Abstract
Purpose
Recurrence of colon cancer, which affects nearly 50% of patients treated by conventional therapeutics, is thought to be due to re-emergence of chemotherapyresistant cancer stem/stem-like cells (CSCs). Therefore, development of therapeutic strategies for targeted elimination of CSCs would be a novel strategy. The current study examines whether diflourinated-curcumin (CDF), a novel analog of the dietary ingredient of curcumin, in combination with 5-fluorouracil and oxaliplatin (5-FU + Ox), the mainstay of colon cancer chemotherapeutic, would be effective in eliminating colon CSCs.
Methods
Multiple methodologies that include real-time RT-PCR, Western blot, MTT assay, caspase-3 activity, colono-sphere formation, Hoechst-33342 dye exclusion and NF-κB-ELISA were used.
Results
We observed that CDF together with 5-FU + Ox were more potent than curcumin in reducing CD44 and CD166 in chemo-resistant colon cancer cells, accompanied by inhibition of growth, induction of apoptosis and disintegration of colonospheres. These changes were associated with downregulation of the membrane transporter ABCG2 and attenuation of EGFR, IGF-1R, and NF-κB signaling consistent with inactivation of β-catenin, COX-2, c-Myc and Bcl-xL and activation of the pro-apoptotic Bax.
Conclusions
Our results suggest that CDF together with the conventional chemotherapeutics could be an effective treatment strategy for preventing the emergence of chemoresistant colon cancer cells by eliminating CSCs.
Keywords: β-catenin, chemo-resistance, NF-κB, oxaliplatin, 5-fluorouracil
INTRODUCTION
Despite recent advances, nearly 50% of patients with colorectal carcinoma (CRC), the third deadliest cancer in the US, will develop recurrent disease, underscoring the need for improved therapies (1). Response to 5-Fluorouracil (5-FU) or 5-FU plus Oxaliplatin (5-FU + Ox) or FOLFOX, the current backbone of colorectal cancer chemotherapeutics, is often incomplete, resulting in cancer recurrence contributing to overall poor survival of patients diagnosed with CRC (2–4). Although the reasons for this recurrence are not fully understood, a growing body of evidence suggests that this could in part be due to enrichment of chemotherapy-resistant cancer stem-like cells (CSCs) that retain the limitless potential to regenerate (5). Development of therapeutic strategies that specifically target CSCs is, therefore, warranted. Furthermore, the continued use of chemotherapy can lead to additional toxicities, some of which may be fatal. Therefore, validation of a non-toxic agent(s) that could improve upon the current chemotherapeutic regimen(s) would be highly desirable.
Curcumin [diferuloylmethane; I,7-bis-(4-hydroxy-3-methoxyphenyl)-1 ,6-heptadiene-3,5-dione], the major pigment in turmeric powder that possesses anti-inflammatory and anti-oxidant properties (6) with no discernable toxicity, has been shown to inhibit the growth of transformed cells and colon carcinogenesis at the initiation, promotion and progression stages in carcinogen-induced rodent models (7–9). Recently, we reported that curcumin synergizes with the combination treatment of 5-fluorouracil (5-FU) and oxaliplatin (hereafter referred to as 5-FU + Ox), the mainstay of colon cancer chemotherapy, to inhibit the growth of colon cancer cells in vitro (10). Curcumin has also been found to synergize with dasatanib, a specific inhibitor of c-Src tyrosine kinases, to inhibit the growth of colon cancer cells in vitro and also caused regression of intestinal adenomas in APCmin−/+ mice (11). Various independent studies have shown that the combination treatment of curcumin with a variety of chemotherapy drugs (i.e., cisplatin, danorubicin, doxorubicin, and vinscristine) enhances the cellular accumulation of these drugs, thereby increasing the cells’ sensitivity to the chemotherapeutics (12). These findings strongly indicate that curcumin or its derivative holds a great promise as an anti-cancer agent, and, given a strong link between the CSCs and chemoresistance, this could be utilized to target CSCs. Indeed, we have recently observed that curcumin either alone or in combination with 5-FU + Ox is highly effective in reducing colon CSCs in vitro (13).
However, the use of curcumin as a therapeutic agent has met with considerable skepticism because of its poor bioavailability. Since as much as 75% of curcumin is excreted in the feces (14) and also undergoes rapid inactivation due to glucuronidation (15), several strategies have been developed to improve the biological activity of curcumin (16–19), but none had proved to be successful. This issue has been addressed by slowing down the rapid metabolism of curcumin by preparing its Knoevenagel condensates and their metal complexes and, further, generating the fluoro- analog of curcumin termed Diflourinated-Curcumin (referred to as CDF) that exhibits increased metabolic stability (20,21). The CDF has also been found to exhibit superior growth-inhibitory properties to the parental compound curcumin (20,21). In view of our recent observation that curcumin either alone or together with 5-FU and oxaliplatin reduced colon CSCs (13), the current investigation was undertaken to compare the effectiveness of CDF with curcumin in inhibiting the growth of 5-FU + Ox-resistant colon cancer cells (hereafter referred to as chemo-resistant cells) with particular reference to formation and disintegration of colonospheres that are highly enriched in colon cancer stem-like cells (CSCs) (22). In addition, regulatory mechanisms for CDF-induced inhibition of colon cancer chemo-resistant cells were examined by analyzing the events of β-catenin and NF-κB signaling.
MATERIALS AND METHODS
Drugs and Reagents
Curcumin, protease inhibitor cocktail, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and all other chemicals were obtained from Sigma (St. Louis, MO). Rabbit anti-p-IGF-1R (Tyr 1161), mouse anti-Bcl-xL, rabbit anti-Bax, mouse anti-β-catenin and rabbit anti-ABCG2 antibodies were obtained from Santa Cruz Biotechnology Inc., Santa Cruz, CA. Rabbit anti-p-EGF-Receptor (Tyr 1173), rabbit anti-c-Myc, rabbit anti-phospho-β-catenin and rabbit anti-cleaved caspase-3 antibodies were the products of Cell Signaling, Danvers, MA, and the mouse anti-β-actin antibodies were purchased from Chemicon International, Billerica, MA. Enhanced Chemiluminescence (ECL) kit for detection of proteins was obtained from Amersham Biosciences/Amersham Pharmacia Biotech (Piscataway, NJ). Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), phosphate saline buffer (PBS), Hanks’ balanced salt solution (HBSS) and antibiotic/ antimycotic reagents were obtained from GIBCO-Invitrogen (Carlsbad, California). Human colon cancer HCT-116 and HT-29 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA).
Cell Culture and Generation of 5-FU + Oxaliplatin-Resistant Colon Cancer Cells
The cells were maintained in tissue culture flasks in a humidified incubator at 37°C in an atmosphere of 95% air and 5% CO2. Medium was supplemented with 10% FBS and 1% antibiotic/antimycotic agents. Medium was changed three times a week, and cells were passaged using 0.5% trypsin/EDTA.
Unless otherwise stated, 5-FU + Ox-resistant (referred to as chemo-resistant cells) were generated by exposing HCT-116 and HT-29 cells to the combination of 5-Fluorouracil (5-FU) and oxaliplatin (Ox) at clinically relevant doses and schedules as described previously (13). Initially, HCT-116 or HT-29 cells were incubated with the combination of 25 µM 5-FU and 0.625 µM oxaliplatin for one week. The adherent cells, which survived the 5-FU + Ox insult, were subjected to trypsin/EDTA treatment and allowed to grow in normal DMEM for two weeks. The surviving cells were then split and gradually exposed to incremental doses of 5-FU and Ox to maximal concentrations of 250 µM 5-FU and 6.25 µM Ox for two to three weeks for each treatment period. The 5-FU + Ox-resistant (chemo-resistant cells) cells were maintained in normal culture medium containing 50 µM 5-FU + 1.25 µM oxaliplatin. The medium was changed three times a week, and the cells were passaged using trypsin/EDTA.
Cell Growth Inhibition Assay
The growth of chemo-resistant HCT-116 and HT-29 cells was assessed by 3-(4,5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay as described previously (22). Briefly, the cells were dispersed by trypsin-EDTA , subsequently re-suspended in DMEM containing 10% FBS and seeded into 96-well culture plates with six to eight replicates. After 24 h of plating, they were incubated for 48 h in the absence (control) or presence of testing agents, described in the legends to figures. The reaction was terminated by adding 20 µl of 5-mg/ml stock of MTT into each well. After 3 to 4 h at 37°C,the medium was removed and formazon crystals dissolved by adding 0.1 ml of dimethyl sulfoxide (DMSO). The intensity of the color was measured at 570 nm. All values were compared to the corresponding controls, and the experiments were repeated multiple times.
Colonosphere Formation and Disintegration Assay
The ability of chemo-resistant colon cancer cells to form spheres in suspension was evaluated as described by Liu et al. (23), with slight modifications (22). Briefly, primary colonospheres were generated by incubating the limited number of chemo-resistant HCT-116 and HT-29 cells at a concentration of 100 cells per 200 µL in serum-free stem cell medium (SCM) containing DMEM/F12 (1:1) supplemented with B27 (Life Technologies, Gaithersburg, MD), 20 ng/ml EGF (Sigma, St Louis, MO), 10 ng/ml fibroblast growth factor (Sigma), and antibiotic-anti-mycotic agent in 24-well ultra low-attachment plates (Corning Inc, Lowell, MA) for five days. The average number of colonospheres formed in each well after five days was calculated by counting them using light microscopy (20X objective). In all experiments the results were expressed as percent colonospheres formed with respect to untreated solvent controls.
For colonospheres disintegration assay, the agents were added into the wells after five days of the formation of spheroids. The spheroids were followed for next five days to analyze for disintegration. The extent of disintegration was photographed.
Secondary Sphere Formation Assay
Self-renewing/regeneration abilities of the chemo-resistant sphere-derived cells were analyzed for secondary colonosphere formation in the absence of any agent. Primary colonospheres formed over a period of five days in SCM containing 50 µM 5-FU and 1.25 µM Ox with or without any of the testing agent were collected by centrifugation, dissociated with 0.05% trypsin/EDTA, and subsequently passed through a 40 µM sieve to obtain single cell suspensions (24). An equal number of cells obtained from primary colonosphere culture were plated (100 cells/ 200 µL in SCM) into each of the 96 ultra low-attachment wells. The secondary colonospheres formed after five days were recorded for their number and size by light microscopy.
Bright Field Microscopy
Colon cancer cells were seeded in six-well plates at a density of 4×104 cells per well and incubated overnight to allow the cells to adhere. The cells were treated with the listed agents and incubated for the periods as described above. The cells were washed in PBS and photographed using bright field microscopy (10 × magnification). Images were taken with Olympus DP72 camera on a computer equipped with DP2-BSW microscope digital-imaging software. Three images of each treatment were taken from randomly chosen fields, and a representative image was selected for display in the figure.
Extreme Limiting Dilution Analysis
Extreme limiting dilution analysis (ELDA) was performed as described by Hu and Smyth (25). Briefly, single cell suspension obtained from the chemo-resistant cells was plated at a concentration of 1000, 100, 10 and 1 cell(s) per 200 µl SCM (24 well for each dilution) in 96-well ultra-low attachment and incubated for five days. At the end of five days, the number of wells showing formation of colonospheres was counted. The frequency of sphere-forming cells in a particular cell type was determined using ELDA web-tool available at http://bioinf.wehi.edu.ac/software/elda.
Hoechst 33342 Dye Exclusion Assay
Single cell suspension obtained from parental and chemoresistant cells were washed with PBS (three times) and stained with Hoechst 33342 or H342 (5 µg/ml, Sigma-Aldrich Inc., St. Louis, MO) for 45 min at 37°C in HBSS buffer (GIBCO-Invitrogen Corp., Carlsbad, California), vortexing gently every 15 min. The controls were treated with an inhibitor of membrane ABCG2 transporter protein, verapamil (Sigma, 50 µM) for ten minutes at room temperature prior to the addition of H342. The stained cells were collected, washed with PBS and re-suspended in 3 ml of PBS containing 2 µg/ml of propidium iodide, and subsequently analyzed by flow cytometer- FACS Vantage SE/DiVa SORP (BD Biosciences, San Jose, CA) with alldigital electronics and octagon- and trigon-shaped detector arrays. Excitation of 100 mW at 488 nm was provided by a 177-G argon ion laser (Spectra-Physics, Mountain View, CA), and 200 mW of all-lines UV (351–365 nm) was provided by an Innova 90-5 argon ion laser (Coherent, Palo Alto, CA). Forward and side laser scatter were detected from 488 nm excitation. H342 and propidium iodide fluorescence from UV excitation was split into “blue” and “red” wavelengths by a 505 nm long pass dichroic with a 450/50 bandpass (425–475 nm) filter in front of the “blue” detector and a 630 nm long pass filter in front of the “red” detector. Cell population showing H342 Bright (H342Br) and H342 Low (H342Lo) was determined, and the ratio of H342Lo/H3342Br was calculated to evaluate the dye-efflux capacities of the cells. The gating of H342Lo and H342Br cells was based on a verapamil control. Dead cells were gated out based on positive staining with propidium iodide (22).
Western Blot Analysis
Western blot analysis was performed essentially according to our standard protocol (13,22). Briefly, the cells were solubilized in lysis buffer (50 mM Tris, 100 mM NaCl, 2.5 mM EDTA, 1% Triton X-100, 1% Nonidet P-40, 2.5 mM Na3VO4, 25 µg/ml aprotinin, 25 µg/ml leupeptin, 25 µg/ml pepstatin A, and 1 mM phenylmethylsulfonyl fluoride). After clarification at 10,000 g for 15 min, the supernatant was used for Western blot analysis. In all analyses, protein concentration, determined by the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA), was standardized among the samples. Aliquots of cell lysates containing 50 µg of protein were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis. After electrophoresis, proteins were transferred electrophoretically onto supported polyvinylidene difluoride membrane (Millipore Corp, Bedford, MA), incubated for 1 h at room temperature with blocking buffer, TBS-T (20 mM Tris, pH7.6, 100 µM NaCl, 0.1% Tween-20) and 5% nonfat dry milk with gentle agitation. After washing the membranes with TBS-T, they were incubated overnight with primary antibodies (1:1,000 dilutions) in TBS-T buffer containing 5% milk at 4°C. The membranes were washed with TBS-T, subsequently incubated with appropriate secondary antibodies (1:5,000 dilutions) in TBS-T/ 5% milk for 1–2 h at room temperature. The membranes were washed again with TBS-T, and the protein bands were visualized by enhanced chemiluminescence (ECL) detection system (Amersham, Piscataway, NJ). The membranes were exposed to Amersham Hyper Film ECL (GE Healthcare, Buckinghamshire, UK). The membranes were stripped (2×for 15 min at 55°C) in stripping buffer containing 100 mM 2-mercaptoethanol, 2% sodium dodecyl sulfate, and 62.5 mM Tris-HCl pH 6.7, and re-probed for β-actin. All Western blots were performed at least three times for each experiment.
Isolation of RNA and Quantitative Polymerase Chain Reaction Analysis
Total RNA was extracted from the parental and chemoresistant HCT-116 cells using RNA-STAT solution (Tel Test, Friendswood, TX) according to the manufacturer’s instruction. RNA was treated with DNase I to remove contaminating genomic DNA and subsequently purified using RNAeasy Mini Kit (Qiagen, Valencia, CA). RNA concentration was measured spectrophotometrically at an optical density of 260 nm.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using the GeneAmp RNA PCR Kit (Applied Biosystems, Foster City, CA). Briefly, 1 µg of purified RNA was reverse-transcribed in the presence of 2.5 mM MgCl2, 1×RT-PCR buffer, 1 mM dNTPs, 10 mM dithiothreitol, 10 U of RNase inhibitor, 1.25 µM random hexamers, and 15 U of MultiScribe Reverse Transcriptase in a final reaction volume of 20 µl. After mixing the components, the mixture was briefly centrifuged and incubated at 25°C for 10 min for hybridization. The reactions were carried out at 42°C for 15 min in a GeneAmp PCR System 9600 (Perkin-Elmer) and then by cooling to 4°C. The RT reactions were subjected to quantitative PCR amplification. Five microliters of complementary DNA products were amplified with SYBR Green Quantitative PCR Master Mix (Applied Biosystems). PCR primers were used as follows: CD44 forward: 5′- aaggtggagcaaacacaacc-3′ and reverse: 5′-aactgcaatgcaaactgcaag-3′, CD166 forward: 5′-tagcaggaatgcaactgtgg-3′ and reverse: cgcagacatag tttccagca- 3′, and β-actin forward: 5′-cccagcacaatgaagaat caa-3′ and reverse 5′-acatctgctggaaggtggtggac-3′. Reactions were carried out in Applied Biosystems 7500 Real-Time PCR System. The conditions for RT-PCR running were as follows: for activating the DNA polymerase, hot start was performed for 10 min at 95°C, and then cycling at 95°C for 15 s and 60°C for 1 min for a total of 40 cycles (13).
Nuclear Factor Kappa B (NF-κB) Binding Assay
Nuclear extract was prepared from the parental and chemo-resistant HCT-116 cells using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific, Rockford, Illinois, USA), and the protein concentrations were determined spectrophotometrically. NF-κB p65 DNA binding activity was assessed in the nuclear extracts using Trans-AM NF-κB p65 transcription factor assay kit (Active Motif North America, Carlsbad, California, USA) according to the manufacturer’s instructions. Ten micrograms of nuclear extracts were added to 96-well plates coated with an oligonucleotide containing the nuclear factors consensus site. The binding of NF-κB to DNA was visualized by means of anti-p65 antibody, which specifically recognizes activated NF-κB. Antibody binding was measured spectrophotometrically. The specificity of NF-κB activation was determined by competition experiments using wild-type and mutant consensus oligonucleotides provided with the kit. The data represent the average ±SD of three separate binding assays and are expressed as percent of NF-κB activation relative to treatment controls.
Assessment of Apoptosis and Caspase-3 Activity
Apoptosis was quantitatively evaluated by acridine orange-ethidium bromide (AO-EtBr) staining as described previously (26). Briefly, the cells were washed once with cold 1× PBS and resuspended in 1× PBS (0.5×106 to 1×106 cells/mL). Cell suspension (10 µL) was stained with 10 µL of acridine orange/ethidium bromide mixture according to the manufacturer’s instructions. Within 5 min of addition of the AO-EtBr mixture, aliquots containing 300 to 500 cells were counted under a fluorescent microscope with blue-green filter. The percentage of apoptotic cells, as defined by cytoplasmic and nuclear shrinkage and chromatin condensation or fragmentation, was determined.
The activity of caspase-3, an enzyme that initiates apoptosis, was determined in the cell lysates using the colorimetric assay kit from the BioVision Research Products (Mountain View, CA) according to the manufacturer’s instruction. The assay is based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrate DEVD-pNA (27).
Statistical Analysis
Unless otherwise stated, data are expressed as mean ±SD of six observations. Where applicable, the results were analyzed using paired t-test. p<0.05 was designated as the level of significance.
RESULTS
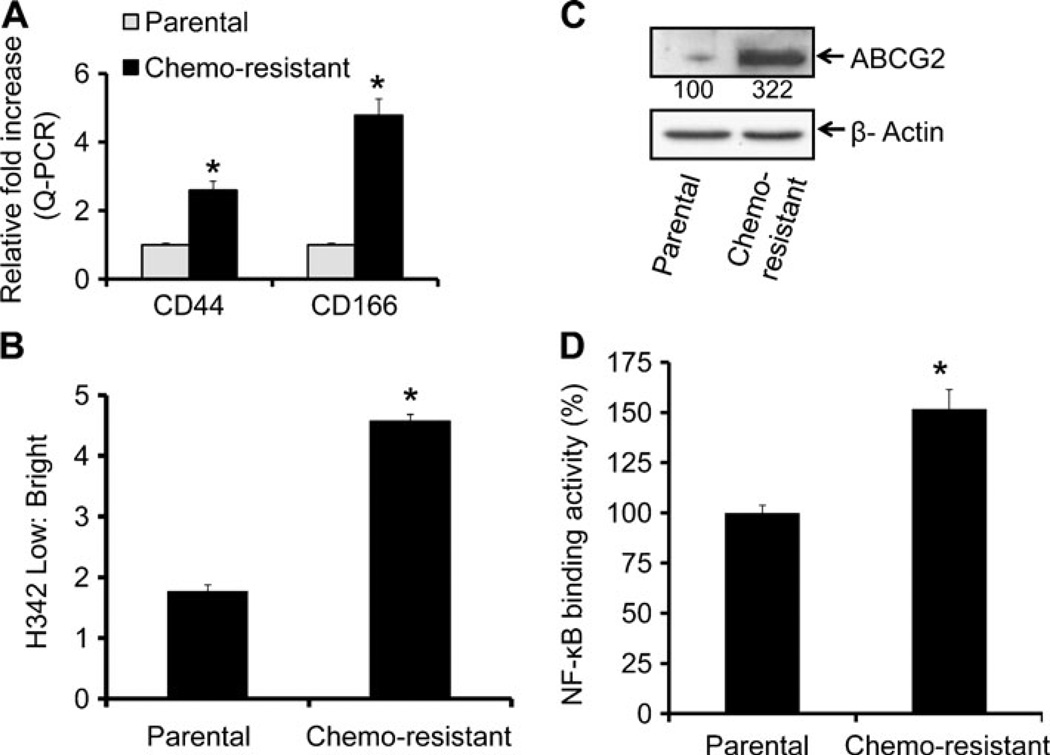
Recent evidence suggests that the cancer cells that are resistant to chemotherapy demonstrate characteristics similar to those of CSCs (28,29). Previously, we reported the predominance of CSCs in the chemo-resistant (5-FU + Ox-resistant) colon cancer cells that displayed elevated levels of various CSC markers (13). Consistent with our previous findings, we observed that the chemo-resistant HCT-116 cells displayed 3–5-fold higher mRNA levels of CD44 and CD166, compared to the corresponding parental cells (Fig. 1a). When the drug efflux ability between the chemo-resistant and parental HCT-116 cells was determined by examining the exclusion of H342 dye, it was found to be 2.5-fold higher in chemo-resistant cells compared to parental cells (Fig. 1b). This was accompanied by a 3-fold increase in ABCG2 expression (Fig. 1c), a member of the superfamily of ATP-binding cassette (ABC) transporters whose primary function is to transport various molecules across the intra- and extra-cellular membranes (30). Further, these changes were associated with a 1.5-fold increase in the activity of NF-κB (Fig. 1d).
Fig. 1.
Chemo-resistant colon cancer HCT-116 cells display cancer stem cell properties. (A) Real time-quantitative RT-PCR (qRT-PCR) analysis showing increased levels of mRNAs of colon CSCs markers- CD44 and CD166 in chemo-resistant cells as compared to parental control cells. (B) The quantitation of flow cytometry analysis of H342 dye exclusion showing higher exclusion by chemo-resistant HCT-116 cells, compared to parental cells. (C) Western blots showing increased expression of ABCG2 in chemo-resistant HCT-116 cells, compared to the corresponding parental cells. (D) The NF-κB DNA binding activity in parental and chemo-resistant cells. Results are average of three experiments with standard deviation. The values are normalized to parental cells. *p<0.001, compared to the corresponding treatment controls.
Functional CSCs possess sphere-forming ability when present in limited numbers under serum-free conditions and are known to exhibit higher drug efflux capacity. Using the in vitro sphere formation as a surrogate for tumor formation, functional CSCs in chemo-resistant cells were validated by employing extreme limiting dilution analysis (ELDA) and statistically analyzing the frequency of sphere forming cells (Table 1). Results revealed a 2-fold greater frequency of sphere-forming cells of the chemo-resistant cells, compared to the parental cancer cells (Table 1).
Table 1.
Extreme Limiting Dilution Analysis of Colonospheres Forming Frequency of Parental and Chemo-Resistant HCT-116 Cells
| Number of cells/well | Number of wells plated | Number of wells showing colonospheres |
|
|---|---|---|---|
| HCT-116 Parental | HCT-116 Chemoresistant | ||
| 1,000 | 24 | 24 | 24 |
| 100 | 24 | 24 | 24 |
| 10 | 24 | 24 | 24 |
| 1 | 24 | 5 | 13 |
| Sphere forming frequency (95% CI) | 1/4 (1/6–1/3) | 1/2 (1/3–1/2) | |
| p value | < 0.05 | ||
Data are pooled from three independent experiments for each.
CI confidence interval
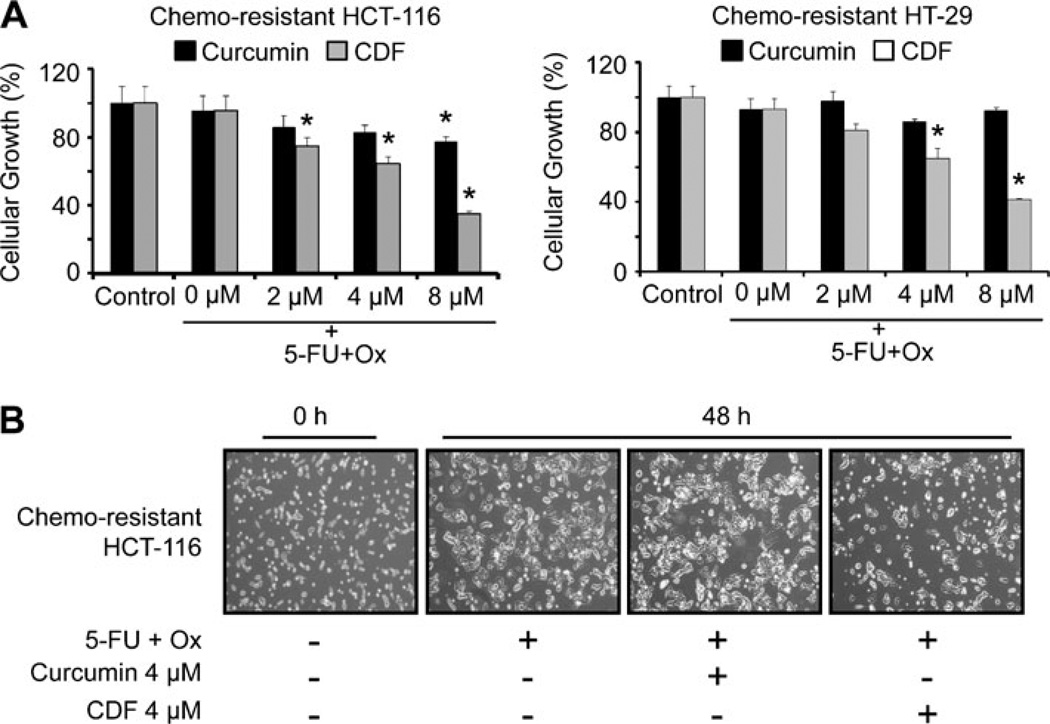
One of the objectives of our current investigation was to compare the efficacy of curcumin with CDF on growth inhibition of chemo-resistant colon cancer cells when combined with chemotherapy of 5-FU + Ox. The chemoresistant HCT-116 and HT-29 cells were analyzed for cellular growth following incubation with 5-FU + Ox alone or in combination with increasing concentrations (up to 8 µM) of either CDF or curcumin. A significant inhibition of 40–70% in cellular growth of chemo-resistant cells was observed when the chemo-resistant HCT-116 or HT-29 cells were incubated for 48 h with the combination of 4 or 8 µM CDF and 5-FU + Ox, whereas the same dose of curcumin caused ≤20% inhibition (Fig. 2a). In contrast, the combination therapy caused only a 25–30% inhibition of growth of non-transformed intestinal epithelial cells (IEC-6) cells which are derived from weanling rats (data not shown). It should also be stated here that a greater inhibition of cellular growth of chemo-resistant HCT-116 cells by CDF compared to curcumin was associated with a corresponding loss of adherent cells (Fig. 2b). Interestingly, further treatment of the chemo-resistant HCT-116 cells with the combination of 5-FU and Ox caused a slight increase in adherent cells, compared with the controls (Fig. 2b).
Fig. 2.
Cellular growth inhibition. (A) Effect of 5-FU + Ox alone or in combination with increasing concentrations of either CDF or curcumin on the growth of chemo-resistant HCT-116 and HT-29 cancer cells as determined by MTT assay after 48 h of exposure. (B) Representative photomicrographs showing the effects of 5-FU + Ox alone or in combination with low dose (4 µM) of either curcumin or CDF on the growth of chemo-resistant HCT-116 cells after 48 h exposure. Each value represents mean±SD of six observations. *p<0.001, compared to the corresponding treatment controls. The 5-FU + Ox treatment represents the combination of 50 µM 5-Fluorouracil and 1.25 µM Oxaliplain, and these concentrations were used in all subsequent experiments.
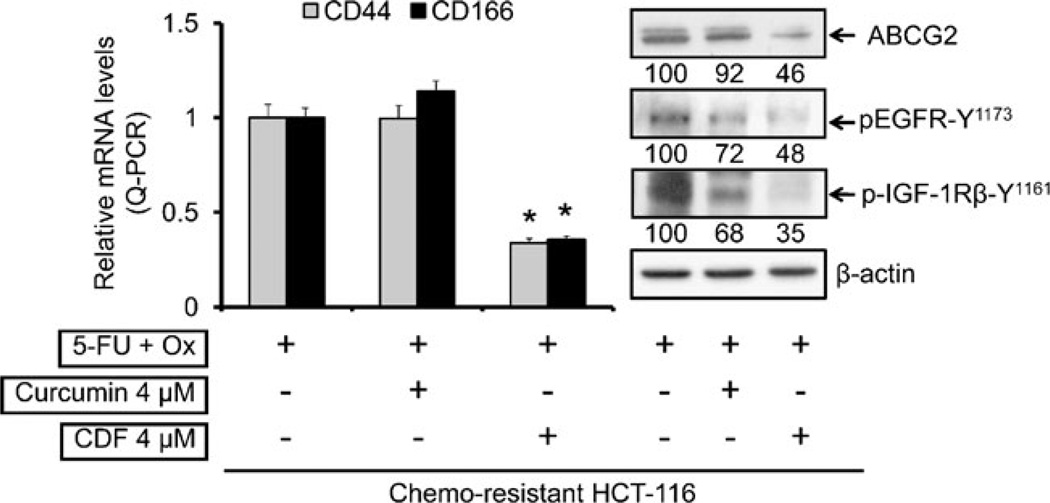
In the next set of experiments, changes in the expression of CSC markers, CD44 and CD166 in chemo-resistant HCT-116 cells in response to CDF or curcumin together with 5-FU + Ox were examined. Quantitative Real Time-PCR (qRT-PCR) analysis was employed. We observed that while 4 µM curcumin together with 5-FU + Ox caused no appreciable change in mRNA levels of either CD44 or CD166, the same combination treatment with 4 µM CDF caused over 70% inhibition of CD44 and CD166 expression (Fig. 3, left panel). As has been observed for CD44 and CD166, the expression of ABC transporter protein ABCG2 in chemo-resistant HCT-116 cells was inhibited by more than 50% in response to the combination of 4 µM CDF and 5-FU + Ox, compared to the 5-FU + Ox-treated controls (Fig. 3, right panel). Curcumin was less effective in this setting.
Fig. 3.
Quantitative real-time PCR (qRT-PCR) analysis showing mRNA expression of colon CSC markers CD44 and CD166 (left panel) and changes in protein levels of ABCG2, pEGFR-Y1173 and p-IGF-1R in chemo-resistant HCT-116 cells in response to 5-FU + Ox alone or in combination with either curcumin or CDF (right panel); β-actin (loading control) levels is also shown. The numbers represent percent of corresponding control normalized to β-actin. *p<0.001, compared to the corresponding treatment controls.
Earlier, we reported a marked activation of EGFR and IGF-1R, accompanied by elevated expression of CD44 and CD166 in HCT-116 cells that survived the 48 h treatment of 5-FU + Ox (referred to as FOLFOX) (31), indicating the presence of the growth factor receptors in chemo-surviving cells that are enriched in colon CSCs (13). To determine whether the current treatment strategy would also affect the functioning of the growth factor receptors, the levels of phosphorylated (activated) form of EGFR and IGF-1R were examined. Our results demonstrated that although curcumin or CDF in combination with 5-FU + Ox attenuated the activation of EGFR and IGF-1R, the magnitude of inhibition was much higher with CDF (50–65%) than that noted for curcumin (∼30%) (Fig. 3, right panel).
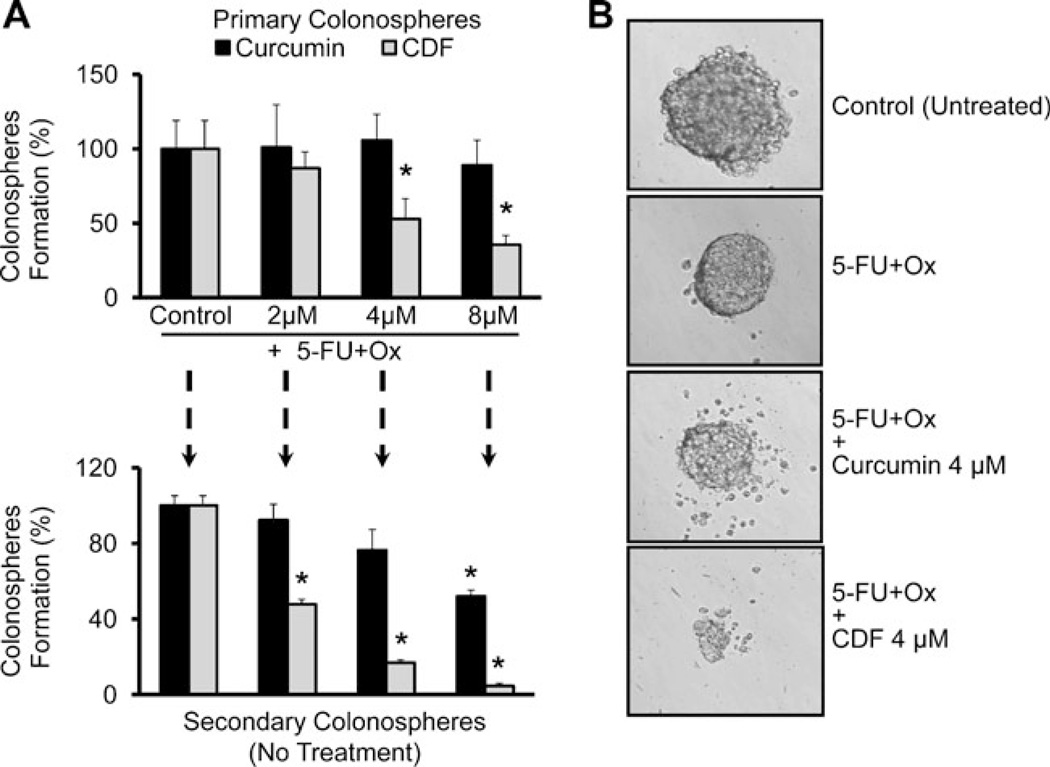
In the next set of experiments, the effects of 5-FU + Ox either alone or in combination with increasing concentrations of curcumin or CDF on the functional properties of chemo-resistant colon cancer cells were examined by determining their ability to form primary and secondary colonospheres. Primary colonosphere formation assay was conducted to assess the sphere-forming abilities of the chemoresistant cells in response to the current treatment strategies. However, to evaluate the colonospheresregenerating ability (a measure of self-renewal) of the chemo-resistant cells, the primary colonospheres, obtained from different treatments, were dispersed into a single cell suspension and subsequently cultured to generate secondary colonospheres in the absence of any treatment. Results revealed that CDF in combination with 5-FU + Ox was superior to curcumin in inhibiting the growth of primary as well as secondary colonospheres (Fig. 4a). These results indicate that the treatment strategy with CDF not only possesses a superior inhibitory effect on the growth of sphereforming cells but also shows a better post-treatment inhibition of the self-renewing capacities of the sphere derived from chemo-resistant cells (Fig. 4a). A similar phenomenon was also observed with respect to disintegration of colonospheres, where we found CDF together with 5-FU + Ox caused a much greater disintegration than that caused by 5-FU + Ox alone or the combination of 5-FU + Ox and curcumin (Fig. 4b).
Fig. 4.
Formation of primary and secondary colonospheres by the chemo-resistant HCT-116 cells is inhibited by CDF. (A) Effect of 5-FU + Ox alone or in combination with increasing concentrations of either CDF or curcumin on the formation primary colonospheres by chemo-resistant HCT-116 cells (upper panel). The primary colonospheres obtained from respective treatments were then dissociated into single cell suspension, subsequently subjected to secondary sphere formation in the absence of any treatment. To assess post-treatment self-renewing abilities of the colonosphere-derived cells, an equal number of these cells, diluted in stem cell media, were plated in 96-well low attachment plates for secondary colonosphere generation in the absence of any agent (lower panel). (B) Representative photomicrographs showing the extent of colonosphere disintegration in chemo-resistant HCT-116 cells in the absence (control-untreated) or presence of 5-FU + Ox alone or in combination with low dose of either curcumin or CDF. *p<0.001, compared to the corresponding treatment controls.
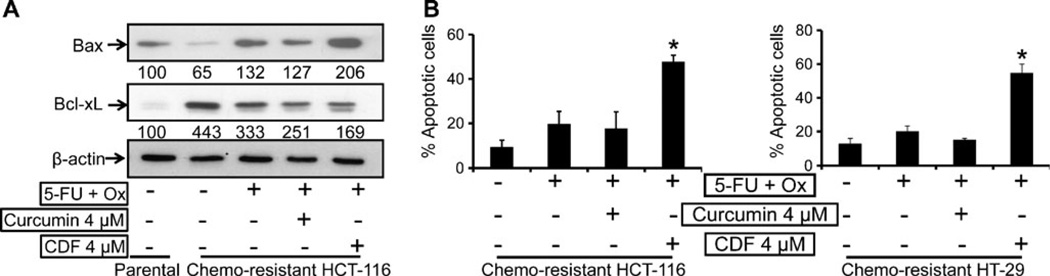
To determine the underlying mechanisms for changes in viability of chemo-resistant the current treatment strategies, we examined the expression of anti-apoptotic Bcl-xL and pro-apoptotic Bax proteins. The chemo-resistant HCT-116 cells showed a significant 30–40% reduction in the levels of pro-apoptotic Bax protein, whereas the levels of anti-apoptotic Bcl-xL were increased by about 400% when compared with the corresponding values of the parental cells (Fig. 5a). However, in response to the combination of 4 µM CDF and 5-FU + Ox, the expression of pro-apoptotic protein Bax in the chemo-resistant HCT-116 cells was increased by about 100%, whereas the same treatment with curcumin caused a mere 27% increase in Bax protein when compared with the corresponding control (Fig. 5a). On the other hand, the expression of the anti-apoptotic Bcl-xL was decreased by 150–250% in response to the combination of CDF or curcumin and 5-FU + OX when compared with the corresponding values of the chemo-resistant cells treated with only 5-FU + Ox (Fig. 5a). Further, the combination treatment of CDF and 5-FU + OX caused a staggering 5–6-fold increase in apoptosis of both HCT-116 and HT-29 chemo-resistant cells (Fig. 5b).
Fig. 5.
(A) Western blot showing changes in the levels of pro-apoptotic Bax, and anti-apoptotic Bcl-xL in untreated parental as compared to chemo-resistant HCT-116 cells in response to the absence or presence of 5-Fu + Ox alone or in combination with either CDF or curcumin. The numbers represent percent of corresponding control normalized to β-actin. (B) Induction of early apoptosis as determined by acridine-orange/ethidium-bromide staining in chemo-resistant HCT-116 and HT-29 cells after incubation of cells in the absence or presence of 5-Fu + Ox alone or in combination with either CDF or curcumin. Values are mean±SD of 4 experiments. *p<0.001, compared to the corresponding treatment control.
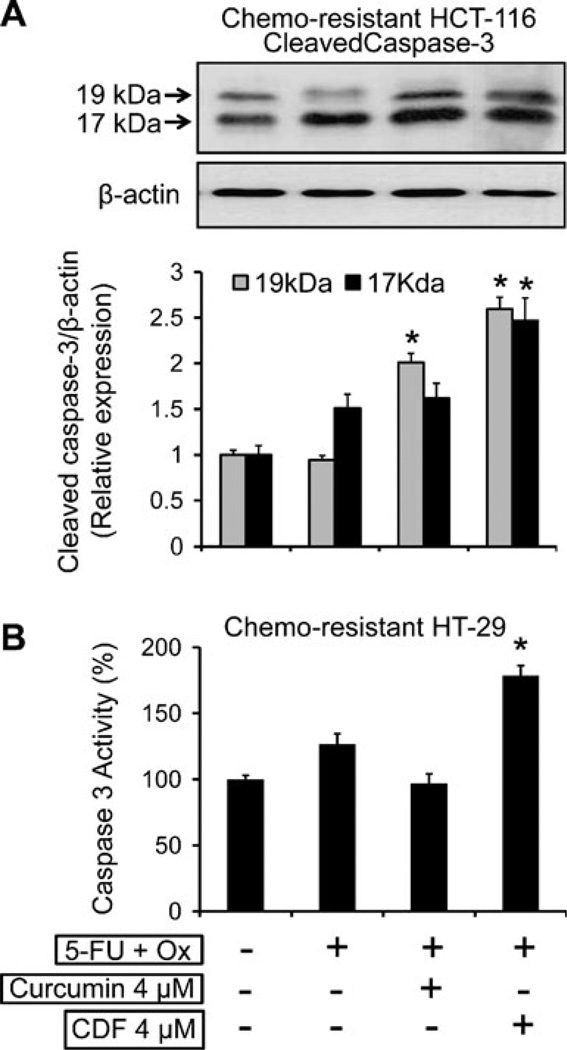
It is known that following induction of apoptosis, proteolytic cleavage of procaspase-3 occurs to generate an active caspase-3 fragment, which targets key modulators of the apoptotic pathway. To corroborate our observation of induction of apoptosis, we examined the levels of cleaved caspase-3 in chemo-resistant HCT-116 cells by Western blot and the activity of caspase-3 in chemo-resistant HT-29 cells in response to the current treatment strategies. Our results revealed a 1.7–2.5-fold increase in the levels of cleaved caspase fragments (19 kDa and 17 kDa, Fig. 6a) and also a similar induction (∼ 1.7-fold increase) in caspase-3 activity following the combination of CDF and 5-FU + Ox (Fig. 6b). On the other hand, the combination of curcumin and 5-Fu + Ox was ineffective in inducing caspase 3 activity (Fig. 6b).
Fig. 6.
(A) Western blot analysis showing changes in the protein expression of cleaved caspase-3 in chemo-resistant HCT-116 cells after 48 h incubation of cells in the absence or presence of 5-Fu + Ox alone or in combination with either CDF or curcumin, β-actin (loading control) levels are also shown (upper panel), and changes in the levels of 19 kDa and 17 kDa fragments of cleaved caspase-3 relative to control normalized to β-actin, as determined by densitometry analysis which, are depicted in the histogram (lower panel). (B) Changes in the caspase-3 activity in the chemo-resistant HT-29 cells. Values are mean±SD of 4 experiments. p < 0.001, compared to the corresponding treatment control.
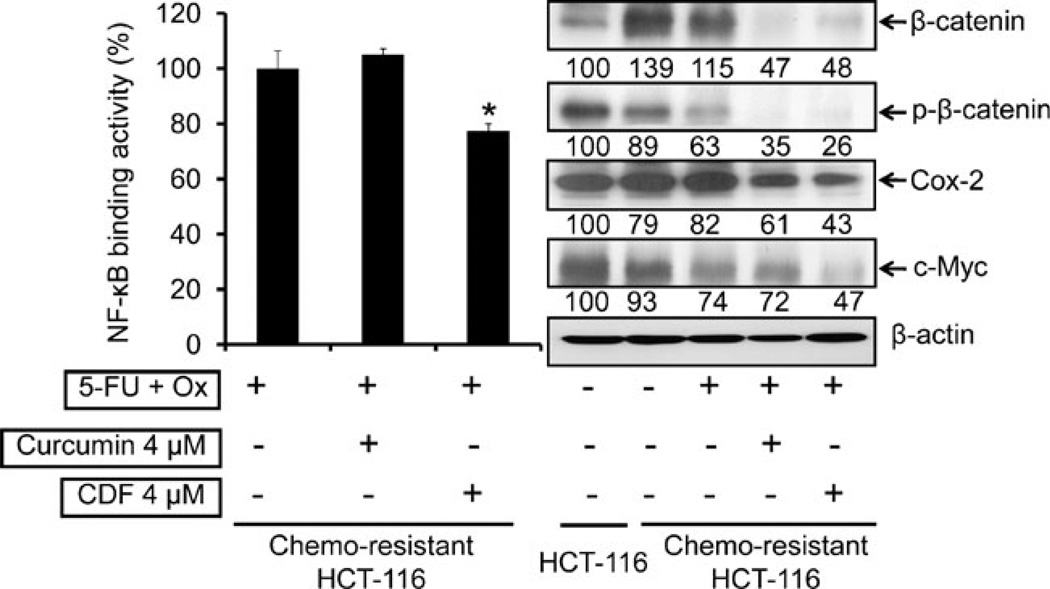
Further regulatory events were evaluated by examining the changes in the activation of NF-κB and β-catenin along with the expression of the downstream effectors Cox-2 and c-Myc. The results revealed that 4 µM CDF, but not curcumin in combination with 5-FU + Ox, caused a 30% reduction in NF-κB activity in chemo-resistant HCT-116 cells, compared with the controls (Fig. 7, left panel). In addition, we observed a 40% increase in the levels of β-catenin in chemo-resistant HCT-116 cells, compared to the parental cells (Fig. 7, right panel). In contrast, a significant 60% reduction of β-catenin was noted following treatment with the combination of CDF and 5-FU + Ox (Fig. 7, right panel). Further, studies revealed that both curcumin and CDF treatments were alike in inhibiting the activation of P-catenin, as evidenced by a complete loss of phosphorylated form of the protein (Fig. 7, right panel). However, CDF was little more effective in inhibiting the expression of Cox-2 and c-Myc when compared with corresponding levels in 5-FU + Ox-treated controls (Fig. 7, right panel).
Fig. 7.
Effect of 5-FU + Ox alone or in combination with either curcumin or CDF on NF-κB DNA binding activity (left panel) and changes in the levels β-catenin, p-β-catenin, Cox-2 and c-Myc, in chemo-resistant HCT-116 cells (right panel); the levels of β-actin (loading control) are also shown. The numbers represent percent of corresponding control normalized to β-actin. *p<0.001, compared to the corresponding treatment control.
DISCUSSION
There is an emerging body of evidence suggesting that tumor cells that are resistant to chemotherapy represent a subpopulation of cells of the original tumor. These chemo-resistant cells, which are molecularly and phenotypically distinct, are also referred to as tumor-initiating cells, tumor-promoting cells or, more commonly, cancer stem cells or cancer stem-like cells (CSC) (Ref (32)). To study the role of CSCs and emergence of chemo-resistance in colon cancer, we have generated 5-FU + Ox-resistant (referred to as chemo-resistant) HCT-116 and HT-29 colon cancer cells by exposing them to increasing doses of the combination of 5-FU and oxaliplatin (referred to as 5-FU + Ox) (13). The newly developed chemo-resistant colon cancer cells were found to exhibit remarkably high levels of CSC markers CD44, CD166 and CD133, suggesting that they are highly enriched in CSCs. Since under the extreme limiting dilution (ELDA) in serum-free conditions (stem cell media) only stem-like cells are believed to divide to form spheroids, ELDA showed a 2-fold increase in the frequency of sphere-forming ability of the cells confirming enrichment of CSCs in chemo-resistant cells (25,33,34). Taken together, the results suggest the preponderance of cancer stem-like cells in the chemo-resistant HCT-116 cells.
In our pursuit to identify agents that may eliminate CSCs, we studied the effect of curcumin either alone or in combination with 5-FU + Ox on the inhibition of cell growth of chemo-resistant colon cancer cells and found these strategies to be effective with high concentrations of curcumin (13). However, the fact that bioavailability of curcumin is very poor has led to the synthesis of analogs or development of formulations of agent(s) to improve upon its bioavailability. CDF, a newly developed analog of curcumin with a greater bioavailability than curcumin, has also been shown to exhibit much greater growth-inhibitory properties than curcumin (20,21). Moreover, recent studies from our laboratory also showed that CDF was much more superior in the killing of gemcitabine-resistant pancreatic cancer cells with epithelial-to-mesenchymal phenotype that is reminiscent of CSCs (35). This prompted us to undertake the current investigation where we compared the effectiveness of curcumin with CDF in inhibiting the growth of chemo-resistant colon cancer cells that are highly enriched in CSCs. Our current data demonstrated that CDF was indeed more effective than curcumin in killing/eliminating chemo-resistant colon cancer cells along with CSCs. The latter is evident from decreased expression of markers of colon CSCs, such as CD44, CD133 and CD166, as has been documented previously (22,36,37). This inference is further supported by the observation that in chemo-resistant colon cancer cells, the CDF also caused a marked inhibition in the expression of ABCG2, a transporter protein which is highly expressed in the stem cell membranes (2). The ABCG2 protein has been implicated as a key regulator in the maintenance of stem cells in various human cancer cell lines, such as those derived from breast, colon, ovary and gastric cancers (29,38–40). It has been well established that ABCG2 is responsible for protecting cells against xenobiotics or endogenous catabolites that may be toxic under unfavorable hypoxic conditions by active efflux activity, and such intrinsically-resistant stem cells are understood to contribute towards resistance to chemotherapy (2,29). Our results suggest that CDF-mediated inhibition in the expression of ABCG2 in chemo-resistant colon cancer cells may, in fact, lead to a higher retention of chemotherapeutic/cytotoxic drugs, which may result in greater cell death. This is supported by our observation that CDF, in comparison with curcumin, causes a greater induction of apoptosis as evidenced by increased staining of acridine-orange by the chemo-resistant colon cancer cells. Moreover, increased levels of pro-apoptotic Bax and cleaved casapsae-3 fragments, a key executioner of downstream apoptotic pathway as well as increased caspase-3 activity along with a concomitant reduction of anti-apoptotic protein, Bcl-xL, were noted.
Furthermore, we have observed that CDF, but not curcumin, at a low concentration of 4 µM causes inhibition and disintegration of the colonospheres that were previously shown to contain over 80% of CD44-positive cells (22). These results are consistent with the observation that CDF is a superior agent in inhibiting the growth of CSCs in colon cancer, a phenomenon similar to that observed with drugresistant pancreatic cancer (35). Additional support comes from the observation that secondary sphere formation by CDF-treated colonosphere-derived cells is greatly inhibited in the absence of further treatment with CDF. In view of the fact that secondary sphere formation represents self-renewing ability of the CSCs (22,41), our observation of decreased secondary formation by CDF-treated primary colonosphere-derived cells suggests that CDF in combination with 5-FU + Ox could be an effective therapeutic strategy for elimination of colon cancer recurrence.
Although, the precise regulatory mechanisms in support of the CDF-mediated inhibition of growth of chemoresistant colon cancer cells are not fully understood, our observation showed that CDF greatly inhibits the activation of EGFR and IGF-1R, which we have shown to be implicated in the development of chemo-resistance, suggesting that CDF may act through the inactivation of EGFR and IGF-1R signaling pathways (13,31,42). This inference is supported by the observation that CDF inhibits the activation of β-catenin as well as NF-κB, both of which are downstream of both EGFR and IGF-1R signaling (43–45). The fact that CDF but not curcumin at 4 µM concentration inhibits the expression of c-Myc and Cox-2, which are downstream effectors of β-catenin and NF-κB signaling, respectively, further indicates a superior efficacy of CDF than curcumin in inhibiting the growth of CSC-enriched chemo-resistant colon cancer cells.
In conclusion, our observations demonstrate that the chemo-resistant colon cancer cells display increased activity of β-catenin and NF-κB, along with higher expression of c-Myc and Cox-2, and ABCG2, which is involved in drug exclusion. We have also demonstrated that chemo-resistant colon cancer cells are highly enriched in CSCs, as evidenced by the increased expression of CD44 and CD166 and their ability to form spheres under ELDA. Our data also suggest that CDF in combination with 5-FU + Ox is more effective than curcumin in inhibiting the growth of chemo-resistant colon cancer cells that are enriched in CSCs. Taken together, our results suggest a potential therapeutic role for CDF in preventing the emergence of chemo-resistant colon cancer cells by reducing/eliminating the CSCs, which could become a novel strategy for improving the overall survival of patients diagnosed with colon cancer.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health/National Institute on Aging (5RO1 AG014343) and the Department of Veterans Affairs (A.P. N.M.) and from the National Cancer Institute, NIH (3RO1 CA131151-02) (F.H.S).
Contributor Information
Shailender Singh Kanwar, Veterans Affairs Medical Center, Wayne State University, Detroit, Michigan 48201, USA; Department of Internal Medicine, Wayne State University, Detroit, Michigan 48201, USA.
Yingjie Yu, Veterans Affairs Medical Center, Wayne State University, Detroit, Michigan 48201, USA; Department of Internal Medicine, Wayne State University, Detroit, Michigan 48201, USA.
Jyoti Nautiyal, Veterans Affairs Medical Center, Wayne State University, Detroit, Michigan 48201, USA; Department of Internal Medicine, Wayne State University, Detroit, Michigan 48201, USA; Department of Pathology, Detroit, Michigan 48201, USA.
Bhaumik B. Patel, Veterans Affairs Medical Center, Wayne State University, Detroit, Michigan 48201, USA Department of Internal Medicine, Wayne State University, Detroit, Michigan 48201, USA; Karmanos Cancer Institute, Wayne State University, Detroit, Michigan 48201, USA.
Subhash Padhye, Dr. D.Y. Patil University, Pune 411018, India.
Fazlul H. Sarkar, Department of Internal Medicine, Wayne State University, Detroit, Michigan 48201, USA Department of Pathology, Detroit, Michigan 48201, USA; Karmanos Cancer Institute, Wayne State University, Detroit, Michigan 48201, USA.
Adhip P. N. Majumdar, Email: a.majumdar@wayne.edu, Veterans Affairs Medical Center, Wayne State University, Detroit, Michigan 48201, USA; Department of Internal Medicine, Wayne State University, Detroit, Michigan 48201, USA; Karmanos Cancer Institute, Wayne State University, Detroit, Michigan 48201, USA; VA Medical Center, Research Service, 4646 John R; Room-B4238, Detroit, Michigan 48201, USA.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 3.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 4.Neugut AI, Lautenbach E, Abi-Rached B, Forde KA. Incidence of adenomas after curative resection for colorectal cancer. Am J Gastroenterol. 1996;91:2096–2098. [PubMed] [Google Scholar]
- 5.Odoux C, Fohrer H, Hoppo T, Guzik L, Stolz DB, Lewis DW, et al. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008;68:6932–6941. doi: 10.1158/0008-5472.CAN-07-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toda S, Miyase T, Arichi H, Tanizawa H, Takino Y. Natural antioxidants. III. Antioxidative components isolated from rhizome of Curcuma longa L. Chem Pharm Bull (Tokyo) 1985;33:1725–1728. doi: 10.1248/cpb.33.1725. [DOI] [PubMed] [Google Scholar]
- 7.Huang MT, Wang ZY, Georgiadis CA, Laskin JD, Conney AH. Inhibitory effects of curcumin on tumor initiation by benzo[a] pyrene and 7, 12-dimethylbenz[a]anthracene. Carcinogenesis. 1992;13:2183–2186. doi: 10.1093/carcin/13.11.2183. [DOI] [PubMed] [Google Scholar]
- 8.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- 9.Rao CV, Simi B, Reddy BS. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis. 1993;14:2219–2225. doi: 10.1093/carcin/14.11.2219. [DOI] [PubMed] [Google Scholar]
- 10.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–273. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 11.Nautiyal J, Banerjee S, Kanwar SS, Yu Y, Patel BB, Sarkar FH, et al. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int J Cancer. 2010 doi: 10.1002/ijc.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pateland BB, Majumdar AP. Synergistic role of curcumin with current therapeutics in colorectal cancer: minireview. Nutr Cancer. 2009;61:842–846. doi: 10.1080/01635580903285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar APN. Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl Oncol. 2009;2:321–328. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahlstromand B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 15.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 16.Labbozzetta M, Baruchello R, Marchetti P, Gueli MC, Poma P, Notarbartolo M, et al. Lack of nucleophilic addition in the isoxazole and pyrazole diketone modified analogs of curcumin; implications for their antitumor and chemosensitizing activities. Chem Biol Interact. 2009;181:29–36. doi: 10.1016/j.cbi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77–103. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 19.Zambre AP, Kulkarni VM, Padhye S, Sandur SK, Aggarwal BB. Novel curcumin analogs targeting TNF-induced NF-kappaB activation and proliferation in human leukemic KBM-5 cells. Bioorg Med Chem. 2006;14:7196–7204. doi: 10.1016/j.bmc.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 20.Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, et al. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking. Pharmacokinetics and tissue distribution in mice. Pharm Res-Dord. 2009;26:2438–2445. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padhye S, Yang H, Jamadar A, Cui QC, Chavan D, Dominiak K, et al. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm Res. 2009;26:1874–1880. doi: 10.1007/s11095-009-9900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212. doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huand Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Nautiyal J, Yu Y, Aboukameel A, Kanwar SS, Das JK, Du J, et al. ErbB-inhibitory protein: a modified ectodomain of epidermal growth factor receptor synergizes with dasatinib to inhibit growth of breast cancer cells. Mol Cancer Ther. 2010;9:1503–1514. doi: 10.1158/1535-7163.MCT-10-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majumdarand AP, Du J. Phosphatidylinositol 3-kinase/Akt signaling stimulates colonic mucosal cell survival during aging. Am J Physiol Gastrointest Liver Physiol. 2006;290:G49–G55. doi: 10.1152/ajpgi.00106.2005. [DOI] [PubMed] [Google Scholar]
- 28.Richand JN, Bao S. Chemotherapy and cancer stem cells. Cell Stem Cell. 2007;1:353–355. doi: 10.1016/j.stem.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Anand Y, Ongkeko WM. ABCG2: the key to chemoresistance in cancer stem cells? Expert Opin Drug Metab Toxicol. 2009;5:1529–1542. doi: 10.1517/17425250903228834. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 31.Patel BB, Gupta D, Elliott AA, Sengupta V, Yu Y, Majumdar AP. Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res. 2010;30:319–325. [PMC free article] [PubMed] [Google Scholar]
- 32.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human coloncancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 34.Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Yeung TM, Gandhi SC, Wilding JL, Muschel R, Bodmer WF. Cancer stem cells from colorectal cancer-derived cell lines. Proc Natl Acad Sci USA. 2010;107:3722–3727. doi: 10.1073/pnas.0915135107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sukachand A, Ivanov E. Formation of spherical colonies as a property of stem cells. Cell Tissue Biol. 2007;1:476–481. [Google Scholar]
- 38.Allenand JD, Schinkel AH. Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2) Mol Cancer Ther. 2002;1:427–434. [PubMed] [Google Scholar]
- 39.Katayama R, Koike S, Sato S, Sugimoto Y, Tsuruo T, Fujita N. Dofequidar fumarate sensitizes cancer stem-like side population cells to chemotherapeutic drugs by inhibiting ABCG2/BCRP-mediated drug export. Cancer Sci. 2009 doi: 10.1111/j.1349-7006.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meissner K, Heydrich B, Jedlitschky G, Meyer zu, Schwabedissen x, Mosyagin I, Dazert P, et al. The ATP-binding cassette transporter ABCG2 (BCRP), a marker for side population stem cells, is expressed in human heart. J Histochem Cytochem. 2006;54:215–221. doi: 10.1369/jhc.5A6750.2005. [DOI] [PubMed] [Google Scholar]
- 41.Kakarala M, Brenner D, Korkaya H, Cheng C, Tazi K, Ginestier C, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu D, Jing T, Liu B, Yao J, Tan M, McDonnell TJ, et al. Overexpression of ErbB2 blocks taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol Cell. 1998;2:581–591. doi: 10.1016/s1097-2765(00)80157-4. [DOI] [PubMed] [Google Scholar]
- 43.Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/ Wnt and activation of p53 signaling pathways. BMC Cancer. 2010;10:238. doi: 10.1186/1471-2407-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dihlmannand S, Doeberitz MVK. Wnt/beta-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int J Cancer. 2005;113:515–524. doi: 10.1002/ijc.20609. [DOI] [PubMed] [Google Scholar]
- 45.Ali S, Banerjee S, Schaffert JM, El-Rayes BF, Philip PA, Sarkar FH. Concurrent inhibition of NF-kappa B, cyclooxygenase-2, and epidermal growth factor receptor leads to greater anti-tumor activity in pancreatic cancer. J Cell Biochem. 2010;110:171–181. doi: 10.1002/jcb.22523. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]