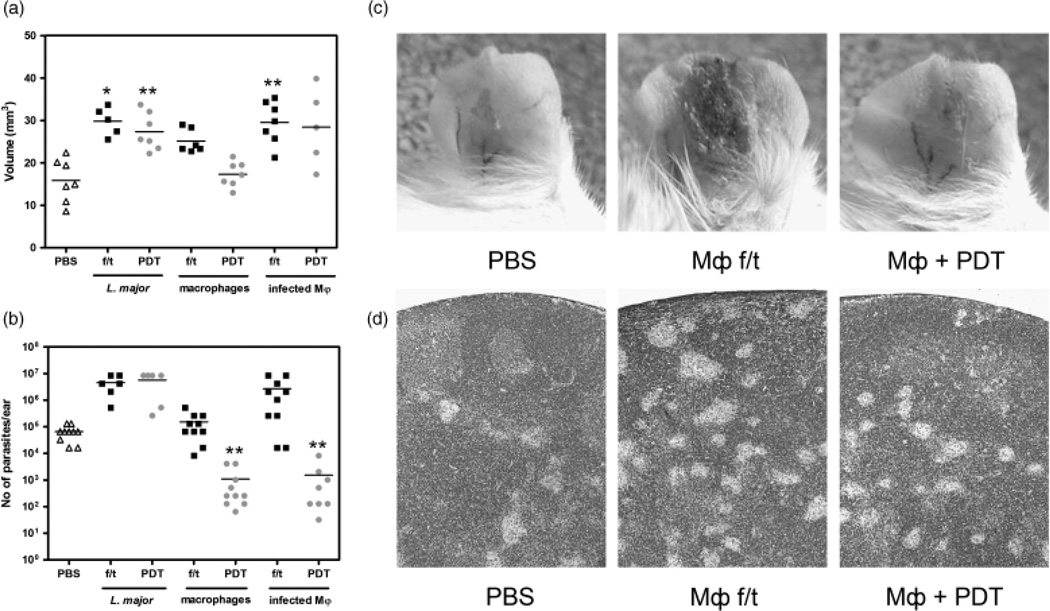
BALB/c mice received three vaccinations with PDT-treated or thaw/ frozen Leishmania parasites, MΦ, or L. major-infected MΦ (1×106 cells in 100 µl of PBS per mouse subcutaneously) over an interval of 2 weeks. Two weeks after the final booster, mice were infected with L. major. Measurable lesions were observed at week 1 in all groups. At week 3, the lesions in the mice vaccinated with Leishmania parasite or necrotic cells were significantly larger than those of the PBS control mice (Fig. 2a). Only the group of mice receiving the PDT-treated, apoptotic MΦ had the same level of inflammation as the control mice. By contrast, after vaccination with necrotic MΦ, the CL lesions exhibited a severe inflammatory reaction with hemorrhagic crusting on top of ulcerative lesions (Fig. 2c). The overall health of mice that received necrotic cells deteriorated to the point of cachexia, and by week 5 all of these mice had to be sacrificed due to the polyorgan pathology.
Fig. 2.
The lysate of PDT-treated, apoptotic MΦ. diminishes the load of Leishmania major. (a) The volume of CL lesions 3 weeks after infection in mice of the vaccine group. Mice were vaccinated with PDT-treated or thaw/frozen cells. Each mark represents an individual ear, and the dash represents the mean for each group. Two independent experiments.*P < 0.05, **P < 0.01 (b) The parasitic burdens were assessed by a serial dilution assay 3 weeks postinfection. Each mark represents an individual ear, and the dash represents the mean for each group. **P < 0.01. (c) Representative photos of mouse ears at 3 weeks post-infection in mice of the vaccine groups. (d) Histological picture of the draining (submandibular) lymph nodes demonstrates a different number of the infectious granulomata. H&E, × 100.
The average parasite number in the group of PDT-treated, apoptotic MΦ was 60.7 times less than that in the PBS group (P < 0.01) (Fig. 2b). Vaccination with the necrotic cells was not protective: the same level of parasites was observed after vaccination with necrotic MΦ and an even higher parasite burden was found after inoculation with necrotic Leishmania cells or necrotic infected MΦ. Histologic analysis of the draining (submandibular) lymph nodes confirmed these findings (Fig. 2d): in relation to PBS-treated mice, the number of infectious granulomata was visually lower with PDT-treated, apoptotic MΦ and higher after necrotic MΦ. We concluded that vaccination with PDT-treated, apoptotic MΦ was beneficial in reducing parasitic burden with some effect on the overall local inflammation.
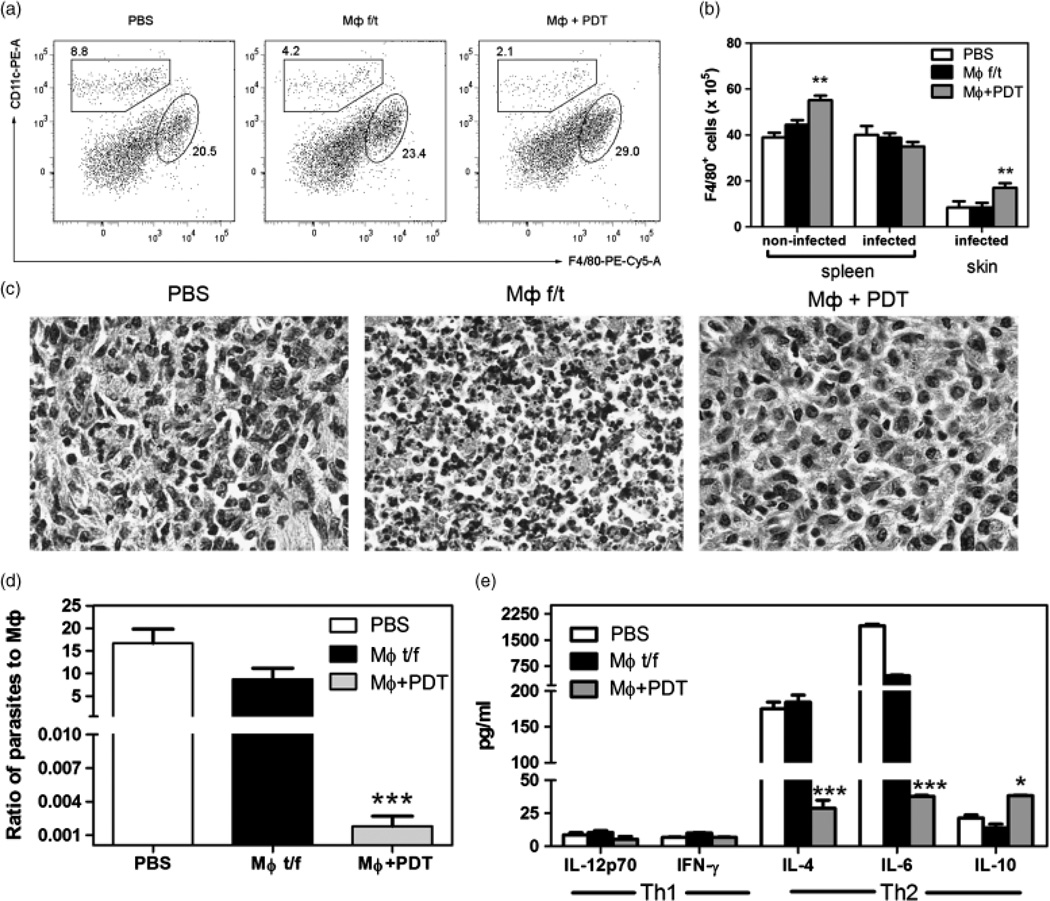
Vaccination with PDT-induced apoptotic MΦ increased the number of MΦ in CL lesions and diminished T helper type-2 (Th2) cytokines
A higher number of MΦ was observed in spleens and CL lesions of mice that received the PDT-treated, apoptotic MΦ than in mice receiving necrotic MΦ or PBS (Fig. 3a and b). The histological assessment of CL lesions confirmed our findings, revealing a dense neutrophilic infiltrate in the lesions of mice vaccinated with necrotic MΦ, in contrast to the almost homomor-phous macrophagal infiltrates in the case of vaccination with PDT-treated, apoptotic MΦ. Control mice receiving PBS only demonstrated typical mixed lymphocytic-macrophagal granulomatous infiltrate (Fig. 3c).
Fig. 3.
Vaccination with PDT-treated, apoptotic MΦ increased the number of MΦ and suppressed Th2 immune response. (a) Dot plots for F4/80 and CD11c expression in the spleens of vaccinated non-infected mice. (b) Absolute numbers of F4/804cells in and ears with the CL lesions in vaccinated non-infected or infected mice. Mean ± SD for each group. ** P < 0.01 in comparison with MΦ f/t. (c) Histological picture of the CL lesions of vaccinated mice 3 weeks after infection. H&E, × 800. (d) Ratio of parasites to macrophages in the CL ears of vaccinated mice 3 weeks after infection. (e) Cytokine production in the CL lesions of vaccinated mice 3 weeks after infection. **P < 0.05, ** P < 0.001 in comparison with PBS.
An increase in the number of MΦ was accompanied by changes in the ratio of parasites to MΦ. After vaccination with PBS or necrotic MΦ, there were 10–15-fold more parasites than MΦ (15 : 1) in CL lesions. Conversely, after vaccination with PDT-treated, apoptotic MΦ, there were more MΦ in the CL lesions than parasites (Fig. 3d): mice that received three injections of PDT-treated, apoptotic MΦ had 554.5±30.6 MΦ for every parasite (1 : 0.06).
While the mice of the PDT-treated, apoptotic MΦ group revealed no changes in the level of IL-12p70 and IFN-γ, significantly lower levels of IL-4 and IL-6 were observed, when compared with the necrotic MΦ or the PBS groups (Fig. 3e). A twofold increase in the level of IL-10 was also observed in mice that received PDT-treated, apoptotic MΦ compared with the PBS group. We have measured the level of IL-17 in the CL lesions obtained 3 weeks after a different regimen of vaccination. The amount of IL-17 was 1.5 ± 0.5 pg/ml in the control group of mice receiving PBS, IL-17 was 0.8 ± 0.5 pg/ml in the group of mice vaccinated with PDT-treated, apoptotic MΦ, and IL-17 was 38.9 ± 3.2 pg/ml in the group of mice vaccinated with necrotic MΦ.
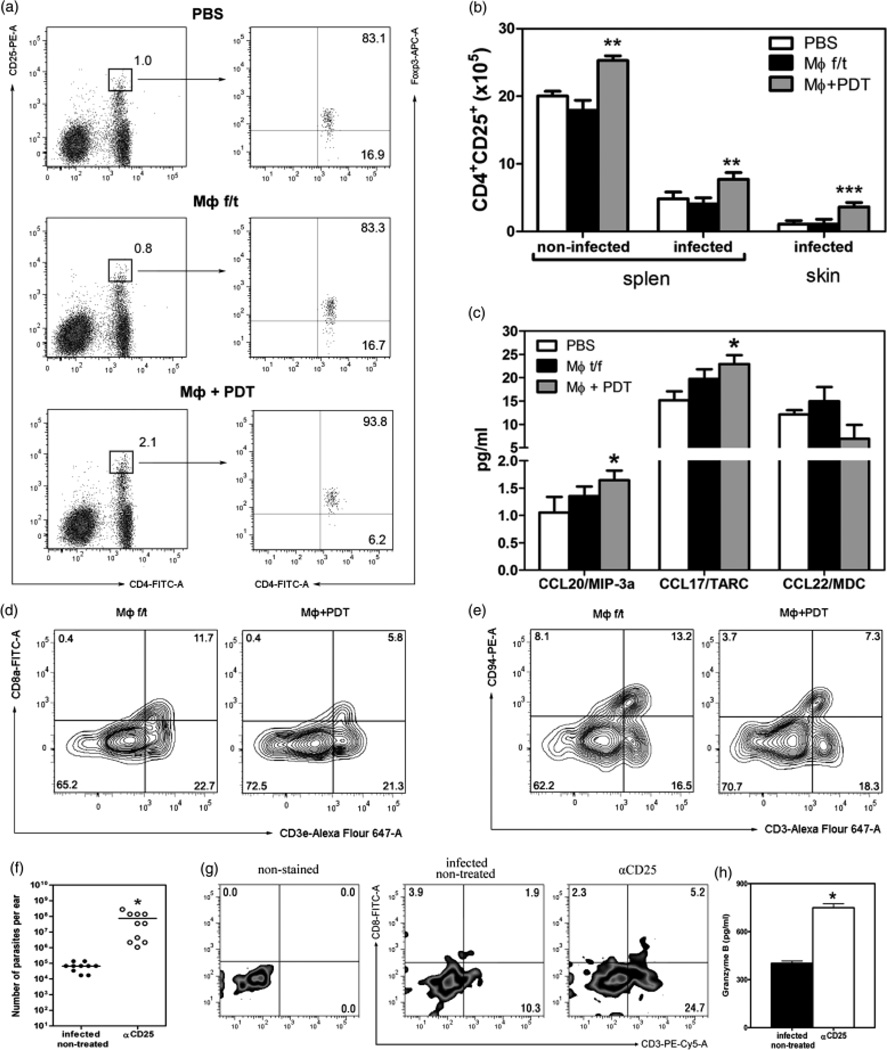
Vaccination with PDT-treated, apoptotic MΦ increased the number of Treg and decreased the percentage of CD8 T and NK cells
We found twice as many CD4+ CD25+ Foxp3+ (Treg) cells in the group of mice that received the PDT-treated, apoptotic MΦ than in the PBS group (Fig. 4a). The CL lesions of mice that received apoptotic MΦ accumulated more CD4+ CD25+ Foxp3+ cells than the groups of comparison (Fig. 4b). A positive correlation was established between the number of Treg and the number of MΦ in the skin (r = 0.93, P < 0.001).
Fig. 4.
Vaccination with PDT-treated, apoptotic MΦ affected the number of Treg, CD8 Tand NKT cells (vaccinated mice 3 weeks after infection). (a) Percentage of CD4+ CD25high in the spleens. (b) The absolute number of CD4+ CD25+ in the spleen and skin. **P < 0.01, ***P < 0.001. Five mice per groups of three independent experiments. (c) Chemokines in the CL lesions. *P < 0.05. (d) CD3 and CD8 in the spleens of vaccinated non-infected mice. Five mice per group. (e) CD3 and CD94 in the spleens of vaccinated non-infected mice. CD25-depletion in infected mice. (f) Parasite load. *P < 0.001 (g) CD3 and CD8a in the CL lesions after CD25 depletion. (h) The granzyme B in the CL lesions after CD25 depletion. Ten ears per sample. *P < 0.05.
CCR4 and CCR6 signaling has been demonstrated to guide Treg to sites of inflammation, where they can attenuate T-cell activation (19, 20). Lesional Langerhan’s cells and macrophages are the source of the chemokines CCL17 and CCL22, the ligand for CCR4 (19). Virtually all peripheral blood CD4+ CD25+ Foxp3+ Treg express high levels of the chemokine receptor CCR4, and 73% of Treg express CCR6 (20). Supernatants from the CL lesions revealed significantly higher level of CCL20 (ligand for CCR6) and CCL17 (but not CCL22) in the group of mice that received the apoptotic MΦ, than in the control group that was injected with PBS (Fig. 4c).
Mice vaccinated with PDT-treated, apoptotic MΦ displayed a 2.3-fold decrease in the percentage of CD8+ T cells and a 2.6-fold decrease in the percentage of NK cells in the spleen as compared with the necrotic MΦ group (Fig. 4d and e). No difference was observed in the percentage of CD3+ γSTCR+ cells (data not shown). The low level of Granzyme B in the CL lesions was found to be 295.43 ±35.94 pg/ml in the apoptotic MΦ mice as compared with 401.61 ± 15.00 pg/ml in infected non-treated mice.
A significant increase in the load of Leishmania parasites in CD25-depleted mice was associated with an increase of CD8+ T cells in the leishmanial granulomata (Fig. 4f and g), which was accompanied by a 1.86-fold increase in the amount of Granzyme B (Fig. 4h).
Treg are responsible for suppression of inflammation and reduction of parasitic burden after vaccination with PDT-treated, apoptotic MΦ
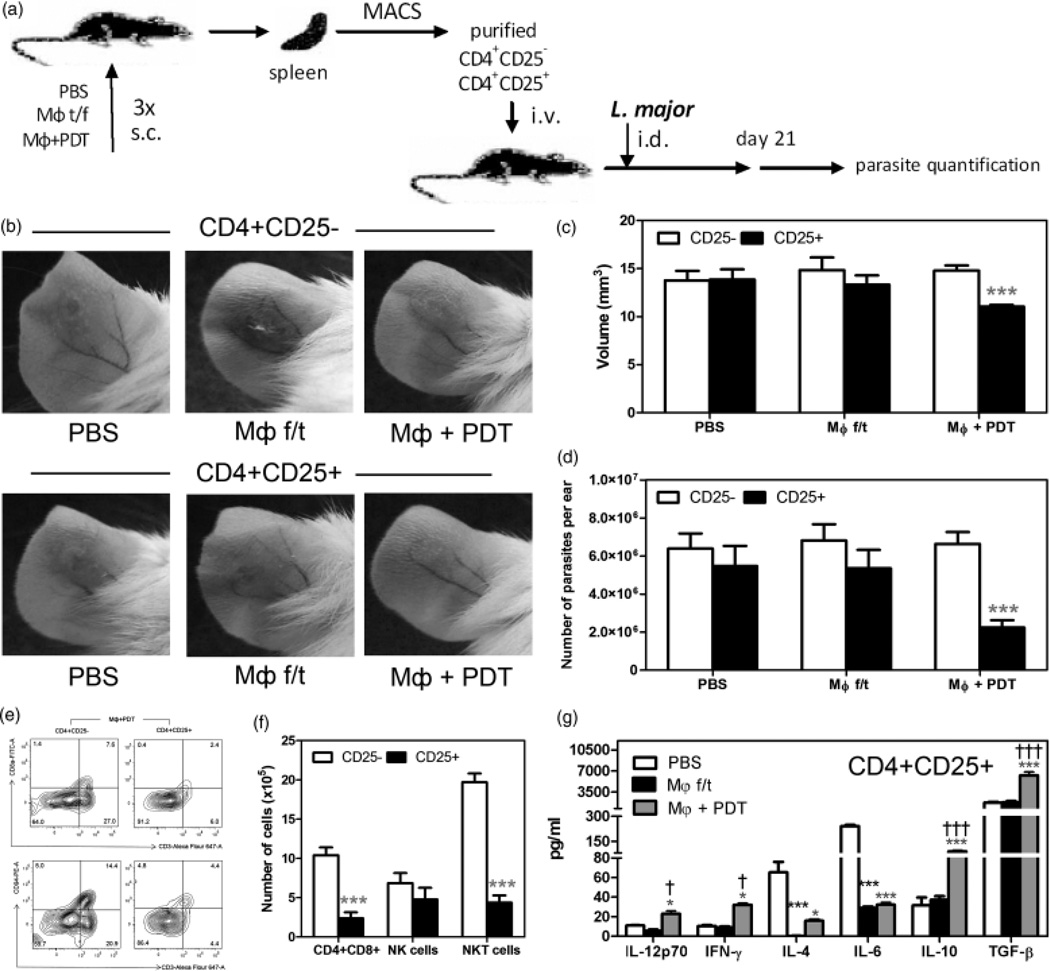
To test our hypothesis that Treg induced by PDT-treated, apoptotic MΦ had a protective effect on the course of CL, we isolated CD4+ CD25− and CD4+ CD25+ cells from the spleens of mice that received vaccinations with necrotic or PDT-treated, apoptotic MΦ. The cells were adoptively transferred (1×106 cells/mouse) into naïve BALB/c mice followed by L. major infection a day later (Fig. 5a). Mice receiving Treg from the mice vaccinated with PDT-treated, apoptotic MΦ showed significantly smaller CL lesions 3 weeks after infection (Fig. 5b and c) and lower parasitic burden (Fig. 5d) than mice that received necrotic MΦ or PBS injection.
Fig. 5.
Adoptive transfer of Treg from mice vaccinated with apoptotic MΦ decreased the parasite burden and overall inflammation. (a) Schema of experiment. (b) The CL lesions of mice that underwent adoptive transfer. (c) The volume of CL lesions. ***P < 0.001 in comparison with CD4+ CD25−. (d) The parasitic burden. (e) CD3, CD8, and CD94 in the spleen after the adoptive transfer of cells from mice vaccinated with PDT-treated, apoptotic MΦ. (f) The absolute number of effector cells in the CL lesions in mice of adoptive transfer group. ***P < 0.001 in comparison with CD4+ CD25−. (g) Cytokines in the CL lesions after the adoptive transfer of CD4+ CD25+. *P < 0.05, ***P < 0.001 in comparison with PBS; †P < 0.05, †††P < 0.001 in comparison with MΦ f/t.
Consistent with our previous findings, the percentage of CD8+ T cells was significantly reduced after the adoptive transfer of MΦ apoptosis-induced Treg in comparison with mice that received CD4+ CD25− cells from the same group of mice vaccinated with apoptotic MΦ (Fig. 5e and f). While the number of NK cells remains to the same in the group of CD4+ CD25− and CD4+ CD25− cells, the number of NKT cells was reduced 4.5 times.
The statistically higher level of IL-10 and TGF-β was observed in the group of PDT-treated, apoptotic MΦ (Fig. 5g). Suppression of Th2 cytokines (IL-4 and IL-6) was presented in both experimental groups, while an activation of Th1 cytokines (IL-12p70 and IFN-γ) was a feature only of the PDT-treated, apoptotic MΦ group.
Taken together, these results confirmed that Treg induced by PDT-treated, apoptotic MΦ but not necrotic MΦ were responsible for diminishing the inflammation and parasitic burden in mice infected with L. major.
Discussion
In the present study, we found that vaccination with apoptotic MΦ was beneficial for the limitation of CL progression by induction of Treg. The use of a vaccine that is unrelated to Leishmania parasites to induce a protective immune response is unique in its creation of an environment conducive to the development of Treg. In our study, vaccination with PDT-treated, apoptotic MΦ appeared to increase the number of Treg. Partial protection was conferred by the inhibition of IL-4, and IL-6 cytokines and the depletion of CD3+CD8+ and NKT cells.
What becomes evident from this study is the influence of Treg on Th1/Th2 dichotomy. Vaccination with PDT-treated, apoptotic MΦ had a protective effect due to its inhibition of Th2 cytokine production. While an activation of the Th1-immune response was observed in an adoptive transfer experiment with CD4+CD25+ splenocytes from mice vaccinated with PDT-treated, apoptotic MΦ, direct vaccination did not result in a bust of Th1. Treg have been shown to control Th1 immune response in C57BL/6 mice (12), or Th2 cell response in BALB/c (21). For the successful inhibition of the cytokine production and proliferation of differentiated and established T cells, Treg should be pre-activated (22). Because of the non-specific nature of our vaccine (MΦ were not exposed to Leishmania parasites), we hypothesize that the suppression observed after Treg induction may also be non-specific for an active immune response. BALB mice develop non-protective Th2 responses and suffer progressive disease after infection with L. major. These outcomes depend on the production of IL-4 and IL-13 (23–25). IL-4 inhibits leishmanicidal activity of infected MΦ by decreasing nitric oxide generation (26). In contrast, the Th1 host immune response is an essential prerequisite for protection against Leishmania (27, 28). IL-12p70 stimulates NK and T cells to produce IFN-γ, which induces leishmanicidal nitric oxide (29, 30). It would be valuable to see whether after vaccination with apoptotic MΦ, C57BL/6 mice would still maintain a resistance to Leishmania infection.
A high level of TGF-β was observed in the CL lesions after the adoptive transfer of CD4+ CD25+ cells isolated from mice vaccinated with PDT-treated, apoptotic MΦ. Previous reports have emphasized that tolerogenic Treg cells require TGF-β in vivo to carry out their suppression of the Th2 immune response (31), while the blockage of membrane-bound TGF-β nullifies its protective effect (32). This effect has also been observed in the low-dose peptide tolerance therapy of lupus, where the low-dose peptide simultaneously induced Treg cells and suppressed Th17 cells by increasing TGF-β and decreasing IL-6 production (33). Thus, in our case, TGF-β that was induced by vaccination with the apoptotic (but not necrotic) MΦ could be responsible for the suppression of Th2 cytokines in the CL lesions. Comparing the protective potential of Th2 cytokines and TGF-β in adoptive immunity against Salmonella, Kochetkova et al. (34) found that the introduction of anti-TGF-β mAb nearly completely neutralized the effect provided by the adoptively transferred CD4+ T cells, while anti-IL-4 monoclonal antibody treatment was beneficial only on 60%. Thus, while TGF-β seems to be essential for protection, other mechanisms may exert an additive effect in restraining pathogens.
We found a hyperergic immune response in case of vaccination with necrotic MΦ. The CL lesions developed quickly with massive purulent exudation and tissue destruction. Moreover, the overall health of mice that received necrotic cells deteriorated to the point of cachexia, and by week 5 all of these mice had to be sacrificed due to the polyorgan pathology. A dense monomorphous neutrophilic infiltrate was observed in the lesions of mice vaccinated with necrotic MΦ. While IL-6 was reduced in comparison with the control, overall the cytokines IL-12p70, IFN-γ and IL-4 were at a similar level. IL-17 was upregulated in this case, which could explain the recruitment of neutrophils at the place of inflammation. Treg may interfere with this process (35, 36). Recent publications indicate that IRF4 may play an important role in the development of both Th2 and Th17 cells (37). It was shown that IRF4 expression segregates in Th2 cells between IL-10 high and low cells (38). The discrepancy in IL-10 and IL-4 secretion between the two groups in our experiments (high level of IL-10 and low level IL-4 in the group of PDT-treated, apoptotic MΦ vs. low level of IL-10 and high level of IL-4 in the group of necrotic MΦ) could be explained by different IRF4 expression, and future study will be required to prove this hypothesis.
It was interesting to learn that apoptotic MΦ not exposed to Leishmania could induce Treg. Such a finding had not been reported previously for Leishmania infection. As the main function of Treg is to suppress the immune response, their final effect will strictly depend on the environment in which they arise. The Th2 immune response dominates in BALB/c mice during CL; hence, Treg activation would be beneficial, while suppression of the protective Th1 immune response in C57BL/6 mice may delay parasite clearance.
In this study, we found an increase of CD4+CD25+Foxp3+ Treg cells and MΦ, along with a decrease of CD8+ T cells and NKT cells after vaccination with PDT-treated, apoptotic non-infected MΦ. It has been shown previously that Treg can suppress the proliferation of CD8+ cells in vitro (39–42), and that in vivo Treg are capable of interfering with the proliferation of CD8+ cells (21). As we demonstrated, the removal of Treg transiently aggravated the disease by releasing CD8+ cells from Treg suppression for MΦ killing, which was in accordance with previous publications (43).
The induction and maintenance of the tolerance is a highly developing field in the therapy of autoimmune conditions (33). While the significance of an autoimmune component in CL is disputable, the field of infectious immunology may offer some new insights into the mechanisms of immune surveillance and immuoregulation. We have shown that apoptotic MΦ could be potent Treg elicitors. This strategy could be suitable for developing supportive therapy for transplant patients, where induction of Treg cells would be beneficial for the suppression of donor-organ rejection.
In summary, we demonstrated that PDT-treated, apoptotic MΦ have a protective effect on BALB/c mice by diminishing the load of parasites during experimental murine CL. While a significant role in Leishmania control is played by the timely switch between Th1 and Th2, this change may be under the regulation of Treg cells.
Summary statement
Tolerance is a state of immunological non-reactivity to an antigen resulting from a previous exposure to the same antigen. While it protects from attacks of the immune system against self-antigens, in case of intracellular parasitism tolerance, it could be a beneficial for parasites facilitating their escape from the immune surveillance. We found that vaccination with the apoptotic, but not necrotic non-infected macrophages was beneficial for the restriction of parasitic replication. This anti-tolerogenic process was mediated by Treg activation and by the depletion of CD3+CD8+ and NKT cells.
Acknowledgements
We are grateful to Mary Ann McDowell, PhD (University of Notre Dame, Notre Dame, IN, USA), for providing us with the L. major V1 strain. We are appreciative of the assistance of Andrea Johnston, Ezra Mirvish and Sue McCann with the manuscript preparation. We thank Kasper Hoebe (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA) and Thomas A. Ferguson (Department of Ophthalmology and Visual Sciences, Washington University School of Medicine, St. Louis, MO, USA) for critically reading our manuscript. This work was supported by the Department of Defense Medical Free Electron Laser Program Grant No. FA9550-04-1-0079 (to T. Hasan), Harvard Skin Disease Research Center Pilot and Feasibility Grant (to O. Akilov).
Footnotes
Conflicts of interest:
None declared.
References
- 1.Viorritto IC, Nikolov NP, Siegel RM. Autoimmunity versus tolerance: can dying cells tip the balance? Clin Immunol. 2007;122:125–134. doi: 10.1016/j.clim.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001;276:47379–47386. doi: 10.1074/jbc.M107678200. [DOI] [PubMed] [Google Scholar]
- 3.Ball DJ, Luo Y, Kessel D, Griffiths J, Brown SB, Vernon DI. The induction of apoptosis by a positively charged methylene blue derivative. J Photochem Photobiol B. 1998;42:159–163. doi: 10.1016/s1011-1344(98)00061-x. [DOI] [PubMed] [Google Scholar]
- 4.Noodt BB, Rodal GH, Wainwright M, et al. Apoptosis induction by different pathways with methylene blue derivative and light from mitochondrial sites in V79 cells. Int J Cancer. 1998;75:941–948. doi: 10.1002/(sici)1097-0215(19980316)75:6<941::aid-ijc18>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Kayes SG. Nonspecific allergic granulomatosis in the lungs of mice infected with large but not small inocula of the canine ascarid, Toxocara canis. Clin Immunol Immunopathol. 1986;41:55–65. doi: 10.1016/0090-1229(86)90051-6. [DOI] [PubMed] [Google Scholar]
- 6.Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunol. 2006;28:515–523. doi: 10.1111/j.1365-3024.2006.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrade ZA, Reed SG, Roters SB, Sadigursky M. Immunopathology of experimental cutaneous leishmaniasis. Am J Pathol. 1984;114:137–148. [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lym-phoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 9.Godkin A, Ng WF, Gallagher K, Betts G, Thomas HC, Lechler RI. Expansion of hepatitis C-specific CD4+CD25+ regulatory T cells after viral clearance: a mechanism to limit collateral damage? J Allergy Clin Immunol. 2008;121:1277–1284. doi: 10.1016/j.jaci.2008.01.070. e1273. [DOI] [PubMed] [Google Scholar]
- 10.Guilliams M, Bosschaerts T, Herin M, et al. Experimental expansion of the regulatory T cell population increases resistance to African trypanosomiasis. J Infect Dis. 2008;198:781–791. doi: 10.1086/590439. [DOI] [PubMed] [Google Scholar]
- 11.Vojdani A, Erde J. Regulatory T cells, a potent immunoregulatory target for CAM researchers: modulating tumor immunity, autoimmunity and alloreactive immunity (III) Evid Based Complement Alternat Med. 2006;3:309–316. doi: 10.1093/ecam/nel047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD41CD251 regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 13.Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged ‘‘silent’’ phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000;165:969–977. doi: 10.4049/jimmunol.165.2.969. [DOI] [PubMed] [Google Scholar]
- 14.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 15.Flamant S, Lebastard M, Pescher P, Besmond C, Milon G, Marchal G. Enhanced cloning efficiency of mouse bone marrow macrophage progenitors correlates with increased content of CSF-1 receptor of their progeny at low oxygen tension. Microbes Infect. 2003;5:1064–1069. doi: 10.1016/j.micinf.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Belkaid Y, Kamhawi S, Modi G, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson-Miller CL, May RD, Tomaszewski J, et al. Differential toxicity of camptothecin, topotecan and 9-aminocamptothecin to human, canine, and murine myeloid progenitors (CFU-GM) in vitro. Cancer Chemother Pharmacol. 1997;39:467–472. doi: 10.1007/s002800050600. [DOI] [PubMed] [Google Scholar]
- 18.Schaeppi U, Fleischman RW, Cooney DA. Toxicity of camptothecin (NSC-100880) Cancer Chemother Rep. 1974;5:25–36. [PubMed] [Google Scholar]
- 19.Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25 high Foxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 21.Aseffa A, Gumy A, Launois P, MacDonald HR, Louis JA, Tacchini-Cottier F. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+T cells. J Immunol. 2002;169:3232–3241. doi: 10.4049/jimmunol.169.6.3232. [DOI] [PubMed] [Google Scholar]
- 22.Stassen M, Jonuleit H, Muller C, et al. Differential regulatory capacity of CD25+T regulatory cells and preactivated CD25+T regulatory cells on development, functional activation, and proliferation of Th2 cells. J Immunol. 2004;173:267–274. doi: 10.4049/jimmunol.173.1.267. [DOI] [PubMed] [Google Scholar]
- 23.Louis JA, Gumy A, Voigt H, Launois P, Rocken M. The use of the murine model of infection with Leishmania major to reveal the antagonistic effects that IL-4 can exert on T helper cell development and demonstrate that these opposite effects depend upon the nature of the cells targeted for IL-4 signaling. Pathol Biol (Paris) 2003;51:71–73. doi: 10.1016/s0369-8114(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 24.Locksley RM, Louis JA. Immunology of leishmaniasis. Curr Opin Immunol. 1992;4:413–418. doi: 10.1016/s0952-7915(06)80032-4. [DOI] [PubMed] [Google Scholar]
- 25.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 26.Vouldoukis I, Becherel PA, Riveros-Moreno V, et al. Interleukin-10 and interleukin-4 inhibit intracellular killing of Leish-mania infantum and Leishmania major by human macrophages by decreasing nitric oxide generation. Eur J Immunol. 1997;27:860–865. doi: 10.1002/eji.1830270409. [DOI] [PubMed] [Google Scholar]
- 27.Raupach B, Kaufmann SH. Immune responses to intracellular bacteria. Curr Opin Immunol. 2001;13:417–428. doi: 10.1016/s0952-7915(00)00236-3. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res Immunol. 1995;146:423–431. doi: 10.1016/0923-2494(96)83011-2. [DOI] [PubMed] [Google Scholar]
- 29.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 30.Assreuy J, Cunha FQ, Epperlein M, et al. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major . Eur J Immunol. 1994;24:672–676. doi: 10.1002/eji.1830240328. [DOI] [PubMed] [Google Scholar]
- 31.Montagnoli C, Fallarino F, Gaziano R, et al. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J Immunol. 2006;176:1712–1723. doi: 10.4049/jimmunol.176.3.1712. [DOI] [PubMed] [Google Scholar]
- 32.Gregg RK, Jain R, Schoenleber SJ, et al. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173:7308–7316. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- 33.Kang HK, Liu M, Datta SK. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J Immunol. 2007;178:7849–7858. doi: 10.4049/jimmunol.178.12.7849. [DOI] [PubMed] [Google Scholar]
- 34.Kochetkova I, Trunkle T, Callis G, Pascual DW. Vaccination without autoantigen protects against collagen II-induced arthritis via immune deviation and regulatory T cells. J Immunol. 2008;181:2741–2752. doi: 10.4049/jimmunol.181.4.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaffar Z, Ferrini ME, Girtsman TA, Roberts K. Antigen-specific Treg regulate Th17-mediated lung neutrophilic inflammation, B-cell recruitment and polymeric IgA and IgM levels in the airways. Eur J Immunol. 2009;39:3307–3314. doi: 10.1002/eji.200939498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baba N, Rubio M, Sarfati M. Interplay between CD45RA+ regulatory T cells and TNF-alpha in the regulation of human Th17 differentiation. Int Immunol. 2010;22:237–244. doi: 10.1093/intimm/dxp130. [DOI] [PubMed] [Google Scholar]
- 37.Brustle A, Heink S, Huber M, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 38.Ahyi AN, Chang HC, Dent AL, Nutt SL, Kaplan MH. IFN regulatory factor 4 regulates the expression of a subset of Th2 cytokines. J Immunol. 2009;183:1598–1606. doi: 10.4049/jimmunol.0803302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+T cells regulate virus-specific primary and memory CD8+T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kursar M, Bonhagen K, Fensterle J, et al. Regulatory CD4+CD25+T cells restrict memory CD8+T cell responses. J Exp Med. 2002;196:1585–1592. doi: 10.1084/jem.20011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai Z, Li Q, Wang Y, et al. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+T cells via a CD30-dependent mechanism. J Clin Invest. 2004;113:310–317. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+T cells contribute to the control of memory CD8+T cells. Proc Natl Acad Sci USA. 2002;99:8832–8837. doi: 10.1073/pnas.132254399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji J, Masterson J, Sun J, Soong L. CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J Immunol. 2005;174:7147–7153. doi: 10.4049/jimmunol.174.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]