Abstract
This study investigated the expression and clinicopathological significance of CD9 in gastrointestinal stromal tumor (GIST). Immunohistochemistry staining for CD9 was performed on tumor tissues from 74 GIST patients. The correlation with clinicopathological features, risk classification and prognosis was analyzed. CD9-positive staining comprised 59.5% (44/74) of the GIST patients. The CD9-positive expression rate of the sample was significantly associated with diameter (P = 0.028), mitotic counts (P = 0.035), risk classification (P = 0.018) and three-year recurrence-free survival (RFS) (P < 0.001). Cox proportional hazards regression (HR = 0.352; P = 0.015) showed that CD9 is an independent factor for post-operative RFS. The subgroup analysis showed that CD9 expression in gastric stromal tumor (GST) is significantly associated with diameter (P = 0.031), risk classification (P = 0.023) and three-year RFS (P = 0.001). The Cox proportional hazards regression (HR = 0.104; P = 0.006) also showed that CD9 is an independent factor for RFS of GST. However, CD9 expression does not have a statistically significant correlation with clinicopathological features, risk classification, and prognosis in non-GST. In conclusion, CD9 expression in GIST appears to be associated with the recurrence and/or metastasis of GIST patients, especially in GST, which may indicate the important role of CD9 in the malignant biological behavior and prognosis of GST.
Keywords: Gastrointestinal Stromal Tumors, Gastric Stromal Tumor, CD9, Immunohistochemistry, Prognosis
INTRODUCTION
Knowledge on the etiology, pathogenesis, diagnosis and treatment of gastrointestinal stromal tumors (GIST) have reached maturity in recent years. GIST prognosis has been also improved significantly. However, the reason that only certain parts of GIST patients relapse or metastasize after operation in the same site remains unclear. Moreover, non-gastric stromal tumor (GST), intestinal and extra-gastrointestinal stromal tumor (EGIST) relapses or metastasizes more easily than GST, even with the same diameter and mitotic counts, which is a phenomenon that should be explored (1). Although metastasis mainly spreads through blood and implantation metastasis in GIST, the mechanism of metastasis remains unclear, and current studies on the mechanism of metastasis in GIST are limited.
CD9 protein that belongs to the transmembrane 4 super-family (TM4SF) is a transmembrane glycoprotein. This protein is present in various tumors, which indicates that CD9 expression is inversely correlated with recurrence and/or metastasis, and can be utilized as a prognostic marker (2, 3).
This study investigates CD9 expression in GIST and analyzes its correlation with clinicopathological significance, risk classification and prognosis.
MATERIALS AND METHODS
Patients and tissue samples
Surgical samples were selected from West China Hospital, Sichuan University of China between January 2002 and June 2010. The following inclusion criteria were used: pathologically confirmed negative surgical margins (R0) after resection, immunohistochemically confirmed CD117-positive tumor; no pre-operative imatinib mesylate (IM) treatment; no severe systemic disease or combined tumor. The exclusion criteria were as follows: less than 18 yr of age; pregnant or breastfeeding patients; with severe systemic disease or combined tumor; and poor compliance.
Immunohistochemistry
Immunohistochemical staining was performed on 4 µm paraffin sections. The sections were soaked through water washing and then incubated in 3% H2O2 methanol solution for 10 min. Soaked in 0.01 M/L EDTA (pH = 9.0) and repaired in a separated water environment for 10 min through pressure heating. Then, blocked with goat serum for 20 min at room temperature. Rabbit monoclonal CD9 antibody (purchased from Abcam Ltd., Cambridge, UK) was applied overnight with a dilution of 1:300 at 4℃ and incubated for 30 min with biotinylated goat anti-mouse immunoglobulin G. The sections was detected with a secondary antibody for 30 min at room temperature and visualized through incubation by using DAB (1:50 dilution) for 5 min to 10 min. The sections was then counterstained, dehydrated and mounted.
The stained specimens were reviewed by two pathologists who had no knowledge of the clinical status of the patients. The sections were scored semi-quantitatively based on previously described method (4), which considered cell staining intensity and percentage. Intensities were classified as 0 (no staining), 1 (weak staining), 2 (distinct staining), and 3 (very strong staining). The cell staining percentage as classified as 0 (< 10%), 1 (10% to 25%), 2 (26% to 50%), 3 (51% to 75%) and 4 ( > 75%). The total score for each specimen was calculated by using the following equation: total score = score of intensity multiplied by score of percentage. Specimens with a total score > 3 were classified as CD9-positive (+), whereas specimens with total score ≤ 3 were classified as CD9-negative (-).
Data and statistical analysis
The last follow-up was conducted on December 31, 2012. Recurrence-free survival (RFS) is the date of operation to the date of recurrence and/or distant metastasis, which is the endpoint for GIST patients. Patients who survived without recurrence and/or metastasis were censored on the date of the last follow-up. The data were evaluated by using SPSS version 18.0. Measurement data were expressed as mean ± standard deviation. Categorical data from different groups were compared by using the chi-square test or Fisher's exact test. Cumulative RFS was estimated by using the Kaplan-Meier method with a one-sided log-rank test. Hazard regression model and 95% confidence interval (CI) were described based on the Cox proportion HR model. P < 0.05 was considered statistically significant.
Ethics statement
The study protocol was approved by the institutional review board of West China Hospital, Sichuan University, China (No. 2013-64), and an informed consent was obtained from every of the patients.
RESULTS
Characteristics of the patients
A total of 74 patients included 50 males and 24 females, with a mean age of 52.9 ± 12.2 (from 29 to 84) yr. The pathological types included 38 GST, 23 intestinal GIST, and 13 EGIST. Based on The NCCN guideline for risk classification of GIST (5), 10 tumors were low risk, 11 were intermediate risk, and 53 were high risk.
CD9 expression in GIST and the relationship between CD9 expression and clinicopathological features of GIST
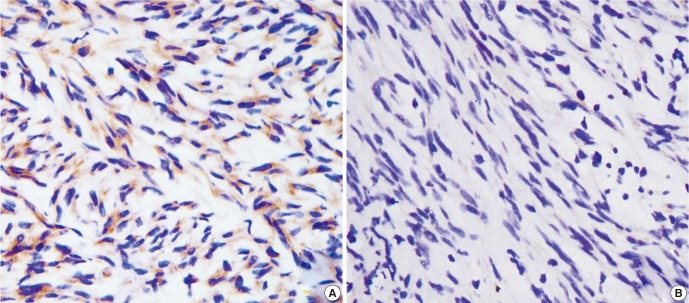
Fig. 1A shows that the immunohistochemical positive reaction product of CD9 was mainly localized in the cytoplasm and/or cell membrane. About 59.5% (44/74) of the 74 GIST specimens were classified as CD9-positive. By contrast, 40.5% (30/74) were classified as CD9-negative (Fig. 1B).
Fig. 1.
CD9 immunohistochemistry in GIST. (A) CD9-positive expression in low-risk GIST (×400), (B) CD9-negative expression in high-risk GIST (×400).
Table 1 summarizes the immunoreactivities in GIST. No significant association was observed between CD9 expression and age (P = 0.333), sex (P = 0.712), and location (P = 0.769) when CD9 expression was compared with various clinical features. However, a highly significant association was found between CD9 expression and tumor diameter (P = 0.028), mitotic count (P = 0.035), and risk classification (P = 0.018).
Table 1.
CD9 expression and clinicopathological features of GIST
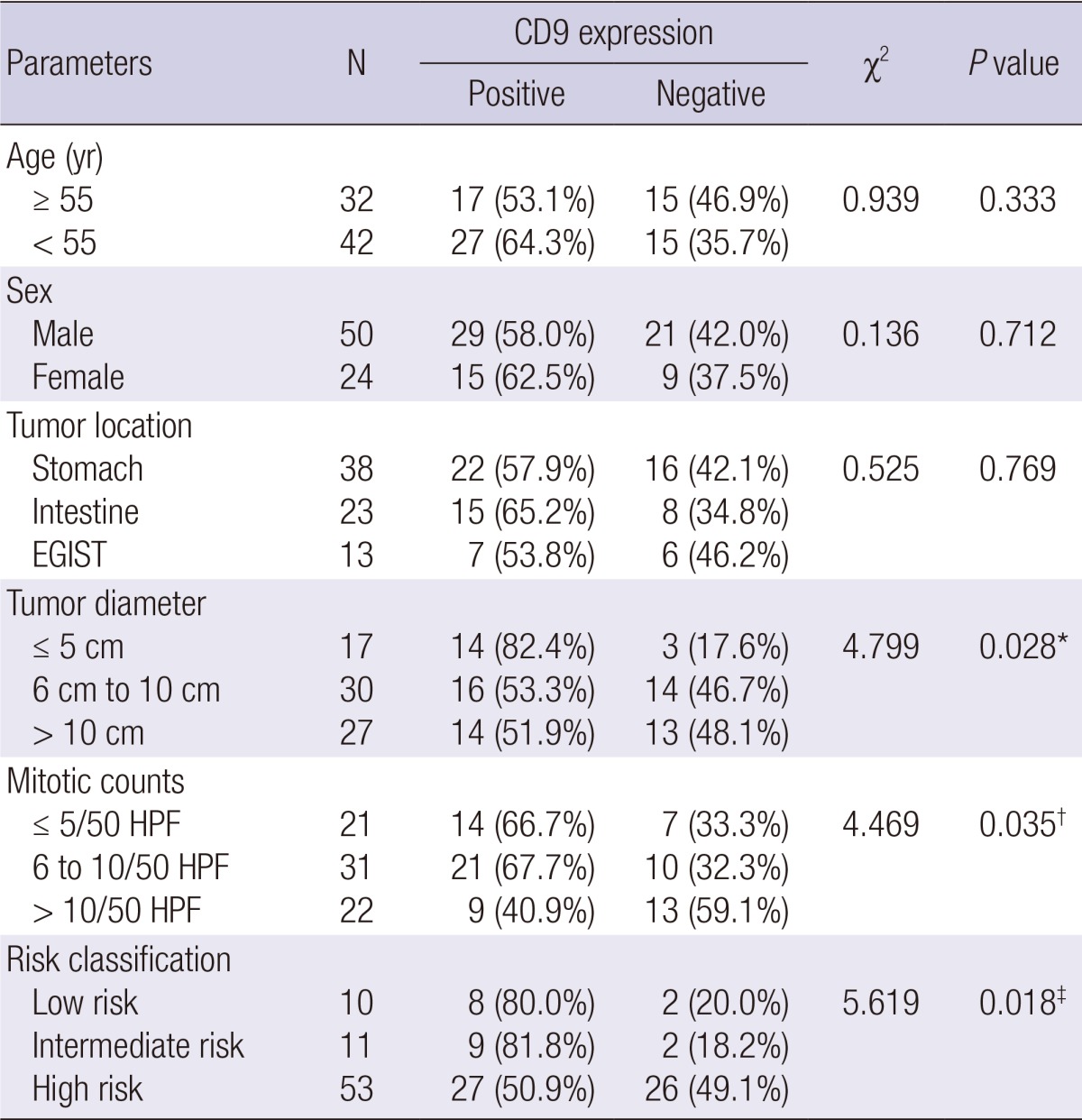
*Tumor diameter ≤ 5 cm group compared with > 5 cm group (6 to 10 cm group and > 10 cm group); †mitotic counts ≤ 10/50 HPF group (≤ 5 and 6 to 10/50 HPF group) compared with > 10/50 HPF group; ‡low-/intermediate-risk group compared with high-risk group. EGIST, Extra-gastrointestinal stromal tumor; HPF, high power field.
Relationship between CD9 expression and RFS
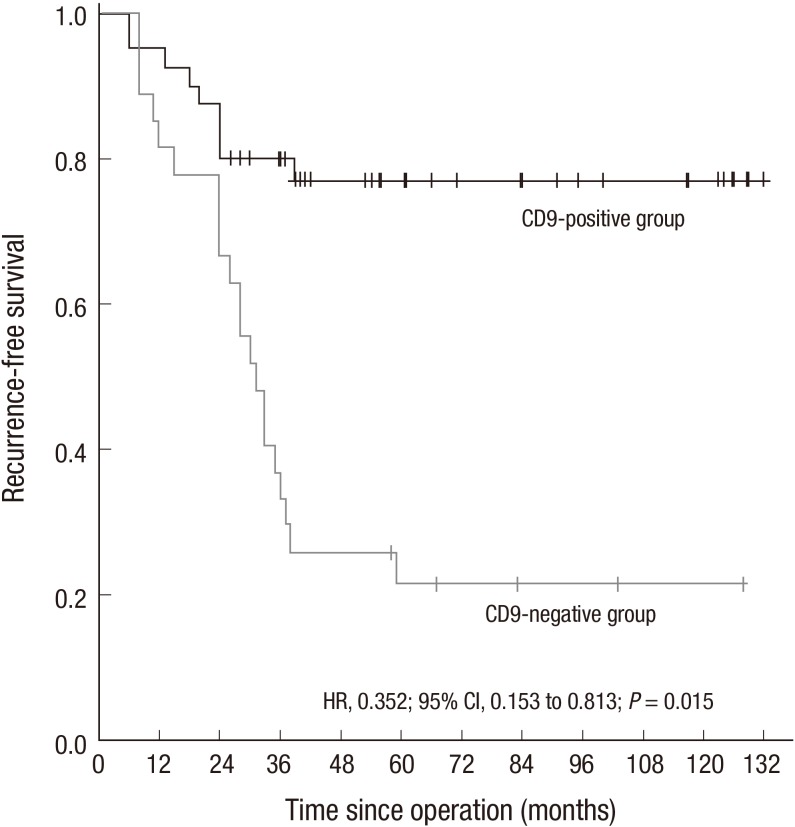
The 74 patients were followed up regularly through telephone and outpatient visits. The average follow-up time was 49 months (6 to132 months). 9 patients recurred, 16 patients had a distant metastasis, 5 patients recurred and merging with a distant metastasis. After excluding seven patients who received IM treatment post-operation and three who underwent a follow-up time of < three years post-operation, sixty-four patients left, who underwent at least 36 months of follow-up without post-operative IM adjuvant treatment. In the 64 patients, the three-year RFS rate was 78.4% (29/37) in 37 patients with CD9-positive expression compared with 33.3% (9/27) in 27 patients with CD9-negative expression (P < 0.001). The Cox proportion hazards regression (HR, 0.352; 95% CI: 0.153 to 0.813; P = 0.015) showed that CD9 expression is an independent prognostic factor of RFS. The cumulative RFS curve of 64 patients in relation to CD9 expression showed that the CD-positive group has a significantly better cumulative RFS rate compared with the group with CD9-negative expression (P = 0.015) (Fig. 2).
Fig. 2.
Kaplan-Meier estimates of RFS between CD9-positive GIST and CD9-negative GIST group. The hazard ratio for RFS in the CD9-positive group was 0.352 (95% CI, 0.153 to 0.813; P = 0.015) compared with the CD9-negative group. RFS curves were constructed using the Kaplan-Meier method. Differences between the curves were tested for statistical significance using Log-rank statistics.
The relationship of CD9 expression in GST with clinicopathological features, risk classification and RFS
Table 2 shows the lack of significant association between CD9 expression and age (P = 0.578), sex (P = 0.542), and mitotic count (P = 0.645) in the 38 GSTs. However, a highly significant association was observed between CD9 expression and tumor diameter (P = 0.031) and risk classification (P = 0.023).
Table 2.
CD9 expression and clinicopathological features of GST
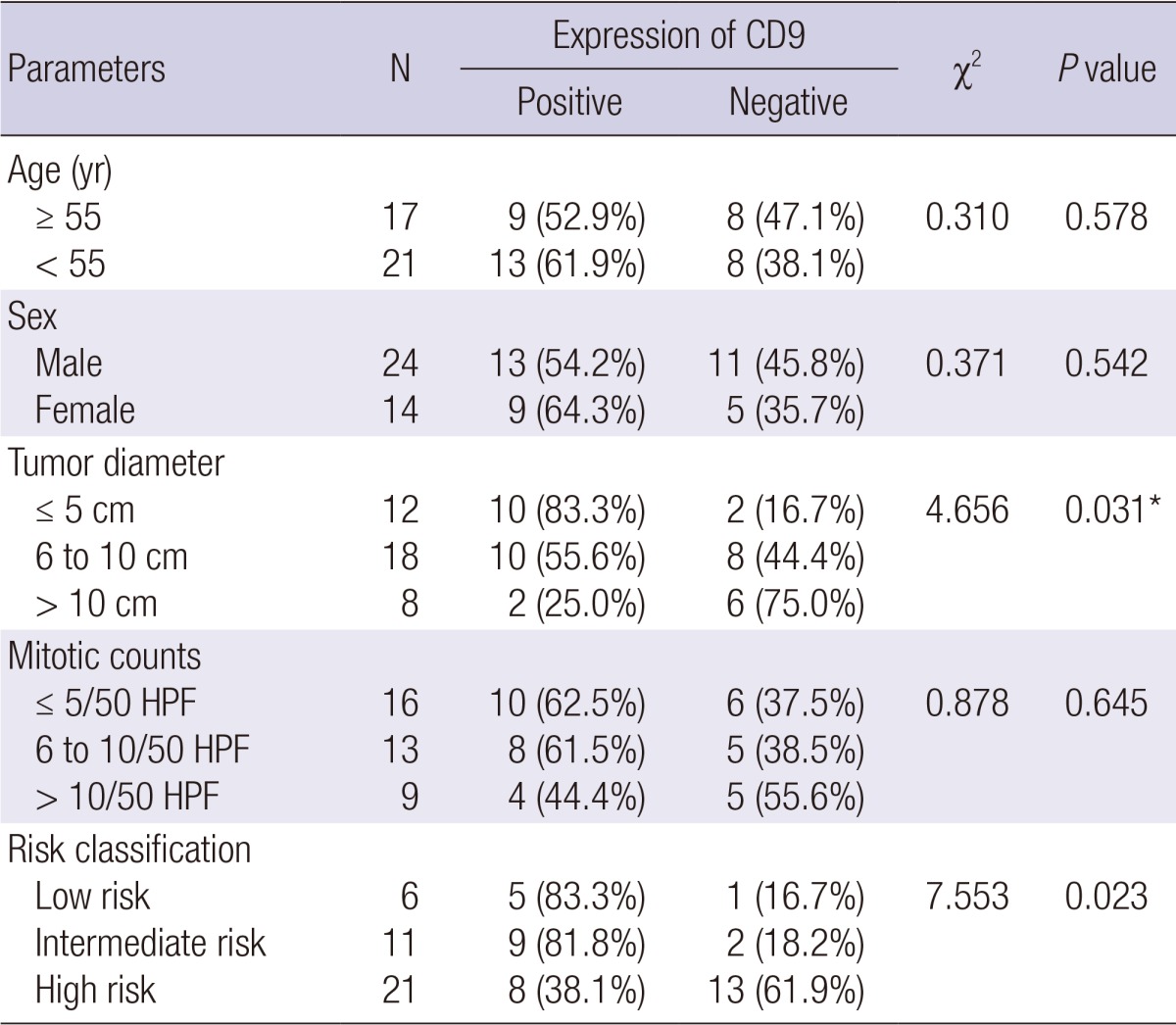
*Tumor diameter ≤ 5 cm group compared with > 5 cm group (6 to 10 cm group and > 10 cm group). HPF, high power field.
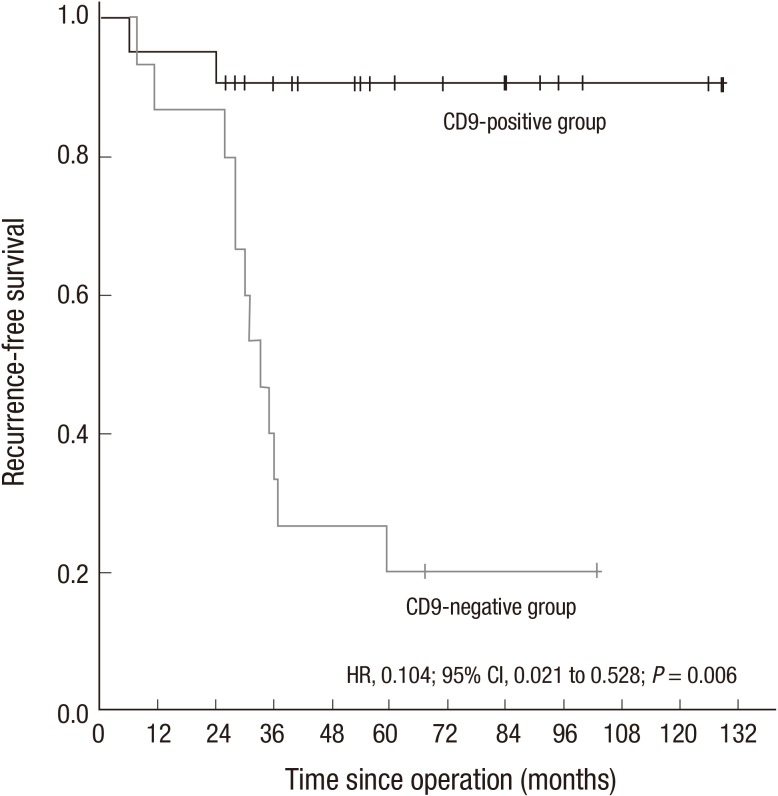
Two patients who received IM treatment post-operation and three patients who underwent a follow-up time of < three years post-operation were excluded. The three-year RFS rate of the remaining 33 patients was 88.9% (16/18) in 18 patients with CD9-positive expression compared with 33.3% (5/15) in 15 patients with CD9-negative expression (P = 0.001). The Cox proportion hazards regression (HR, 0.104; 95% CI: 0.021 to 0.528; P = 0.006) showed that CD9 expression was also an independent prognostic factor of RFS for GST. The cumulative RFS curve showed that the RFS of patients with CD-positive expression group was significantly better than that of the CD9-negative expression group (P = 0.006) (Fig. 3).
Fig. 3.
Kaplan-Meier estimates of RFS between CD9-positive GST and CD9-negative GST group. The hazard ratio for RFS in the CD9-positive group was 0.104 (95% CI, 0.021 to 0.528; P = 0.006) compared with the CD9-negative group. RFS curves were constructed using the Kaplan-Meier method. Differences between the curves were tested for statistical significance using Log-rank statistics.
The relationship of CD9 expression in non-GST with clinicopathological features, risk classification and RFS
No significant relationship was observed between CD9 expression and clinicopathological features, risk classification, and operative three-year RFS (P > 0.05) of the 36 non-GST patients. The Cox proportion hazards regression (HR, 0.710; 95% CI: 0.214 to 2.353; P = 0.575) showed that CD9 expression was not an independent prognostic factor.
DISCUSSION
Despite complete resection, nearly half of the primary GIST recurred or had a metastasis, and showed poor outcome (6). Joensuu found that non-GST relapsed or metastasized more easily than GST even with the same diameter and mitotic count. However, the reason behind this phenomenon remains unclear because the mechanism is yet to be classified.
IM has significantly improved the prognosis of advanced or high-risk GIST patients (7). However, secondary resistance caused by gene amplification or secondary gene mutation caused by prolonged IM treatment has became the main factor that affects prognosis (8). An in-depth study of the mechanism of recurrence and/or metastasis may screen patients with high risk of recurrence or metastasis post-operation and provide a basis for implementing individualized treatment to improve prognosis.
Tumor recurrence and/or metastasis is a complex process accompanied with multiple genes and regulated by the interaction of a number of genes, adhesion molecules, enzymes, and protein. Epigenetic changes occur in small GISTs, such as 14q, 15q, and 22q, which result in malignant transformation and metastasis (9-11). Okamoto et al. (12) found that the methylative level of tumor suppressor genes, such as RASSF1A, P1, CDH1, and MGMT4, is correlated with tumor malignancy. Thus, this study examined whether some proteins play important roles in the mechanism of recurrence and/or metastasis in GIST.
CD9, which belongs to TM4SF, can inhibit proliferation and metastasis by inhibiting the activation of the Wnt signaling pathway (13), the degradation of transforming growth factor α (TGF-α) (14), and the secretion of metalloproteinase (15). CD9 downregulation is a poor prognostic marker in various cancers and is correlated with tumor invasion and metastasis in gastric cancer (16), lung cancer (17), and bladder cancer (18). Setoguchi et al. (19) found that CD9 in metastatic liver GIST is downregulated by micro-array and PCR. The present study employed immunohistochemistry analysis in detecting CD9 expression to investigate its correlation with clinicopathological features, risk classification, and prognosis.
In our study, forty-four (59.5%) cases are CD9 positive. Positive reaction product was mainly localized in the cytoplasm and/or cell membrane, similar to other reports (20, 21). The positive rates of CD9 were higher in cases with ≤ 5 cm diameter, ≤ 10/50 HPF mitotic count, and low-/intermediate-risk group than in cases with > 5 cm diameter, > 10/50 HPF mitotic counts, and high-risk group (P < 0.05). These results suggest that reduced CD9 expression plays an important role in tumor progression and malignant behaviors. Comparing the RFS of CD9-positive with CD9-negative group, the universal analysis showed that the postoperative three-year RFS rate of the CD9-positive group is higher than that of the CD9-negative group (78.4% vs 33.3%, P < 0.001). Multivariate analysis (HR, 0.352; 95% CI, 0.153 to 0.813; P = 0.015) showed that CD9 expression is an independent predictor of RFS. It indicates CD9 is an important role in the invasion process and metastasis in GIST, the loss of CD9 increases the risk of recurrence or metastasis. Thus, CD9 may be a favorable predictor of tumor progression or aggressive behavior in GIST.
Researchers are increasingly focusing on the role of CD9 in recurrence or metastasis of GIST. Setoguchi et al. (19) confirmed that the postoperative five-year RFS rate of the CD9-positive group is higher than that of the CD9-negative group in GST. However, a statistically significant relationship was not found between these two groups in intestinal GIST. This finding is attributed to the different functions and signal transduction pathways in different organs (22).In our study, the subgroup analysis of GST in this study showed that the positive rate of CD9 expression of the ≤ 5 cm group is higher than that of the > 5 cm group (P = 0.031). The CD9-positive rate declined when risk increased (P = 0.023). The postoperative three-year rate of the CD9-positive group was higher than that of the CD9-negative group (88.9% vs 33.3%, P = 0.001). However, no significant difference was found between CD9 expression and clinicopathological features risk classification and RFS in non-GST. This finding is consistent with the findings of Setoguchi. Thus, unlike non-GST, it indicates that CD9 expression may be organ specific, CD9 plays an more important role in the progression and/or metastasis of GST.
In conclusion, CD9 expression is closely related to the diameter, risk classification, and RFS of GIST, especially in GST. CD9 expression is downregulated during the malignant transformation process or metastasis in GST. This mechanism of CD9 could be a potential prognostic marker in GST and may guide individualized treatment for patients.
ACKNOWLEDGMENTS
We would like to acknowledge the services of Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, which assisted us throughout the duration of the study.
Footnotes
The present study was supported by the Science and Technology Supporting Plan of Sichuan Province (No. 2012SZ0006).
The authors have no conflicts of interest to disclose.
References
- 1.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Buim ME, Lourenço SV, Carvalho KC, Cardim R, Pereira C, Carvalho AL, Fregnani JH, Soares FA. Downregulation of CD9 protein expression is associated with aggressive behavior of oral squamous cell carcinoma. Oral Oncol. 2010;46:166–171. doi: 10.1016/j.oraloncology.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Nakazawa Y, Sato S, Naito M, Kato Y, Mishima K, Arai H, Tsuruo T, Fujita N. Tetraspanin family member CD9 inhibits Aggrus/podoplanin-induced platelet aggregation and suppresses pulmonary metastasis. Blood. 2008;112:1730–1739. doi: 10.1182/blood-2007-11-124693. [DOI] [PubMed] [Google Scholar]
- 4.Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, Mansfield P, Ajani J, Xie K. Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res. 2004;10:4109–4117. doi: 10.1158/1078-0432.CCR-03-0628. [DOI] [PubMed] [Google Scholar]
- 5.Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8:S1–S41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan I, You YN, Shyyan R, Dozois EJ, Smyrk TC, Okuno SH, Schleck CD, Hodge DO, Donohue JH. Surgically managed gastrointestinal stromal tumors: a comparative and prognostic analysis. Ann Surg Oncol. 2008;15:52–59. doi: 10.1245/s10434-007-9633-z. [DOI] [PubMed] [Google Scholar]
- 7.Kang YK, Kim KM, Sohn T, Choi D, Kang HJ, Ryu MH, Kim WH, Yang HK. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. J Korean Med Sci. 2010;25:1543–1552. doi: 10.3346/jkms.2010.25.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang WL, Conley A, Reynoso D, Nolden L, Lazar AJ, George S, Trent JC. Mechanisms of resistance to imatinib and sunitinib in gastrointestinal stromal tumor. Cancer Chemother Pharmacol. 2011;67:S15–S24. doi: 10.1007/s00280-010-1513-8. [DOI] [PubMed] [Google Scholar]
- 9.Breiner JA, Meis-Kindblom J, Kindblom LG, McComb E, Liu J, Nelson M, Bridge JA. Loss of 14q and 22q in gastrointestinal stromal tumors (pacemaker cell tumors) Cancer Genet Cytogenet. 2000;120:111–116. doi: 10.1016/s0165-4608(00)00212-0. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Liou CP, Tseng HH, Jan YJ, Li CF, Tzeng CC. Deletions of chromosome 1p and 15q are associated with aggressiveness of gastrointestinal stromal tumors. J Formos Med Assoc. 2009;108:28–37. doi: 10.1016/S0929-6646(09)60029-2. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Du X, Lazar AJ, Pollock R, Hunt K, Chen K, Hao X, Trent J, Zhang W. Genetic aberrations of gastrointestinal stromal tumors. Cancer. 2008;113:1532–1543. doi: 10.1002/cncr.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamoto Y, Sawaki A, Ito S, Nishida T, Takahashi T, Toyota M, Suzuki H, Shinomura Y, Takeuchi I, Shinjo K, et al. Aberrant DNA methylation associated with aggressiveness of gastrointestinal stromal tumour. Gut. 2012;61:392–401. doi: 10.1136/gut.2011.241034. [DOI] [PubMed] [Google Scholar]
- 13.Huang CL, Liu D, Masuya D, Kameyama K, Nakashima T, Yokomise H, Ueno M, Miyake M. MRP-1/CD9 gene transduction downregulates Wnt signal pathways. Oncogene. 2004;23:7475–7483. doi: 10.1038/sj.onc.1208063. [DOI] [PubMed] [Google Scholar]
- 14.Imhof I, Gasper WJ, Derynck R. Association of tetraspanin CD9 with transmembrane TGF{alpha} confers alterations in cell-surface presentation of TGF{alpha} and cytoskeletal organization. J Cell Sci. 2008;121:2265–2274. doi: 10.1242/jcs.021717. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y, Tachibana I, Takeda Y, Yamane H, He P, Suzuki M, Minami S, Kijima T, Yoshida M, Kumagai T, et al. Absence of CD9 enhances adhesion-dependent morphologic differentiation, survival, and matrix metalloproteinase-2 production in small cell lung cancer cells. Cancer Res. 2006;66:9557–9565. doi: 10.1158/0008-5472.CAN-06-1131. [DOI] [PubMed] [Google Scholar]
- 16.Soyuer S, Soyuer I, Unal D, Ucar K, Yildiz OG, Orhan O. Prognostic significance of CD9 expression in locally advanced gastric cancer treated with surgery and adjuvant chemoradiotherapy. Pathol Res Pract. 2010;206:607–610. doi: 10.1016/j.prp.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Kohmo S, Kijima T, Otani Y, Mori M, Minami T, Takahashi R, Nagatomo I, Takeda Y, Kida H, Goya S, et al. Cell surface tetraspanin CD9 mediates chemoresistance in small cell lung cancer. Cancer Res. 2010;70:8025–8035. doi: 10.1158/0008-5472.CAN-10-0996. [DOI] [PubMed] [Google Scholar]
- 18.Mhawech P, Herrmann F, Coassin M, Guillou L, Iselin CE. Motility-related protein 1 (MRP-1/CD9) expression in urothelial bladder carcinoma and its relation to tumor recurrence and progression. Cancer. 2003;98:1649–1657. doi: 10.1002/cncr.11698. [DOI] [PubMed] [Google Scholar]
- 19.Setoguchi T, Kikuchi H, Yamamoto M, Baba M, Ohta M, Kamiya K, Tanaka T, Baba S, Goto-Inoue N, Setou M, et al. Microarray analysis identifies versican and CD9 as potent prognostic markers in gastric gastrointestinal stromal tumors. Cancer Sci. 2011;102:883–889. doi: 10.1111/j.1349-7006.2011.01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan J, Zhu GZ, Niles RM. Expression and function of CD9 in melanoma cells. Mol Carcinog. 2010;49:85–93. doi: 10.1002/mc.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou Q, Xiong L, Yang Z, Lv F, Yang L, Miao X. Expression levels of HMGA2 and CD9 and its clinicopathological significances in the benign and malignant lesions of the gallbladder. World J Surg Oncol. 2012;10:92. doi: 10.1186/1477-7819-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55. doi: 10.1038/nrc2543. [DOI] [PubMed] [Google Scholar]