Abstract
The mammalian endoplasmic reticulum (ER)-to-cytosol degradation pathway for disposal of misfolded proteins is an attractive target for therapeutic intervention in diseases that are characterized by impaired protein degradation. The ability to do so is hampered by the small number of specific inhibitors available and by our limited understanding of the individual steps involved in this pathway. Cells that express a class I major histocompatibility complex (MHC) heavy chain-enhanced green fluorescent protein (EGFP) fusion protein and the human cytomegalovirus protein US11, which catalyzes dislocation of the class I MHC EGFP reporter, show only little fluorescence. Treatment with proteasome inhibitors increases their fluorescence by stabilizing EGFP-tagged MHC class I molecules. We used this change in signal intensity as a readout to screen a chemical library of 16,320 compounds and identified two structurally related compounds (eeyarestatin I and II) that interfered with the degradation of both EGFP-heavy chain and its endogenous unmodified class I MHC heavy chain counterpart. Eeyarestatin I also inhibited degradation of a second misfolded type I membrane protein, T-cell receptor α. Both compounds stabilize these dislocation substrates in the ER membrane, without preventing proteasomal turnover of cytosolic substrates. The new inhibitors must therefore interfere with a step that precedes proteasomal degradation. The use of eeyarestatin I thus allows the definition of a new intermediate in dislocation.
INTRODUCTION
Proteins that enter the secretory pathway acquire their secondary and tertiary structure in the endoplasmic reticulum (ER) in a carefully controlled sequence of events. In the ER, the nascent polypeptide is subjected to modifications that include signal-peptide cleavage, N-linked glycosylation and oligosaccharide trimming. Folding of proteins is guided by chaperones such as BiP and the lectins calnexin and calreticulin. Oxidoreductases control the formation of disulfide bonds between the correct pairs of cysteine residues (Frand et al., 2000; High et al., 2000), to stabilize the folded structure. As a consequence of imperfections in protein folding, some polypeptides never attain their native conformation, and such terminally misfolded proteins are singled out by a quality control process in the ER (Bonifacino and Weissman, 1998; Schubert et al., 2000). The destruction of misfolded proteins takes place mostly in the cytosol. Therefore, these substrates must cross the ER membrane before their degradation. Ubiquitination is a prerequisite for the export of many misfolded proteins from the ER and can occur while these substrates are still associated with the membrane (Hampton et al., 1996; Hiller et al., 1996; Knop et al., 1996; Biederer et al., 1997; Bordallo et al., 1998; Shamu et al., 2001; Tiwari and Weissman, 2001; Zhang et al., 2001). The Sec61 complex, involved in cotranslational protein import into the ER, was identified as a channel that can also mediate export of misfolded proteins out of the ER (Wiertz et al., 1996b; Bays et al., 2001; Ye et al., 2001; Bays and Hampton, 2002; Jarosch et al., 2002; Rabinovich et al., 2002). This process is referred to as dislocation (Wiertz et al., 1996a). The driving force for the ATP-dependent extraction (Wiertz et al., 1996b) of proteins from the ER membrane is probably provided by a complex of p97/Cdc48 with Npl4 (Bays et al., 2001; Ye et al., 2001; Bays and Hampton, 2002; Jarosch et al., 2002; Rabinovich et al., 2002). After their arrival in the cytosol, glycoproteins are deglycosylated by an N-glycanase and ultimately destroyed by the proteasome (Hirsch and Ploegh, 2000; Hirsch et al., 2003).
ER-to-cytosol transport of proteins is not restricted entirely to misfolded ER-proteins. In fact, the dislocation of an ER-resident type I membrane protein to the cytosol was first described as a mechanism used by the human cytomegalovirus to destroy class I major histocompatibility complex (MHC) heavy chains (Tortorella et al., 2000). In the absence of any other viral products, the human cytomegalovirus-encoded gene product US2 selectively targets folded class I MHC molecules and induces the dislocation of MHC class I heavy chains from the ER to the cytosol, in a chain of events that resembles the degradation of aberrant proteins (Wiertz et al., 1996a,b; Tortorella et al., 1998). US11 catalyzes a similar sequence of events. These viral proteins must use elements of the ER quality control system for their own purpose, and therefore US2- and US11-mediated degradation of class I MHC heavy chains, used by the virus to escape immune attack, is a useful model to study the ER quality control machinery.
Studies of the ER-to-cytosol degradation pathway in the mammalian system are hampered by the small number of inhibitors that interfere specifically with each of the individual steps. Inhibition of ER-mannosidase I stabilizes only some misfolded glycoproteins (Tokunaga et al., 2000). Manipulation of redox potential by addition of alkylating reagents, such as N-ethylmaleimide or diamide, blocks the delivery of misfolded proteins from the ER to the cytosol (Tortorella et al., 1998). However, alkylating agents modify many different targets and lack the specificity required for detailed studies on quality control in the ER. Several commonly available proteasome inhibitors inhibit only the final step in protein turnover (Lee and Goldberg, 1998). In mammalian cells, few steps upstream of ubiquitin conjugation and proteolysis have been identified as targets for specific inhibitors. To further our understanding of dislocation in mammalian cells, a genetic approach might help identify essential players, either through isolation of mutants in mammalian cells, never an easy task, or by exploiting the more advanced analysis in yeast and attempt to identify essential components by homology. As we show here, small molecule inhibitors might also be used to dissect the process in mammalian cells, an approach referred to as chemical genetics (Kuruvilla et al., 2002).
We performed a high-throughput screen to identify compounds that interfere with the turnover of a class I MHC heavy chain-enhanced green fluorescent protein fusion protein (EGFP-HC) as the reporter substrate, because this fusion protein is still targeted for US2- and US11-mediated degradation (Fiebiger et al., 2002). Inhibition of EGFP-HC degradation in US11-expressing cells with proteasome inhibitors causes the accumulation of EGFP-HC and results in an increase in fluorescence (Fiebiger et al., 2002). Interference with any other step in dislocation or degradation should likewise increase the fluorescence signal emitted by these cells. Such a screen requires continuing protein synthesis and should yield compounds that do not impair cell viability. In a chemical library of 16,320 compounds, we identified two structurally related compounds (eeyarestatin I and II) that inhibit degradation of three dislocation substrates: EGFP-HC, wild-type class I heavy chain, and T-cell receptor α (TCRα), by retaining them in the ER. The mode of inhibition of eeyarestatin is distinct from that of inhibitors of the proteasome and defines a new step in the degradation pathway of type I membrane proteins.
MATERIALS AND METHODS
Cell Lines and Antibodies
The generation and culture of U373, US2EGFP-HC, and US11EGFP-HC cell lines were performed as described previously (Fiebiger et al., 2002). Polyclonal anti-class I MHC heavy chain serum and anti-green fluorescent protein (GFP) serum were used as described previously (Fiebiger et al., 2002). W6/32 was used to define properly folded MHC class I molecules (Parham et al., 1979). Polyclonal anti-protein disulfide isomerase (PDI) serum was generated from bacterially expressed human PDI (Fiebiger et al., 2002). Both the soluble and signal sequence-containing TCRα were expressed in human embryonic kidney (HEK)-293 cells by transient transfection using Lipofectamine according to the manufacturer's instructions (Hirsch et al., 2003). Digestion of the wild-type TCRα construct with KpnI and XmaI and religation after mung bean digestion generated an expression vector for TCRα lacking its N-terminal signal sequence. TCRα was immunoprecipitated as described previously (Huppa and Ploegh, 1997).
High-Throughput Screen
US11EGFP-HC cells were seeded at a density of 8000 cells/well in a 384-well assay plate (black, clear bottom, tissue culture treated; Nalge Nunc International, Naperville, IL) and maintained at 37°C, 5% CO2. The chemical library (Divers E set, Chembridge Chemical, provided by the Institute for Chemistry and Biology, Harvard Medical School, Boston, MA) was assayed in 384-well plates. We examined a total of 51 plates containing 16,320 compounds. The compounds were dissolved in dimethyl sulfoxide (5 mg/ml), and 40 nl of each compound was robotically transferred to the assay plate by pin transfer. After a 16-h incubation at 37°C, 5% CO2, the cells were washed with 100 μl of phosphate-buffered saline (PBS) three times using a plate washer (Elx405; Bio-Tek Instruments, Winooski, VT), followed by the addition of 50 μl of PBS to each well. Using the LJL Analysts plate reader (Molecular Devices, Sunnyvale, CA), the fluorescent signal from each well was measured (excitation, 485 nm; emission, 530 nm). All compounds were examined in duplicate. Compounds were identified as positive when they induced at least a 1.5-fold increase in fluorescent signal compared with cells treated with the solvent control.
Chemical Compounds
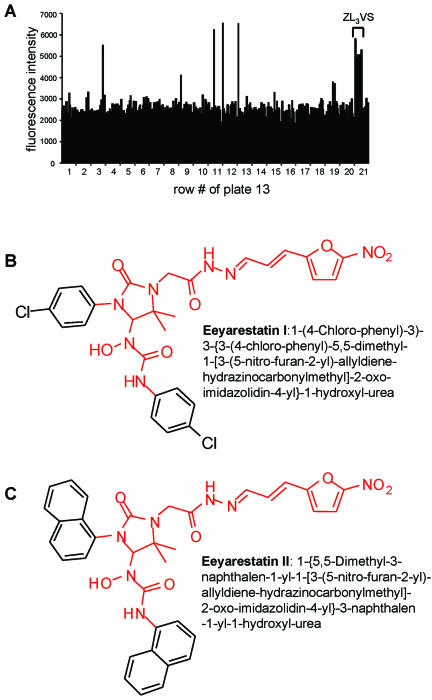
Eeyarestatin I. 1-(4-Chloro-phenyl)-3-{3-(4-chloro-phenyl)-5,5-dimethyl-1-[3-(5-nitro-furan-2-yl)-allyldiene-hydrazinocarbonylmethyl]-2-oxo-imidazolidin-4-yl}-1-hydroxyl-urea.
Eeyarestatin II. 1-{5,5-Dimethyl-3-naphthalen-1-yl-1-[3-(5-nitro-furan-2-yl)-allyldiene-hydrazinocarbonylmethyl]-2-oxo-imidazolidin-4-yl}-3-naphthalen-1-yl-1-hydroxyl-urea.
Pulse-Chase Analysis, Immunoprecipitation and Immunoblotting
Cells were starved in methionine-/cysteine-free DME for 45 min at 37°C. Cells were metabolically labeled with 500 μCi of [35S]methionine/cysteine (1.200 Ci/mM; PerkinElmer Life Sciences, Boston, MA)/ml at 37°C for the times indicated. Pulse-chase experiments, cell lysis, and immunoprecipitations were performed as described previously (Rehm et al., 2001). The immunoprecipitates were analyzed by SDS-PAGE followed by fluorography (Ploegh, 1995). Endoglycosidase H and N-glycanase (Sigma/RBI, Beverly, MA) digestions were performed as described by the manufacturers. Immunoblots were performed with SDS-lysates of inhibitor-treated or untreated U373EGFP/HC, US2EGFP/HC, and US11EGFP/HC cells as described previously (Fiebiger et al., 2002). Labeling of cysteine proteases of the cathepsin family was performed with DCG-04 as described previously (Lennon-Dumenil et al., 2002).
Subcellular Fractionation and Fluorometric Analysis
Subcellular fractionation of metabolically labeled cells was performed as described previously (Tortorella et al., 1998). Briefly, cells were suspended in 50 mM Tris, pH 7.4, 250 mM sucrose and homogenized using a ball-bearing homogenizer with a gap width of 12 μm. The homogenate was subjected to high-speed centrifugation to isolate different particulate fractions. The fluorescent signal from subcellular fractions was measured as described previously (Fiebiger et al., 2002). The 100,000-g supernatant is defined as the cytosol fraction and the 100,000-g pellet as the membrane fraction. We also used cells exposed to ZL3VS (5 μM) or eeyarestatin I (8 μM) to prepare homogenates and subcellular fractionations. Fluorescence of EGFP-HC chimeric molecules was determined using an ISS KS multifrequency phase fluorimeter (ISS Fluorescence Analytical Instrumentation, Champaign, IL) at 490 nm (excitation) and 515 nm (emission).
Flow Cytometry
Flow cytometry of the green fluorescent reporter construct in living cells was performed by FACS (FACSCalibur; BD Biosciences, Mountain View, CA) and evaluated with CellQuest software (BD Biosciences). Mean fluorescence intensity correlates with the amount of cellular EGFP-HC.
Immunostaining and Confocal Microscopy
Immunofluorescence experiments were performed essentially as described (Fiebiger et al., 2002). Cells were allowed to attach to slides before incubation with various inhibitors over night: ZL3VS (Wiertz et al., 1996a) (5 μM), eeyarestatin I or II (8 μM). Dimethyl sulfoxide was used as solvent control. After fixation with 3.7% paraformaldehyde for 20 min at room temperature, immunohistochemistry was performed in a 0.5% saponin (wt/vol)/3% bovine serum albumin (wt/vol)/PBS solution as described previously (Fiebiger et al., 2002). The monoclonal antibody mAb W6/32 reacts with properly folded MHC class I and was used to define the localization of MHC class I molecules. Anti-mouse Alexa Fluor 568 (Molecular Probes, Eugene, OR) was used as the fluorescent secondary antibody. DNA staining was performed with 4,6-diamidino-2-phenylindole. Microscopy was performed on an MRC1024 confocal laser scanning microscope (Bio-Rad, Hercules, CA).
Immuno-electron Microscopy (EM)
Ultrathin cryosections were prepared and labeled according to the method of Tokuyasu (1973). In brief, cells were rinsed once PBS and removed from the dish with 0.5 mM EDTA in PBS. One milliliter of the cell suspension was layered on top of a 200-μl cushion of 4% paraformaldehyde (in 0.1 M Na-phosphate buffer, pH 7.4) and pelleted for 3 min at 3000 rpm. The supernatant was carefully removed and fresh 4% paraformaldehyde added. After 2-h fixation at room temperature, cell pellets were washed with PBS containing 0.2 M glycine to quench any free aldehyde groups from the fixative. Before freezing in liquid nitrogen, cell pellets were infiltrated with 2.3 M sucrose in PBS for 15 min. Frozen samples were sectioned at -120°C, the sections were transferred to Formvar-carbon–coated copper grids and floated on PBS until the immunogold labeling was carried out. The gold labeling of EGFP-HC was carried out at room temperature with an anti-GFP antiserum followed by 10-nm protein A-gold. Contrasting/embedding of the labeled grids was carried out on ice in 0.3% uranyl acetate in 2% methyl cellulose for 10 min. The grids were examined in a 1200EX transmission electron microscope (JEOL, Tokyo, Japan), and images were recorded at a primary magnification of 20–30,000×.
RESULTS
Identification of Chemical Compounds That Inhibit US11-mediated Degradation of EGFP-HC
In cells that express EGFP-HC and US11 (US11EGFP-HC), inhibition of the proteasome induces the accumulation of fluorescent chimeric molecules (Fiebiger et al., 2002). Regardless of the step in the pathway that is targeted, any unknown compound that interferes with US11-mediated heavy chain degradation should stabilize EGFP-HC and similarly increase fluorescence. Changes in fluorescence intensity can therefore be used as the readout in a high-throughput screen for compounds that might interfere with US11-mediated degradation.
We determined the optimal cell number to be 8000 US11EGFP-HC cells/well, as calibrated on cells exposed to ZL3VS (5 μM; Fiebiger et al., 2002), the reference substance for inhibition of proteasomal activity (our unpublished data). Incubation of cells with library compounds for 16 h was optimal for maintaining cell viability. In this setting, we screened in duplicate the 16,320 compounds of the Chembridge compound library. Figure 1A depicts the results obtained for a typical plate where four chemical compounds significantly increased the EGFP signal. In total, 40 of the 16,320 compounds of the library induced at least a twofold increase in EGFP fluorescence.
Figure 1.
High-throughput screen of a chemical compound library identified eeyarestatin I and II as inhibitors of US11-mediated degradation of EGFP-HC. (A) Representative fluorescent readout of a single plate of US11EGFP-HC cells treated with 320 compounds from the chemical library (see MATERIALS AND METHODS). The proteasome inhibitor ZL3VS was used as a reference substance. Inhibition of US11-mediated EGFP-HC degradation induces an increase in EGFP fluorescence. Compounds that induced at least a twofold increase in fluorescence signal were considered for further analysis as potential inhibitors. Chemical structure of the two inhibitors eeyarestatin I (B) and eeyarestatin II (C) are shown. The portion common to the two compounds is indicated in red.
Active compounds were reinvestigated by treatment of US11EGFP/HC, US2EGFP/HC, and U373 cells (control) with the relevant compounds for 16 h, followed by flow cytometry (our unpublished data). After this second round of screening, only two structurally related compounds, hereafter referred to as eeyarestatin I and II (EERST I and II), specifically induced EGFP fluorescence in US2EGFP-HC and US11EGFP-HC but not in control cells (our unpublished data). Signals obtained with the other compounds are likely attributable to autofluorescence of the substances. The structural similarity of the compounds eeyarestatin I and II (Figure 1, B and 1C) increases the likelihood that they inhibit a specific step in EGFP-HC degradation.
Treatment with the New Inhibitors Induces a Time-dependent Accumulation of EGFP-HC in the Presence of US11
We analyzed the induction of fluorescence in inhibitor-treated US11EGFP-HC cells. Cells were treated with eeyarestatin I (8 μM), eeyarestatin II (8 μM), or ZL3VS (5 μM) for up to 16 h and EGFP fluorescence was measured by flow cytometry (Figure 2A). In ZL3VS-treated samples (Figure 2A, open circles), a significant increase of fluorescence is observed as early as 4 h after treatment and continues to increase hereafter. In cells treated with eeyarestatin I (Figure 2A, closed squares) fluorescence accumulates more slowly, but after 16 h is comparable in intensity to that obtained with ZL3VS. Despite the modest induction of EGFP accumulation during shorter incubation times, eeyarestatin I is nonetheless a potent inhibitor of EGFP-HC degradation. Comparable results were obtained for US2EGFP-HC cells (our unpublished data).
Figure 2.
Inhibition of proteasomal degradation by eeyarestatin I and eeyarestatin II induces accumulation of properly folded, fluorescent EGFP-HC in US11EGFP-HC. (A) Flow cytometric quantitation of the induction of green reporter fluorescence. Cells were incubated with eeyarestatin I (EERSTI, 8 μM, closed diamonds), eeyarestatin II (EERSTII, 8 μM, open diamonds), or ZL3VS (5 μM, open circles) for the indicated time periods (ordinate). Mean fluorescence intensity was measured by FACS (abscissa). Results are representative of three experiments. (B) Eeyarestatin I does not prevent proper folding of class I MHC molecules. U373 cells untreated, treated with EERSTI or ZL3VS were pulse labeled with [35S]methionine for 15 min and chased for up to 30 min. Cells were lysed in NP-40 lysis buffer and immunoprecipitated with W6/32, a mAb that recognizes properly folded class I molecules only. The immunoprecipitates were analyzed by SDS-PAGE (12.5%). Properly folded glycosylated MHC class I complexes [HC(+)CHO] can be recovered regardless of inhibitor treatment.
The differences in kinetics of induction of fluorescence by ZL3VS and eeyarestatin I suggest that eeyarestatin I may require a metabolic conversion to be active, an issue that remains to be explored. Eeyarestatin II seems to be a weaker inhibitor than either ZL3VS or eeyarestatin I (Figure 2A). Because of the lesser potency of eeyarestatin II, we conducted all further experiments on eeyarestatin I.
Eeyarestatin I Does Not Impede the Assembly of Class I MHC Molecules in Control Cells
The class I MHC molecule is a dimeric complex comprised of a class I heavy chain and the soluble protein β2-microglubulin. Does eeyarestatin I prevent the assembly and folding of class I MHC molecules? If so, it might affect recognition by US2 (Gewurz et al., 2001b) or US11 and explain the observed fluorescence. U373 cells untreated or treated with ZL3VS or eeyarestatin I were metabolically labeled with [35S]methione for 15 min and chased for up to 30 min. Properly folded class I MHC molecules were recovered from NP-40 cell lysates by using W6/32, a mAb that recognizes properly folded class I molecules (Figure 2B). Properly folded class I molecules were recovered from lysates of untreated, ZL3VS-treated, and eeyarestatin I-treated U373 cells throughout the chase period. The increased recovery of class I molecules at the 30-min chase period confirms the continued assembly of class I molecules. Therefore, we conclude eeyarestatin I does not prevent membrane insertion via the Sec61 apparatus, folding or induces misfolding of newly synthesized glycoproteins in the ER.
Degradation of EGFP-HC and Endogenous Class I Heavy Chains Is Blocked by Eeyarestatin I
The effect of eeyarestatin I treatment on expression levels of MHC class I heavy chains in US2EGFP-HC and US11EGFP-HC cells was assessed by immunoblotting by using HC-10, a mAb that recognizes unfolded class I MHC heavy chain (Stam et al., 1986; Sernee et al., 1998). Low levels of class I heavy chains were present in lysates from untreated cells compared with cells treated with either inhibitor (Figure 3A). In agreement with earlier observations (Fiebiger et al., 2002), glycosylated [HC(+)CHO; Figure 3A] and deglycosylated class I heavy chain [HC(-)CHO; Figure 3A] are present in ZL3VS-treated cells. In contrast, only the glycosylated class I heavy chains accumulate in eeyarestatin I-treated cells (Figure 3A). The EGFP-HC behaves similarly to the endogenous class I MHC heavy chains (our unpublished data).
Figure 3.
Eeyarestatin I blocks the degradation of US2- and US11-induced class I heavy chains and EGFP-HC. (A) Steady-state levels of EGFP-HC in US2EGFP-HC and US11EGFP-HC cells. Immunoblots of SDS-lysates from equal numbers of untreated (lanes 1 and 4), EERSTI-(8 h, 8 μM; lanes 2 and 4), and ZL3VS-treated (8 h, 5 μM; lanes 3 and 6) cells (0.25 × 106) were performed with HC10. The glycosylated heavy chains [HC(+)CHO] and deglycosylated intermediates [HC(-)CHO] are indicated. An anti-PDI immunoblot was performed as a loading control. US11EGFP-HC cells were pulse labeled with [35S]methionine for 15 min and chased up to 60 min after various treatment conditions (untreated [lanes 1–3], EERSTI [4 h, 8 μM; lanes 4–6], ZL3VS [4 h, 10 μM; lanes 7–9]). Cells were lysed followed by immunoprecipitation with anti-GFP serum (B; lanes 1–9) or with anti-class I heavy chain serum (C; lanes 1–9). The immunoprecipitates were analyzed by SDS-PAGE (12.5%). The positions of migration of EGFP-HC, endogenous class I heavy chain [HC(+)CHO] and deglycosylated intermediate [HC(-)CHO] are indicated.
We do not suggest that the block in dislocation imposed by eeyarestatin I is necessarily complete. Consequently, MHC class I molecules that escape the eeyarestatin I block would be rapidly destroyed by proteasomal degradation. Thus, the amount of glycosylated HC that accumulates does not correspond to the extent of HC synthesis by these cells. Because US2 and US11 use subtle different means of dislocation, we also do not expect eeyarestatin I to affect the US11 and US2 pathways to the same extent (compare Figure 3A, lanes 2 and 5, with 3B, lanes 3 and 6).
Next, we compared the fate of newly synthesized heavy chains in the absence or presence of inhibitors in a pulse-chase experiment. EGFP-HC molecules were recovered from SDS-lysates with a polyclonal anti-GFP serum (Figure 3C). Endogenous class I heavy chains were immunoprecipitated with a polyclonal anticlass I heavy chain serum that recognizes unfolded class I molecules (Figure 3D). In the absence of inhibitors, we observed rapid loss of EGFP-HC (Figure 3C, lanes 1–3) and endogenous heavy chains (Figure 3D, lanes 1–3). In ZL3VS-treated cells, EGFP-HC is stabilized throughout the 60-min chase period (Figure 3C, lanes 4–12). Two populations of endogenous class I heavy chains are recovered from ZL3VS-treated cells, the glycosylated heavy chains [HC(+)CHO] and the deglycosylated intermediate [HC(-)CHO] (Figure 3D, lanes 4–6). The presence of the deglycosylated heavy chain is diagnostic for ER-to-cytosol dislocation.
In US11EGFP-HC cells treated with eeyarestatin I, heavy chain molecules are stabilized to a significant degree throughout the chase period (Figure 3, C and D). We did not recover deglycosylated class I heavy chains after eeyarestatin I treatment, in agreement with our immunoblotting data (Figure 3D). Thus, eeyarestatin I also inhibits US11-mediated degradation of newly synthesized class I MHC heavy chains. The lack of deglycosylated intermediates further suggests that eeyarestatin I interferes with a step required for dislocation before the heavy chain reaching the cytosol. The results obtained for eeyarestatin I in pulse-chase experiments were confirmed in US2EGFP-HC cells (our unpublished data).
We addressed the possibility that eeyarestatin I inhibits the N-glycanase responsible for the initial deglycosylation event. In vitro N-glycanase activity assays (Hirsch et al., 2003) demonstrate that eeyarestatin I does not prevent cleavage of N-linked glycans from TCRα (Hirsch and Ploegh, unpublished data). Therefore, we conclude that eeyarestatin I does not interfere with the deglycosylation of glycoproteins, but rather it must target a different step.
EGFP-HC and Endogenous Class I Heavy Chains Accumulate in the ER of Eeyarestatin I-treated Cells
To examine whether eeyarestatin I inhibits dislocation of class I MHC heavy chains from the ER, we examined the intracellular localization of EGFP-HC and endogenously expressed class I heavy chains. Localization of intracellular EGFP-HC was studied in US11EGFP-HC cells treated with ZL3VS or eeyarestatin I and analyzed by confocal laser scanning microscopy (Figure 4A). The cellular distribution of EGFP-HC is entirely different in ZL3VS- and eeyarestatin I-treated cells. In ZL3VS-treated cells, EGFP-HC accumulates in the ER lumen as well as the cytoplasm (our unpublished data; Fiebiger et al., 2002). In contrast, upon eeyarestatin I treatment EGFP-HC localizes in a distinct juxtanuclear area (Figure 4A, first panel). Eeyarestatin I-treated US11EGFP-HC cells were stained with W6/32, a mAb that recognizes properly folded MHC class I molecules (Figure 4A, third panel). EGFP-HC molecules (Figure 4A, second panel) accumulate mostly as properly folded, W6/32-reactive molecules. The W6/32-reactive forms of EGFP-HC most likely correspond to ER-resident heavy chains, because they reside at a location that is predicted to be the ER lumen (Figure 4A, second and fourth panel). Because the immunofluorescence images do not resolve the ambiguity of colocalization in a definitive manner, we performed fractionation experiments and immuno-EM to provide further evidence that the localization of the HC accumulated upon eeyarestatin I-treatment of cells and proteasomal inhibition are distinct.
Figure 4.
Intracellular distribution of EGFP-HC in ZL3VS-treated and eeyarestatin I-treated US2EGPF-HC and US11EGPF-HC cells. (A) Localization of EGFP-HC and endogenous class I molecules in US11EGFP/HC cells treated with EERSTI (16 h) was analyzed by confocal laser scanning microscopy (see MATERIALS AND METHODS). EGFP-HC molecules (all panels, green) accumulate in a distinct juxtanuclear region (first panel, nucleus labeled with 4,6-diamidino-2-phenylindole in blue). Properly folded class I molecules were detected using the mAb W6/32 (third and fourth panel, red). The merged image (fourth panel) of panel 2 and 3 shows a distinct pattern of localization for EGFP-HC and properly folded class I molecules. (B) Fluorometric quantitation of cytosolic fractions from US11EGFP-HC cells. Fluorescent signal (490-nm excitation;, 515-nm emission) was measured from cytosolic fractions (see MATERIALS AND METHODS) of US11EGFP-HC cells treated with ZL3VS (5 μM), eeyarestatin I (8 μM), and untreated (UT) U373 and US11EGFP-HC cells. The fluorescent signal was plotted as fluorescent units (abscissa). (C) US2EGFP/HC cells were pulse labeled with [35S]methionine for 15 min and chased up to 45 min in the absence or presence of inhibitors (EERSTI, 8 μM or ZL3VS, 5 μM). Cells from the 45-min chase period were homogenized and subjected to subcellular fractionation (MATERIALS AND METHODS). EGFP-HC and class I molecules were recovered from whole lysate, 100,000-g supernatant (100-kg sup), and 100,000-g pellet (100-kg pellet) by using an anticlass I heavy chain serum and analyzed by SDS-PAGE (12.5%). Positions of glycosylated EGFP-HC [EGFP-HC(+)CHO], glycosylated endogenous class I heavy chains [HC(+)CHO], deglycosylated EGFP-HC [EGFP-HC(-)CHO], and deglycosylated endogenous class I heavy chains [HC(-)CHO] are indicated.
The fluorescent properties of EGFP allow for quantitative assessment of the distribution of EGFP-HC molecules in subcellular fractions (Fiebiger et al., 2002). Untreated, ZL3VS- and eeyarestatin I-treated US11EGFP-HC and U373 control cells were homogenized and the cytosol was collected after high-speed centrifugation (Fiebiger et al., 2002). The cytosol from ZL3VS-treated cells contains fluorescent EGFP-HC intermediates (Fiebiger et al., 2002; Figure 4B), whereas the fluorescence signal in the cytosol of eeyarestatin I-treated cells is comparable with levels found in control cells that lack US11 (Figure 4B). The cytosolic fraction of eeyarestatin I-treated cells is therefore devoid of fluorescent EGFP-HC molecules.
Next, metabolically labeled eeyarestatin I-treated US2EGFP-HC cells were subjected to subcellular fractionation and immunoprecipitation (Figure 4C). Cells were treated with a solvent control, ZL3VS, or eeyarestatin I; metabolically labeled for 15 min; and chased for 45 min. Cells harvested at the 45-min chase point were homogenized and fractionated into a 100-kg supernatant (cytosol) and 100-kg pellet (microsomal pellet). EGFP-HC and endogenous class I heavy chains were precipitated from cell lysates or fractions with an anti-class I heavy chain serum. Glycosylated EGFP-HC and class I MHC heavy chain molecules were recovered exclusively from the membrane fraction of ZL3VS-treated cells (Figure 4C). The cytosol from ZL3VS-treated cells contained the deglycosylated class I MHC heavy chain intermediates (Figure 4C; Fiebiger et al., 2002). Eeyarestatin I-treated US2EGFP-HC cells contain no such intermediates in the cytosol. The glycosylated EGFP-HC and class I heavy chains are detected exclusively in the 100-kg membrane pellet obtained from eeyarestatin I-treated (Figure 4C).
To provide images at higher resolution, capable of distinguishing rigorously between cytoplasm and ER, we analyzed the distribution of EGFP-HC by EM, by using a polyclonal anti-GFP serum detected by protein A labeled with 10-nm gold particles (Figure 5). In untreated US11EGFP-HC cells, little background fluorescence was observed (Fiebiger et al., 2002) and accordingly barely any anti-GFP immunoreactive material was seen (our unpublished data; Figure 5B). In US11EGFP-HC cells treated with ZL3VS, we observed staining with the anti-GFP serum throughout the cytoplasm. These immuno-EM data confirm our biochemical observations (this study; Fiebiger et al., 2002). We have also observed earlier a similar cytoplasmic distribution of dislocated class I HC in US2 cells treated with proteasome inhibitors (Wiertz et al., 1996b). Gold particles were evenly distributed throughout the ZL3Vs-treated cells (Figure 5, left). As expected, gold particles were also observed in apposition with intracellular membranes, for the most parts the staining was confined to the cytoplasmic matrix. In striking contrast, in cells treated with eeyarestatin I (Figure 5, right), we observed little, if any, cytoplasmic staining and the majority of gold particles was found in proximity of intracellular membranes.
Figure 5.
Localization of EGFP-HC in US11EGFP-HC cells by immuno-electron microscopy. (A) US11EGFP-HC cells treated with EERSTI (8 μM) or ZL3VS (5 μM) for 8 h were fixed and labeled with anti-GFP serum followed by protein A conjugated to 10-nm gold. Left, ZL3VS-treated cells; right, EERSTI-treated cells. N, nucleus. (B) Higher magnification of A (see size bars). The arrow heads indicate gold particles not associated with membranes.
In conclusion, eeyarestatin I inhibits US2- and US11-mediated class I degradation by preventing the dislocation of the class I MHC molecules from the ER to the cytosol.
Eeyarestatin I Inhibits Proteasomal Degradation of Glycosylated TCRα Chains in the Cytosol
Can eeyarestatin I inhibit degradation of other misfolded ER-resident proteins? We studied the degradation of a substrate that has served as a paradigm for this pathway: the α-subunit of the TCRα (Bonifacino et al., 1991; Huppa and Ploegh, 1997). TCRα protein is degraded in a proteasome-dependent manner when expressed in the absence of the TCRβ and the CD3 subunits required for the formation of a functional TCR. HEK-293 cells were transiently cotransfected with constructs encoding wild-type TCRα and a cytosolic version of the protein lacking its signal sequence. The signal sequence-less TCRα was included to yield a form of TCRα that is obligatorily cytoplasmic and more rapidly degraded than membrane anchored TCRα (Figure 6A). Stability of both soluble and membrane inserted TCRα was analyzed in pulse-chase experiments in untreated (Figure 6A), ZL3VS-(Figure 6B), or eeyarestatin I-treated (Figure 6B) transfectants. After 16 h of treatment, transfectants were metabolically labeled for 15 min and chased up to 2 h. TCRα was recovered from lysates by immunoprecipitation (Huppa and Ploegh, 1997). In the absence of inhibitors, we observe the rapid loss of both forms of the fully glycosylated TCRα and the cytosolic, nonglycosylated TCRα during the chase period (Figure 6A). Treatment with ZL3VS stabilizes full-length and cytosolic TCRα, demonstrating that both forms are degraded by the proteasome (Figure 6B). Eeyarestatin I selectively inhibits the degradation of full length TCRα throughout the chase (Figure 6C), whereas the cytosolic TCRα disappears with kinetics comparable with that observed in untreated cells (Figure 6C). The slight loss in molecular weight of the ER form of TCRα reflects the occurrence of mannose trimming of the protein (Figure 6C), a process unaffected by eeyarestatin I. These results show that eeyarestatin I inhibits the ER-to-cytosol degradation pathway of several type I membrane proteins. Importantly, these data confirm that degradation of proteins in the ER is impaired by inclusion of eeyarestatin I, without affecting the turnover of proteins in the cytosol.
Figure 6.
Eeyarestatin I blocks the degradation of ER-resident TCRα but not cytosolic TCRα. Wild-type TCRα and a cytosolic TCRα were transiently cotransfected into HEK-293 cells. Stability of both proteins was analyzed in pulse-chase experiments in untreated (A), ZL3VS-(B), or EERSTI-treated (C) transfectants. After 16 h of treatment, cells were metabolically labeled for 15 min and chased up to 180 min. TCRα was recovered from cell lysates using anti-TCRα serum. The immunoprecipitates were analyzed by SDS-PAGE (12.5%). The autoradiograms were quantified and percentage of modified TCRα (open squares) and cytosolic TCRα (closed squares) were plotted (abscissa) against the chase time points (ordinate). The respective graphs represent the degradation kinetics of ER-resident TCRα (open squares) and cytosolic TCRα (closed squares).
Eeyarestatin I Does Not Block the Degradation of an N-End Rule Ubiquitin/Proteasome Substrate or Interfere with Catalytic Activity of the Proteasome
The N-end rule links the stability of a protein to the identity of its N terminus. Polypeptides equipped with stabilizing amino acids such as methionine are less likely to be degraded by the proteasome than proteins that carry a destabilizing bulky or hydrophobic amino acid at their N terminus. We examined whether eeyarestatin I can inhibit the degradation of a cytosolic protein that contains a destabilizing amino acid at its N terminus. To that end we used a chimeric molecule comprised of ubiquitin fused to the N terminus of GFP (Ub-XGFP) in which an arginine (Ub-RGFP) or methionine (Ub-MGFP) residue has been engineered at its N terminus (Dantuma et al., 2000). When Ub-XGFP is expressed in the cytosol, ubiquitin is cleaved from the fusion molecule by an ubiquitin-specific protease, generating a GFP molecule that contains either an arginine (destabilizing) or a methionine (stabilizing) residue at its N terminus. U373 cells that stably express Ub-RGFP were treated with eeyarestatin I and ZL3VS for 16 h. Cell lysates were subjected to SDS-PAGE and anti-GFP immunoblotting (Figure 7A). In eeyarestatin I-treated cells, a minimal amount of GFP was detected from cells that express Ub-RGFP compared with cells treated with the proteasome inhibitor ZL3VS or with cells that express Ub-MGFP (Figure 7A). These results demonstrate that eeyarestatin I does not inhibit degradation of N-end rule substrates and is therefore unlikely to interfere with proteasomal activity more generally.
Figure 7.
Further characterization of the inhibitory properties of Eeyarestatin I. (A) EERSTI does not block the degradation of an N-end rule substrate. U373 cells expressing an unstable GFP molecule, RGFP, were treated with a solvent control (ut, lane 2), EERSTI (lane 3), or ZL3VS (lane 4) for 16 h. Lysates were subjected to SDS-PAGE (12%) and analyzed by anti-GFP immunoblotting. Lysates from cells that express a stable version of GFP, MGFP, were used as a control (lane 1). An anti-PDI immunoblot was performed as a loading control. (B) EERSTI does not modify the enzymatic subunits of the proteasome. Lysates from untreated (ut, lane 1), ZL3VS-treated (lane 2), or EERSTI-treated (lane 3) US11EFGP-HC cells were incubated with AdaL3VSbiot (10 μM) for 1 h at 37°C. Binding of AdaL3VSbiot to the active proteasome subunits (β1, β2, and β5) was visualized after SDS-PAGE and immunoblotting with streptavidin-HRP. (C) EERSTI does not interfere with the activity of lysosomal proteases. pH 4.5 lysates from untreated (ut, lane 1), LHVS-treated (50 μM, lane 2), or EERSTI-treated (lane 3) were incubated with DCG-04 for 1 h at 37°C. Binding of DCG-04 to active cysteine proteases was visualized after SDS-PAGE and immunoblotting with streptavidin-HRP. In contrast to active site inhibitor LHVS, EERSTI did not impair the DCG-04 labeling of active cathepsin B (Cat B).
We directly addressed the question whether eeyarestatin I modifies the active sites of the proteasome. Cell lysates from untreated, ZL3VS- and eeyarestatin I-treated US11EFGP-HC were prepared as described previously (Kessler et al., 2001) and incubated with the proteasome inhibitor AdaL3VSbiot (10 μM) for 1 h at 37°C (Kessler et al., 2001). Inclusion of the compound AdaL3VSbiot results in modification of residual active proteasomal β subunits (Kessler et al., 2001). If proteasome inhibition occurs, residual labeling with AdaL3VSbiot is reduced. Binding of AdaL3VSbiot to the active proteasome subunits was visualized after SDS-PAGE and immunoblotting with streptavidin-horseradish peroxidase (HRP). As expected, proteasomes from cells pretreated with ZL3VS could not be modified subsequently by AdaL3VSbiot (Figure 7B), because all their active sites had been covalently modified. Preincubation with eeyarestatin I did not affect the labeling pattern obtained with AdaL3VSbiot (Figure 7B). These data confirm that eeyarestatin I is not a proteasome inhibitor.
Interference of eeyarestatin I with the activity of lysosomal proteases was analyzed by comparing its ability to interfere with active cysteine protease labeling, by using the active site directed probe DCG-04 (Lennon-Dumenil et al., 2002). As opposed to LHVS (50 μM), a cysteine protease inhibitor (Fiebiger et al., 2001), eeyarestatin I did not inhibit detection of the lysosomal cysteine protease cathepsin B (Figure 7C). This experiment further shows that eeyarestatin I acts unlike alkylating agents and does not interfere with cellular pH or redox potential.
DISCUSSION
Protein degradation is an essential part of normal cellular physiology (Lee and Goldberg, 1998). Indeed, the failure to destroy aberrantly folded proteins that accumulate in the ER may lead to disease (Aridor and Hannan, 2000, 2002). The normal pathway of quality control includes the removal of such misfolded proteins from the ER to the cytosol, and subsequently its destruction. Several proteins implicated in disease are believed to be retained in the ER and destroyed because of relatively minor defects in folding. The ER-to-cytosol degradation pathway is therefore an attractive target for therapeutic intervention. Interference with this process requires a detailed knowledge of the identity and regulation of the key players in this process. So far, the proteasome is the only structure in this pathway that can be targeted specifically with inhibitors (Kisselev and Goldberg, 2001).
Some viral strategies to avoid immune detection, like the US2- or US11-mediated degradation of class I MHC heavy chains, follow the principal steps of ER-to-cytosol transport and degradation of misfolded proteins (Tortorella et al., 2000). We exploited US11-mediated degradation of fluorescent-tagged heavy chains in a high throughput screen to identify specific inhibitors of glycoprotein turnover. We identified two structurally related compounds (eeyarestatin I and II) that inhibited degradation of EGFP-HC, of which eeyarestatin I was the more potent. Eeyarestatin I inhibited degradation of both wild-type class I MHC heavy chain and TCRα.
The first step in the dislocation process must involve recognition of the substrate and its successful discrimination from properly folded molecules. We have argued that viral proteins such as US2 direct otherwise perfectly folded class I MHC molecules to the cytoplasm for degradation (Gewurz et al., 2001a) and may thus use a common and highly specific mode of substrate recognition. After recognition of the substrate, subsequent steps must involve its recruitment to the channel via which expulsion from the ER occurs, a step that may be preceded by chaperone-catalyzed unfolding, although complete unfolding may not be a prerequisite (Fiebiger et al., 2002; Tirosh et al., 2003). The movement of a type I membrane protein from the ER to the cytoplasm is believed to require a translocation channel such as Sec 61, although other as yet to be identified protein complexes may be involved in this stage as well. Cytoplasmic exposure of portions of the dislocation substrate allows ubiquitin conjugation, whereas the substrate remains membrane attached, followed finally by extraction from the ER itself. The cytoplasmic exposure of substrates to be destroyed may be accomplished by (partial) transport of the substrates through a channel or by discontinuities of the ER itself. The Cdc48/p97complex may be crucial in the extraction of substrates from the ER and prepare them for proteasomal degradation (Hiller et al., 1996; Ye et al., 2001; Bays and Hampton, 2002; Jarosch et al., 2002; Rabinovich et al., 2002).
Accumulation of misfolded proteins in neurodegenerative disorders is accompanied by the formation of characteristic cellular deposits, called aggresomes (Kopito, 2000). The formation of such aggresomes upon treatment with an inhibitor could pose a major obstacle for potential in vivo application of such compounds. We observed that the EGFP-HC that accumulates upon treatment with eeyarestatin I does not form classical aggresomes (Garcia-Mata et al., 1999) (our unpublished data). Although vimentin filaments show a tendency to collapse around accumulating EGFP-HC after long periods of eeyarestatin I treatment, class I MHC heavy chains retain their properly folded W6/32 reactive state and remain readily extractable with NP-40 (our unpublished data).
The juxtanuclear location of the accumulated EGFP-HC suggests that the ER may be compartmentalized upon treatment with eeyarestatin I, with a particular ER substructure as a receptacle for these protein deposits. Our observation that the degradation of not only EGFP-HC, but also that of TCRα is affected by eeyarestatin I excludes the class I MHC/US11 interaction as its immediate target.
What are the likely targets of eeyarestatin I located in the ER? The activity of mannosidase I does not seem to be impaired, as inferred from the reduction of MW seen in TCRα, a reaction diagnostic of N-linked glycan processing (Figure 7). The formation of W6/32 reactive class I MHC molecules in control cells is not affected by eeyarestatin I, indicating that neither folding nor assembly of a multisub-unit protein in the ER is inhibited by eeyarestatin I (Figure 2B). This result distinguishes the action of eeyarestatin I from that of alkylating agents such as N-ethylmaleimide, or of agents that affect redox potential, such as diamide, all of which impair folding and assembly reactions.
We established that eeyarestatin I affects neither proteasomal proteolysis, nor the activity of lysosomal cysteine proteases. The latter observation again distinguishes eeyarestatin I from alkylating agents. When we examined the ability of eeyarestatin I to interfere with ubiquitin conjugation, as assessed by analysis of cells transfected with hemagglutinin-ubiquitin and exposed to eeyarestatin I, we saw no differences between eeyarestatin I-treated cells and controls, suggesting that ubiquitin conjugation is most likely not the target of eeyarestatin I, although this remains to be confirmed for bona fide ER-resident dislocation substrates.
These data leave the following possibilities for the identity of eeyarestatin I's targets: the steps involved in substrate recognition, recruitment to the dislocation channel or (partial) unfolding in the course of dislocation.
The unfolding requirement for dislocation remains to be settled unambiguously. For proteasomal destruction, substrates must be unfolded. We have no evidence for the existence of folded structures in the cytoplasm with the exception of the fusion partners used to generate the reporter constructs. Indeed, both EGFP and DHFR (Fiebiger et al., 2002; Tirosh et al., 2003), when fused to the class I MHC heavy chain, retain their folded characteristics upon arrival in the cytoplasm, or refold with kinetics too fast to allow resolution of partially (un)folded intermediates (Fiebiger et al., 2002; Tirosh et al., 2003). Proteasome inhibitors cause accumulation of the deglycosylated, at least partially folded, dislocation substrates in the cytosol. In cells treated with eeyarestatin I, fluorescence microscopy, immuno-EM, and subcellular fractionation all show that EGFP-HC accumulates predominantly in a defined area close to the nucleus and that the cytosol is devoid of EGFP-HC. Immunoprecipitations of subcellular fractions from eeyarestatin I-treated cells confirm that fully glycosylated heavy chains accumulate exclusively inside the ER. We conclude that EGFP-HC cannot be efficiently dislocated from the ER into the cytosol by US2 or US11 in eeyarestatin I-treated cells and is protected from degradation. Similar results were obtained when we examined the effect of eeyarestatin I on the degradation of TCRα.
Synthesis of derivatives of eeyarestatin I will now be required to generate compounds that can be cross-linked to its target or that can be used as a ligand to identify the target of eeyarestatin I. Regardless of its identity, the application of eeyarestatin I has allowed us to distinguish a new intermediate in the dislocation of type I membrane proteins.
Acknowledgments
During this study, Edda Fiebiger was supported by an APART (the Austrian Program for Advanced Research and Technology) Fellowship of the Austrian Academy of Sciences. Christian Hirsch is a fellow of the Boehringer Ingelheim Fonds, Germany. This study was supported by National Institutes of Health Grant 5R37-AI33456.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–07–0506. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–07–0506.
References
- Aridor, M., and Hannan, L.A. (2000). Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic 1, 836-851. [DOI] [PubMed] [Google Scholar]
- Aridor, M., and Hannan, L.A. (2002). Traffic jams II: an update of diseases of intracellular transport. Traffic 3, 781-790. [DOI] [PubMed] [Google Scholar]
- Bays, N.W., and Hampton, R.Y. (2002). Cdc48-Ufd1-Npl 4, stuck in the middle with Ub. Curr. Biol. 12, R366-R371. [DOI] [PubMed] [Google Scholar]
- Bays, N.W., Wilhovsky, S.K., Goradia, A., Hodgkiss-Harlow, K., and Hampton, R.Y. (2001). HRD4/NPL4 is required for the proteasomal processing of ubiquitinated ER proteins. Mol. Biol. Cell 12, 4114-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer, T., Volkwein, C., and Sommer, T. (1997). Role of Cue1p in ubiquitination and degradation at the ER surface. Science 278, 1806-1809. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., Cosson, P., Shah, N., and Klausner, R.D. (1991). Role of potentially charged transmembrane residues in targeting proteins for retention and degradation within the endoplasmic reticulum. EMBO J. 10, 2783-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J.S., and Weissman, A.M. (1998). Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu. Rev. Cell Dev. Biol. 14, 19-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordallo, J., Plemper, R.K., Finger, A., and Wolf, D.H. (1998). Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Mol. Biol. Cell 9, 209-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantuma, N.P., Lindsten, K., Glas, R., Jellne, M., and Masucci, M.G. (2000). Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nat. Biotechnol. 18, 538-543. [DOI] [PubMed] [Google Scholar]
- Fiebiger, E., Meraner, P., Weber, E., Fang, I.F., Stingl, G., Ploegh, H., and Maurer, D. (2001). Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells. J. Exp. Med. 193, 881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebiger, E., Story, C., Ploegh, H.L., and Tortorella, D. (2002). Visualization of the ER-to-cytosol dislocation reaction of a type I membrane protein. EMBO J. 21, 1041-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand, A.R., Cuozzo, J.W., and Kaiser, C.A. (2000). Pathways for protein disulphide bond formation. Trends Cell Biol. 10, 203-210. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata, R., Bebok, Z., Sorscher, E.J., and Sztul, E.S. (1999). Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J. Cell Biol. 146, 1239-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz, B.E., Gaudet, R., Tortorella, D., Wang, E.W., Ploegh, H.L., and Wiley, D.C. (2001a). Antigen presentation subverted: structure of the human cytomegalovirus protein US2 bound to the class I molecule HLA-A2. Proc. Natl. Acad. Sci. USA 98, 6794-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz, B.E., Wang, E.W., Tortorella, D., Schust, D.J., and Ploegh, H.L. (2001b). Human cytomegalovirus US2 endoplasmic reticulum-lumenal domain dictates association with major histocompatibility complex class I in a locus-specific manner. J. Virol. 75, 5197-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton, R.Y., Gardner, R.G., and Rine, J. (1996). Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Mol. Biol. Cell 7, 2029-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High, S., Lecomte, F.J., Russell, S.J., Abell, B.M., and Oliver, J.D. (2000). Glycoprotein folding in the endoplasmic reticulum: a tale of three chaperones?. FEBS Lett. 476, 38-41. [DOI] [PubMed] [Google Scholar]
- Hiller, M.M., Finger, A., Schweiger, M., and Wolf, D.H. (1996). ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science 273, 1725-1728. [DOI] [PubMed] [Google Scholar]
- Hirsch, C., Blom, D., and Ploegh, H.L. (2003). A role for N-glycanase in the cytosolic turnover of glycoproteins. EMBO J. 22, 1036-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, C., and Ploegh, H.L. (2000). Intracellular targeting of the proteasome. Trends Cell Biol. 10, 268-272. [DOI] [PubMed] [Google Scholar]
- Huppa, J.B., and Ploegh, H.L. (1997). In vitro translation and assembly of a complete T cell receptor-CD3 complex. J. Exp. Med. 186, 393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosch, E., Taxis, C., Volkwein, C., Bordallo, J., Finley, D., Wolf, D.H., and Sommer, T. (2002). Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat. Cell Biol. 4, 134-139. [DOI] [PubMed] [Google Scholar]
- Kessler, B.M., Tortorella, D., Altun, M., Kisselev, A.F., Fiebiger, E., Hekking, B.G., Ploegh, H.L., and Overkleeft, H.S. (2001). Extended peptide-based inhibitors efficiently target the proteasome and reveal overlapping specificities of the catalytic beta-subunits. Chem. Biol. 8, 913-929. [DOI] [PubMed] [Google Scholar]
- Kisselev, A.F., and Goldberg, A.L. (2001). Proteasome inhibitors: from research tools to drug candidates. Chem. Biol. 8, 739-758. [DOI] [PubMed] [Google Scholar]
- Knop, M., Finger, A., Braun, T., Hellmuth, K., and Wolf, D.H. (1996). Der1, a novel protein specifically required for endoplasmic reticulum degradation in yeast. EMBO J. 15, 753-763. [PMC free article] [PubMed] [Google Scholar]
- Kopito, R.R. (2000). Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 10, 524-530. [DOI] [PubMed] [Google Scholar]
- Kuruvilla, F.G., Shamji, A.F., Sternson, S.M., Hergenrother, P.J., and Schreiber, S.L. (2002). Dissecting glucose signalling with diversity-oriented synthesis and small-molecule microarrays. Nature 416, 653-657. [DOI] [PubMed] [Google Scholar]
- Lee, D.H., and Goldberg, A.L. (1998). Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 8, 397-403. [DOI] [PubMed] [Google Scholar]
- Lennon-Dumenil, A.M., Bakker, A.H., Maehr, R., Fiebiger, E., Overkleeft, H.S., Rosemblatt, M., Ploegh, H.L., and Lagaudriere-Gesbert, C. (2002). Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J. Exp. Med. 196, 529-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham, P., Barnstable, C.J., and Bodmer, W.F. (1979). Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J. Immunol. 123, 342-349. [PubMed] [Google Scholar]
- Ploegh, H.L. (1995). Current Protocols in Protein Science, New York: John Wiley & Sons.
- Rabinovich, E., Kerem, A., Frohlich, K.U., Diamant, N., and Bar-Nun, S. (2002). AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol. Cell. Biol. 22, 626-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm, A., Stern, P., Ploegh, H.L., and Tortorella, D. (2001). Signal peptide cleavage of a type I membrane protein, HCMV US11, is dependent on its membrane anchor. EMBO J. 20, 1573-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert, U., Anton, L.C., Gibbs, J., Norbury, C.C., Yewdell, J.W., and Bennink, J.R. (2000). Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404, 770-774. [DOI] [PubMed] [Google Scholar]
- Sernee, M.F., Ploegh, H.L., and Schust, D.J. (1998). Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol. Immunol. 35, 177-188. [DOI] [PubMed] [Google Scholar]
- Shamu, C.E., Flierman, D., Ploegh, H.L., Rapoport, T.A., and Chau, V. (2001). Polyubiquitination is required for US11-dependent movement of MHC class I heavy chain from endoplasmic reticulum into cytosol. Mol. Biol. Cell 12, 2546-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam, N.J., Spits, H., and Ploegh, H.L. (1986). Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J. Immunol. 137, 2299-2306. [PubMed] [Google Scholar]
- Tirosh, B., Furman, M.H., Tortorella, D., and Ploegh, H.L. (2003). Protein unfolding is not a prerequisite for endoplasmic reticulum-to-cytosol dislocation. J. Biol. Chem. 278, 6664-6672. [DOI] [PubMed] [Google Scholar]
- Tiwari, S., and Weissman, A.M. (2001). Endoplasmic reticulum (ER)-associated degradation of T cell receptor subunits. Involvement of ER-associated ubiquitin-conjugating enzymes (E2s). J. Biol. Chem. 276, 16193-16200. [DOI] [PubMed] [Google Scholar]
- Tokunaga, F., Brostrom, C., Koide, T., and Arvan, P. (2000). Endoplasmic reticulum (ER)-associated degradation of misfolded N-linked glycoproteins is suppressed upon inhibition of ER mannosidase I. J. Biol. Chem. 275, 40757-40764. [DOI] [PubMed] [Google Scholar]
- Tokuyasu, K.T. (1973). A technique for ultracryotomy of cell suspensions and tissues. J. Cell Biol. 57, 551-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella, D., Gewurz, B.E., Furman, M.H., Schust, D.J., and Ploegh, H.L. (2000). Viral subversion of the immune system. Annu. Rev. Immunol. 18, 861-926. [DOI] [PubMed] [Google Scholar]
- Tortorella, D., Story, C.M., Huppa, J.B., Wiertz, E.J., Jones, T.R., Bacik, I., Bennink, J.R., Yewdell, J.W., and Ploegh, H.L. (1998). Dislocation of type I membrane proteins from the ER to the cytosol is sensitive to changes in redox potential. J. Cell Biol. 142, 365-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiertz, E.J., Jones, T.R., Sun, L., Bogyo, M., Geuze, H.J., and Ploegh, H.L. (1996a). The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84, 769-779. [DOI] [PubMed] [Google Scholar]
- Wiertz, E.J., Tortorella, D., Bogyo, M., Yu, J., Mothes, W., Jones, T.R., Rapoport, T.A., and Ploegh, H.L. (1996b). Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384, 432-438. [DOI] [PubMed] [Google Scholar]
- Ye, Y., Meyer, H.H., and Rapoport, T.A. (2001). The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414, 652-656. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Nijbroek, G., Sullivan, M.L., McCracken, A.A., Watkins, S.C., Michaelis, S., and Brodsky, J.L. (2001). Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol. Biol. Cell 12, 1303-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]