Abstract
Objective
Determine if Eustachian Tube (ET) function (ETF) tests can identify ears with physician-diagnosed ET dysfunction (ETD) in a mixed population at high sensitivity and specificity and define the inter-relatedness of ETF test parameters.
Methods
ETF was evaluated using the Forced-Response, Inflation-Deflation, Valsalva and Sniffing tests in 15 control ears of adult subjects after unilateral myringotomy (Group I) and in 23 ears of 19 adult subjects with ventilation tubes inserted for ETD (Group II). Data were analyzed using logistic regression including each parameter independently and then a step-down Discriminant Analysis including all ETF test parameters to predict group assignment. Factor Analysis operating over all parameters was used to explore relatedness.
Results
The Discriminant Analysis identified 4 ETF test parameters (Valsalva, ET opening pressure, dilatory efficiency and % positive pressure equilibrated) that together correctly assigned ears to Group II at a sensitivity of 95% and a specificity of 83%. Individual parameters representing the efficiency of ET opening during swallowing showed moderately accurate assignments of ears to their respective groups. Three factors captured approximately 98% of the variance among parameters, the first had negative loadings of the ETF structural parameters, the second had positive loadings of the muscle-assisted ET opening parameters and the third had negative loadings of the muscle-assisted ET opening parameters and positive loadings of the structural parameters.
Discussion
These results show that ETF tests can correctly assign individual ears to physician-diagnosed ETD with high sensitivity and specificity and that ETF test parameters can be grouped into structural-functional categories.
Keywords: Adults, Eustachian tube, Function, Tests, Sensitivity, Specificity
INTRODUCTION
The Eustachian tube (ET) represents a potential communication between the middle ear (ME) and nasopharynx. While usually closed, the ET lumen is opened periodically for short period of times by contraction of the tensor veli palatini muscle (mTVP) with perhaps the assistance of the levator veli palatini muscle (mLVP)1. These transient, muscle-assisted ET openings allow for the gradient driven, exchange of gas between the ME and nasopharynx2. Such gas transfers decrease the extant ME-ambient pressure gradient that is constantly being perturbed by changes in atmospheric pressure and by changes in ME pressure secondary to diffusive gas transfer from the ME to mucosal blood2. Experiments in monkeys (and other animal species) show that an inability to open the ET causes the successive development of ME under-pressures (ref. ambient), ME mucosal inflammation and effusion accumulation in the normally air-filled ME cavity3. This presentation is similar, if not identical, to that for otitis media with effusion (OME), a common disease in infants and children that also occurs in adults4,5. When persisting as a chronic condition (COME), OME secondary to ET dysfunction (ETD) is a primary cause of hearing loss in the population and is associated with other complications such as balance disturbances6 and speech and language delays in children7.
A large number of tests have been developed to assess ET function (ETF) for purposes of diagnosing the presence/absence of ETD and identifying the underlying cause of any observed dysfunction8–13. Because the most information-rich tests require a non-intact tympanic membrane (TM) which is uncommon in “normal” subjects, and all tests require an effusion-free ME which is uncommon in subjects with ME disease in the absence of a ventilation tube (VT), the result for those tests are usually presented as a distribution for the outcome parameters in the subset of the affected population(s) with functional VTs without reference to an age-matched “normal” population4,14,15. Alternatively, where tests can be done in effusion-free ears with and without a history of ME disease, the information is limited to Yes/No detections of ET opening during a specified maneuver and, usually, the frequency of positive assignments is compared between “affected” and “control” populations or between different test methods in either population12,16.
These types of comparisons at the population level do not translate well to the clinical setting where the focus of testing is on the individual patient and, specifically, on a diagnosis of the presence or absence of ETD, an identification of the underlying cause(s) of ETD and, where possible, the development of a treatment plan to improve ETF and “cure” ETD-related diseases. However, there is little support in the literature that any ETF test is used with regularity in clinical practice or that clinician’s base their decision-making on those test results, even when available.
In this study, we used a broad test panel to evaluate ETF in a “diseased” group of adults with VTs inserted for physician-diagnosed ETD with or without concurrent OME and in a “control” group of adults without extant or a positive history for ETD or ME disease after surgical perforation of the TM by myringotomy. We first determined the sensitivity and specificity of the ETF test parameters alone, and in combination, with respect to the correct assignment of ears to the ETD group and then explored the general relatedness among the ETF test parameters included in the panel. The study was designed to evaluate the “proof of principal” that ETF tests can accurately identify “affected” (and non-affected) ears with high sensitivity and specificity. This has direct applications to the clinical diagnosis of extant ETD on presentation and to documentation of improved ETF after specific interventions. A more complete understanding of the structural-functional information captured by the ETF test parameters included in our panel may lead to diagnostic methods that can access the cause of the ETD in individual ears and to the development of targeted interventions specifically tailored to a given type of ETD.
METHODS
Screening Procedures
Otherwise healthy, male and female subjects, greater than or equal to 18 years old and of any self-assigned race as is consistent with NIH rules were recruited for study participation by advertisement and by referral from otology practices that treat adults with ETD in the Greater Pittsburgh Area. All persons presenting as candidates for enrollment signed an IRB-approved informed consent and then provided general demographic information, were screened by history and physical examination for satisfaction with inclusion/exclusion criteria, and their MEs were examined using pneumatic otoscopy and tympanometry to document the presence/absence of a patent VT(s) and to rule out the presence of extant ME effusion/otorrhea.
Group Definition
Fifteen subjects with no extant symptoms/diagnoses of ETD or ME disease and without a significant past history of those conditions were enrolled as control subjects (Group I). Five, 3 and 2 of these subjects reported a positive history for allergic rhinitis, sinusitis and gastro-esophageal reflux disease (GERD), respectively. The average age for this Group was 30.0 (range 19.3 to 47.9) years; 8 were male and 7 female, and 9 identified their race as being White, 5 as Black and 1 as Asian. These subjects were further screened for normal hearing by clinical audiometry and for no known adverse reactions to lidocaine or epinephrine. The TMs of each subject were visualized through a speculum with the aid of an operating microscope and 4% lidocaine with epinephrine was applied topically to one TM. After approximately 20 minutes, a 3–4 mm radial incision was made in the anterior-inferior quadrant of that TM using a myringotomy knife4. After the procedure, a unilateral non-patent TM was confirmed by pneumatic otoscopy and tympanometry. The myringotomy was performed on the right TM in 12 and on the left TM in 3 subjects, with the sidedness of the procedure chosen by the surgeon based on technical ease and clear visualization of the TM. After the procedure and follow-up testing, these subjects were examined weekly by otoscopy and tympanometry until the incised TM had healed and then had repeat audiologic testing to confirm no procedure-related change in hearing threshold. All TMs healed without incident or complications and none of the ears had a significant change in hearing threshold.
Nineteen screened subjects with extant, functional bilateral (n=4) or unilateral (n=15, 8 with left 7 with right) VTs inserted by their otologist to treat “ETD” were enrolled into Group II. The average age for the group was 35.2 (range 18.3 to 60.4) years old, 8 were male and 11 female, and 16 identified their race as being White, 2 as Black and 1 as Asian. From subject history and medical record (available for 14 subjects) review, the VT was inserted into 3 Group II TMs (all unilateral cases) for an isolated diagnosis of ETD defined by a “hyper-inflated” ME that could not be cleared in 1 case and by symptoms of tinnitus, ME “fullness” and “muffled” hearing in 2 cases. VTs were inserted into the remaining 20 Group II TMs (4 bilateral, 12 unilateral) for a combined diagnosis of OME/ME effusion secondary to ETD (unspecified). None of the 3 isolated ETD cases had a past history of COME or of acute/recurrent acute otitis media but, of the 20 ears with a combined ETD/OME diagnosis, all had a history of COME and 10 had a history of acute/recurrent acute otitis media. Of the 19 Group II subjects, 11 reported a history of ME disease in childhood (10 with VTs inserted) and 10, 6 and 4 reported a positive history for allergic rhinitis, sinusitis and GERD, respectively. There is no evidence in the medical records or by subject history that any of these co-morbidities were specifically targeted for treatment in an attempt to resolve the ETD/OME prior to VT insertion. The study protocol was approved by the University of Pittsburgh Institutional Review Board (REN1210013).
ETF Testing Methods
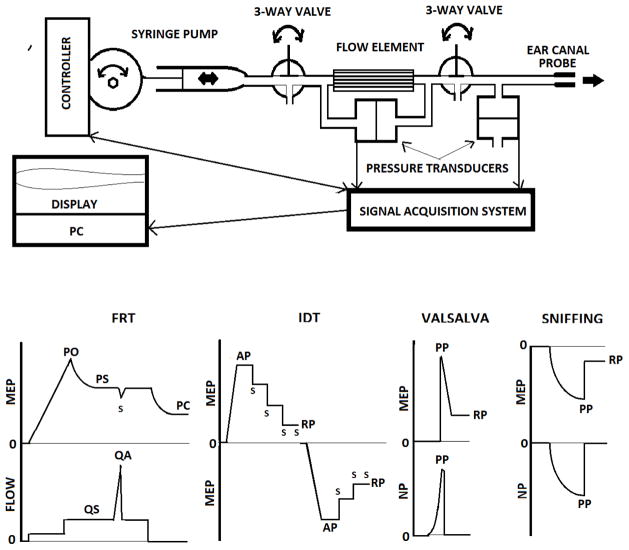
ETF in the 15 Group I ears with a TM perforation and in the 23 Group II ears with functional VTs was evaluated using a panel of 4 test protocols; the Forced-Response test (FRT), the Inflation-Deflation test (IDT), the Sniffing test and the Valsalva test. The instrument used for ETF testing was developed by us and consists of an ear-canal probe coupled serially by tubing to a SDX01D4 differential pressure transducer (Honeywell, Golden Valley, MN USA), via a 3-way valve to a flow sensor (Respiratory Flowhead 1L MLT1l, AD Instruments, Bella Vista, NSW Australia) and via a second 3-way valve to a variable-speed, constant flow pump (Harvard Apparatus Pump 22, Harvard Apparatus, Holliston, MA USA) with a controller (Syringe Pump Controller ver. 1.2, National Instruments, Austin, TX USA); and a nasal probe coupled by tubing to a SDX01D4 differential pressure transducer (Honeywell, Golden Valley, MN USA). The transducer signals are routed via a PL3504 PowerLab 4/35 data acquisition system to a PC running Lab Chart software 7.3.6 (AD Instruments, Bella Vista, NSW Australia) for real-time display of waveforms and data storage (See Figure 1a for schematic and 1b for idealized waveforms).
Figure 1.
Schematic diagram of the instrument used to test ETF (a), and idealized ME pressure (MEP) and trans-ET Flow versus time waveforms for the Forced-Response test (FRT), MEP versus time waveforms for the Inflation-Deflation test (IDT), and MEP and nasal pressure (NP) versus time waveforms for the Valsalva and Sniffing tests (b). “S” indicates a swallow. PO=ET opening pressure, PS=ET steady-state pressure, PC=ET closing pressure, AP=applied pressure, PP=peak pressure, RP=residual pressure, QS=steady-state flow and QA=peak flow during a swallow. See Methods for a complete description.
For the FRT, the ear-canal probe was sealed into the test ear. Both valves of the test instrument were opened and the constant flow pump delivered an air-flow of ≈11 ml/min to the ME. This increased ME pressure to passively force open the ET lumen (Opening pressure-PO). Continued delivery of the air-flow usually resulted in a semi-stable system pressure (PS) where trans-ET flow (QS) approximated the applied flow rate. At steady-state, the subject was instructed to swallow which is associated with contraction of the mTVP and mLVP. Activity of those muscles causes a change in ET lumen diameter reflected as an increased or decreased trans-ET air-flow (QA=maximum air-flow during a swallow). The pump was then turned off allowing the ET to passively close at a residual ME pressure (PC). This test sequence was then repeated at an applied air-flow rate of ≈23 ml/min. Throughout the test, system pressure and flow were continuously recorded. These waveforms were analyzed by two blinded investigators who identified and recorded PO, PC, PS, QS and QA and calculated 2 derived parameters; passive ET resistance (RS=PS/QS) and ET dilatory efficiency (DE=QS/QA) for both flow rates. The data were reconciled and the following parameters entered into the database for analysis: PO, PC, RS and DE at each air-flow rate and the ratio of the ET resistance at the 11 and 23 ml/min flow rates (RS11/RS23). PO and PC are measures of the passive forces that act to maintain a closed ET lumen, RS is a measure of the ease of trans-ET air-flow, and RS11/RS23 is a measure of ET compliance. Together, these parameters characterize the structural properties of the ET. In contrast, DE is a measure of the functional efficiency of muscle-assisted ET lumen dilation independent of surface adhesive forces; i.e. a functional property of the ET8.
For the IDT, the ear-canal probe was sealed into the test ear. Both valves were opened, ME pressure was increased at ≈11 cc/min to an overpressure of ≈200 daPa (ref. ambient), the valves were closed to reduce system volume, and the subject was asked to swallow repeatedly at a normal rate to a residual pressure (RP; the ME pressure at which further swallowing did not cause a ME pressure change). Then, ME pressure was reduced to ambient by venting the system to the atmosphere and the procedure was repeated at an applied ME under-pressure of ≈200 daPa. The parameters for analysis were the percent differences (SW+, SW−) between the applied ME over- or under-pressure and the respective residual pressures divided by the applied pressure. These two parameters are measures of the efficiency of muscle-assisted opening of the closed ET lumen; i.e. a functional property of the ET1.
For the Sniffing and Valsalva tests, the probe was sealed into the ear-canal, ME pressure was set at 0 daPa (ref. ambient) and the valves were closed to reduce system volume. Nasopharyngeal pressure was measured by the pressure sensor connected to a nasal olive held against one naris. The subject was asked to perform a forcible “sniff” and the pressures in the nasopharynx and ME during the maneuver and any residual ME pressure were recorded. The test was repeated if the minimum nasopharyngeal pressure was greater than −400 daPa. ME system pressure was reduced to 0 daPa, the upstream valves closed and the subject was asked to perform the Valsalva maneuver with both nares blocked and those parameters were again recorded. The test was repeated if the maximum nasopharyngeal pressure was less than 400 daPa. These tests were scored as positive if the change in ME pressure during the maneuver was at least 10% of the maximum nasopharyngeal pressure (peak pressure=PP) recorded during the Valsalva test (Val) or at least 10% of the minimum nasal pressure during the Sniffing test (SNF). Both of these tests are effort-dependent and susceptible to false negative results4,17. A negative Valsalva test may evidence a physical obstruction of the ET lumen and a positive Sniffing test was suggested to evidence “ET closing failure”16. We do not assign these parameters ad hoc to a structure/function category.
Statistical Methods
Table I lists the ETF test parameters included in the statistical analyses and, for each, the associated ETF test, a tentative assignment to a category (structural/functional) and a brief description of the measure. First, we defined the subset of parameters whose values were different between the two groups. There, the between-group difference for each parameter was evaluated for statistical significance using a Student’s t test if measured as a continuous variable or using a Chi-Square test if measured as a binomial variable, both evaluated at alpha=0.05. The sensitivity and specificity of each parameter alone to assign test ears to Group II were determined using Logistic Regression based on the receiver operating characteristic curve for parameters measured as a continuous variable or using a standard 2 × 2 contingency table for parameters measured as a binomial variable. The set of individual parameters that were identified as significant predictors of group assignment in these analyses were entered together into a logistic regression equation to determine if that set had a higher sensitivity and specificity for assignment to Group II than those for the individual parameters. We also attempted to identify a minimized subset of parameters that maximized between-group discrimination by entering all parameters into a step-down Discriminant Function Analysis. Finally, we used Factor Analysis with Varimax Rotation to explore the dimensionality of the set of ETF test parameters and to determine if there are a limited number of factors that group the parameters into meaningful subsets.
TABLE I.
Description and Categorization of Each Eustachian Tube Function Test Parameter (See Methods)
| PARAMETER | UNITS | TEST1 | CATEGORY | DESCRIPTION2 |
|---|---|---|---|---|
| VAL | Yes/No | Valsalva | Unclassified | NP to ME trans-ET gas flow at high NP over-pressures |
| SNF | Yes/No | Sniffing | Unclassified | ME to NP trans-ET gas flow at high NP under-pressures |
| PO#3 | (da/Pa) | FRT | Structural | ME pressure at which a closed ET is forced open |
| PC# | (da/Pa) | FRT | Structural | ME pressure at which an open ET passively closes |
| RS# | (daPa/ml/min) | FRT | Structural | ET resistance at a specified air-flow (intra-ET pressure/trans-ET flow) |
| RS23/RS11 | None | FRT | Structural | Ratio of ET resistances recorded at 23 and 11 ml/min (ET Compliance) |
| DE# | None | FRT | Functional | Dilatory Efficiency of muscle-assisted ET opening |
| SW+ | % | IDT | Functional | Percent applied ME over-pressure equilibrated by swallowing |
| SW− | % | IDT | Functional | Percent applied ME under-pressure equilibrated by swallowing |
FRT=Forced-Response Test, IDT=Inflation-Deflation Test
NP=Nasopharynx, ME=Middle Ear, ET=Eustachian Tube
# =Value recorded at a specified flow rate (11 or 23 ml/min)
The more simple analyses for each individual parameter used the total available dataset for all tested ears. However, Discriminant Function Analysis and Factor Analysis require a complete matrix of entries for all ears and parameters. Three Group I and 4 Group II tests did not satisfy this criterion and were not included yielding a sample size of 12 Group I and 19 Group II ears for those analyses. All analyses were done using the NCSS 2007 statistical software package (Kaysville, Utah).
RESULTS
For each parameter, Table II lists the average and standard deviation of the measures for the two groups, the Student’s t or chi-square value and associated probability level for the between-group comparison, the percent variance explained by a logistic regression equation that included the parameter as a predictor of group assignment and the sensitivity and specificity of the parameter for assigning ears to Group II. The between-group difference was significant for all test parameters classified as functional measures of ETF; the percent applied over- (SW+) and under-pressure (SW−) equilibrated by swallowing and the ET dilatory efficiencies recorded at the two flow rates (DE11 and DE23); but for no parameter classified as a structural measure of the ET. One of the two uncategorized parameters, the ability to force open the ET at high nasopharyngeal over-pressure (VAL), was also significantly different between groups. In all cases, the values associated with a more efficient ETF characterized Group I when compared to Group II ears. Individually, those 5 parameters had sensitivities ranging from 55% to 79% and specificities ranging from 58% to 85% for assigning ears to the ETD group. To determine if this combination of parameters was a better predictor of group assignment than any individual parameter, a logistic regression including the 5 parameters as predictors was run. The r2 of that regression equation was 0.52 and the sensitivity and specificity for assigning ears to the ETD group were 84% and 83%, respectively.
TABLE II.
For each test Parameter, the Average (AVG) and Standard Deviation (STD) of the Measure for the Control and Eustachian Tube Dysfunction (ETD) Groups, the Student’s t (T) or Chi-Square (χ2) Value and Associated Probability (P) for the Between-Group Comparison, and the R2 Value, Sensitivity (SENS) and Specificity (SPEC) of a Logistic Regression Equation including the Parameter as the Sole Predictor for Correct Assignment of Ears to the ETD Group.
| PARAMETER1 | ETD | CONTROL | T/χ2 | P | r2 | SENS | SPEC | ||
|---|---|---|---|---|---|---|---|---|---|
| AVG | STD | AVG | STD | ||||||
| Continuous | Variables | ||||||||
| SW+ | 0.57 | 0.36 | 0.94 | 0.1 | −3.43 | 0.002 | 0.411 | 75 | 65 |
| SW− | 0.33 | 0.38 | 0.73 | 0.23 | −3.35 | 0.002 | 0.279 | 73 | 58 |
| PO11 | 323.4 | 223.3 | 335.1 | 90.8 | −0.19 | 0.853 | 0.001 | ND3 | ND |
| PC11 | 85.8 | 103.4 | 81.9 | 51.1 | 0.13 | 0.897 | 0.000 | ND | ND |
| RS11 | 15.11 | 11.89 | 13.36 | 3.81 | 0.53 | 0.598 | 0.006 | ND | ND |
| DE11 | 3.16 | 3.08 | 8.7 | 7.4 | −3.07 | 0.004 | 0.205 | 79 | 58 |
| PO23 | 334.1 | 155.2 | 297.2 | 96.0 | 0.8 | 0.428 | 0.016 | ND | ND |
| PC23 | 69.0 | 59.0 | 57.6 | 30.0 | 0.67 | 0.505 | 0.013 | ND | ND |
| RS23 | 8.76 | 5.92 | 7.18 | 2.57 | 0.87 | 0.393 | 0.013 | ND | ND |
| DE23 | 2.48 | 2.25 | 4.51 | 2.83 | −2.44 | 0.021 | 0.115 | 79 | 58 |
| RS11/RS23 | 1.86 | 0.66 | 1.97 | 0.52 | −0.51 | 0.613 | 0.014 | ND | ND |
| Binomial | Variables | ||||||||
| VAL2 | 0.38 | 0.5 | 0.83 | 0.39 | 6.3 | 0.012 | ND | 55 | 85 |
| SNF 2 | 0.19 | 0.4 | 0.17 | 0.39 | 0.03 | 0.865 | ND | 52 | 51 |
See Table I for Parameter Definitions
Binomial Variables Expressed as a Frequency and Compared using a Chi-Square Test.
ND=Not Defined
Table III lists the minimum set of parameters identified by the Step-Down Discriminant Analysis that maximizes the accuracy of the model for assignment of ears to the 2 groups. Four parameters were members of that set; 2 functional measures, the percent ME over-pressure equilibrated by swallowing (SW+) and the dilatory efficiency recorded at an air-flow rate of 11 ml/min (DE11); 1 structural measure, the forced opening pressure of the ET at an air-flow rate of 11 ml/min (PO11), and 1 unclassified measure, the ability to force open the ET at high nasopharyngeal over-pressures (VAL). For this set of parameters, the sensitivity and specificity of the model equation for correct assignment of ears to Group II were 95% and 83%, respectively.
TABLE III.
The Minimal Set of Parameters that Maximize Group Discrimination Identified by a Step-Down Discriminant Function Analysis and the Associated Sensitivity and Specificity of the Model for Assignment of Ears to the Eustachian Tube Dysfunction (ETD) group
| Parameter-Selection Summary | |||
|---|---|---|---|
| Parameter1 | % Change In Lambda | F-Value | P-Level |
| SW+ | 23.78 | 9.05 | 0.005 |
| DE11 | 16.3 | 5.26 | 0.030 |
| PO11 | 17.25 | 5.42 | 0.028 |
| VAL | 15.35 | 5.08 | 0.032 |
| Predicted | ||||
|---|---|---|---|---|
| ETD | Control | Total | ||
| Actual | ETD | 18 | 1 | 19 |
| Control | 2 | 10 | 12 | |
| Total | 20 | 11 | 31 | |
| Sensitivity | 0.95 | |||
| Specificity | 0.83 | |||
See Table I for Parameter Definitions
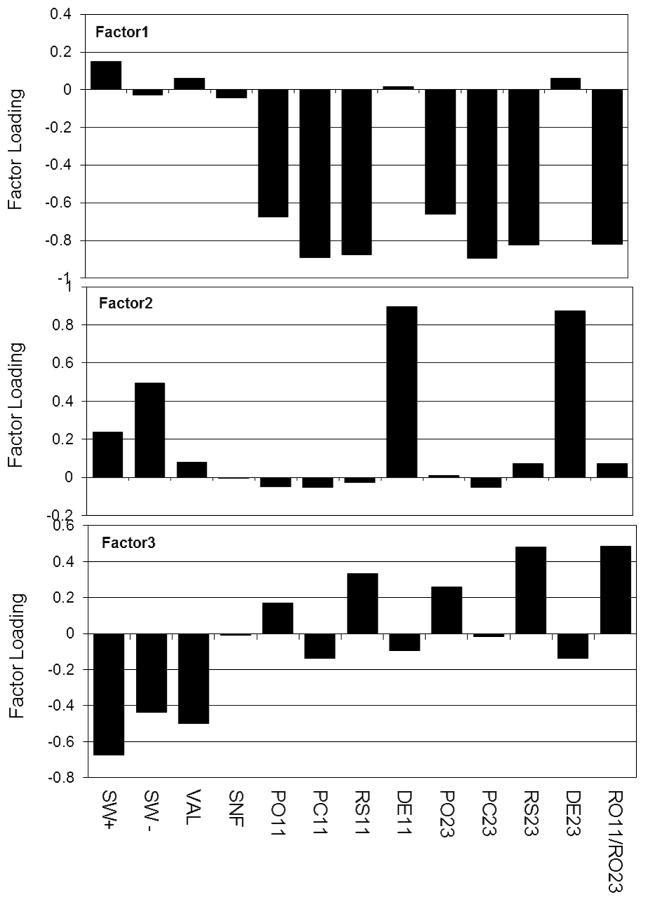
Three primary factors accounted for 98% of the variance among parameters; Factor 1=56%, Factor 2=23% and Factor 3=20%. Figure 2 shows a bar chart of the absolute loadings of each ETF test parameter on the three factors. Examination of Factors 1 and 2 suggest an interpretation consistent with expectation and, specifically, that most of the test parameters are dichotomized into two structural-functional domains. There, those parameters believed to be associated with the structural properties of the ET such as the forced ET opening (PO11, 23) and closing (PC11, 23) pressures, ET resistance (RO11, 23) and ET compliance (RS11/RS23) loaded negatively on Factor 1; while those parameters believed to be associated with the functional properties of the ET, such as the ET dilatory efficiency (DE11, 23) and the percent of applied ME over- (SW+) and under-pressures (SW−) equilibrated by swallowing loaded positively on Factor 2. In contrast, the two parameters that are not easily assigned to a given category, the ability of large nasopharyngeal over- (VAL) and under-pressures (SNF) to change ME pressure did not load on either factor. The structure of Factor 3 is more complicated, with a negative loading of most functional measures of the ET and a positive loading of most structural measures of the ET. Our interpretation is that Factor 3 represents a negative interaction between the two groups of parameters such that the structural properties of the ET constrain the efficiency of the functional measures of the ETF.
Figure 2.
Absolute loading of the ETF test parameters onto each of the three factors identified by Factor Analysis after Varimax Rotation. Factors are listed in an arbitrary sequence corresponding to those that capture structural measures of the ET (Factor 1), functional measures of the ET (Factor 2) and mixed measures (Factor 3). See Table I for parameter definitions.
DISCUSSION
ETF refers to the efficiency of ET openings with respect to maintaining an approximate ME-ambient pressure balance while protecting the ME from nasopharyngeal pressures and pathogens2. Adequate ETF is a prerequisite for normal hearing and continued ME health1. Most evidence suggests that there is a constitutive ETF imposed by the physical composition (e.g. distribution of elastic fibers in the periluminal tissues), mechanical properties (e.g. compliance and hysteresis of the periluminal tissues) and geometry (e.g. mTVP/mLVP-ET vector relationships) of the ET system; i.e. the structural properties of the ET18–20. In young children, constitutive ETF improves with growth and development as the ET system matures20,21 and, at all ages, constitutive ETF is downgraded during periods with extant co-morbid disease conditions that provoke inflammation in or around the ET such as viral upper-respiratory tract infection, allergic rhinitis and GERD22–24. ETD is an extant ETF insufficient to maintain ME pressure greater than the critical under-pressure that precipitates pathology (e.g. OME) and can be either constitutive or secondary (compromised ETF attributable to co-morbidies)2. ETD can be caused by an extrinsic (e.g. impinging adenoids, ET lumen obstruction during palatal elevation) or intrinsic (e.g. luminal inflammation, high periluminal tissue pressures) mechanical obstruction of the ET lumen or by a functional ET obstruction attributable to inefficient muscle-assisted dilation of the ET lumen (e.g. atrophic mTVP, inefficient mTVP-ET vector relationships)11,25.
Because of the important role played by ETD in the pathogenesis of common ME diseases such as OME and the low efficacy of standard medical treatments for promoting long-term resolution of those disease conditions, there has long been an interest in developing tests to evaluate ETF for purposes of diagnosing the presence/absence of ETD, identifying the type and cause of diagnosed ETD and targeting interventions to improve ETF and resolve the consequent ME disease1,8–13. To date, these efforts have been largely confined to the research setting with little evidence that any of the available ETF tests has been adopted for use in clinical decision-making.
In that regard, a number of ETF tests has been described1. Simple, non-invasive tests such as sonotubometry, barotubometry and the 9-step test can determine if the ET opens during specified maneuvers by detecting the presence/absence of signal transmission through the open ET, but provide no information regarding the underlying cause of any observed ET opening failure12,17,26,27. Alternatively, more complex, multi-protocol tests that require a non-intact TM can assess the efficiency of ET opening by swallowing and other maneuvers, “rule-out” certain causes of ETD and provide information that may relate to the physical and mechanical properties of the ET that constrain constitutive ETF (see above)4,8. However, the inter-relatedness of the various parameters for these tests has not been well characterized and existing interpretations of test results are based primarily on theory without strong empirical foundation.
Most past work on ETF tests focused on describing the test protocol, defining test-retest reproducibility and documenting a difference in the test measure(s) between populations with and without ME disease and/or suspected ETD8,12,14,16. While providing evidence that ETD as measured by each test is prevalent in populations “at risk” for ETD/ME disease, those results cannot be used to support the clinical utility of the test under study which requires a focus on ETF in the individual patient/ear. Recently, we began to explore the clinical utility of the more comprehensive ETF testing protocols that require a non-intact TM. For example, we completed a study wherein we enrolled children aged 3–6 years of age with VTs secondary to COME, repeatedly tested the children using an abbreviated FRT while the VTs were functional and evaluated the MEs for COME recurrence after the VTs became non-functional. For the population, there was no evidence of a patterned change in any of the ETF parameters over the period of time with functional VTs19. One FRT parameter, ET dilatory efficiency, when combined with sex, race and duration of VT functionality predicted disease recurrence at a sensitivity of 82% and a specificity of 77% (Mandel et. al., In Press, Laryngoscope, 2013). These results support the prognostic capabilities of this test protocol and hold promise for other clinical applications of ETF tests.
In the present study, we continue our research on the clinical utility of ETF testing by determining if a broad panel of ETF test parameters, alone or in combination, can identify individual ears with physician-diagnosed ETD and functional VTs within a mixed population of affected and control subjects. Because the test protocols require a non-intact TM which is not typical for ears without recent ME disease, a unilateral TM perforation was created in the control subjects by performing a myringotomy. Using standard between-group statistical comparison procedures, measures of the 4 parameters believed to reflect the efficiency of ET opening during swallowing (SW+, SW−, DE11,23), but none of those believed to reflect the structural properties of the ET, were significantly different between the two groups. Values for each parameter indicative of better ETF characterized the control group. When each of the 4 parameters was entered alone into an accuracy analysis, all had a sensitivity of approximately 75% and a specificity of approximately 60% for ear assignment to the ETD group. The percent of subjects who could opened their ET at high nasopharyngeal over-pressures (VAL) was also significantly different between the two groups and that measure had a sensitivity of 55% and a specificity of 85% for ear assignment to the ETD group. When combined, these 5 parameters correctly assigned ears to the ETD group at an 84% sensitivity and an 83% specificity which are comparable to those for other clinical tests/examinations (e.g. pneumatic otoscopy, tympanometry) used by ENT clinicians in their diagnosis of ME diseases28.
An ideal diagnostic test would include the minimal number of test protocols (parameters) required to maximize the information needed for group assignment. We explored this issue using a step-down Discriminant function algorithm operating on all available data. The resulting model included 4 parameters that together had a sensitivity and specificity for detecting ETD of 95% and 83%, respectively. This set was a subset of the parameters that were independent predictors of group assignment (SW+, DE11 and VAL) with an additional parameter (PO11). The higher sensitivity and specificity of this maximized discriminatory model when compared to the 5 parameter model and the difference in set elements for the two models suggest that the individual parameters capture a degree of redundant information and that interactions between parameter subclasses add important information for group discrimination.
From theory, we previously classified the test parameters as either measuring muscle-assisted ET opening efficiency or characterizing ET structure. We used Factor Analysis to test empirically the validity of those parameter assignments. Consistent with expectation, 2 independent factors were identified with high loadings of the presumed structural (Factor 1) or functional (Factor 2) parameters, respectively. A third factor was identified that can be interpreted as a negative interaction between the structural and functional parameters such that the former constrains the efficiency of the latter. Of interest, the parameters associated with the Valsalva and Sniffing tests did not load on either of the independent factors, though the Valsalva test parameter did load onto the interaction factor. These results support our suggestion that the test protocols used in this study capture redundant information with respect to the efficiency of ET opening, the structure of the ET system and the inter-play between ET structure and function.
The results of the present study establish an empirical classification of the relatedness among the measured ETF test parameters and support our tested “proof of principal” that certain combinations of ETF test parameters can accurately identify ears with “ETD” in a mixed population with high sensitivity and specificity. These results have clinical applications for identifying ears with ETD and evaluating the efficacy of selected interventions (medical treatments/surgical procedures) with respect to improving ETF. While it is expected that these test protocols can “rule-out” certain causes of ETD such as physical obstruction of the ET lumen, it is not expected that they will be able to distinguish between secondary and constitutive ETD which would require an intervention with an efficacious medication targeting the suspected co-morbidity and then retesting.
There are two caveats to our interpretation of the study results. First, the relatively small sample sizes for the two groups may have allowed us to develop a Discrimant Function equation that includes a parameter subset and assigns sensitivity and specificity estimates relevant only to the studied population. Future application of that type of statistical model to larger populations will be required to determine if the model developed here is generalizable or needs to be modified. Second, when assigning specificity and sensitivity to a diagnostic test, an independent “gold standard” is needed to define the presence/absence of the “true” disease state. Conceptually, ETD is an extant ETF insufficient to maintain ME pressure greater than the critical under-pressure that precipitates pathology, but there is no accepted method to diagnose ETD based on a defined set of symptoms and signs without recourse to testing. Here, we used VTs inserted for a physician diagnosis of ETD to define “true” state positive (ETD) and a negative history of ME diseases to define “true” state negative conditions2. There, we assume that practitioners have a shared, unarticulated, experiential conception of ETD that they use in diagnosing the condition. As described in the Methods section, the ETD group had a diverse ME disease history which introduces the possibility that ETF in Group II was not homogenous but included subsets of ears with different types of ETD and, perhaps, with normal ETF. However, the high accuracy of our test protocols to assign ears to the “true” disease state and the agreement between theory and experiment with respect to which parameters have the greatest discriminatory power suggests that the possible intra-group heterogeneity was not realized.
Acknowledgments
This study was supported in part by a grant from the National Institutes of Health (P50 DC007667), and by the Hamburg and Eberly Endowments to the Division of Pediatric Otolaryngology, University of Pittsburgh. These sources provided funding for the study, but did not have input into the design, analyses or interpretation of the data.
References
- 1.Bluestone CD, Doyle WJ. Anatomy and physiology of eustachian tube and middle ear related to otitis media. J Allergy Clin Immunol. 1988 May;81(5 Pt 2):997–1003. doi: 10.1016/0091-6749(88)90168-6. [DOI] [PubMed] [Google Scholar]
- 2.Doyle W. Middle ear pressure regulation. In: Rosowski J, Merchant S, editors. The Function and Mechanics of Normal, Diseased and Reconstructed Middle Ears. The Hague, The Netherlands: Kugler Publications; 2000. [Google Scholar]
- 3.Doyle WJ. Functional eustachian tube obstruction and otitis media in a primate model. A review. Acta Otolaryngol Suppl. 1984;414:52–57. doi: 10.3109/00016488409122882. [DOI] [PubMed] [Google Scholar]
- 4.Swarts JD, Alper CM, Mandel EM, Villardo R, Doyle WJ. Eustachian tube function in adults without middle ear disease. Ann Otol Rhinol Laryngol. 2011 Apr;120(4):220–225. doi: 10.1177/000348941112000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson MJ. Otitis media with effusion. Lippincotts Prim Care Pract. 1997 May-Jun;1(2):168–171. [PubMed] [Google Scholar]
- 6.Casselbrant ML, Villardo RJ, Mandel EM. Balance and otitis media with effusion. Int J Audiol. 2008 Sep;47(9):584–589. doi: 10.1080/14992020802331230. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa CT. Conductive hearing loss and speech development. J Allergy Clin Immunol. 1988 May;81(5 Pt 2):1015–1020. doi: 10.1016/0091-6749(88)90171-6. [DOI] [PubMed] [Google Scholar]
- 8.Cantekin EI, Saez CA, Bluestone CD, Bern SA. Airflow through the eustachian tube. Ann Otol Rhinol Laryngol. 1979 Sep-Oct;88(5 Pt 1):603–612. doi: 10.1177/000348947908800504. [DOI] [PubMed] [Google Scholar]
- 9.Honjo I, Kumazawa T, Honda K, Shimojo S. Electromyographic study of patients with dysfunction of the Eustachian tube. Arch Otorhinolaryngol. 1979;222(1):47–51. doi: 10.1007/BF00456338. [DOI] [PubMed] [Google Scholar]
- 10.Mathew GA, Kuruvilla G, Job A. Dynamic slow motion video endoscopy in eustachian tube assessment. Am J Otolaryngol. 2007 Mar-Apr;28(2):91–97. doi: 10.1016/j.amjoto.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H, Miura M, Honjo I, Fujita A. Cause of eustachian tube constriction during swallowing in patients with otitis media with effusion. Ann Otol Rhinol Laryngol. 1996 Sep;105(9):724–728. doi: 10.1177/000348949610500910. [DOI] [PubMed] [Google Scholar]
- 12.Bhat VK, Kumar PR, Nag M, Hegde J. Comparison of a eustachian barotubometer with a tympanometer to evaluate eustachian tube function in chronic suppurative otitis media. J Otolaryngol Head Neck Surg. 2009 Aug;38(4):456–461. [PubMed] [Google Scholar]
- 13.Handzel O, Poe D, Marchbanks RJ. Synchronous endoscopy and sonotubometry of the eustachian tube: a pilot study. Otol Neurotol. 2012 Feb;33(2):184–191. doi: 10.1097/MAO.0b013e3182423242. [DOI] [PubMed] [Google Scholar]
- 14.Swarts JD, Bluestone CD. Eustachian tube function in older children and adults with persistent otitis media. Int J Pediatr Otorhinolaryngol. 2003 Aug;67(8):853–859. doi: 10.1016/s0165-5876(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 15.Alper CM, Losee JE, Mandel EM, Seroky JT, Swarts JD, Doyle WJ. Postpalatoplasty eustachian tube function in young children with cleft palate. Cleft Palate Craniofac J. 2012 Jul;49(4):504–507. doi: 10.1597/11-065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falk B, Magnuson B. Evacuation of the middle ear by sniffing: a cause of high negative pressure and development of middle ear disease. Otolaryngol Head Neck Surg. 1984 Jun;92(3):312–318. doi: 10.1177/019459988409200313. [DOI] [PubMed] [Google Scholar]
- 17.Falk B, Magnuson B. Eustachian tube closing failure in children with persistent middle ear effusion. Int J Pediatr Otorhinolaryngol. 1984 May;7(2):97–106. doi: 10.1016/s0165-5876(84)80034-8. [DOI] [PubMed] [Google Scholar]
- 18.Beery QC, Doyle WJ, Cantekin EI, Bluestone CD. Longitudinal assessment of Eustachian tube function in children. Laryngoscope. 1979 Sep;89(9 Pt 1):1446–1456. doi: 10.1002/lary.5540890910. [DOI] [PubMed] [Google Scholar]
- 19.Doyle WJ, Mandel EM, Seroky JT, Swarts JD, Casselbrant ML. Reproducibility of the forced response test in children with chronic otitis media with effusion. Otol Neurotol. 2013 Jan;34(1):16–21. doi: 10.1097/MAO.0b013e31827853f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyle WJ, Swarts JD. Eustachian tube-tensor veli palatini muscle-cranial base relationships in children and adults: an osteological study. Int J Pediatr Otorhinolaryngol. 2010 Sep;74(9):986–990. doi: 10.1016/j.ijporl.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bylander A. Comparison of eustachian tube function in children and adults with normal ears. Ann Otol Rhinol Laryngol Suppl. 1980 May-Jun;89(3 Pt 2):20–24. doi: 10.1177/00034894800890s308. [DOI] [PubMed] [Google Scholar]
- 22.Friedman RA, Doyle WJ, Casselbrant ML, Bluestone C, Fireman P. Immunologic-mediated eustachian tube obstruction: a double-blind crossover study. J Allergy Clin Immunol. 1983 May;71(5):442–447. doi: 10.1016/0091-6749(83)90459-1. [DOI] [PubMed] [Google Scholar]
- 23.Heavner SB, Hardy SM, White DR, McQueen CT, Prazma J, Pillsbury HC., 3rd Function of the eustachian tube after weekly exposure to pepsin/hydrochloric acid. Otolaryngol Head Neck Surg. 2001 Sep;125(3):123–129. doi: 10.1067/mhn.2001.116448. [DOI] [PubMed] [Google Scholar]
- 24.McBride TP, Doyle WJ, Hayden FG, Gwaltney JM., Jr Alterations of the eustachian tube, middle ear, and nose in rhinovirus infection. Arch Otolaryngol Head Neck Surg. 1989 Sep;115(9):1054–1059. doi: 10.1001/archotol.1989.01860330044014. [DOI] [PubMed] [Google Scholar]
- 25.Bluestone CD. Eustachian Tube Structure, Function, Role in Otitis Media. Hamilton, Ontario: BC Decker Inc; 2005. [Google Scholar]
- 26.Di Martino EF, Nath V, Telle A, Antweiler C, Walther LE, Vary P. Evaluation of Eustachian tube function with perfect sequences: technical realization and first clinical results. Eur Arch Otorhinolaryngol. 2010 Mar;267(3):367–374. doi: 10.1007/s00405-009-1074-9. [DOI] [PubMed] [Google Scholar]
- 27.McBride TP, Derkay CS, Cunningham MJ, Doyle WJ. Evaluation of noninvasive eustachian tube function tests in normal adults. Laryngoscope. 1988 Jun;98(6 Pt 1):655–658. doi: 10.1288/00005537-198806000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Nozza RJ, Bluestone CD, Kardatzke D, Bachman R. Identification of middle ear effusion by aural acoustic admittance and otoscopy. Ear Hear. 1994 Aug;15(4):310–323. doi: 10.1097/00003446-199408000-00005. [DOI] [PubMed] [Google Scholar]