Abstract
The growth of natural biominerals is often tightly regulated by surface adsorption and subsequent incorporation of proteins into the crystal structure. Understanding how macromolecules intercalate into inorganic crystal lattices and how incorporation affects protein structure is crucial to learning how to engineer biomimetic materials with advanced properties, yet knowledge about the molecular-level interactions between organic guests and inorganic hosts remains sparse. Here we have used fluorescence resonance energy transfer (FRET) to probe conformational changes of a macromolecule as it adsorbs to, and becomes incorporated within, a biomineral crystal. Calcium oxalate monohydrate (COM) was used as a model due to its large size and kinetic stability under a wide range of pH values. Since the conformation of the extracellular matrix protein fibronectin (Fn) is highly sensitive to local ion concentrations, major conformational changes can be observed by FRET, as Fn senses and responds to varying local ionic conditions. When transferred from a physiological buffer to a supersaturated solution, Fn's crossed-over dimeric arms separate, indicating a weakening of the electrostatic interactions which otherwise stabilize the compact conformation of the protein. Fn returns to a more compact state when binding to the flat (–1 0 1) surface of the crystal, suggesting that Fn might sense a zone of ion depletion right at the interface of the growing crystal. As the crystal begins to grow around the absorbed protein, the dimeric Fn arms separate again, potentially driven by interactions with the newly formed charged step edges forming around it during the embedding process. FRET thus reveals for the first time how local changes in the electrostatic environment during the growth of a biomineral can cause major alterations in protein conformation. The insights derived using FRET and atomic force microscopy (AFM) could stimulate novel ways to tailor and tune the properties of organic–inorganic composites by exploiting dynamically changing electrostatic guest–host interactions.
Keywords: Fluorescence resonance energy transfer, (FRET), Protein adsorption, Protein, incorporation, Biominerals
1. Introduction
The conformation that proteins assume as they are incorporated into biominerals has a fundamental impact on crystal attributes, including their nucleation kinetics [1], morphology [2], polymorphism [3,4]and materials properties [5]. Some proteins can become interred within biominerals without affecting the integrity of single inorganic crystals [3,6–8]. Understanding how such large molecules become incorporated into a crystal lattice is crucial to tailoring the properties of inorganic materials. Atomic force microscopy (AFM) was one of the first techniques that probed protein conformation and interactions with growing biomineral crystals. Single globular proteins bound to specific calcite planes [9] were imaged, to demonstrate protein attachment to the step edges of growing calcite [10,11]. Globular proteins were shown to attach to calcite edges, slowing and eventually stopping step growth, and atomic resolution measurements of the final lattice structure showed that a direct change from calcite to aragonite occurred in the presence of these soluble proteins [12].
More recently, thermodynamic and solid state NMR has been used to examine protein and biomineral surface interactions by probing interactions between the biomineral hydroxyapatite (HAP) and the saliva protein statherin, a protein known to inhibit HAP nucleation [13,14], and it was found that the interaction of statherin with HAP induces formation of secondary structure, while statherin in solution remains unstructured. While probing protein conformations by AFM or NMR remains limited to surface-bound states, probing peptides or protein conformations during or after the embedding process has mainly been done by demineralization, followed by scanning electron microscopy (SEM) [15,16], histo-chemical labeling [17], or TEM [18]. These studies have provided data about the roles of major functional groups of macromolecular matrices in directing biomineralization processes and enhancing mechanical properties, as well as providing techniques for mapping location and distribution of proteins within biominerals. Immuno-gold labeling, in combination with SEM, has also been used on fractured calcium carbonate to identify protein types and their distribution within the crystals [19]. However, fluorescence resonance energy transfer (FRET), which has been extensively applied in biomedical sciences and many other fields, has never been applied to the absorption and incorporation of macromolecules into a biomineral.
Calcium oxalate monohydrate (COM) was used as a model biomineral system because it can be grown to comparatively large crystal sizes, has a relatively simple crystal structure and well-understood phase behavior, and is physiologically significant as the primary mineral component in human kidney stones [20]. Furthermore, its growth kinetics are insensitive over a wide range of pH values [21], which is important since a macromolecule carrying charges may alter local effective pH conditions [22–24].
Although a number of urinary proteins have already been shown to interact with COM and influence growth kinetics, morphology and cell attachment [25–27], how they bind and incorporate remains an open question. Stereospecificity between macromolecules and COM has been demonstrated for the urinary protein nephrolcalcin [25] and glycosaminoglycans [27], which are thought to preferentially bind to the (–101) face. It has also been demonstrated that proteins do incorporate into single crystals of COM both in vivo and in vitro[16], and that such intercrystalline proteins may play important roles in facilitating the breakdown of kidney stones, particularly after cell attachment and phagocytosis. Interestingly, COM crystals can upregulate the expression of Fn by renal epithelial cells, which may subsequently play a dual role in both preventing COM aggregation [28] and regulating the endocytosis of early COM crystals [29–31]. Fn is postulated to be a potent COM binding molecule[32], but little isknownabout its attachment to and incorporation into these crystals.
Fibronectin (Fn), a cell adhesion protein, was used as a model protein since it is known to undergo major changes of its quaternary structure in response to changes in ionic strength and electrostatic surface interactions [33–44]. Focusing on Fn is of major physiological interest since it exists as a compact dimer in blood, interstitial fluids, and urine, and is assembled by cells into fibrillar networks that define extracellular matrix and connective tissues [45]. Fn furthermore plays a regulatory role in early phases of bone formation [46,47].
Molecular interaction studies with biomineral surfaces revealed that they are dominated by electrostatics rather than specific protein or lipid/lattice interactions [24,48]. Fn has a number of recognition sites that can bind to solid surfaces, other extracellular proteins and cells, including cell integrin binding via the Arg–Gly–Asp (RGD) loop and the proximal synergy site (for review see [49]). Exposure of these sites is regulated by conformational changes within the protein [50], and when adsorbed to surfaces is highly sensitive to the surface chemistry [42].
FRET, the non-radiative energy transfer from a donor to an acceptor [51], has frequently been applied as a “spectroscopic ruler” [52–54] to study layer thicknesses (for review see [55]), protein/ligand and protein/membrane interactions, as well as to examine protein conformational changes, with broad applications in biological research [56–58]. Here we apply FRET to investigate conformational changes as a macromolecule both adsorbs from a supersaturated solution to a well defined single crystal surface, and intercalates during crystallization. Resonant dipole coupling between a donor and acceptor can occur if their distance is less than 10 nm. Fn, however, is composed of more than 54 domains exhibiting an end-to-end distance of 120-140 nm, even when the secondary structure of all domains is intact [34,59]. To ensure that the FRET signature is sensitive to a broad range of conformational changes, Fn was labeled on average with 7 donors and 4 acceptors per molecule [41,44,60]. We thus sacrificed the ability to measure absolute distances, but gained sensitivity to probing major changes in the average donor-acceptor distances, from a tightly folded quaternary structure, to breaking the quaternary structure, to loosing secondary structure and upon denaturation [34,41,44,59]. To prevent intermolecular FRET, FRET-labeled Fn was always highly diluted with unlabeled Fn.
Here, we investigate changes in Fn conformation by FRET and AFM. The acceptor versus donor fluorescence intensity (IA/ID) was measured for Fn either adsorbed to mature crystals or incorporated during the growth of COM. This was compared to the IA/ID ratio probed for Fn under partial denaturing conditions [44,60]. These results suggest a model in which the protein's compact conformation is disrupted by supersaturated solution conditions, as well as short-range electrostatic interactions on the surface of a growing mineral crystal, ultimately revealing unexpectedly complex conformational changes during the process of incorporation.
2. Materials and methods
2.1. Protein labeling
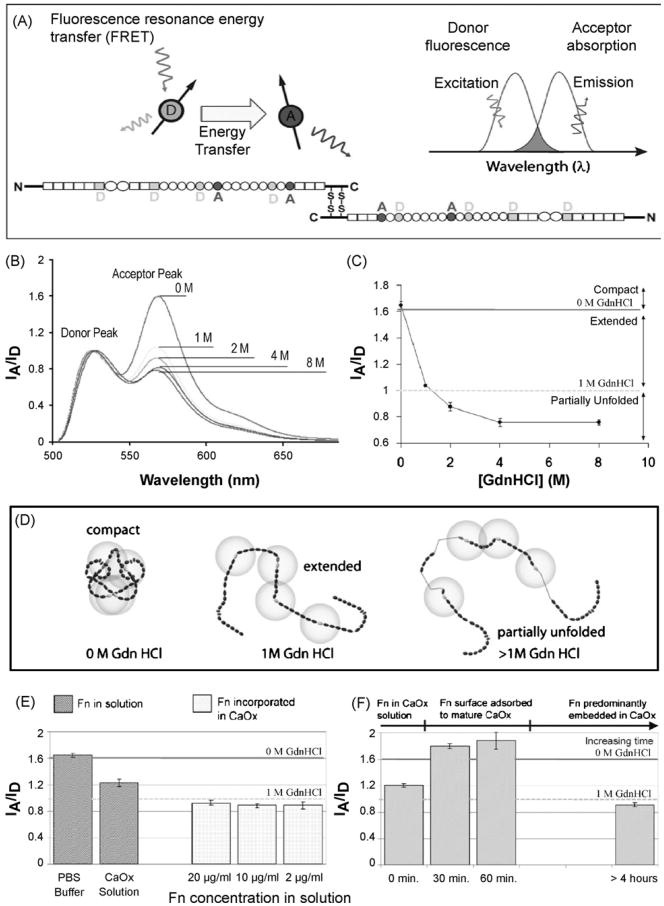
Human plasma fibronectin (Chemion) was labeled by conjugation of fluorescent dyes to specific protein residues, as described previously [41,60] and shown in Fig. 1A. Briefly, Fn was specifically conjugated with the acceptor dye, Alexa 546 maleimide (Molecular Probes), on the free cysteine residues in modules FnIII7 and FnIII15, resulting in four possible sites per dimeric Fn protein (Fig. 1A). Amine residues of unknown location were randomly labeled with Oregon Green 488 succinimidyl ester. There are over 80 amine residues per Fn dimer.The results reported here are all from a single batch yielding an average of 6 donors to 3.8 acceptors per dimeric Fn protein. Consistent results were obtained using several other batches of labeled Fn.
Fig. 1.
Conformational changes of fibronectin (Fn) probed by FRET. (A) Fn was specifically conjugated with the acceptor dye, Alexa 546 maleimide, on the free cysteine residues in modules FnIII7 and FnIII15, resulting in 4 possible sites per dimeric Fn protein. Amine residues were randomly labeled with the donor dye, Oregon Green 488 succinimidyl ester. (B) Fluorescence spectra of FRET-labeled fibronectin (Fn-D/A) in buffer normalized to the donor peak. Spectra were measured at 0M, 1 M, 2M, 4M and 8M GdnHCl. The residual acceptor peak at 8M GdnHCL is due to direct excitation of the acceptor since the raw spectra as monitored on the microscope stage are reported not corrected for residual cross-talk between the excitation and emission channel. (C) Reference curve probing the—FRET (IA/ID) ratio as a function of GdnHCL concentration for Fn in solution. Please note that the raw spectra are shown, not corrected for residual crosstalk between the excitation and emission channel. (D) Schematic sketch of putative Fn conformations in solution [44]. When IA/ID >1.6, Fn-D/A is in a compact conformation. When 1.6 >IA/ID > 1.0, Fn-D/A is partially extended due to the reduced electrostatic interactions that stabilize the contact between the crossed-over dimer arms of Fn. When 1.0 >IA/ID > 0.88, Fn-D/A is extended. When 0.88 >IA/ID > 0.76, Fn-D/A is partially unfolded. When IA/ID < 0.76, Fn-D/A is unfolded (denatured). Energy transfer between donors and acceptors bound to free cysteines are limited to within approximately double the Förster radius (∼12 nm), denoted by gold circles around Fn III7 and Fn III15. Fn in solution assumes a compact conformation stabilized through ionic interactions between dimer arms [39]. To prevent energy transfer between adjacent Fn molecules, only 5% of the Fn used was labeled for FRET, and the remainder was unlabeled. (E) Intramolecular energy transfer (IA/ID) for Fn in solution and for Fn incorporated in COM crystals which were grown in the presence of the protein. IA/ID decreases for Fn suspended in supersaturated calcium oxalate solution, indicating that Fn has adopted a partially extended conformation (1.6 >IA/ID> 1.0). IA/ID further decreases for Fn incorporated into COM (1.0 >IA/ID > 0.88), suggesting a fully extended, but not unfolded, conformation. IA/ID is consistent for batches of 5% Fn-D/A+ 95% Fn-unlabeled added to supersaturated calcium oxalate crystallizing solutions with concentrations of 20,10 and 2 μg/ml prior to COM nucleation. Data are averages ±SD for at least 10 separate crystals. (F) Energy transfer (IA/ID) for 10 μg/ml Fn (5% Fn-D/A+ 95% Fn-unlabeled) adsorbed to mature COM crystals as a function of time. For Fn adsorbed directly to crystals in situ, IA/ID increases over time until it stabilizes in the >1.6 range, which is similar to energy transfer seen in buffer and indicates a compact conformation. IA/ID for crystals remaining in supersaturated solution in separated crystallizing dishes over time eventually decreases indicating that Fn is extended (1.0 >IA/ID > 0.88) as it becomes incorporated within crystals. Data are averages ±SD for at least 10 separate crystals.
2.2. Crystal growth
Calcium oxalate monohydrate (CaC2O4-H2O or COM) crystals were grown at room temperature (22–25 °C) from supersaturated solutions prepared by mixing appropriate amounts of stock solutions consisting of salts (CaCl2-2H2O and K2C2O4-H2O, Aldrich) dissolved in ultrapure water (18 MΩ cm resistivity). The CaCl2 stock was added drop-wise to a 10mmoll–1 ionic strength K2C2O4 solution resulting in an equimolar mixture with [Ca2+]=[C2O42–] = 220 μmoll–1. Spontaneous precipitation occurs at this molarity, which has relative supersaturation (σ) values of 1.44 as calculated by a speciation program similar to EQUIL [61]. Crystals were grown on cover slips that were placed in either 20 ml clean glass vials or in Petri dishes for at least 48 h. The majority of the COM crystals had a characteristic hexagonal prismatic shape [62], with sizes ranging from 10 μm × 5 μm to 60 μm × 20 μm. Prior to crystal growth experiments, labeled Fn was mixed with various concentrations of unlabeled Fn to ensure that only intermolecular energy transfer occurred. A ratio of 95% unlabeled and 5% labeled Fn-D/A was well below the threshold at which we noticed intermolecular energy transfer, yet this ratio still gave sufficient signal intensity to be detected by the system.
In adsorption experiments, Fn in concentrations ranging from 0.02 to 20 μg/ml was added directly to supersaturated solutions that included mature crystals, and Fn was allowed to adsorb for 1 to 4h. In crystallization experiments, mixed crystals of protein and calcium oxalate were grown as described above except that Fn was pipetted into the calcium oxalate solution prior to crystal nucleation in concentrations ranging from 0.02 to 20 μg/ml (with 95% unlabeled, 5% Fn-D/A). These adsorption and crystallization experiments were repeated in glass vials, in Petri dishes, and in situ, directly on the microscope.
2.3. Characterization of labeled fibronectin in calcium oxalate solution
A range of concentrations of labeled Fn in fresh calcium oxalate solution (σ = 1.44) was measured in 40 μl droplets on a coverslip using the imaging system described below. The data given here are for a concentration of 45 μg/ml in calcium oxalate solution at a relative supersaturation of σ = 1.44.
2.4. FRET imaging
Fluorescence imaging and spectroscopy were performed in this laboratory with an inverted epi-fluorescence microscope (Nikon TE 2000) and a 512×512 back-illuminated liquid nitrogen CCD camera (TEK512; Princeton Instruments, Trenton, NJ). Energy transfer was visualized with a FITC cube/filter combination that passes only the donor excitation and transmits both donor/acceptor emissions [41,60]. Images were taken in conjunction with emission spectra, which provided additional data and illustrated how efficiency depends on the protein conformation. Imaged light was converted to spectra using Metamorph software (Universal Imaging Corporation) and further analyzed using Igor Pro (Wavemetrics). A background was automatically subtracted from each spectrum.
2.5. FRET from Fn under denaturing conditions solution
As seen in Fig. 1B-D, FRET is sensitive to a wide range of conformational changes of Fn that occur upon denaturing the protein in increasing concentrations of Guanidinium Hydrochloride (GdnHCl). Since we did not correct our spectra for any crosstalk, the residual acceptor peak at 8 M GdnHCL is due to direct excitation of the acceptor. Fn began to extend from its compact conformation in buffer solution, to an intermediate range of conformations at 0–1.5 M GdnHCl which have previously been attributed to the disruption of ionic interactions within the protein, including those that stabilize the two dimers. At concentrations above 1.5 M GdnHCl, Fn modules began to lose their β sheet structure, and finally Fn became fully denatured at 8 M GdnHCl.
2.6. AFM
Scanning probe microscopy measurements were taken in air using at room temperature in non-contact mode with a Digital Mul-timode IIIa with an E scanner (Digital Instruments, Santa Barbara, USA).
3. Results
3.1. FRET from Fn in buffer, under denaturing conditions and in supersaturated calcium oxalate solution
To investigate how ionic conditions alter the conformation of Fn in solution, FRET was probed for Fn in PBS buffer and under denaturing conditions, as well as in supersaturated calcium oxalate solution. Fluorescence spectra of FRET-labeled fibronectin (Fn-D/A) in buffer normalized to the donor peak were measured at 0 M, 1 M, 2M, 4M and 8M GdnHCl (Fig. 1B,C). The residual acceptor peak at 8 M GdnHCL was due to direct excitation of the acceptor since the raw spectra as monitored on the microscope stage are reported uncorrected for residual cross-talk between the excitation and emission channel. As supported by circular dichroism spectra [35,41,44,63,64], Fig. 1D shows putative Fn conformations in solution [44], from the compact conformation in solution or the blood, to a partially extended structure that results when the intramolecular electrostatic interactions that stabilize the contact between the crossed-over dimer arms of Fn are broken by competing ions, to, finally, partially denatured states. When Fn was suspended in calcium oxalate solution with an ionic strength of 10 mM (NaCl), a slight decrease in IA/ID was observed as compared to Fn in PBS buffer (Fig. 1E). It has been found previously [34,41] that 1 M NaCl causes an expansion of Fn which is not associated with a loss of secondary structure. This suggests that the ionic strength of the supersaturated solution caused a slight disruption of electrostatic interactions between the two arms of the protein, concomitant with the reduction in FRET.
3.2. Fn adsorption to the flat planes of mature single crystals of calcium oxalate probed by FRET
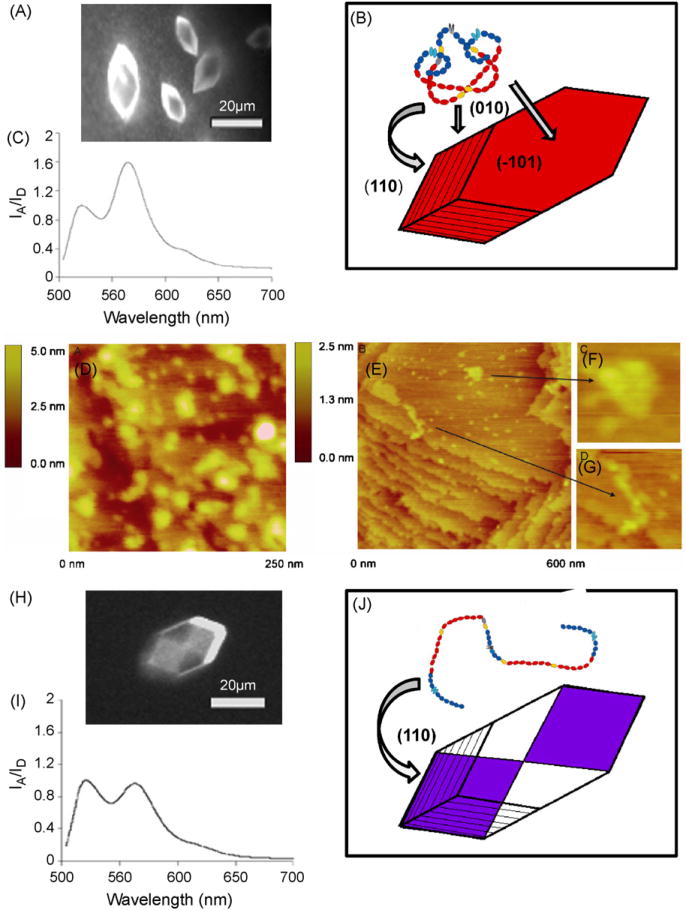
Fn was found to adsorb directly to mature crystals of COM, and the FRET ratio was not a function of Fn concentration (Fig. 1F). A high ratio of IA/ID was measured on the (–101), (0 1 0) and apical faces of COM, with no significant difference in intensities between the different faces. The increase in IA/ID upon adsorption to the crystal implies that Fn initially adopts a conformation that is more compact than that in supersaturated calcium oxalate solution, approaching the IA/ID measured for the tightly folded conformation seen in PBS buffer. After the protein was left to adsorb to mature crystals over several hours (with no depletion of crystallizing solution) energy transfer was observed to decrease, implying that the dimeric arms of Fn become separated as it is embedded into a growing crystal. A representative fluorescence image and emission spectra are shown in Fig. 2A–C, along with a schematic sketch of the crystal and compact (adopted from [39]). The data suggest that while the globular Fn structure may be slightly disrupted in supersaturated calcium oxalate crystallizing solution, it assumes a tightly compact conformation as it adsorbs to the various faces of COM. The FRET data then give first indications that the protein might transition to a more extended conformation as it is incorporated over time into a growing crystal, a conclusion that is further corroborated when COM is nucleated in the presence of labeled Fn (see below).
Fig. 2.
Imaging of fibronectin adsorbed to and incorporated within single COM crystals. (A) Fluorescent image of Fn adsorbed to mature COM. (B) Representative energy transfer (IA/ID) spectrum for 10 μg/ml Fn (5% Fn-D/A + 95% Fn-unlabeled) adsorbed to mature COM crystals for 20min (C) Schematic illustration of protein adsorption to all exposed faces of COM and the compact conformation that Fn adopts once it is adsorbed to the surface. (D) AFM image (250 nm × 250 nm) of the (–101) face of mature COM with Fn adsorbed for 1 h and adhering to terraces in a compact form. (E) AFM image (600 nm × 600 nm) of 0.04 μg/ml unlabeled Fn adsorbed to COM and left in crystallizing solution for 3 h.(F) Zoomed image (120 nm × 120 nm) of Fn extended between two step edges. (G) Zoomed image (50 nm × 50 nm) of compact Fn resting on a wide terrace. (H) Fluorescent image of Fn incorporated into COM along fast growing (110) apical planes. (I) Representative energy transfer (IA/ID) spectrum for 20 μg/ml Fn(5% Fn-D/A + 95% Fn-unlabeled) incorporated into COM during crystallization. (J) Schematic illustration of the “hourglass” pattern indicative of incorporation along (110) and the extended conformation that Fn adopts as it is becomes incorporated into a growing crystal.
3.3. AFM of Fn adsorbed to single calcium oxalate crystals
Mature COM crystals that were exposed for 1 h to a supersaturated solution containing Fn (in concentrations ranging from 2 to 5 μg/ml) were rinsed, dried and placed in the AFM under ambient conditions. AFM non-contact mode measurements confirmed Fn frequently adsorbed as compact, globular structures, 20–30 nm in width, resting on the wide step terraces found on the (–1 01) faces of mature COM (Fig. 2D). Naturally AFM could not be used to image protein already interred within the single crystal. However, AFM images were also taken of mature COM crystals that first adsorbed Fn and then were allowed to rest in supersaturated solution for several hours. These measurements exhibited regions where Fn was in an extended conformation between step edges (Fig. 2E and G), but remained in globular, compact form where it rested on the wide step terraces (Fig. 2F).
3.4. Calcium oxalate crystals grown in the presence of FRET-labeled Fn
As investigated previously, Fn incorporates into growing COM over the full range of protein concentrations measured here [65]. At higher concentrations (generally ≥ 1 μg/ml) protein incorporation exhibited intersectoral zoning, appearing as a fluorescent “hourglass” shaped region within the crystal (Fig. 2H) and denoting incorporation along the fast growing apical planes. At lower protein concentrations, Fn still incorporated, but appeared more diffuse and the hourglass pattern was less clear. Overgrowth of protein by COM was confirmed by partial dissolution of fluorescent “hourglass” patterned crystals, followed by additional examination with the fluorescence microscope. IA/ID was measured from several points on at least 10 crystals per sample, and the from these mixed crystals were consistent over the range of protein concentrations (Fig. 1E). This insensitivity of the IA/ID ratio to the packing density of FRET-labeled FN and further confirms intramolecular FRET where the probe molecules were sufficiently diluted by unlabeled Fn. The IA/ID ratio of Fn incorporated into COM crystals was significantly lower than that from the protein in PBS buffer and in calcium oxalate solution (Fig. 2I). These results suggest that Fn is gradually converted back to a more extended conformation during the embedding process (Fig. 2J). The degree of Fn extension seen in COM crystals is similar to that measured in 1–2M GdnHCl in solution. Previous work has shown that GdnHCl at these concentrations causes Fn to open up in solution [41,44].
4. Discussion
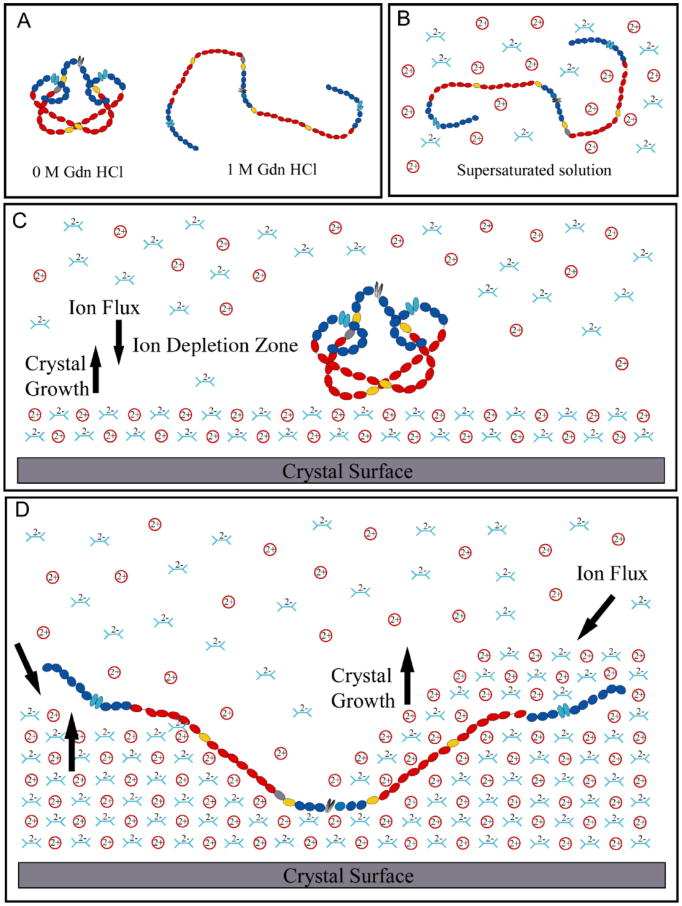
Major conformational changes can be observed by FRET as Fn senses and responds to alterations in the local ionic conditions during biomineralization. When transferred from physiological buffer to supersaturated solution conditions, Fn loses its compact globular conformation and its dimeric arms partially separate (Fig. 1E). Unexpectedly, Fn returns to a more compact state after binding to the flat (–101), (0 1 0) or (0 1 0) COM crystal surfaces under super-saturated conditions, as confirmed by two independent techniques, FRET (Fig. 2A–C) and AFM (Fig. 2D–G). Finally, FRET indicates that Fn's dimeric arms open up again during the embedding process as the COM crystal grows around it (Fig. 2H–J). Under physiological buffer conditions, electrostatic interactions stabilize the dimeric arms of Fn in a compact quaternary structure [39,60], while high salt concentrations are known to disrupt these interactions [39]. Breaking those intramolecular Fn arm–arm interactions facilitates major movement of those segments which will reduce or even eliminate the intramolecular energy transfer between them. The fact that Fn is observed by FRET to undergo major conformational changes indicates that Fn responds to major alterations of the local chemistry and electrostatic conditions as function of time, as well as its position within the crystal. Our data indicate that supersaturated Ca oxalate solutions may affect Fn conformation similar to 1–2M GdnHCl in physiological buffer (Fig. 3A and B). Based on FRET and supported by AFM experiments, we suggest the following mechanisms regarding the surface binding and embedding process.
Fig. 3.
Schematic model illustrating how Fn might interact with a growing COM crystal. (A) Schematic sketch of putative Fn conformations in GdnHCl solution. (B) The ionic nature of the supersaturated crystallizing solution may disrupt the electrostatic bonds just enough to partially extend the protein. (C) The protein reverts to its most compact conformation once adsorbed to a charged, but electrostatically neutral COM face. The large protein blocks ions in solution from accessing the growing crystal surface, creating a zone of ion depletion and returning the protein to its compact, globular form. (D) As the crystal continues to grow, charged step edges may come in direct contact with the protein, transitioning from layer to layer growth to roughening caused by adding layers next to a large protein. The presence of the charged step edges poses a mechanical strain on the protein and results in the extension of Fn as growing planes surround it and it becomes embedded within the crystal.
First, as Fn adsorbs to the (–1 0 1) or (0 1 0) face of COM crystals, it adopts a more compact structure. FRET and AFM have previously been used to probe conformational changes in Fn on hydrophilic and hydrophobic surfaces [40,41] and both types of measurements have indicated that Fn retains a compact form on hydrophobic surfaces but adopts a slightly more extended conformation on hydrophilic glass. Charge-charge interactions between Fn and negatively charged hydrophilic glass disrupt electrostatic interactions that stabilize the compact state, allowing Fn's arms to partially extend. Here, the binding of Fn to the flat (–1 0 1) or (0 1 0) faces of mature COM crystals caused a sharp increase in IA/ID, implying the protein assumes a more compact globular conformation (confirmed by AFM). Fn's unexpected return to a more compact conformation on the densely charged surfaces of COM may indicate that it senses a zone of ion depletion, which are typically generated if the local growth rate is transport limited (Fig. 3C).
Second, Fn extends as it is incorporated into a growing crystal. What might be the underpinning mechanism that again leads to the opening up of Fn after becoming incorporated into the crystal? The presence of a protein on a flat crystal will locally perturb the crystal growth kinetics. At the point of closest contact, the growth will be stopped while transport of calcium and oxalate ions proceeds with reduced rate in the proximity of the protein. Step edges will appear in the close proximity of Fn, and since the two building blocks of COM, calcium and oxalate ions, are both charged, the step edges present uncompensated charges as sketched in Fig. 3D. Because Fn carries a significant amount of positive and negative charges, these complementary Fn charges may be attracted to the step edges.
The electrostatic interaction of Fn with charges on these gradually emerging COM step edges could alter the conformation of Fn as the COM crystal starts growing around it. Since the surface chemistry can have a direct impact on Fn's conformation [40-42,66-71], the reduced FRET upon embedding suggests that environmental changes ultimately result in the disruption of intramolecular interactions that might be replaced by altering electrostatic guest-host interactions. Fn has a diameter of 20–30 nm in its compact globular form and after complete arm separation, and approximate length of 120–160 nm with a diameter of 2.5–3 nm as probed here by AFM (Fig. 2D-G) and reported in the literature [59,63,64,72,73]. AFM measurements of the (–101) face of COM have revealed wide (≈200 nm) stepped terraces with an average step height of 0.65 ± 0.23 nm, a value within the range of the elemental step height (∼6Å) [62,74]. Therefore, when Fn binds to COM in its globular form, it can interact with up to six stacked planes, and multiple step edges, as the crystal continues to grow around it.
As found previously for fluorophores and other proteins [65], the presence of Fn does not alter the morphology of single COM crystals: COM commonly nucleates as elongated hexagonal plates with major faces characterized by the (–1 0 1), (0 1 0) and (110) planes and exposes a dense network of both positive (Ca2+) and negative charges (Ox2–) [75]. Nor does the presence of Fn during crystal nucleation alter the final COM morphology (unlike proteins such as nephrocalcin, which preferentially binds to (–1 01) planes and inhibits growth in that direction [25]), indicating that the protein is not binding with charge/subunit specificity [48]. This is further supported by the data, which indicate that Fn binds to all faces of mature COM (Fig. 2A) and preferentially incorporates, along the (110) or (1 2 0) apical faces during single crystal nucleation in the presence of the resulting in the observed fluorescent “hourglass” shape (Fig. 2H and J) that has been seen previously with several unbound dye molecules, as well as Protein G [65]. Proteins extracted from mineralized plant tissue have also been found to preferentially interact with these apical (1 1 0) planes during COM nucleation, and it has been shown that these fast growing apical planes present crystal faces with balanced charge and (Ox2–) anions that emerge at oblique angles without geometrically defined stereochemistry [76].
4.1. Implications of how the electrostatic sensitivity of macromolecules could be utilized to tailor properties of inorganic host crystals
When utilizing macromolecules to tailor the properties of single inorganic crystals, several different modes of guest–host interactions might be considered. Peptides and proteins that specifically recognize selective crystal faces might be exploited to induce oriented crystal growth, alter crystal shape or switch the crystal habit [10,77–79]. In contrast, proteins that primarily interact electrostatically [80] might be exploited to alter specific material properties without changing the crystal structure. As shown previously, Fn becomes embedded without perturbing the overall shape of COM crystals, nor does it cause the formation of microscopically visible defects [65]. Due to its condensation to a more compact structure when positioned at the crystal/solution interface, Fn might initially block a comparatively small surface area from further growth. During the subsequent incorporation process, separation of the arms ultimately allows single Fn molecules to interact with a large number of COM unit cells such that Fn is finally interlaced without altering the local COM structure. This could have a major impact on stabilizing the mechanical properties of biominerals, particularly when proteins are used that can elongate to more than 100nm in length just by eliminating quaternary structure.
In summary, this is the first time that FRET has been applied to probe protein–biomineral interactions. In fact, our data demonstrate that a FRET-labeled protein can be used as an optical sensor to probe local ionic conditions which could be of major interest for other (bio)mineralization studies. FRET thus sheds first light into how local changes in the ionic environment as crystals grow can cause major alterations in protein conformations.
Acknowledgments
We are grateful to Dr. Dong Qin of the University of Washington Nanotech User Facility for assistance with AFM imaging. The work was supported by a UW Center for Nanotechnology Graduate Research Award, an NSF Integrative Graduate Education and Research Traineeship (NSF-IGERT) Fellowship (LAT) and the PNNL/UW Joint Institute for Nanoscience. This article is submitted in honor of Prof. Hans Kuhn's 90th birthday.
References
- 1.Addadi L, Berman A, Moradian-Oldak J, Weiner S. Tuning of crystal nucleation and growth by proteins: molecular interactions at solid–liquid interfaces in biomineralization. Croatica Chem Acta. 1990;63:539–544. [Google Scholar]
- 2.Addadi L, Weiner S. Interactions between acidic proteins and crystals: stere-ochemical requirements in biomineralization. Proc Natl Acad Sci USA. 1985;82:4110–4114. doi: 10.1073/pnas.82.12.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belcher AM, Wu XH, Christensen RJ, Hansma PK, Stucky GD, Morse DE. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature. 1996;381:56–58. [Google Scholar]
- 4.Falini G, Albeck S, Weiner S, Addadi L. Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science. 1996;271:67–69. [Google Scholar]
- 5.Berman A, Addadi L, Kvick A, Leiserowitz L, Nelson M, Weiner S. Intercalation of sea urchin proteins in calcite: study of a crystalline composite material. Science. 1990;250:664–666. doi: 10.1126/science.250.4981.664. [DOI] [PubMed] [Google Scholar]
- 6.Berman A, Addadi L, Weiner S. Interactions of sea-urchin skeleton macro-molecules with growing calcite crystals—a study of intracrystalline proteins. Nature. 1988;331:546–548. [Google Scholar]
- 7.Aizenberg J, Albeck S, Weiner S, Addadi L. Crystal–protein interactions studied by overgrowth of calcite on biogenic skeletal elements. J Cryst Growth. 1994;142:156–164. [Google Scholar]
- 8.Aizenberg J, Ilan N, Weiner S, Addadi L. Intracrystalline macromolecules are involved in the morphogenesis of calcitic sponge spicules. Connect Tissue Res. 1996;34–5:17–23. doi: 10.3109/03008209609005269. [DOI] [PubMed] [Google Scholar]
- 9.Wierzbicki A, Sikes CS, Madura JD, Drake B. Atomic force miscrosopy and molecular modeling of protein and peptide binding to calcite. Calcif Tissue Int. 1994;54:133–141. doi: 10.1007/BF00296064. [DOI] [PubMed] [Google Scholar]
- 10.Walters DA, Smith BL, Belcher AM, Paloczi GT, Stucky GD, Morse DE, Hansma PK. Modification of calcite crystal growth by abalone shell proteins: an atomic force microscope study. Biophys J. 1997;72:1425–1433. doi: 10.1016/S0006-3495(97)78789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BL, Paloczi GT, Hansma PK, Levine RP. Discerning nature's mechanism for making complex biocomposite crystals. J Cryst Growth. 2000;211:116–121. [Google Scholar]
- 12.Thompson JB, Paloczi GT, Kindt JH, Michenfelder M, Smith BL, Stucky G, Morse DE, Hansma PK. Direct observation of the transition from calcite to aragonite growth as induced by abalone shell proteins. Biophys J. 2000;79:3307–3312. doi: 10.1016/S0006-3495(00)76562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goobes G, Goobes R, Shaw WJ, Gibson JM, Long JR, Raghunathan V, Schueler-Furman O, Popham JM, Baker D, Campbell CT, Stayton PS, Drobny GP. The structure, dynamics, and energetics of protein adsorption—lessons learned from adsorption of statherin to hydroxyapatite. Magn Reson Chem. 2007;45:S32–S47. doi: 10.1002/mrc.2123. [DOI] [PubMed] [Google Scholar]
- 14.Masica DL, Gray JJ. Solution- and adsorbed-state structural ensembles predicted for the statherin–hydroxyapatite system. Biophys J. 2009;96:3082–3091. doi: 10.1016/j.bpj.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petit H, Davis WL, Jones RG. A scanning electron microscopic study of the inorganic and organic matrices comprising the mature shell of Amblema, a fresh-water mollusc. Tissue Cell. 1980;12:581–593. doi: 10.1016/0040-8166(80)90046-4. [DOI] [PubMed] [Google Scholar]
- 16.Ryall RL, Fleming DE, Doyle IR, Evans NA, Dean CJ, Marshall VR. Intracrys-talline proteins and the hidden ultrastructure of calcium oxalate urinary crystals: implications for kidney stone formation. J Struct Biol. 2001;134:5–14. doi: 10.1006/jsbi.2001.4363. [DOI] [PubMed] [Google Scholar]
- 17.Nudelman F, Gotliv BA, Addadi L, Weiner S. Mollusk shell formation: mapping the distribution of organic matrix components underlying a single aragonitic tablet in nacre. J Struct Biol. 2005;153:176–187. doi: 10.1016/j.jsb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Lee SW, Choi CS. The correlation between organic matrices and biominerals (myostracal prism and folia) of the adult oyster shell, Crassostrea gigas. Micron. 2007;38:58–64. doi: 10.1016/j.micron.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Marin F, Pokroy B, Luquet G, Layrolle P, De Groot K. Protein mapping of calcium carbonate biominerals by immunogold. Biomaterials. 2007;28:2368–2377. doi: 10.1016/j.biomaterials.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Mandel N. Mechanism of stone formation. Semin Nephrol. 1996;16:364–374. [PubMed] [Google Scholar]
- 21.Bunker BC, Rieke PC, Tarasevich BJ, Campbell AA, Fryxell GE, Graff GL, Song L, Liu J, Virden JW, McVay GL. Ceramic thin-film formation on functionalized interfaces through biomimetic processing. Science. 1994;264:48–55. doi: 10.1126/science.264.5155.48. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin S. The electrostatic properties of membranes. Ann Rev Biophys Chem. 1989;18 doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- 23.Xiao XD, Vogel V, Shen YR. Probing the proton excess at interfaces by second harmonic generation. Chem Phys Lett. 1989;163:555–559. [Google Scholar]
- 24.Lochhead ML, Letellier SR, Vogel V. Quantitative assessment of the electrostatic contributions to surface-directed mineralization. J Chem Phys B. 1997;101:10821–10827. [Google Scholar]
- 25.Deganello S. Interaction between nephrocalcin and calcium oxalate monohy-drate: a structural study. Calcif Tissue Int. 1991;48:421–428. doi: 10.1007/BF02556456. [DOI] [PubMed] [Google Scholar]
- 26.Clark RH, Campbell AA, Klumb LA, Long CJ, Stayton PS. Protein electrostatic surface distribution can dictate whether calcium oxalate crystal growth is promoted or inhibited. Calcif Tissue Int. 1999;64:516–521. doi: 10.1007/s002239900642. [DOI] [PubMed] [Google Scholar]
- 27.Shirane Y, Kurokawa Y, Miyashita S, Komatsu H, Kagawa S. Study of inhibition mechanisms of glycosaminoglycans on calcium oxalate monohydrate crystals by atomic force microscopy. Urol Res. 1999;27:426–431. doi: 10.1007/s002400050131. [DOI] [PubMed] [Google Scholar]
- 28.Tsujihata M, Miyake O, Yoshimura K, Kakimoto KI, Takahara S, Okuyama A. Fibronectin as a potent inhibitor of calcium oxalate urolithiasis. J Urol. 2000;164:1718–1723. [PubMed] [Google Scholar]
- 29.Lieske JC, Toback FG. Regulation of renal epithelial cell endocytosis of calcium oxalate monohydrate crystals. Am J Physiol. 1993;264:F800–F807. doi: 10.1152/ajprenal.1993.264.5.F800. [DOI] [PubMed] [Google Scholar]
- 30.Tsujihata M, Yoshimura K, Tsujikawa K, Tei N, Okuyama A. Fibronectin inhibits endocytosis of calcium oxalate crystals by renal tubular cells. Int J Urol. 2006;13:743–746. doi: 10.1111/j.1442-2042.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 31.Tsujikawa K, Tsujihata M, Tei N, Yoshimura K, Nonomura N, Okuyama A. Elucidation of the mechanism of crystal–cell interaction using fibronectin-overexpressing Madin–Darby canine kidney cells. Urol Int. 2007;79:157–163. doi: 10.1159/000106331. [DOI] [PubMed] [Google Scholar]
- 32.Kramer G, Steiner GE, Prinz-Kashini M, Bursa B, Marberger M. Cell-surface matrix proteins and sialic acids in cell-crystal adhesion; the effect of crystal bindingonthe viabilityofhuman CAKI-1 renal epithelial cells. BJU Int. 2003;91:554–559. doi: 10.1046/j.1464-410x.2003.04139.x. [DOI] [PubMed] [Google Scholar]
- 33.Williams EC, Janmey PA, Ferry JD, Mosher DF. Conformational states of fibronectin. Effects of pH, ionic strength, and collagen binding. J Biol Chem. 1982;257:14973–14978. [PubMed] [Google Scholar]
- 34.Erickson HP, Carrell NA. Fibronectin in extended and compact conformations. Electron microscopy and sedimentation analysis. J Biol Chem. 1983;254 [PubMed] [Google Scholar]
- 35.Rocco M, Carson M, Hantgan R, McDonagh J, Hermans J. Dependence of the shape of the plasma fibronectin molecule on solvent composition. Ionic strength and glycerol content. J Biol Chem. 1983;258:14545–14549. [PubMed] [Google Scholar]
- 36.Tooney NM, Mosesson MW, Amrani DL, Hainfeld JF, Wall JS. Solution and surface effects on plasma fibronectin structure. J Cell Biol. 1983;97(6):1686–1692. doi: 10.1083/jcb.97.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjoberg B, Pap S, Osterlund E, Osterlund K, Vuento M, Kjems J. Solution structure of human plasma fibronectin using small-angle X-ray and neutron scattering at physiological pH and ionic strength. Arch Biochem Biophys. 1987;255(2):347–353. doi: 10.1016/0003-9861(87)90402-4. [DOI] [PubMed] [Google Scholar]
- 38.Benecky MJ, Wine RW, Kolvenbach CG, Mosesson MW. Ionic-strength- and pH-dependent conformational states of human plasma fibronectin. Biochemistry. 1992;30:4298–4306. doi: 10.1021/bi00231a028. [DOI] [PubMed] [Google Scholar]
- 39.Johnson KJ, Sage H, Briscoe G, Erickson HP. The compact conformation of fibronectin is determined by intramolecular ionic interactions. J Biol Chem. 1999;274:15473–15479. doi: 10.1074/jbc.274.22.15473. [DOI] [PubMed] [Google Scholar]
- 40.Bergkvist M, Carlsson J, Oscarsson S. Surface-dependent conformations of human plasma fibronectin adsorbed to silica, mica, and hydrophobic surfaces, studied with use of atomic force microscopy. J Biomed Mater Res. 2003;64A:349–356. doi: 10.1002/jbm.a.10423. [DOI] [PubMed] [Google Scholar]
- 41.Baugh L, Vogel V. Structural changes offibronectin adsorbedtomodel surfaces probed by fluorescence resonance energy transfer. J Biomed Mater Res. 2004;69A:525–534. doi: 10.1002/jbm.a.30026. [DOI] [PubMed] [Google Scholar]
- 42.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A. 2003;66:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 43.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Bio-materials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 44.Smith ML, Gourdon G, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, Vogel V. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007;5:2243–2254. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Couchourel D, Escoffier C, Rohanizadeh R, Bohic S, Daculsi G, Fortun Y, Padrines M. Effects of fibronectin on hydroxyapatite formation. J Inorg Biochem. 1999;73:129–136. doi: 10.1016/s0162-0134(99)00006-9. [DOI] [PubMed] [Google Scholar]
- 47.Daculsi G, Pilet P, Cottrel M, Guicheux G. Role of fibronectin during biological apatite crystal nucleation: Ultrastructural characterization. J Biomed Mater Res. 1999;47:228–233. doi: 10.1002/(sici)1097-4636(199911)47:2<228::aid-jbm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 48.Hanien D, Geiger B, Addadi L. Fibronectin adsorption to surfaces of hydrated crystals. An analysis of the importance of bound water in protein–substrate interactions. Langmuir. 1993;9:1058–1065. [Google Scholar]
- 49.Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Ann Rev Biophys Biomol Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- 50.Little WC, Smith ML, Ebneter U, Vogel V. Assaytomechanically tune and optically probe fibrillar fibronectin conformations from fully relaxed to breakage. Matrix Biol. 2008;27:451–461. doi: 10.1016/j.matbio.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Förster T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann Physik. 1948;2:55. [Google Scholar]
- 52.Kuhn H. Energy transfer in monomolecular layers. Naturwissenschaften. 1967;54:429–435. doi: 10.1007/BF00603138. [DOI] [PubMed] [Google Scholar]
- 53.Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- 54.Lakowicz JR. Principles of Fluorescence Spectroscopy. Third. Plenum Press; New York: 1986. [Google Scholar]
- 55.Kuhn H, Försterling HD, Waldeck DH. Principles of Physical Chemistry. Second. John Wiley & Sons; 2009. [Google Scholar]
- 56.Yang CH, Soll D. Studies of transfer RNA tertiary structure of singlet-singlet energy transfer. Proc Natl Acad Sci USA. 1974;71:2838–2842. doi: 10.1073/pnas.71.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haas E, Wilchek M, Katchalski-Katzir E, Steinberg IZ. Distribution of end-to-end distances of oligopeptides in solution as estimated by energy transfer. Proc Natl Acad Sci USA. 1975;72:1807–1811. doi: 10.1073/pnas.72.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu P, Brand L. Resonance energy transfer: methods and applications. Anal Biochem. 1994;8:1–13. doi: 10.1006/abio.1994.1134. [DOI] [PubMed] [Google Scholar]
- 59.Zenhausern F, Adrian M, Descouts P. Solution structure and direct imaging of fibronectin adsorption to solid surfaces by scanning force microscopy and cryo-electron microscopy. J Electron Microsc (Tokyo) 1993;42:378–388. [PubMed] [Google Scholar]
- 60.Baneyx G, Baugh L, Vogel V. Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc Natl Acad Sci USA. 2001;98:14464–144468. doi: 10.1073/pnas.251422998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ting-Po I, Nancollas GH. EQUIL: a general computational method for the calculation of solution equilibria. Anal Chem. 1972;44:1940–1950. [Google Scholar]
- 62.Tazzoli V. The crystal structures of whewellite and weddellite; re-examination and comparison. Am Miner. 1980;65:327–334. [Google Scholar]
- 63.Erickson HP, Carrell N, McDonagh J. Fibronectin molecule visualized in electron microscopy: A long, thin, flexible strand. J Cell Biol. 1981;91:673–678. doi: 10.1083/jcb.91.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacDonald DE, Markovic B, Allen M, Somasundaran P, Boskey AL. Surface analysis of human plasma fibronectin adsorbed to commercially pure titanium materials. J Biomed Mater Res. 1998;41:120–130. doi: 10.1002/(sici)1097-4636(199807)41:1<120::aid-jbm15>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 65.Touryan LA, Clark RH, Gurney RW, Stayton PS, Kahr B, Vogel V. Incorporation of fluorescent molecules and proteins into calcium oxalate monohydrate singe crystals. J Cryst Growth. 2001;233:380–388. [Google Scholar]
- 66.Lewandowska K, Pergament E, Sukenik CN, Culp LA. Cell-type-specific adhesion mechanisms mediated by fibronectin adsorbed to chemically derivatized substrata. J Biomed Mater Res. 1992;26:1343–1363. doi: 10.1002/jbm.820261007. [DOI] [PubMed] [Google Scholar]
- 67.Iuliano DJ, Saavedra SS, Truskey GA. Effect of the conformation and orientation of adsorbed fibronectin on endothelial cell spreading and the strength of adhesion. J Biomed Mater Res. 1993;27:1103–1113. doi: 10.1002/jbm.820270816. [DOI] [PubMed] [Google Scholar]
- 68.Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halter M, Antia M, Vogel V. Fibronectin conformational changes induced by adsorption to liposomes. J Controlled Release. 2005;101:209–222. doi: 10.1016/j.jconrel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Antia M, Islas LD, Boness DA, Baneyx G, Vogel V. Single molecule fluorescence studies of surface-adsorbed fibronectin. Biomaterials. 2006;27:679–690. doi: 10.1016/j.biomaterials.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 71.Lan MA, Gersbach CA, Michael KE, Keselowsky BG, Garcia AJ. Myoblast proliferation and differentiation on fibronectin-coated self assembled monolayers presenting different surface chemistries. Biomaterials. 2005;26:4523–4531. doi: 10.1016/j.biomaterials.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 72.Liwnicz BH, Sawaya R. Fibronectin. Immunol Sci. 1990;53:537–554. [PubMed] [Google Scholar]
- 73.Leahy DJ, Aukhil I, Erickson HP. 2.0 angstrom crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 74.Sheng XX, Ward MD, Wesson JA. Adhesion between molecules and calcium oxalate crystals: critical interactions in kidney stone formation. J Am Chem Soc. 2003;125:2854–2855. doi: 10.1021/ja029575h. [DOI] [PubMed] [Google Scholar]
- 75.Deganello S, Piro OE. The crystal structure of calcium oxalate monohydrate (whewellite) N Jb Miner Mh. 1981;2:81–88. [Google Scholar]
- 76.Bouropoulos N, Weiner S, Addadi L. Calcium oxalate crystals in tomato and tobacco plants: morphology and in vitro interactions of crystal-associated macromolecules. Chem Eur J. 2001;7:1881–1888. doi: 10.1002/1521-3765(20010504)7:9<1881::aid-chem1881>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 77.Aizenberg J, Hanson J, Ilan M, Leiserowitz L, Koetzle TF, Addadi L, Weiner S. Morphogenesis of calcitic sponge spicules: a role for specialized proteins interacting with growing crystals. FASEB J. 1995;9:262–268. doi: 10.1096/fasebj.9.2.7781928. [DOI] [PubMed] [Google Scholar]
- 78.Weiner S. Biomineralization: a structural perspective. J Struct Biol. 2008;163:229–234. doi: 10.1016/j.jsb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Pokroy B, Kang SH, Mahadevan L, Aizenberg J. Self-organization of a mesoscale bristle into ordered, hierarchical helical assemblies. Science. 2009;323:237–240. doi: 10.1126/science.1165607. [DOI] [PubMed] [Google Scholar]
- 80.Sheinerman FB, Honig B. On the role of electrostatic interactions in the design of protein–protein interfaces. J Mol Biol. 2002;318:161–177. doi: 10.1016/S0022-2836(02)00030-X. [DOI] [PubMed] [Google Scholar]