Table 4.
Amination of 2-halopyridines.[a]
| Entry | Amine | Product (yield [%]) for the reaction with | |||
|---|---|---|---|---|---|
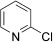 |
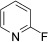 |
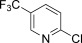 |
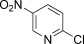 |
||
| 1 | 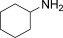 |
(<5) | (6) | 37 (60) | 43 (96) |
| 2 | 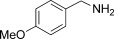 |
(<5) | no reaction | 38 (65) | 44 (70) |
| 3 | 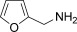 |
(<5) | no reaction | 39 (48; MW 59)[b] | 45 (73) |
| 4 | 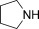 |
34 (<5) | 34 (54) | 40 (53) | 46 (74) |
| 5 |  |
35 (9; MW 25)[b] | 35 (16) | 41 (36) | 47 (87) |
| 6 |  |
36 (21)[c] | 36 (46)[c] | 42 (85) | 48 (76) |
| 7 |  |
no reaction | no reaction | no reaction | 49 (73) |
All reactions were performed with aryl halide (1 equiv.), amine (1 equiv.) and KF (2 equiv.) in water at 100 °C for 17 h.
MW refers to the yield if the reaction was performed in a microwave reactor (300 W) at 175 °C for 1 h (2 h in the case of furfurylamine) with KF (1 equiv.).
Yield reported as an NMR yield calculated from the internal standard using 1,4-dioxane.
