Abstract
Background
Type 1 Diabetes is an autoimmune disease resulting from the destruction of pancreatic beta-cells. One of the main antigens targeted in this auto reactive response is insulin. It has been shown that insulin is expressed in small amounts in the thymus, and more specifically in the medullary thymic epithelial cells (mTECs), which also express a variety of other tissue-specific antigens. This thymic expression enables the maintenance of self-tolerance, and is essential in preventing auto-immune disease. Our laboratory has created a mouse mTEC clonal cell line specifically expressing insulin in order to better understand the regulatory mechanisms of this ectopic expression of insulin. In this study, we compared the insulin expressing cell line to an insulin non-expressing mTEC line by genome-wide expression profiling.
Results
The most important difference was overexpression of CD34 in the insulin expressing clone, confirmed by Real-time Rt-PCR and flow cytometry. Cells in the thymus expressing higher levels of CD34 were found to contain higher levels of insulin and, to a lesser extent, Aire, a master regulator of self-antigen expression in the thymus. The cells expressing CD34 were not enriched in CD80, a known mTEC maturity marker.
Conclusion
CD34 may be a specific marker for functionality, with some specificity for insulin.
Keywords: Autoimmunity, Ifn-γ, Insulin, mTEC, Self-antigens, Thymus, Tnf-α
BACKGROUND
Type 1 diabetes (T1D) is caused by an autoimmune response against the beta cells of the pancreas, which results in the disruption of production of insulin in the body. This autoautoimmune response is thought to be directed against insulin, an important antigen in type 1 diabetes (Janeway et al., 2001). It has previously been shown that small amounts of insulin are expressed in the thymus (Chentoufi et al., 2004; Derbinski et al., 2001). This has also been found to be true for a large number and variety of other tissue-specific antigen (Derbinski et al., 2001). As the thymus, through the action of the medullary thymic epithelial cells (mTECs), is the site for negative selection of self-reactive T cells, this ectopic expression is believed to be essential in this purging process. The level of insulin expression in the human thymus is inversely correlated to susceptibility to type 1 diabetes, as a genetic variant determining lower insulin expression in the thymus confers susceptibility (Vafiadis et al., 1997). Furthermore, the number of functional insulin gene copies in the mouse thymus, inversely correlates to levels of insulin auto-reactive T cells in circulation (Chentoufi and Polychronakos, 2002) and, against the NOD background, diabetes (Thebault-Baumont et al., 2003). The expression of insulin, as well as the level at which it is being expressed, are essential components to establishing and maintaining self-tolerance to this particular antigen. In particular, the lack of expression of insulin specifically and only in the thymus leads to an autoimmune response, and has been demonstrated in a mouse model (Fan et al., 2009). It is now known that the specific location of this distinct expression pattern in the thymus is the medullary epithelial cells (Derbinski et al., 2001). They express a variety of self antigens at low levels, representing many tissues. This unusual expression pattern has been termed “promiscuous” gene expression, in reference to the atypical pattern of genes expressed in this one cell type (Derbinski et al., 2001). Transcription of insulin in the pancreas is known to depend on the transcription factor Pdx1, while in the thymus it has no effect on insulin’s transcription (Palumbo et al., 2006; Danso-Abeam et al., 2013). This lack of dependency on a known regulator of insulin expression in the pancreas demonstrates a distinctive regulatory mechanism in the thymus for this gene.
This regulatory mechanisms that underlies “promiscuous” gene expression are only starting to be uncovered. The AIRE regulator is known to be essential for the expression of a number of self-antigens in the medullary thymic epithelial cells (mTECs) (Anderson et al., 2002). AIRE is mutated in the APECED syndrome (Ahonen, 1985; Nagamine et al., 1997), characterized by a multi-organ autoimmunity including T1D in 10–20% of cases. Additionally, mice lacking the Aire gene, show similar autoimmune responses in numerous organs, and have drastically decreased levels of expression of self antigens in the thymus, including insulin (Anderson et al., 2002). The precise molecular mechanism of Aire action on these genes in the mTECs is not yet entirely known. Recent studies have uncovered binding partners for Aire, enabling a broader understanding of the mechanism by which it induces tissue specific transcription in the thymus (Abramson et al., 2010). Furthermore, the transcription of tissue specific antigens in the thymus is regulated through different mechanisms than those used by the usual tissues where they are expressed (Villasenor et al., 2008). Therefore, uncovering these mechanisms and Aire binding partner function is key in understanding of this aberrant transcription of self-antigens.
Aire has also been shown to correlate with a known maturity marker, CD80, in mTECs (Derbinski et al., 2005). The epithelium of the thymic medulla is comprised of a gradient of maturity levels, whereby not all cells are in the same stage of development. mTECs that are more mature express higher levels of the co-stimulating molecule CD80, as well as Aire (Derbinski et al., 2005). This in turn, leads to higher levels, as well as a wider range, of antigen expression. Therefore, the maturity and development of mTECs have a strong impact on the final expression levels of self-antigens, and ultimately the preservation of self-tolerance.
Only a small proportion of all mTECs express insulin (1–3%, although a value as high as 15% has been reported by Villasenor, 2008) and it is thought that each antigen is represented by, on average, 0.5% mTECs (Anderson et al., 2005; Derbinski et al., 2001). In our laboratory, we have generated a clonal cell line from the medullary thymic epithelium, whereby we can assess insulin-expressing mTECs specifically, as opposed to thymic aggregates used in this field of study (Levi and Polychronakos, 2009; Palumbo et al., 2006). To better understand what makes these cells distinct, we profiled gene expression by microarray of an insulin-expressing clone and one insulin-non-expressing clone. While a few genes were found to be overexpressed in either clone, the outstanding gene that was found to be significantly higher in the insulin-expressing clone was CD34.
METHODS
Microarray
The microarray was performed on RNA extracted (Rneasy Plus Kit, Qiagen, Netherlands) in triplicate from sets of cultured mTECs, either insulin expressing (INS(+)) or insulin non-expressing (INS(−)) clones described in (Palumbo et al., 2006). An Illumina microarray chip (Mouse WG-6 v2.0) was used for expression comparisons. We assessed expression in three biological replicates (separate cultures).
Microarray Statistics
The data from the microarray chip were analysed with FlexArray software (http://genomequebec.mcgill.ca/FlexArray/license.php). They were normalized using the lumi Bioconductor package for Illumina chips. The two clones were then compared by t-test, with false-discovery rate (FDR) correction. Of those that passed the FDR threshold of q< 0.001, only genes above a fold change of 1.15 in the INS(+) or INS(−) were kept for further analysis. The fold change cutoff of 1.15 was chosen based on literature indicating that thymic expression of tissue specific antigens is much more subtle than that in the tissue specifically expressing them (Vafiadis 1997, Derbinski 2001).
Chromosomal Clustering
The chromosomal positions of significant (q<0.001) tissue specific genes was determined and distances between the genes were calculated. The distribution of distances was evaluated for clustering, using the sum of the inverse of distances between consecutive genes on the same chromosome.
where n is the number of pairs of consecutive genes on the same chromosome genome-wide and li the distance between the 5’ ends of the two genes of the ith pair. This metric is sensitive to two or more genes clustering together, additive between chromosomes and minimally penalized by the effect of long distances between clusters or isolated genes. Its statistical significance was tested against a distribution created by randomizing the gene positions on the Illumina array probe coordinates 10,000 times.
Tissue Specificity
The significant genes above the 1.15 fold change were assessed for tissue specificity. The expression profile of the 74 genes was found on BioGPS (biogps.org) using the MOE430 data set. For each gene, tissue specificity was called if levels of expression of 10 times the median were found in less than 5 tissues, as has been defined in previous literature (Derbinski et al., 2005).
Primary mTEC Extraction
Bl6 mice were purchased from Jackson Laboratories (Maine, USA) at 6 weeks of age. Ten mice were sacrificed per experiment and the thymus dissected and placed in PBS. The thymus was cut into quarters or smaller sections, and gently agitated in PBS with a magnetic stirrer to liberate the thymocytes. The PBS was decanted and the thymus sections were covered with 1ml of Collagenase/Dispase and DnaseI (Roche, Mannheim, Germany) solution at a concentration of 125ug/ml and 100ug/ml respectively. The digestion was completed after two sets of 30 minutes, removing the supernatant and adding fresh solution in between the sets. The digested thymus was then centrifuged on a Percoll density gradient at 3700 rpm for 30 minutes (Amersham Biosciences, Uppsala, Sweden), as described in (Chentoufi et al., 2004), and the mTEC fraction was collected and pelleted by centrifugation at 1800rpm for 10 minutes. These studies were approved by the Animal Care Committee of McGill University.
Antibodies and Sorting
All flow cytometry markers were purchased from eBioscience (San Diego, USA) with the exception of UEA-1, which was from Vector Laboratories (Burlingame, USA). The eBioscience Fixable Viability dye eFluor780 was used to assess cell death, and all dead cells were excluded from the pool to be sorted. MTECs were specifically sorted out by negative bone marrow cell markers and positive mTEC markers as described in (Derbinski et al., 2001): CD45lo/UEA-1+/EpCAM+/CD34hi and CD45lo/UEA-1+/EpCAM+/CD34lo separately. Thresholds for CD34 hi and lo was set with a fluorescence minus one (FMO) method, allowing to mark the appropriate gates for the population sort. This method eliminated any background in the positive population.
RNA and Real-Time QPCR
RNA from each subset of sorted cells was extracted by RnAqueous-Micro Kit (Invitrogen, Carlsbad, USA) and reverse-transcribed with Superscipt II (Invitrogen, Carlsbad, USA). Real-time PCR was performed using ABI Taqman probes for 18S, Insulin, Aire and CD80 (Applied Biosystems, Foster City, USA).
Flow cytometry
Bl6 mice were euthanized at 6 weeks of age, and treated as described above. The mTEC fraction was then stained for CD45lo/UEA-1+/EpCAM+/CD34/CD80. The results were the analysed using FlowJo software.
RESULTS
Microarray
An mTEC cultured cell line expressing insulin (INS(+)) and mTEC cell line not expressing insulin (INS(−)) as a control were compared by microarray expression profiling. When comparing the two cell lines, 1914 genes were found to be significant at a q-value lower than 0.001 by the false-discovery method (Table 1) (Hochberg and Benjamini, 1990). Out of these genes 74 were found to be above a 1.15 fold change threshold in overexpression or underexpression in the INS(+) clone. The INS(+) clone had 31 genes overexpressed (Table 1A) while the INS(−) overexpressed the other 43 genes (Table 1B).
Table 1.
| A) Overexpressed genes in INS(+) clone in order of decreasing statistical significance (q<0.001, fold change larger than 1.15) TSA: Tissue specific antigens. | |||||||
|---|---|---|---|---|---|---|---|
| Gene Name | p-value | Fold change |
TSA | Gene Name | p-value | Fold change |
TSA |
| CD34 | 8.31287E-10 | 1.25 | CES5 | 7.64969E-05 | 1.17 | X | |
| RPS4Y2 | 2.5464E-07 | 1.31 | X | POU3F1 | 8.60952E-05 | 1.17 | X |
| DDX3Y | 6.35655E-07 | 1.25 | CDKN1A | 9.46779E-05 | 1.28 | ||
| SLPI | 8.77212E-07 | 1.20 | X | GLIPR1 | 0.000142719 | 1.34 | X |
| 1110018J23RIK | 1.62349E-06 | 1.28 | 6330404C01RIK | 0.000212375 | 1.27 | X | |
| LIMCH1 | 3.98834E-06 | 1.25 | DCXR | 0.000221063 | 1.16 | ||
| NDN | 4.80082E-06 | 1.18 | X | ADH7 | 0.000299863 | 1.36 | X |
| CCNG1 | 8.45852E-06 | 1.24 | X | MDM2 | 0.00038091 | 1.15 | X |
| SFRP2 | 2.21486E-05 | 1.24 | LOC100038894 | 0.000404037 | 1.25 | ||
| PHLDA3 | 2.27858E-05 | 1.17 | BAX | 0.000424174 | 1.16 | X | |
| COL8A1 | 2.83159E-05 | 1.18 | MFAP5 | 0.000427988 | 1.23 | ||
| SUPT16H | 2.87221E-05 | 1.21 | X | LOC100044376 | 0.000562258 | 1.17 | |
| INHBA | 3.2915E-05 | 1.15 | X | SVOP | 0.000916685 | 1.19 | X |
| CASP1 | 4.35817E-05 | 1.16 | ACTA2 | 0.002078199 | 1.29 | ||
| ERDR1 | 5.42239E-05 | 1.24 | LY6A | 0.002744749 | 1.18 | X | |
| FGF5 | 7.42138E-05 | 1.34 | |||||
| B) Underexpressed genes in INS(+) clone in order of decreasing statistical significance (q<0.001, fold change smaller than 0.87) | |||||||
|---|---|---|---|---|---|---|---|
| Gene Name | p-value | Fold change |
TSA | Gene Name | p-value | Fold change |
TSA |
| TXNIP | 8.69064E-08 | 0.86 | 1810014F10RIK | 6.45088E-05 | 0.87 | X | |
| TMEM223 | 3.48217E-07 | 0.80 | NXPH1 | 8.05158E-05 | 0.85 | X | |
| GCAP26 | 6.67668E-07 | 0.84 | 2310014G06RIK | 0.000124063 | 0.87 | ||
| HAL | 7.05042E-07 | 0.77 | X | HR | 0.000194914 | 0.85 | X |
| CD47 | 1.9707E-06 | 0.85 | SCL0003799.1_2 | 0.000197981 | 0.86 | ||
| TSPAN8 | 2.49213E-06 | 0.73 | CYP2S1 | 0.000209385 | 0.85 | ||
| HOXA7 | 2.94151E-06 | 0.74 | X | C2 | 0.000212954 | 0.80 | |
| NKX2-3 | 4.29496E-06 | 0.82 | ITGB4 | 0.00024623 | 0.77 | ||
| CHCHD10 | 5.06008E-06 | 0.74 | BC064033 | 0.000262833 | 0.83 | ||
| FXYD6 | 6.86269E-06 | 0.70 | FXYD3 | 0.000326697 | 0.76 | ||
| MGC41689 | 7.06235E-06 | 0.84 | COL6A1 | 0.000380021 | 0.85 | ||
| GPX7 | 8.70002E-06 | 0.84 | ANGPTL7 | 0.000395528 | 0.66 | X | |
| NDRG1 | 1.08504E-05 | 0.83 | EGR1 | 0.000472283 | 0.86 | X | |
| LOC675899 | 1.13252E-05 | 0.84 | RPP25 | 0.000482431 | 0.84 | X | |
| NDRL | 1.22543E-05 | 0.82 | COL6A2 | 0.000674136 | 0.83 | ||
| 3526401B18RIK | 1.22718E-05 | 0.77 | X | HOXA5 | 0.000691461 | 0.84 | |
| SCD1 | 1.53393E-05 | 0.83 | LGALS2 | 0.000880288 | 0.85 | X | |
| KRT18 | 1.60738E-05 | 0.87 | 9930105H17RIK | 0.000892251 | 0.84 | ||
| 4930533K18RIK | 1.67902E-05 | 0.86 | X | FOS | 0.001291024 | 0.86 | X |
| SCARA3 | 2.32944E-05 | 0.77 | GCH1 | 0.001486611 | 0.86 | X | |
| CTSH | 2.69068E-05 | 0.80 | X | RAB6B | 0.002095833 | 0.86 | X |
| MMP2 | 5.74252E-05 | 0.84 | |||||
Chromosomal Clustering
There have been reports in the literature that tissue-specific antigens in mTECs could be clustering on chromosomal locations, possibly for a locus-control transcriptional regulatory mechanism (Derbinski et al., 2005; Johnnidis et al., 2005)). Although the genes that were found to be differentially expressed in the Derbinski et al study were much higher in number, our results show fewer genes as the comparison is of two single clones. It was still important to assess whether these genes were clustering in chromosomal locations as this could be an important regulatory mechanism for their transcription. The location for the significantly overexpressed tissue specific genes was found and distances between the genes were calculated. The minimal distance found between genes was approximately 4 Mb. Therefore, we found no evidence for clustering in the differentially expressed genes in these cell lines.
Tissue Specificity
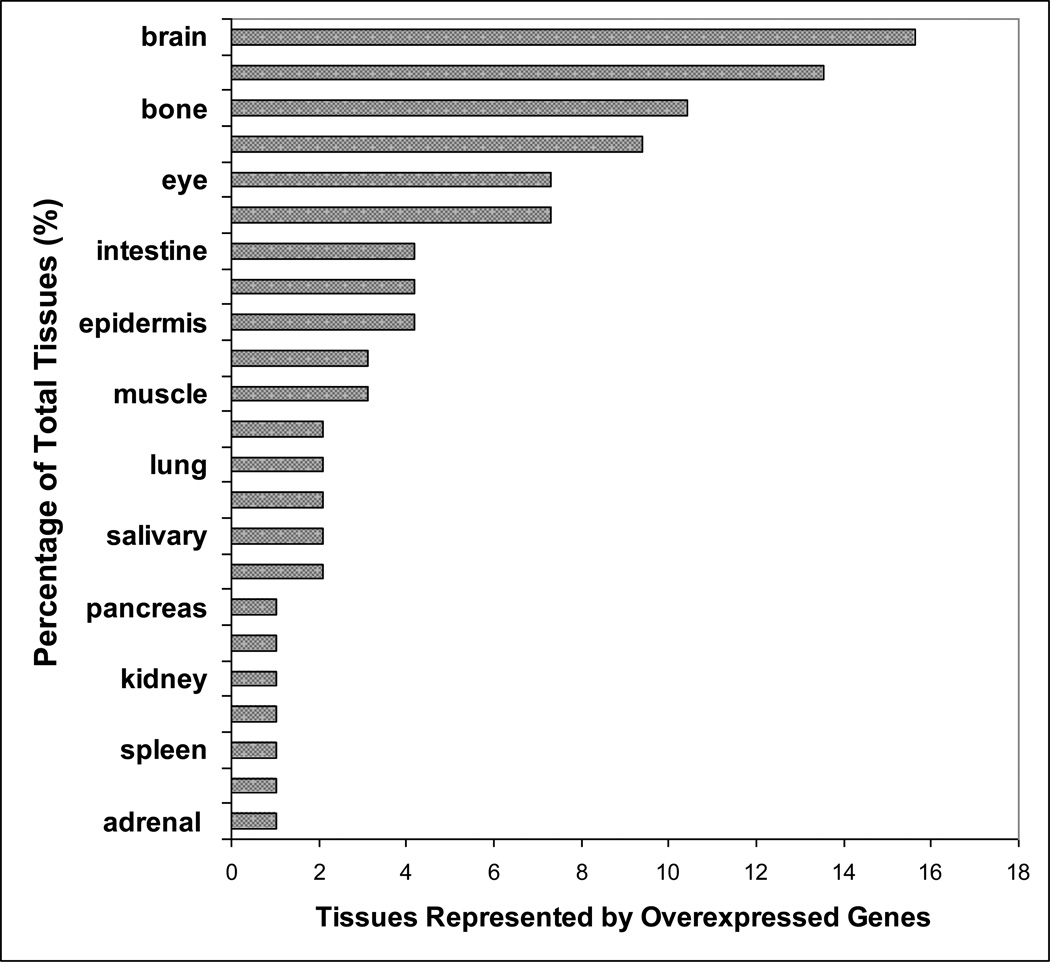
Out of the 74 significantly overexpressed genes assessed, 30 were found to be tissue specific by the BioGPS expression data (Table 1). Various tissues were represented collectively by the 30 tissue specific genes. (Figure 1).
Figure 1.
Tissues represented by the significantly overexpressed tissue specific genes in the microarray analysis of INS(−) and INS(+) mTEC clones. Percentage of each tissue represented by the significant genes is plotted.
Overexpressed genes
The gene with the highest significance (q=0.001, p=8.31E–10) in the microarray analysis, is CD34. This gene was identified as a hematopoietic stem cell maker used most commonly in bone marrow (Simmons et al., 1992) and other types of transplants (Takami et al.). Its function, or even expression, have not until now been examined in the thymus epithelium.
The TXNIP and RPS4Y2 genes were both overexpressed in the insulin non- expressing clone, and are currently known as a tumor suppressor gene (Zhou et al.) and a male specific Y-linked ribosomal protein (Skaletsky et al., 2003), respectively. Their function in the medullary epithelial cells has not been documented, although RPS4Y2 was found to be tissue specific by our criteria, and is most likely expressed as a tissue antigen.
The next most significant gene is Tmem223, a little-studied gene of unknown function. It is overexpressed in the insulin positive clone, and was not found to be tissue specific by our criteria.
Following were DDX3Y and GCAP26, none were found to be tissue specific by our given criteria. DDX3Y was overexpressed in the negative clone and is a highly conserved gene encoding an RNA-helicase that belongs to a large family of helicases (DEAD box helicases). The GCAP26 gene, also known as Spock2, is only known to encode a proteoglycan, but its function has not yet been described. The group that cloned the protein, calling it KIAA0275, found a high expression by Northern blot in the thymus, along with the brain and peripheral leukocytes (Nagase et al., 1996). GCAP26 is overexpressed in the insulin positive clone.
Genes found by the Abramson group to be involved in the regulatory mechanism of self antigen expression and interact with Aire, DNA-PK, TOP2A, and PARP1 were specifically assessed for over or underexpression in our microarray analysis results (Abramson et al. 2010) The fold changes seen in these three genes were minimal and not statistically significant.
Transcription Factors
The 74 significant genes were assessed for possible functions in transcriptional regulation. Out of the 74 genes, 10 genes were found to be transcription factors; FoxJ2, HOXA7, and NKX2-3, NDN, SUPT16H, POU3F1, HR, EGR1, HOXA5, and FOS.
CD34
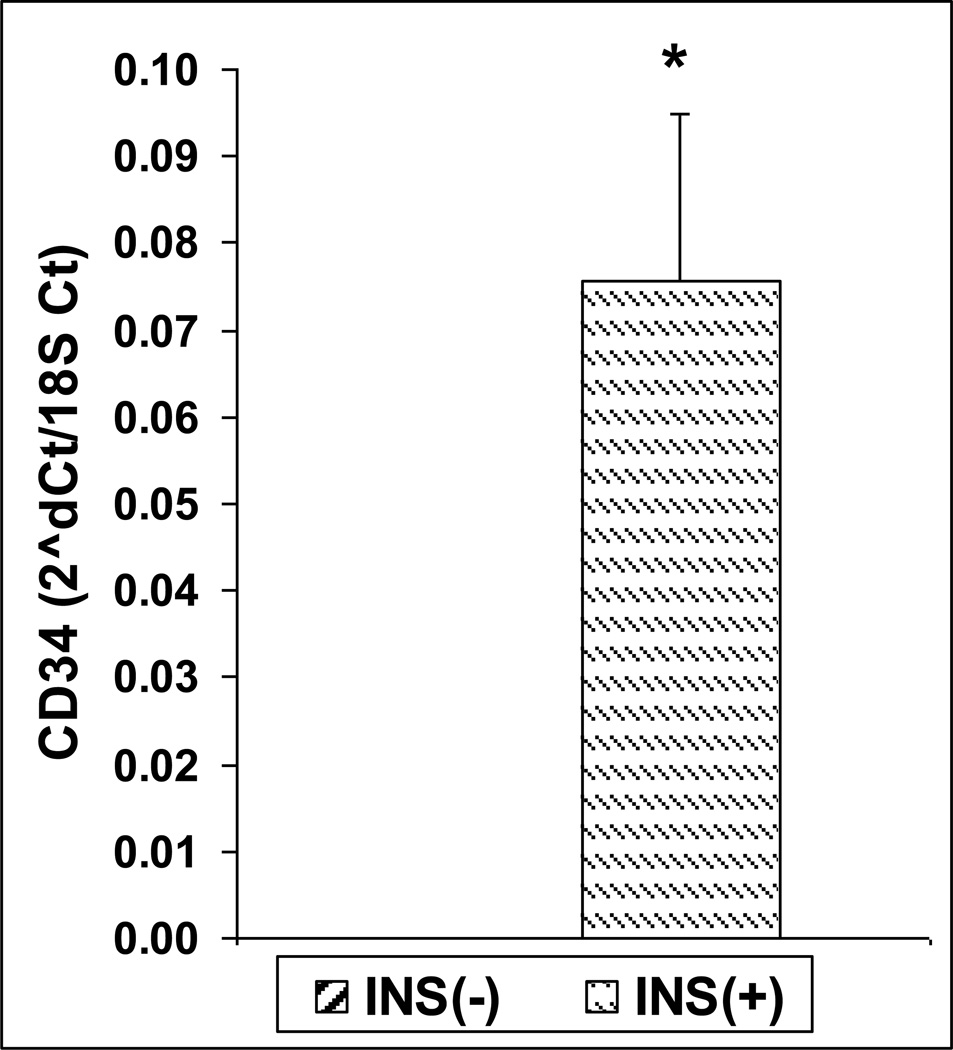
We confirmed the overexpression of CD34 found in the microarray by quantitative real-time PCR (Figure 2), showing higher expression in the insulin positive clone by four orders of magnitude (p<0.01). This was also confirmed by FACS, with higher CD34 staining in the insulin positive clone.
Figure 2.
Expression of CD34 in INS(−) and INS(+) mTEC clones by Real-time Rt-PCR (n=3, p<0.01, t-test).
CD34lo vs. CD34hi Cells
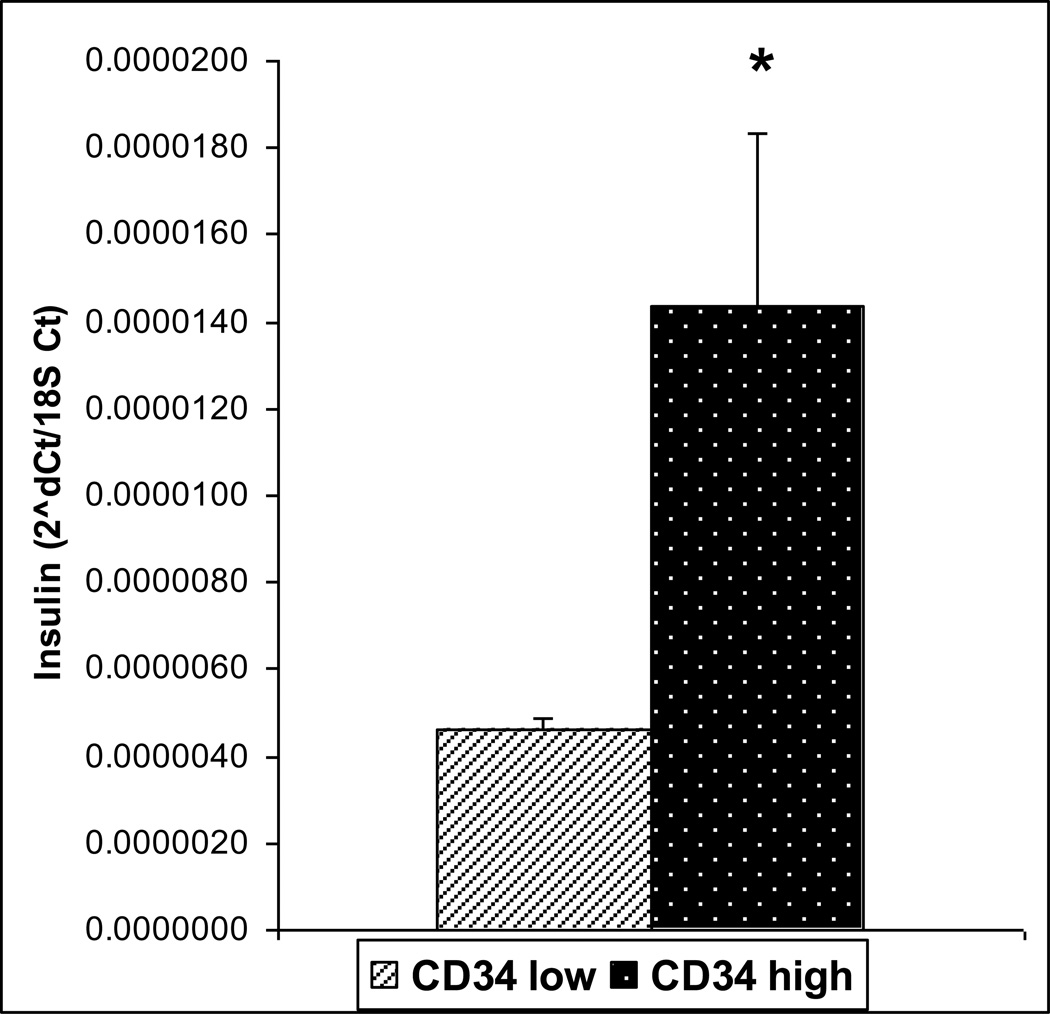
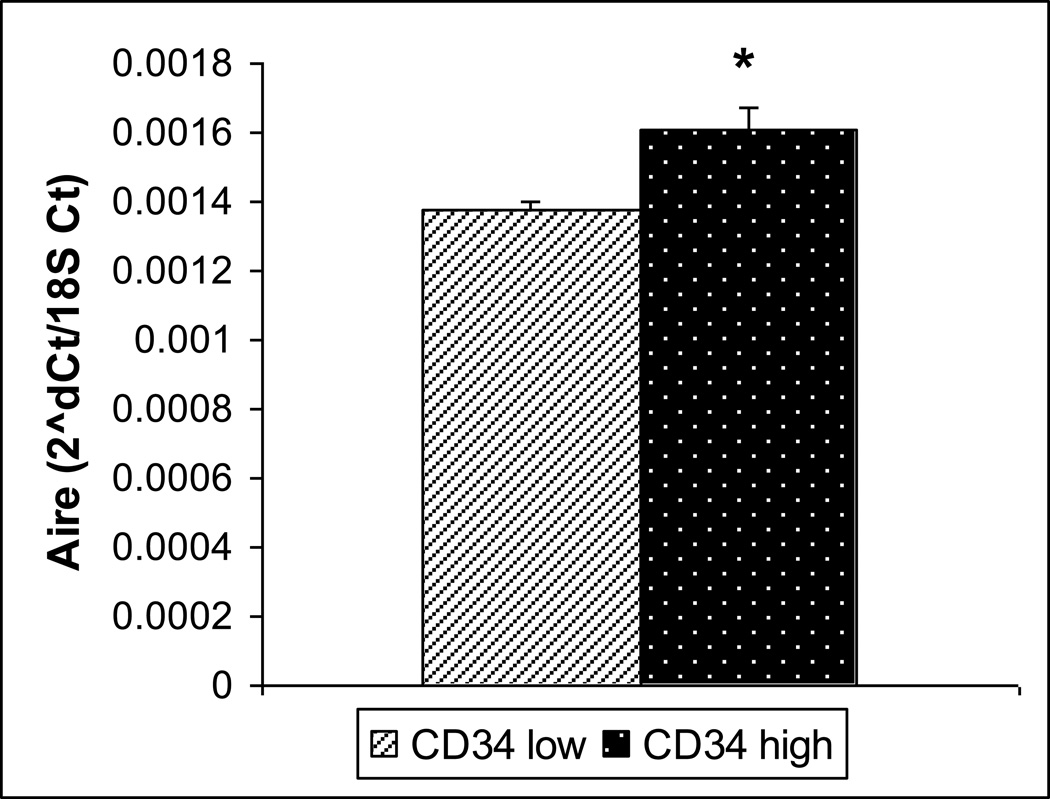
To further investigate the possible role of CD34 and its higher expression in the insulin positive clone, we sorted primary mTECs enriched from a pool of mouse thymi, into CD34 high expressing (CD34hi) and CD34non-expressing or low expressing (CD34lo) samples. Cells positive for mTEC markers (CD45lo/EpCAM+/UEA-1+) were sorted into CD34hi and CD34lo. The CD34hi cells had a higher insulin expression level (p= 0.049) (Figure 3). This also correlated to a significantly higher level of Aire (p=0.024) (Figure 4), but not higher CD80 expression (data not shown).
Figure 3.
Insulin expression in sorted CD34low and CD34hi cells by Real-time Rt-PCR. Insulin was found to be expressed at higher levels in the CD34 hi population. (n=4, p=0.049)
Figure 4.
Aire Expression in sorted CD34 lo and hi cells by Real-time Rt-PCR. Aire was like insulin found to be expressed at higher levels in the CD34 hi population. (n=3, p=0.02)
CD34hi Expressing Cells
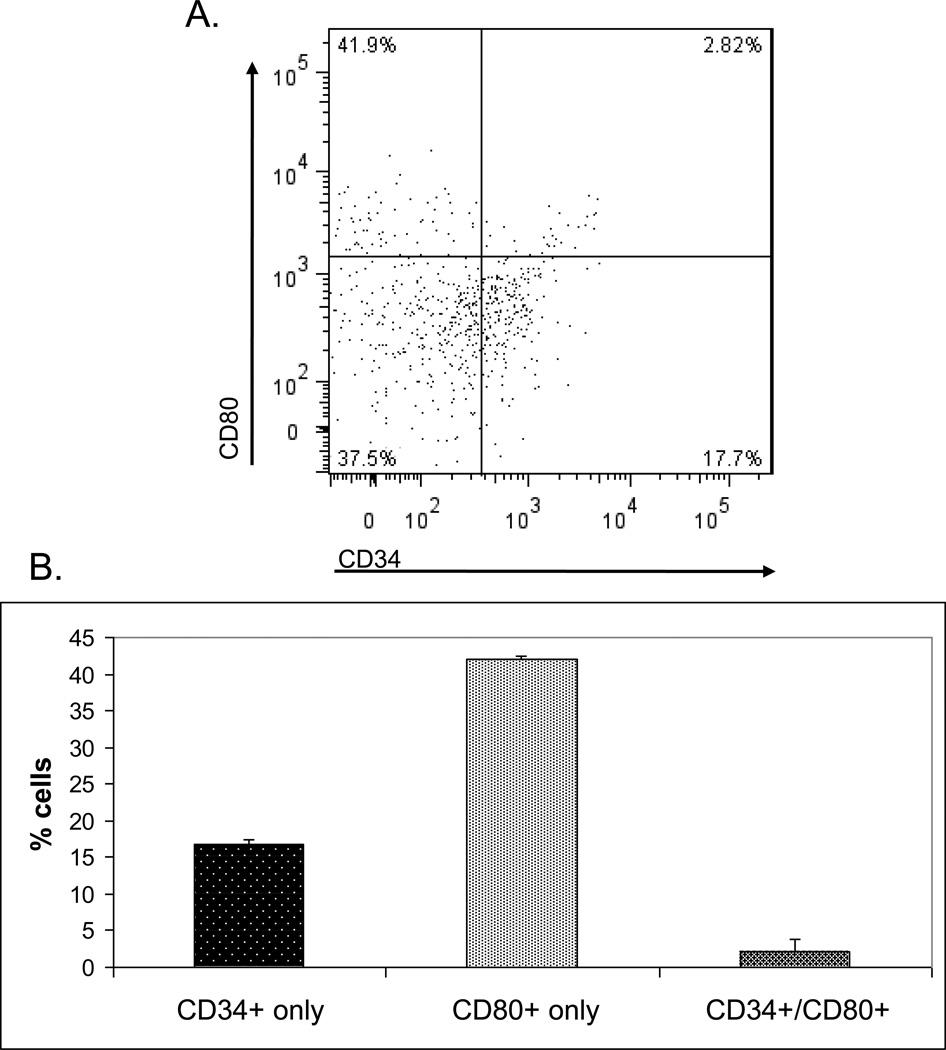
To further characterize the CD34 gene in the thymus, the cells expressing it in higher levels were analysed by FACS. MTECs were isolated from mouse thymi, as mentioned above, and stained for CD45lo/EpCAM+/UEA-1+ expression, in addition to CD34 and CD80 (Figure 5A). The proportions of mTECs expressing CD34, CD80 or both were then assessed (Figure 5B). Cells primarily expressed CD80 alone, with 42% (+/− 0.5%) of the mTEC population. CD34 alone was found in 17% (+/− 0.7%) of mTECs, while CD34+/CD80+ double positives were found in 2% (+/− 1.5%) of the population.
Figure 5.
Populations of mTECs expressingCD34 and CD80 cell surface markers. A) Gates delimiting each population were set based upon fluorescence-minus-one technique.
B) Proportions of cells expressing each CD34, CD80 and both cell surface markers. (Exemplary plot of four separate experiments)
CONCLUSIONS
Studies on specific thymic antigen expression and its regulation have largely been hampered by the lack of a clonal system to study individual cells. As different mTEC lineages express specific antigens, studying the whole mTEC population is nearly impossible to discern how many antigens, as well as which ones, are expressed by a particular mTEC individually. Therefore, the creation of an in vitro model for this type of study was necessary to truly demonstrate the expression profiles of specific cells. As we currently have such an in vitro model for insulin (as well as one that does not express insulin), it was of great importance to compare the expression profiles of these two cell lines, using an expression microarray.
The 30 tissue specific genes out of the significantly overexpressed genes did not cluster on chromosomes, which is at variance with one report that has claimed that overexpressed genes in mTECs tend to cluster (Derbinski et al., 2005). However, their comparison was of mTEC pools with other subsets of thymic cells, such as cortical thymic epithelial cells, or dendritic cells (Derbinski et al., 2005). Our comparison of two clones focuses on one cell lineage and would have been more powerful to detect such clustering. Although this specificity simultaneously limits us to one antigen (insulin) and only one clone that expresses it, it is of considerable importance to be able to discern the expression profiles and in turn the regulatory mechanisms of one mTEC. Due to the vast ectopic thymic antigen expression in whole thymus, this would allow us to pinpoint the exact set of antigens that are being represented by a particular mTEC lineage. We hypothesize that, beyond the general control by AIRE, additional regulatory mechanisms are specific for each antigen. If this specific expression profile is characterized for one cell, it can further aid in the elucidation of a regulatory mechanism that can be applied to other mTECs. Therefore, our analysis centered on tissue-specific antigens and genes encoding proteins with potential for regulating transcription.
Of these 74 genes, 30 were found to be tissue specific; 15 were overexpressed in the insulin expressing clone, and the other 15 in the insulin non-expressing clone. This demonstrates that each cell can have a specific profile of antigens that it expresses with some degree of exclusivity. Our findings do not rule out overlap but simply indicate that the profile of each mTEC may be different. However, more in vitro clones with varying specificities would be needed to assess this particular hypothesis.
CD34 was the gene that was overexpressed in the insulin positive clone by the largest margin. Although the few genes that were found to be overexpressed in either clone may be poorly characterized, or simply not characterized at all for the thymus, they may act either as tissue antigens or perhaps even have some type of regulatory role related to the insulin expression. Their exact function in the thymus, and more specifically in the mTEC clones, still need to be clarified. Therefore, more detailed research into their actions specifically in the thymus is needed. As CD34 was the highest hit on the list, we chose to further explore its role in the thymus.
When mTECs expressing high levels of CD34 were sorted and compared to those that did not express it, or expressed it at low levels, insulin was found to be expressed at much higher levels. This corroborates the microarray data, and it shows the correlation of CD34 and insulin as process that is happening in vivo in the thymic environment. As Aire is also upregulated in these CD34hi cells, it may point to a type of functionality marker, whereby Aire is expressed at a higher level and is regulating antigens, such as insulin, by increasing their expression as well. Whether CD34 is simply a marker or if it plays a direct role in a regulation of negative selection, remains to be determined. In endothelial cells, it attracts adhesion by interacting with L-selectin expressed in T-lymphocytes, a major mechanism for targeting T-cells to the lymph nodes (Baumheter et al., 1993). If its expression in mTECS plays a similar role, this would mediate preferential interaction of Ins+ mTECs with thymocytes undergoing negative selection, indicating a privileged role of insulin among promiscuously expressed antigens. If a hierarchy of importance among self-antigens exists, insulin would appear to have a privileged position.
Cells in the thymus express CD80 as a maturity marker, and mTECs with higher CD80 are noted for the increase in Aire levels as well as transcription of antigens (Derbinski et al., 2005). In our study, primary mTECs expressing CD80 were found to be mainly independent of CD34 expression. mTECs were found to express in highest proportions either one or the other marker separately, with a small subset of cells expressing both CD34 and CD80. This demonstrates the probability that CD34 may be a marker for specific functions, possibly even thymic insulin expression, through a mechanism independent of mTEC maturation for which CD80 is a marker. Insulin was found to be expressed in 1–3% of all mTECs in the thymus and could potentially correspond to the numbers we have seen with CD34 and CD80 co-expression (Chentoufi et al., 2004). Because of the low number (2%) of CD34hi cells within the primary mTEC total population, it is difficult to isolate and characterize them. However, future studies addressing this technical problem are justified, as they might result in interesting insights into the possibility of CD34 being a marker for specific antigens in mTECs, such as insulin. This could in turn open up significantly easier avenues for isolating and further studying specific antigens in the thymus.
Highlights.
-
-
Expression of insulin by specialized cells in the thymus epithelium is needed for self-tolerance.
-
-
We compared the gene-expression profile of a clonal insulin-expressing thymus epithelial cell line, with one that did not express insulin.
-
-
Numerous genes were found to be differentially expressed, many being tissue specific
-
-
CD34 was the most significant result and was overexpressed in the insulin-positive cell clone
-
-
In primary cells high expression of CD34 was a marker for cells strongly expressing insulin.
-
-
Aire, the master regulator of self-antigen expression in the thymus was also expressed at higher levels in the CD34 high population
Acknowledgements
The authors would like to acknowledge Huiqi Qu and Li Quan for the statistical analysis help. D.L. is funded by the Claude Giroud Fund of the Montreal Children’s Research Institute and the DP3 Program of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors have no competing interests.
Contributions of Authors
DL carried out the RNA extractions and sample preparations for the microarray experiment, microarray analysis, confirmation of expression of CD34, and all subsequent FACS and isolation experiments and Real-time PCR quantifications. DL drafted the manuscript. CP conceived the study, participated in its design, supervised the microarray analysis, and participated in the drafting and editing of the manuscript. All authors have read and approved the final manuscript.
References
- Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;1:123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Ahonen P. Autoimmune polyendocrinopathy--candidosis--ectodermal dystrophy (APECED): autosomal recessive inheritance. Clin Genet. 1985;27:535–542. doi: 10.1111/j.1399-0004.1985.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Ve nanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Baumheter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993;262:436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- Chentoufi AA, Palumbo M, Polychronakos C. Proinsulin expression by Hassall's corpuscles in the mouse thymus. Diabetes. 2004;53:354–359. doi: 10.2337/diabetes.53.2.354. [DOI] [PubMed] [Google Scholar]
- Chentoufi AA, Polychronakos C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes. 2002;51:1383–1390. doi: 10.2337/diabetes.51.5.1383. [DOI] [PubMed] [Google Scholar]
- Danso-Abeam D, Staats KA, Franckaert D, Van Den Bosch L, Liston A, Gray DH, Dooley J. Aire mediates thymic expression and tolerance of pancreatic antigens via an unconventional transcriptional mechanism. Eur J. Immunol. 2013;1:75–84. doi: 10.1002/eji.201242761. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 2009;18:2812–2824. doi: 10.1038/emboj.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Janeway C, Travers P, Walport M, Shlomchik M. Immunobiology 5 : the immune system in health and disease. New York: Garland Pub; 2001. [Google Scholar]
- Johnnidis J, Venanzi E, Taxman D, Ting J, Benoist C, Mathis D. Chromosomal clustering of genes controlled by the aire transcription factor. Proc Natl Acad Sci USA. 2005;20:7233–7238. doi: 10.1073/pnas.0502670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D, Polychronakos C. Regulation of insulin gene expression by cytokines and cell-cell interactions in mouse medullary thymic epithelial cells. Diabetologia. 2009;52:2151–2158. doi: 10.1007/s00125-009-1448-y. [DOI] [PubMed] [Google Scholar]
- Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- Nagase T, Seki N, Ishikawa K, Ohira M, Kawarabayasi Y, Ohara O, Tanaka A, Kotani H, Miyajima N, Nomura N. Prediction of the coding sequences of unidentified human genes. VI. The coding sequences of 80 new genes (KIAA0201-KIAA0280) deduced by analysis of cDNA clones from cell line KG-1 and brain. DNA Res. 1996;3:321–329. 341–354. doi: 10.1093/dnares/3.5.321. [DOI] [PubMed] [Google Scholar]
- Palumbo MO, Levi D, Chentoufi AA, Polychronakos C. Isolation and characterization of proinsulin-producing medullary thymic epithelial cell clones. Diabetes. 2006;55:2595–2601. doi: 10.2337/db05-1651. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Satterthwaite AB, Tenen DG, Seed B. Molecular cloning of a cDNA encoding CD34, a sialomucin of human hematopoietic stem cells. J Immunol. 1992;148:267–271. [PubMed] [Google Scholar]
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, Chinwalla A, Delehaunty A, Delehaunty K, Du H, Fewell G, Fulton L, Fulton R, Graves T, Hou SF, Latrielle P, Leonard S, Mardis E, Maupin R, McPherson J, Miner T, Nash W, Nguyen C, Ozersky P, Pepin K, Rock S, Rohlfing T, Scott K, Schultz B, Strong C, Tin-Wollam A, Yang SP, Waterston RH, Wilson RK, Rozen S, Page DC. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- Takami T, Terai S, Sakaida I. Stem cell therapy in chronic liver disease. Curr Opin Gastroenterol. 28:203–208. doi: 10.1097/MOG.0b013e3283521d6a. [DOI] [PubMed] [Google Scholar]
- Thebault-Baumont K, Dubois-Laforgue D, Krief P, Briand JP, Halbout P, Vallon-Geoffroy K, Morin J, Laloux V, Lehuen A, Carel JC, Jami J, Muller S, Boitard C. Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest. 2003;111:851–857. doi: 10.1172/JCI16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, Wickramasinghe S, Colle E, Polychronakos C. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yu Q, Chng WJ. TXNIP (VDUP-1, TBP-2): a major redox regulator commonly suppressed in cancer by epigenetic mechanisms. Int J Biochem Cell Biol. 2003;43:1668–1673. doi: 10.1016/j.biocel.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Villasenor J, Besse W, Benoist C, Mathis D. Ectopic expression of peripheral-tissue antigens in the thymic epithelium: probabilistic, monoallelic, misinitiated. Proc Natl Acad Sci U S A. 2008;41:15854–15859. doi: 10.1073/pnas.0808069105. [DOI] [PMC free article] [PubMed] [Google Scholar]