Abstract
Many aspects of cellular motility and mechanics are cyclic in nature such as the extension and retraction of lamellipodia or filopodia. Inherent to the cycles of extension and retraction that test the environment is the production of mechano-chemical signals that can alter long-term cell behavior, transcription patterns, and cell fate. We are just starting to define such cycles in several aspects of cell motility, including periodic contractions, integrin cycles of binding and release as well as the normal oscillations in motile activity. Cycles of local cell contraction and release are directly coupled to cycles of stressing and releasing extracellular contacts (matrix or cells) as well as cytoplasmic mechanotransducers. Stretching can alter external physical properties or sites exposed by matrix molecules as well as internal networks; thus, cell contractions can cause a secondary wave of mechano-regulated outside-in and internal cell signal changes. In some cases, the integration of both external and internal signals in space and time can stimulate a change in cell state from quiescence to growth or differentiation. In this review we will develop the basic concept of the mechano-chemical cycles and the ways in which they can be described and understood.
Introduction
Recent developments in microscopy and nanotechnology have enabled the analysis of cellular mechanical processes at the subcellular and molecular levels in conjunction with the analysis of the biochemical signal changes that control other cell functions. It is now possible to approach the question of how the mechanical signals interact with the biochemical signaling pathways. As the cyclic actions of kinases and phosphatases are central to intracellular biochemical signaling, we need to identify the central molecular players whose functions are altered by cycles of extension and retraction that produce intermittent forces on matrix contacts. What is emerging is the picture that actin filaments are assembled and disassembled in a treadmilling cycle as the cell extends the leading edge and retracts, and each cycle generates a signal that is integrated over many cycles and locations to produce a signal to alter the cellular state (as reviewed in [1•]). In other words, one mechanical event is often not sufficient to cause a cell to change direction but many events will activate a major change. In a cyclic mechanical function, for example, cell movement can be initiated by the activation of actin filament assembly plus myosin contraction, followed by force generation on junctional complexes (matrix or adjacent cells) and/or movement, ending with inactivation of the primary function [2••]. Alternatively, external forces can cause major alterations in cell signaling often through the same pathways as internal force generation does [3]. Many of the proteins that have been implicated in force and geometry sensing are involved in producing long lasting changes in cell behavior (as recently reviewed in [1•,4,5,6••,7•,8,9,10•,11•]). While it is well recognized that the mechano-chemical signal conversion involves force-induced alterations of protein functions (Figure 1), it remains a major mystery how physical and biochemical factors are coupled, and how they are integrated to enable mechanical sensing.
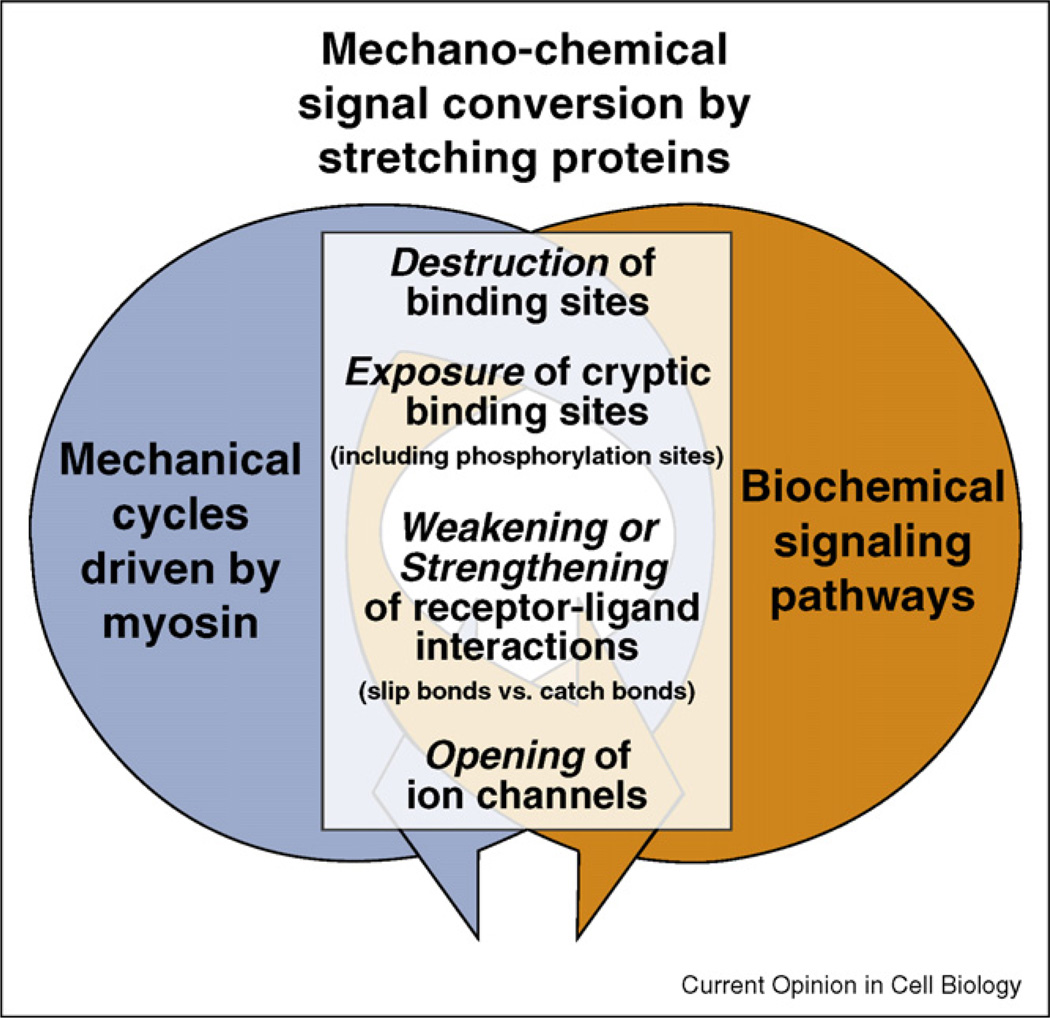
Figure 1.
Mechanical and biochemical signaling networks are coupled through proteins that serve as mechano-chemical signal converters. Protein stretching can cause a multitude of functional changes (as recently reviewed in [6••,12,1•]). Force-induced alterations of the equilibrium structure of proteins can destroy molecular binding motifs, expose cryptic binding sites that are buried in native protein folds ([13,14•,15•,16•,81••,82] and recently reviewed in [17]). This includes exposing phosphorylation sites [18••], force furthermore either accelerates the dissociation of non-covalent bonds or activates catch bonds that bind more tightly when activated by force [19,20••,26••,28,43]. Finally, membrane stretching can lead to the opening of ion channels [6••,21–23].
Tensile mechanical force and biochemical signaling networks are coupled
In most cases, mammalian cell motility involves extension of the plasma membrane by actin polymerization that pushes the membrane outwards, movement of that actin rearward by myosin and the disassembly of actin in a treadmilling cycle (reviewed in [24]). For cells to generate force on extracellular matrices, transmembrane integrins must link the actin cytoskeleton to the matrix and a variety of adhesion linkages have been described at the cytoplasmic tails of the integrins [25•]. Thus, a network of physically coupled proteins is generated that links the outside to the cell interior. Myosin-generated force stresses this protein network and all of its components. Once tensile force breaks the weakest protein–protein linkage, a local relaxation occurs but stress is often restored rapidly by contraction. Important to understanding mechanical cycles is that tensile force acting on receptor-ligand complexes is known to typically weaken the lifetime of non-covalent bonds by accelerating their dissociation rate [26••]. However, to enable long-lived (>10 min) adhesion complexes to form despite the presence of cell forces, components in the complexes must either be remodeled, for example, through the recruitment of additional integrins to the adhesion site [27] (most molecular components of adhesion complexes have exchange half-lives much less than 3 min), and/or form catch bonds with their respective ligands, that is, bonds that hold on more strongly when stretched (as reviewed in [28]). Without force, these complexes fail to assemble or disassemble relatively rapidly. Thus, force is necessary for the typical adhesion complexes that in turn generate a number of biochemical signals.
Integrin binding to matrix as well as to actin, followed by force generation and finally release constitutes just one example [29]. Furthermore, there is often a force-dependent sequential exchange of adhesion linkages over time from one type to another with a clear change in molecular composition and integrin content, meaning that all of the involved proteins must turn over [29]. We understand the roles of a number of proteins in the motility complexes that catalyze actin assembly or disassembly, myosin activation, and actin crosslinking. How those proteins are integrated into the cycle of extension by actin polymerization, rearward movement, and actin disassembly is only poorly understood. Some of the important effects could be the result of force, since actin assembly in stress fibers is linked to force as well as is the size of the adhesion complexes.
One of the obvious problems with the current approach of studying cell functions by removing proteins (knockout or knockdown technologies) is that a motility function based on a series of tightly coupled events will be blocked by the inhibition of any step in the series, for example, blocking either actin polymerization or depolymerization will block the motility cycles described above. An integrated systems description is needed to link the physical and chemical aspects of motile functions (as reviewed in [30–33]). The urgency of recognizing and defining these dependencies is particularly current as evidence is mounting how many diseases involve defects in the cellular mechanoresponse system [17]. Even in systems biology, it has so far not been considered that the physical and chemical aspects of signaling networks are coupled. Proteins, whose functions can be upregulated or inhibited by stretching, play very special roles in the mechano-chemical signal conversion (external proteins like fibronectin [34,35,36••] and internal proteins like p130Cas [18••,37] and talin [38,39]).
A major goal thus has to be to identify all proteins that are part of various force-bearing networks (Figure 2), as well as the detailed structural mechanisms how forces might switch their diverse functions (Figure 1). Transmembrane proteins, such as integrins, anchor cells to their environment and thus physically link the exterior to the cell interior. Mechanical forces generated by myosin contractions can thus be transmitted via actin filaments through the transmembrane adhesion proteins to ECM fibers or neighboring cells, or vice versa. Evidence exists that mechanical forces acting on integrins can accelerate their activation [40–42] and that some might form catch bonds ([40] and reviewed in [28]). The physical linkage between integrins and actin can be formed independently by five cytosolic proteins. Talin, tensin, plectin, filamin, and α-actinin were reported to individually bridge between the various integrins and actin since they have binding sites for both proteins [10• ,25• ,44,45] kindlin-3 requires couples to integrins via additional adaptor proteins [43]. The stretching of talin leads to a reinforcement of the talin–actin linkage through the recruitment of further proteins that are subsequently involved in downstream cell signaling events [39,46–49]. Alternatively, the recruitment and stretching of p130cas regulates cell signaling events due to its phosphorylation that is upregulated when stretched [18••].
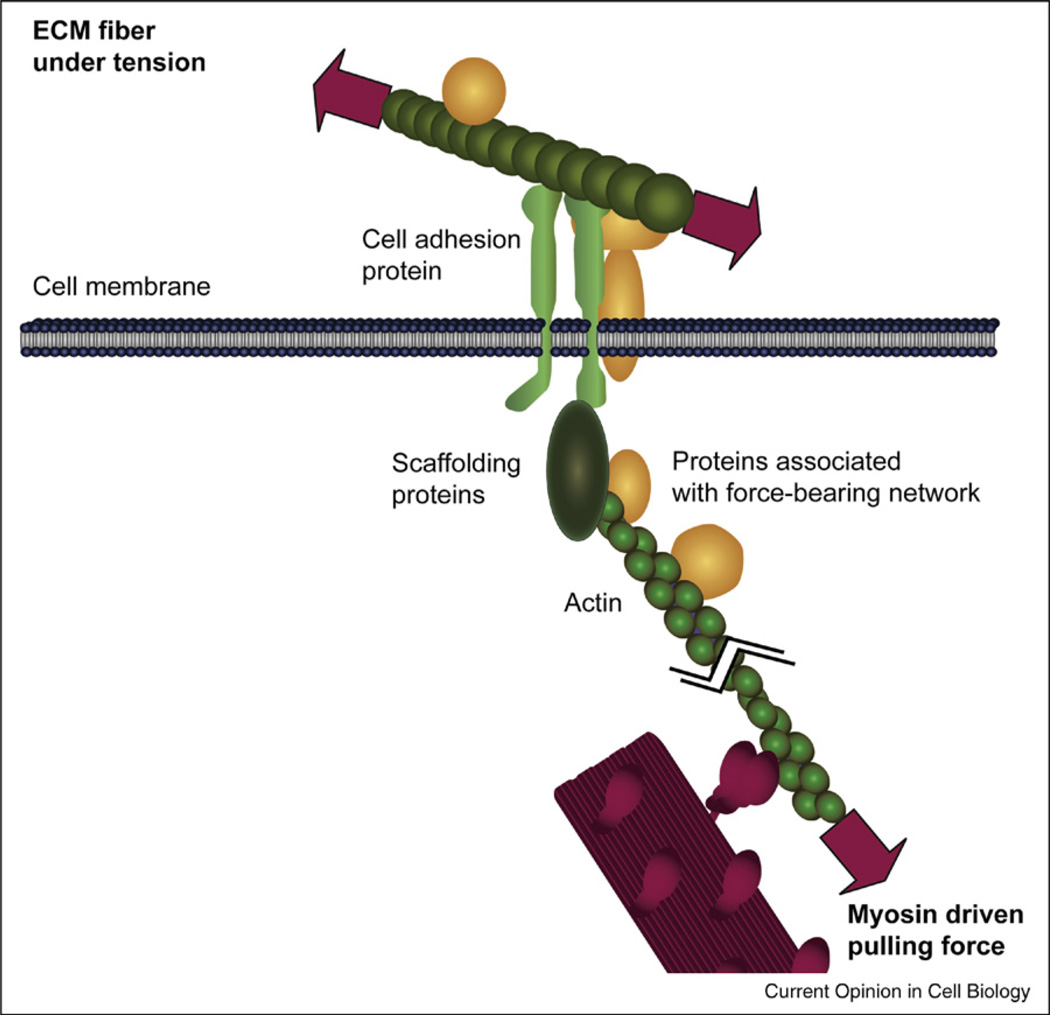
Figure 2.
Force-bearing protein network that links the cytoskeleton to the extracellular matrix. The molecular motor myosin (red) pulls on an actin fiber thereby applying force to a protein network (green) that physically links the cytoskeleton to the extracellular matrix. Diverse proteins that are associated with the various molecules of the force-bearing network are given in yellow. Other force-bearing networks exist too, including those that link cytoskeletal elements to cell-cell junctions.
Mechanisms leading to mechano-chemical signal conversion inside the cell
As the tools became available to study the mechanisms by which protein stretching might alter protein functional states, it became clear that there are many molecules that can act as mechano-chemical signal converters (Figure 1), and that many different mechanisms are at work, two of which we would like to briefly describe below.
Vinculin is recruited to stretched talin
Upon cell adhesion, talin rapidly accumulates in focal contacts before vinculin recruitment [50]. The recruitment of vinculin to cell adhesion sites has been shown to be upregulated by force [46–48] and to correlate with adhesion strengthening [49] and reduced focal adhesion turnover [51]. This indicated that vinculin recruitment to focal adhesions is upregulated by force [46–48,52,53] through the stretching of talin. A recent study has experimentally measured an increase in vinculin binding to the talin rod upon mechanical stretching [54] and a high resolution structural mechanism has been proposed [39].
Since talin’s vinculin binding helices are buried in its native structure, how might tensile mechanical forces activate them? Some key experimental observations [55–57] together with computational simulations [39] provide high resolution structural insights into the force-induced unfolding process of the N-terminal helix bundle of the talin rod. The following model of activation has been derived: When mechanically strained, the tightly packed helix bundle of the talin rod breaks into fragments thereby gradually exposing the buried hydro-phobic surfaces of the five vinculin binding helices [39]. We suggested that a vinculin binding helix becomes ‘activated’, if the buried surface area in mechanically strained talin falls below the buried surface area in complex with vinculin. The vinculin binding helices of talin can then spontaneously swap their association, breaking off from the strained talin and associating with vinculin. We named this the helix swap mechanism [39]. Vinculin recruitment to talin thus initially increases if talin is incorporated into a force-bearing network formed when a cell adheres to a surface or matrix fibrils [39]. However, since each of the vinculin binding helices is exposed to water at a different time point in the unfolding pathway of the talin tail, talin can recruit vinculin in a force-graded response. Since vinculin can bridge talin and actin, it may reinforce the early talin–actin linkage that has been shown previously to be a rather weak bond breaking at a force of 2pN [58].
Increased tyrosine phosphorylation
A new paradigm was established recently that can possibly explain the basis of the increase in tyrosine phosphorylation upon cell stretch through integrins [59] and some of the mechanisms of tyrosine kinase and phosphatase effects on development, cancer, and morphology [4]. Stretching of cytoskeletally attached tyrosine kinase substrates appears to activate their phosphorylation by Src family kinases [60]. In the case of p130Cas, it is found that a partial stretch of the substrate in vitro activates Src-family phosphorylation of p130Cas by manyfold [18••] Because p130Cas is a scaffold protein that binds phosphatases and kinases [61], the actual phosphorylation state upon stretch in vivo may be modulated over time and space. Much more information is needed to determine when and under what conditions p130Cas is phosphorylated and when does that phosphorylation result in a biochemical signal for cell growth or differentiation. The central role that p130Cas plays in breast cancer as the BCAR1 gene indicates that it is an important pathway that warrants further study.
Static versus cyclic external forces acting on integrin junctions
The assembly of adhesion sites is upregulated by force, and some upregulation occurs whether the force is applied by myosin contractions or externally [46]. It is thus interesting that the application of static versus cyclic external forces has already been linked to major differences in biochemical cell signaling changes. For example, laminar shear and cyclic stretch both induced FAK phosphorylation but at different sites, that is, Y576 versus Y397 and Y576 respectively, thereby causing differential effects on Rho versus Rac activation [62]. Cyclic stretch activates RhoA in fibroblasts and thereby induces ROCK-dependent actin assembly [63]. Further downstream, the activation of the extracellular signal-regulated mitogen-activated protein kinase 1/2 (ERK 1/2(MAPK)) is a prototypical marker for integrin-mediated cell responsiveness to mechanical forces. For aortic smooth muscle cells, a transient maximal 3.5-fold increase in phosphorylated ERK 1/2(MAPK) was seen but only if the cells were stretched cyclicly (0.5 and 2.0 Hz). The ERK activation response peaks after 5 min of exposure and decreased to baseline levels after 30 min [64]. In contrast to ERK1/2, cyclic stretch induced a sustained JNK activation followed by a sustained cytokine production and release (IL-8) as reported for alveolar epithelial cells [65].
Cycles of contractility (lamellipodial, whole cell)
Periodic cycles of contraction and extension of active lamellipodia have been correlated with the formation of matrix contacts during contraction and the testing of matrix rigidity (Figure 3). The displacements of cell contacts on pillars is similar in that it is also cyclic, increasing and decreasing over time and the overall force is increasing with rigidity over a wide range of rigidity [66••]. There is evidence for similar cycles in the contraction of collagen fibers [67], the contraction of cytoplasm of cells after microtubule depolymerization [68], and the leading edge of moving cells [69]. It is the effect of these cycles that are of particular interest. Force during the contraction results in an active signaling but why release occurs is not evident.
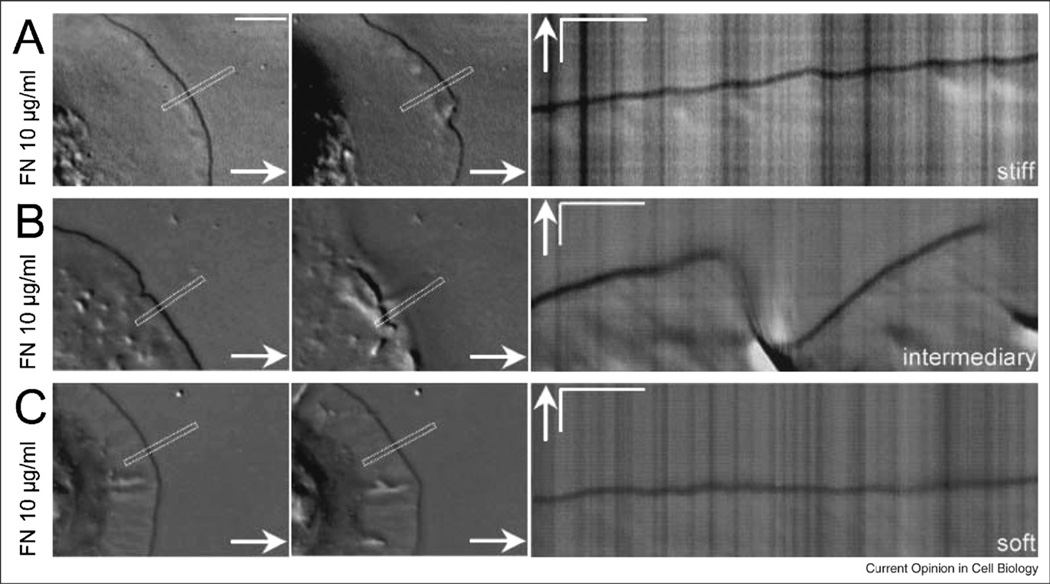
Figure 3.
Micrographs of cells spreading on surfaces of different rigidities show different spreading patterns. (A) DIC images of the first and last point show the extent of spreading of a MEF on a stiff polyacrylamide. The kymograph of the same MEF spreading on a stiff polyacrylamide gel (20% acrylamide, 0.8% bisacrylamide) covalently linked with FN 10 µg/ml shows that periodic contractions were generated. (B) DIC images and kymograph of a MEF spreading on an intermediary stiffness polyacrylamide gel (10% acrylamide, 0.1% bisacrylamide) covalently linked with FN 10 µg/ml. Note the effective protrusion of the leading edge without the generation of periodic contractions and the following global ruffling of the lamella. (C) DIC images and kymograph of a MEF spreading on a soft polyacrylamide gel (10% acrylamide, 0.04% bisacrylamide) covalently linked with FN 10 µg/ml. Note the absence of both periodic contractions and effective protrusion of the leading edge. (Left. Scale bars are equal to 5 m. Right. time bars are equal to 30 s; scale bars are equal to 2 m. Arrows indicate the direction of protrusion.)
In the periodic retractions on rigid substrata, the best studied case so far, there is a clear signal that is generated by the force on the substrate. If cells spread on soft surfaces, then they stop spreading when contraction starts and the velocity of actin contraction increases. We interpret this as an indication that the signal from a soft surface, weakens the adhesion between matrix and the cytoskeleton such that actin contraction velocity is increased and surface traction force is decreased. By contrast, hard surfaces stimulate adhesion formation that leads to further extension and the development of periodic contractions. That signal may be the unfolding of a cytoplasmic protein in the force-bearing linkage from the fibronectin matrix to the actin cytoskeleton [70]. Further, the force transmitted to dendritic actin networks (like those formed in the lamellipodia of motile cells), can cause stress stiffening followed by a regime of reversible stress softening at higher loads [71].
Cell motility drives matrix unfolding and remodeling
Cell motility drives matrix assembly and remodeling, and vice versa, matrix properties regulate cell motility (as reviewed in [1•]). There are a number of common themes that have to be considered to understand different motile functions and include: (1) localized actin filament assembly, myosin filament assembly, contraction, and disassembly; (2) localized adhesion and force generation to produce a rigidity or force-dependent signal; and (3) generation of a dynamic, yet force-bearing cytoskeleton. In all cases where it has been examined, actin filament assembly occurs in a different location from myosin filament assembly but the two are often closely coordinated [2••]. Mechanical continuity that is provided by actomyosin networks has the important property of being adaptable to oscillating force loads. The catch-bond property of some myosins enables tension to be preserved with little ongoing ATP hydrolysis [72•]. Thus, for transient pulls the strain hardening that is observed can be the result of myosin or other catch bonds.
As local contractions occur, the cells apply force via their integrin junctions to extracellular matrix fibers. The forces generated are sufficient to not only stretch but also partially unfold fibrillar fibronectin [35•,36••] and potentially other matrix molecules. If cell contractility is inhibited, refolding of the fibronectin molecules embedded in fibers occurs [35•,36••]. While the refolding of single molecules in aqueous buffers typically occurs on the second to subsecond timescale once the force is released [73], the refolding of fibrillar fibronectin molecules in the presence of serum proteins happens within minutes [74]. Stretching of matrix fibers will not only increase their rigidity, but will also alter the displayed binding sites [75]. Consequently, cell contractions will alter the physical and biochemical microenvironment of the cell. While the significance of the microenvironment in controlling cell function and fate is increasingly recognized [76], little attention has been given to the question how cells can initiate a secondary feed-back loop whereby cell contractions actively alter the cellular microenvironment by stretching and unfolding matrix fibers. Cells start to assemble their own matrix soon after being seeded. As they pull on existing fibers and assemble new ones, the old fibronectin fibers become increasingly more unfolded with age, and old fibers are on average more unfolded than newly deposited fibers [77•] (Figure 4). Only during the first few hours after seeding do we find a direct correlation between the rigidity of the initial substrate and the mechanical strain of matrix fibers, whereby fibrillar fibronectin is more unfolded on rigid than on soft substrates [77•].
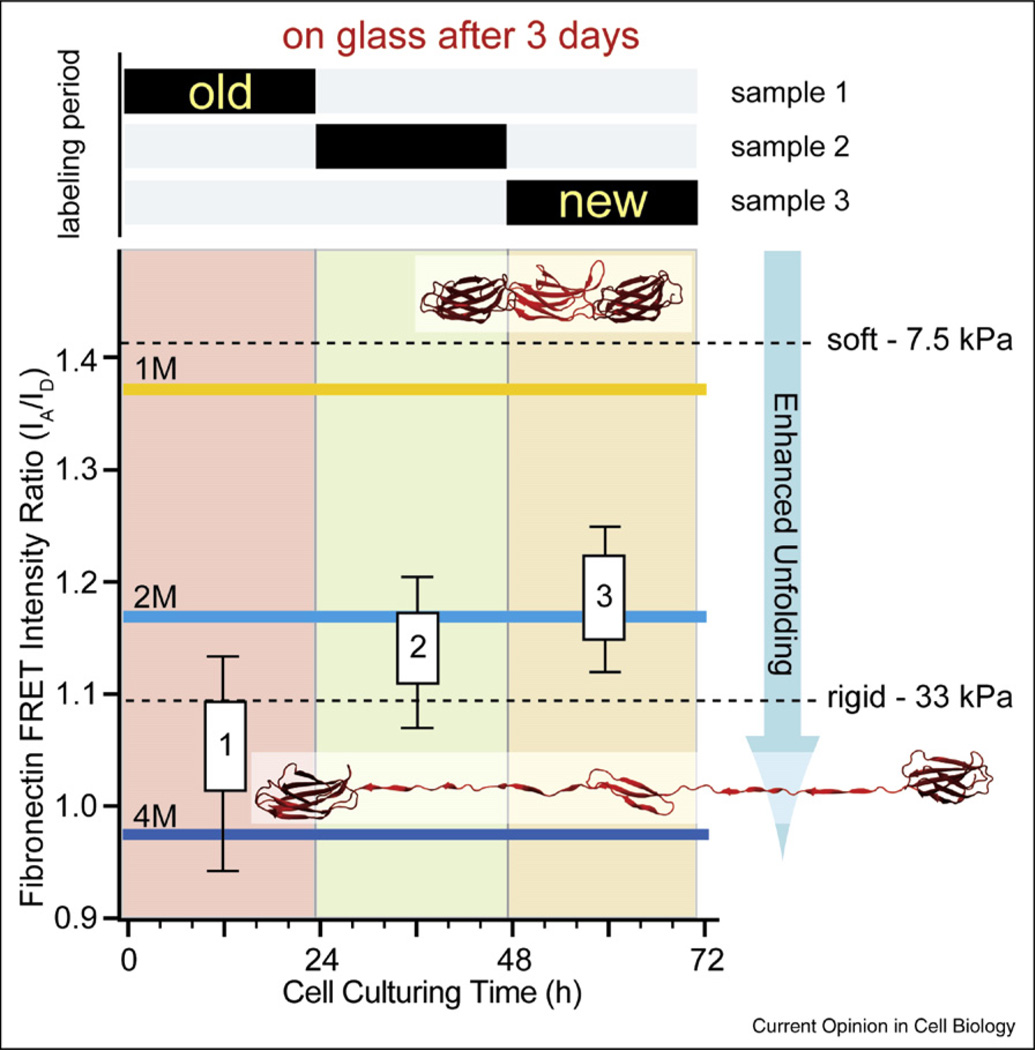
Figure 4.
Fibronectin fibers are progressively more unfolded as the extracellular matrix ages as probed by fluorescence resonance energy transfer (FRET). Fibroblasts were seeded on glass and allowed to assemble matrix for three days in 10% serum. Trace amounts of FRET-labeled fibronectin were added for limited time periods as indicated in the upper bar graph. On average, each labeled fibronectin molecule carried seven donors and four acceptors. The FRET ratios probed in matrix (without optical cross-talk correction) are compared to those measured in solution in the presence of various concentrations of the denaturant GnHCl, where the onset of a loss of secondary structure is seen at 1 M GnHCl and beyond. Fibronectin is completely unfolded at 4 M GnHCl. After three days, the matrix deposited during the first 24 h is highly unfolded while the younger matrix is far less unfolded. Thus, the physical properties of matrix are changing as matrix ages (adopted from [77•]). For a comparison, the FRET ratios of fibronectin matrix assembled by fibroblasts on rigid and soft polyacrylamide surfaces are shown 4 h after cell seeding. As inserts, a fibronectin fragment of three type III modules is shown in a folded state as well as after partial unfolding by tensile mechanical force acting on its termini.
What might the physiological significance of fibronectin unfolding be? Fibronectin has many different recognition sites distributed over its more than 54 domains, some of which bind serum proteins, others ECM proteins and cell adhesion proteins. Mechanical force might switch them on and off by various molecular mechanisms [75]. Since they differ in mechanical stability [78–80] tensile force acting on fibronectin fibers can structurally alter the fibronectin modules in a well-defined sequence, thus allowing a broad range of forces to be translated into a well-defined sequence of biochemical changes. Stretching and module unfolding can destroy molecular binding motifs ([13,75] or activate protein binding by exposing cryptic binding sites buried in native protein folds [15•,81••,82].
Future research is needed to decipher which of the longer term cellular responses are controlled by initial material properties versus those of the self-made matrix, and which cell signaling pathways are affected by matrix unfolding.
Summary
Major questions on how mechanical factors and stimuli, some of which persist for only short periods, can lead to longer term changes in cell behavior remain unanswered. The biochemical signals produced by mechanotransduction must be integrated over space and/or time to give the appropriate graded response of the cell. Transient responses to protein stretching such as increased tyrosine phosphorylation or protein ligand binding can be one element that is integrated perhaps by GTP enhancing factors (GEFs) or MAP kinase cascades to give longer term cellular responses [83,84]. Perhaps the longer term integrated analysis of cell mechanical behavior can help to unravel the molecular mechanisms that underlie these robust yet complex cell fate changes to produce the appropriate regenerative response.
Acknowledgements
We gratefully acknowledge Sheila Luna for the graphics and members of our labs as well as of the Nanotechnology Center for Mechanics in Regenerative Medicine (an NIH Roadmap Nanomedicine Development Center) for numerous discussions that shaped so many of the ideas in this review.
References and recommended reading
Papers of particular interest published within the period of review have been highlighted as:
• of special interest
•• of outstanding interest
- 1. Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. This review details many of the mechanisms of cell mechanosensing and how response mechanisms must integrate the sensory signals
- 2. Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Dobereiner HG, Freund Y, Borisy G, et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. This study characterizes the mechanical cycle of edge extension and retraction that is seen in many cells
- 3.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force, focal adhesion assembly a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 4.Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Lele TP, Kumar S. Brushes cables anchors recent insights into multiscale assembly and mechanics of cellular structural networks. Cell Biochem Biophys. 2007;47:348–360. doi: 10.1007/s12013-007-0013-x. [DOI] [PubMed] [Google Scholar]
- 6. Janmey PA, McCulloch CA. Cell mechanics. integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. This review summarizes many of the recent findings on cell mechanics
- 7. Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. Endothelial cell responses to flow have been well characterized at a molecular level
- 8.Sniadecki NJ, Desai RA, Ruiz SA, Chen CS. Nanotechnology for cell-substrate interactions. Ann Biomed Eng. 2006;34:59–74. doi: 10.1007/s10439-005-9006-3. [DOI] [PubMed] [Google Scholar]
- 9.Graeter SV, Huang J, Perschmann N, Lopez-Garcia M, Kessler H, Ding J, Spatz JP. Mimicking cellular environments by nanostructured soft interfaces. Nano Lett. 2007;7:1413–1418. doi: 10.1021/nl070098g. [DOI] [PubMed] [Google Scholar]
- 10. Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. This article summarizes the many protein interactions reported between adhesion complex proteins
- 11. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. The effect of rigidity on the differentiation of mesenchymal stem cells is reported in this paper
- 12.Kaazempur Mofrad MR, Abdul-Rahim NA, Karcher H, Mack PJ, Yap B, Kamm RD. Exploring the molecular basis for mechanosensation, signal transduction, and cytoskeletal remodeling. Acta Biomater. 2005;1:281–293. doi: 10.1016/j.actbio.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Krammer A, Craig D, Thomas WE, Schulten K, Vogel V. A structural model for force regulated integrin binding to fibronectin’s RGD-synergy site. Matrix Biol. 2002;21:139–147. doi: 10.1016/s0945-053x(01)00197-4. [DOI] [PubMed] [Google Scholar]
- 14. Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - An intimate relationship. Eur J Cell Biol. 2008 doi: 10.1016/j.ejcb.2008.01.012. It is shown here that myofibroblast contractions can directly activate ECM-bound TGF-beta1 thus indicating that the ECM acts as a growth factor storage
- 15. Little WC, Smith ML, Ebneter U, Vogel V. Assay to mechanically tune and optically probe fibrillar fibronectin conformations from fully relaxed to breakage. Matrix Biol. 2008;27:451–461. doi: 10.1016/j.matbio.2008.02.003. The assay presented here illustrates how protein stretching exposes otherwise cryptic protein binding sites
- 16. Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schafer LV, Brandmeier B, Grater F, Grubmuller H, Gaub HE, Gautel M. Mechanoenzymatics of titin kinase. Proc Natl Acad Sci USA. 2008;105:13385–13390. doi: 10.1073/pnas.0805034105. This paper shows that the titin kinase binding of ATP is force-dependent
- 17.Vogel V, Sheetz MP. Mechanical forces matter in health and disease: from cancer to tissue engineering. In: Vogel V, editor. Nanomedicine. VCH: Wiley; 2009. Nanotechnology, vol 5. [Google Scholar]
- 18. Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. This paper defines the effect of molecular stretch on the phosphorylation of p130Cas by Src and Abl family kinases
- 19.Thomas W, Forero M, Yakovenko O, Nilsson L, Vicini P, Sokurenko E, Vogel V. Catch-bond model derived from allostery explains force-activated bacterial adhesion. Biophys J. 2006;90:753–764. doi: 10.1529/biophysj.105.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas WE, Vogel V, Sokurenko E. Biophysics of catch bonds. Annu Rev Biophys. 2008;37:399–416. doi: 10.1146/annurev.biophys.37.032807.125804. This review discusses the proposed emchanisms how increases in tensile stress on certain bonds can increase bond lifetime
- 21.Charras GT, Horton MA. Single cell mechanotransduction and its modulation analyzed by atomic force microscope indentation. Biophys J. 2002;82:2970–2981. doi: 10.1016/S0006-3495(02)75638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SY, Corey DP. TRP channels in mechanosensation. Curr Opin Neurobiol. 2005;15:350–357. doi: 10.1016/j.conb.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci USA. 2007;104:15941–15946. doi: 10.1073/pnas.0707246104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 25. Wiesner S, Lange A, Fassler R. Local call: from integrins to actin assembly. Trends Cell Biol. 2006;16:327–329. doi: 10.1016/j.tcb.2006.05.002. This review summarizes the ways in which the integrins signal to the actin polymerization complex
- 26. Evans EA, Calderwood DA. Forces and bond dynamics in cell adhesion. Science. 2007;316:1148–1153. doi: 10.1126/science.1137592. This article details how bond lifetimes are altered by force
- 27.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Sokurenko EV, Vogel V, Thomas WE. Catch-bond mechanism of force-enhanced adhesion: counterintuitive, elusive, but widespread? Cell Host Microbe. 2008;4:314–323. doi: 10.1016/j.chom.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J Cell Sci. doi: 10.1242/jcs.042127. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 31.Dobereiner HG, Dubin-Thaler BJ, Giannone G, Sheetz MP. Force sensing and generation in cell phases: analyses of complex functions. J Appl Physiol. 2005;98:1542–1546. doi: 10.1152/japplphysiol.01181.2004. [DOI] [PubMed] [Google Scholar]
- 32.Civelekoglu-Scholey G, Orr AW, Novak I, Meister JJ, Schwartz MA, Mogilner A. Model of coupled transient changes of Rac, Rho, adhesions and stress fibers alignment in endothelial cells responding to shear stress. J Theor Biol. 2005;232:569–585. doi: 10.1016/j.jtbi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol. 2005 doi: 10.1016/j.ejcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Baneyx G, Baugh L, Vogel V. Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc Natl Acad Sci USA. 2001;98:14464–14468. doi: 10.1073/pnas.251422998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci USA. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. First indication that the stretching of fibronectin fibers by cell generated forces causes their mechanical unfolding
- 36. Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, Vogel V. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007;5:e268. doi: 10.1371/journal.pbio.0050268. Providing final proof that stretching of fibronectin fibers causes mechanical unfolding of its modules
- 37.Sawada Y, Sheetz MP. Force transduction by Triton cytoskeletons. J Cell Biol. 2002;156:609–615. doi: 10.1083/jcb.200110068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hytonen VP, Vogel V. How force might activate talin’s vinculin binding sites: SMD reveals a structural mechanism. PLoS Comput Biol. 2008;4:e24. doi: 10.1371/journal.pcbi.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puklin-Faucher E, Gao M, Schulten K, Vogel V. How the headpiece hinge angle is opened: new insights into the dynamics of integrin activation. J Cell Biol. 2006;175:349–360. doi: 10.1083/jcb.200602071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Astrof NS, Salas A, Shimaoka M, Chen J, Springer TA. Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry. 2006;45:15020–15028. doi: 10.1021/bi061566o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 44.Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113(Pt20):3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- 45.Brakebusch C, Fassler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003;22:2324–2333. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giannone G, Jiang G, Sutton DH, Critchley DR, Sheetz MP. Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. J Cell Biol. 2003;163:409–419. doi: 10.1083/jcb.200302001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallant ND, Michael KE, Garcia AJ. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol Biol Cell. 2005;16:4329–4340. doi: 10.1091/mbc.E05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DePasquale JA, Izzard CS. Accumulation of talin in nodes at the edge of the lamellipodium and separate incorporation into adhesion plaques at focal contacts in fibroblasts. J Cell Biol. 1991;113:1351–1359. doi: 10.1083/jcb.113.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandrasekar I, Stradal TE, Holt MR, Entschladen F, Jockusch BM, Ziegler WH. Vinculin acts as a sensor in lipid regulation of adhesion-site turnover. J Cell Sci. 2005;118:1461–1472. doi: 10.1242/jcs.01734. [DOI] [PubMed] [Google Scholar]
- 52.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 53.Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Del-Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. doi: 10.1126/science.1162912. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Izard T, Evans G, Borgon RA, Rush CL, Bricogne G, Bois PR. Vinculin activation by talin through helical bundle conversion. Nature. 2004;427:171–175. doi: 10.1038/nature02281. [DOI] [PubMed] [Google Scholar]
- 56.Papagrigoriou E, Gingras AR, Barsukov IL, Bate N, Fillingham IJ, Patel B, Frank R, Ziegler WH, Roberts GC, Critchley DR, et al. Activation of a vinculin-binding site in the talin rod involves rearrangement of a five-helix bundle. EMBO J. 2004;23:2942–2951. doi: 10.1038/sj.emboj.7600285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fillingham I, Gingras AR, Papagrigoriou E, Patel B, Emsley J, Critchley DR, Roberts GC, Barsukov IL. A vinculin binding domain from the talin rod unfolds to form a complex with the vinculin head. Structure (Camb) 2005;13:65–74. doi: 10.1016/j.str.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 58.Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–337. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt C, Pommerenke H, Durr F, Nebe B, Rychly J. Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. J Biol Chem. 1998;273:5081–5085. doi: 10.1074/jbc.273.9.5081. [DOI] [PubMed] [Google Scholar]
- 60.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 61.Geiger B. A role for p130Cas in mechanotransduction. Cell. 2006;127:879–881. doi: 10.1016/j.cell.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 62.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res. 2005;304:40–49. doi: 10.1016/j.yexcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Chiquet M, Tunc-Civelek V, Sarasa-Renedo A. Gene regulation by mechanotransduction in fibroblasts. Appl Physiol Nutr Metab. 2007;32:967–973. doi: 10.1139/H07-053. [DOI] [PubMed] [Google Scholar]
- 64.Goldschmidt ME, McLeod KJ, Taylor WR. Integrin-mediated mechanotransduction in vascular smooth muscle cells: frequency and force response characteristics. Circ Res. 2001;88:674–680. doi: 10.1161/hh0701.089749. [DOI] [PubMed] [Google Scholar]
- 65.Li LF, Ouyang B, Choukroun G, Matyal R, Mascarenhas M, Jafari B, Bonventre JV, Force T, Quinn DA. Stretch-induced IL-8 depends on c-Jun NH2-terminal and nuclear factor-kappaB-inducing kinases. Am J Physiol Lung Cell Mol Physiol. 2003;285:L464–L475. doi: 10.1152/ajplung.00031.2003. [DOI] [PubMed] [Google Scholar]
- 66. Saez A, Buguin A, Silberzan P, Ladoux B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys J. 200589:L52–L54. doi: 10.1529/biophysj.105.071217. This paper shows that cells on pillars of different rigidities will cause the same average displacement
- 67.Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7:157–164. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- 68.Pletjushkina OJ, Rajfur Z, Pomorski P, Oliver TN, Vasiliev JM, Jacobson KA. Induction of cortical oscillations in spreading cells by depolymerization of microtubules. Cell Motil Cytoskeleton. 2001;48:235–244. doi: 10.1002/cm.1012. [DOI] [PubMed] [Google Scholar]
- 69.Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 70.Kostic A, Sheetz MP. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol Biol Cell. 2006;17:2684–2695. doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaudhuri O, Parekh SH, Fletcher DA. Reversible stress softening of actin networks. Nature. 2007;445:295–298. doi: 10.1038/nature05459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo B, Guilford WH. Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. Proc Natl Acad Sci USA. 2006103:9844–9849. doi: 10.1073/pnas.0601255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernandez J, Li H. Force-clamp spectroscopy monitors the folding trajectory of a single protein. Science. 2004;303:1674–1678. doi: 10.1126/science.1092497. [DOI] [PubMed] [Google Scholar]
- 74.Little W, Schwartlander R, Smith MA, Vogel V. unpublished observation [Google Scholar]
- 75.Vogel V. Mechanotransduction involving multimodular proteins converting force into biochemical signals. Ann Rev Biophys Biomol Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- 76.Vessella RL, Pantel K, Mohla S. Tumor cell dormancy: an NCI workshop report. Cancer Biol Ther. 2007;6:1496–1504. doi: 10.4161/cbt.6.9.4828. [DOI] [PubMed] [Google Scholar]
- 77. Antia M, Baneyx G, Kubow KE, Vogel V. Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. Faraday Discsussions. 2008;139:229–249. doi: 10.1039/b718714a. First illustration that new and old fibronectin fibers differ in their physical and thus biochemical properties
- 78.Oberhauser AF, Badilla-Fernandez C, Carrion-Vazquez M, Fernandez JM. The mechanical hierarchies of fibronectin observed with single-molecule AFM. J Mol Biol. 2002;319:433–447. doi: 10.1016/S0022-2836(02)00306-6. [DOI] [PubMed] [Google Scholar]
- 79.Craig D, Krammer A, Schulten K, Vogel V. Comparison of the early stages of forced unfolding for fibronectin type III modules. Proc Natl Acad Sci USA. 2001;98:5590–5595. doi: 10.1073/pnas.101582198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Craig D, Gao M, Schulten K, Vogel V. Tuning the mechanical stability of fibronectin type III modules through sequence variations. Structure (Camb) 2004;12:21–30. doi: 10.1016/j.str.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 81. Ingham KC, Brew SA, Huff S, Litvinovich SV. Cryptic selfassociation sites in type III modules of fibronectin. J Biol Chem. 1997;272:1718–1724. doi: 10.1074/jbc.272.3.1718. First indication that tensile force might expose cryptic binding sites on a protein
- 82.Ingham KC, Brew SA, Erickson HP. Localization of a cryptic binding site for tenascin on fibronectin. J Biol Chem. 2004;279:28132–28135. doi: 10.1074/jbc.M312785200. [DOI] [PubMed] [Google Scholar]
- 83.Liu WF, Nelson CM, Tan JL, Chen CS. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res. 2007;101:e44–e52. doi: 10.1161/CIRCRESAHA.107.158329. [DOI] [PubMed] [Google Scholar]
- 84.Knies Y, Bernd A, Kaufmann R, Bereiter-Hahn J, Kippenberger S. Mechanical stretch induces clustering of beta1-integrins and facilitates adhesion. Exp Dermatol. 2006;15:347–355. doi: 10.1111/j.0906-6705.2006.00422.x. [DOI] [PubMed] [Google Scholar]